Abstract
Purpose
The objectives of this research were to assess the biocompatibility of self-assembled Fe3O4 magnetic nanoparticles (MNPs) loaded with daunorubicin (DNR), ie, (Fe3O4-MNPs/DNR), and to explore their potential application in the treatment of hematologic malignancies.
Methods
A hemolysis test was carried out to estimate the hematologic toxicity of Fe3O4- MNPs/DNR and a micronucleus assay was undertaken to identify its genotoxicity. Fe3O4-MNPs/ DNR were injected intraperitoneally into mice to calculate the median lethal dose (LD50). The general condition of the mice was recorded, along with testing for acute toxicity to the liver and kidneys.
Results
Hemolysis rates were 2.908%, 2.530%, and 2.415% after treatment with different concentrations of Fe3O4-MNPs/DNR. In the micronucleus assay, there was no significant difference in micronucleus formation rate between the experimental Fe3O4-MNPs/DNR groups and negative controls (P > 0.05), but there was a significant difference between the experimental groups and the positive controls (P < 0.05). The LD50 of the Fe3O4-MNPs/DNR was 1009.71 mg/kg and the 95% confidence interval (CI) was 769.11–1262.40 mg/kg, while that of the DNR groups was 8.51 mg/kg (95% CI: 6.48–10.37 mg/kg), suggesting that these nanoparticles have a wide safety margin. Acute toxicity testing showed no significant difference in body weight between the treatment groups at 24, 48, and 72 hours after intraperitoneal injection. The mice were all in good condition, with normal consumption of water and food, and their stools were formed and yellowish-brown. Interestingly, no toxic reactions, including instability of gait, convulsion, paralysis, and respiratory depression, were observed. Furthermore, alanine transaminase, blood urea nitrogen, and creatinine clearance in the experimental Fe3O4-MNPs/ DNR groups were 66.0 ± 28.55 U/L, 9.06 ± 1.05 mmol/L, and 18.03 ± 1.84 μmol/L, respectively, which was not significantly different compared with the control and isodose DNR groups.
Conclusion
Self-assembled Fe3O4-MNPs/DNR appear to be highly biocompatible and safe nanoparticles, and may be suitable for further application in the treatment of hematologic malignancies.
Introduction
Magnetic nanoparticles (MNPs) have been investigated for various biomedical applications, including targeted therapy,Citation1,Citation2 magnetic hyperthermia,Citation3 and contrast enhancement in magnetic resonance imaging.Citation4 The ability to transport anticancer drugs via MNPs to specific parts of the body reduces the side effects of chemotherapy. Our earlier research has shown that magnetic Fe3O4 nanoparticles (Fe3O4-MNPs) combined with daunorubicin (DNR) and 5-bromotetrandrine are able to overcome multidrug resistance in hematologic malignancies, and achieve a satisfactory therapeutic effect, without histologic toxicity to nontarget organs or tissues.Citation5–Citation7 On the other hand, simultaneous use of hyperthermia and low-doses of chemotherapeutic agents decreases tumor growth through targeted cytotoxicity and reduces systemic side effects.Citation8 Therefore, MNPs are expected to enhance therapeutic effects and to reduce side effects when used in combination with conventional cancer treatment. On this basis, we prepared Fe3O4-MNPs loaded with DNR (Fe3O4-MNPs/DNR) as a novel therapeutic agent using chemical coprecipitation and emulsification under ultrasound guidance. It is essential to determine the biocompatibility for in vivo biomedical applications to ensure their safe clinical use,Citation9 and the constructed nanoparticles must have low toxicity and little innate bioreactivity. In this research, we evaluated the biocompatibility and stability of self-assembled Fe3O4-MNPs/DNR by hemolysis testing, micronucleus assay, and detection of median lethal dose (LD50).
Materials and methods
Experimental agents
Ferric chloride, ferrous sulfate, and ammonia water were obtained from Hanguang Chemical Reagent Co, Ltd (Shanghai, China). Albumin was prepared from human plasma obtained from Shanghai RAAS Blood Products Co Ltd (Shanghai, China). Other reagents used included daunorubicin hydrochloride (Pharmacia Italia SpA, Milan, Italy), potassium oxalate (analytical grade; Shanghai First Reagent Factory, Shanghai, China), cyclophosphamide (Jiangsu Hengrui Medicine Co Ltd., Lianyungang, China) methanol (Sinopharm Chemical Reagent Co Ltd, Shanghai, China), Giemsa staining solution (Chroma, Bellows Falls, VT), 4,6-diamidino-2-phenylindole (DAPI) staining solution (Beyotime Institute of Biotechnology, Jiangsu, China), and a T6 ultraviolet-visible spectrophotometer (Beijing Purkinje General Instrument Co Ltd, Beijing, China).
Experimental animals
Kunming mice, age-matched (six weeks of age) and weightmatched (18–22 g), were purchased from the Shanghai National Center for Laboratory Animals. They were maintained in clean facilities. After matching for gender, the mice were randomly assigned to treatment groups for experimental purposes.
Preparation of Fe3O4-MNPs-DNR
Fe3O4-MNPs were prepared by chemical coprecipitation,Citation10 and then Fe3O4-MNPs, DNR, and albumin were mixed in certain proportions with emulsification under ultrasonic guidance.Citation11 After formation of the compound nanoparticles containing the DNR and magnetic materials, solidifying and drying through evaporation, the nanoparticles were preserved at 4°C. The materials were misced bene for 30 minutes in the presence of ultrasound. The effective drug-loading rate was 1%, as calculated by high-performance liquid chromatography and using a fluorescence detector.
Hemolysis test
Blood was obtained from healthy rabbits and anticoagulated with potassium oxalate, at a final concentration of 1.0 mg/mL of blood. The Fe3O4-MNPs/DNR were scrubbed with distilled water twice, lixiviated with saline, with a final concentration reached of 100 mg/mL. Saline was used as the negative control and distilled water was used as the positive control. The materials detected were divided into three concentration groups, ie, 100, 50, and 25 mg/mL. Each group had three test tubes, each tube containing 10 mL leaching liquor of materials, saline, or distilled water, and preheated for 30 minutes at 37°C. Then, 0.2 mL of diluted anticoagulated blood was added to each tube. After incubation for 60 minutes at 37°C, the process of hemolysis was observed macroscopically, the tubes were centrifugated at 2500 rpm for five minutes, the supernatant fluid was assembled, and optical density (OD) values were determined at 545 nm using ultraviolet-visible spectrophotometry. The hemolysis rate was calculated using the mean OD value for each group as follows: hemolysis rate (%) = (mean of compound nanoparticles group – mean of negative control group)/(mean of positive control group – mean of negative control group) × 100%. If the hemolysis rate was less than 5%, the material would have no hemolytic reaction, which fits the requirements of a hemolysis test.
Micronucleus assay
Experimental mice were randomly assigned to 10 groups; the Fe3O4-MNPs/DNR groups were divided into 1.25, 2.50, 3.75, and 5.00 g/kg, the isodose DNR groups calculated using the effective drug-loading rate were set, the positive control group was injected intraperitoneally with cyclophosphamide 100 mg/kg, and the negative control group was injected with an equivalent volume of saline. All experimental animals were given the test materials twice with an interval between of 24 hours. After the second administration of the experimental treatments, the animals were sacrificed.Citation12,Citation13 Bone marrow slides were prepared, immobilized with methanol for 15 minutes, and stained with Giemsa or DAPI for 15 minutes. At least 1000 polychromatic erythrocytes were counted for each mouse, and the rate of formation of polychromatic erythrocytes in the micronucleus was calculated. Statistically significant differences were detected by Poisson distribution.
Determination of LD50
Experimental mice were randomly assigned to treatment groups containing 10 mice per group. The Fe3O4-MNPs/DNR suspension was injected intraperitoneally, with different dosing groups set at 0.1, 0.5, 0.9, 1.2, 1.6, and 2.0 g/kg. Isodose DNR control groups were determined by calculating the effective drug-loading rate. Saline was used as the negative control. All experimental animals were observed continuously for 15 days. Deaths of mice in each group were recorded, and the LD50 of the compound material and DNR was calculated by the Bliss method.
Acute toxicity test
Experimental mice were assigned randomly to three groups, with each group containing six mice. The mice allocated to receive Fe3O4-MNPs/DNR were injected intraperitoneally at a dose of 100 mg/kg (according to the therapeutic dose of DNR), whereas the control group was administered isodose DNR calculated by the effective drug-loading rate. The negative control group was given an equivalent volume of saline. Change in body weight for the mice was recorded at 24, 48, and 72 hours. General physical state, including respiration, eating and movement, was recorded, and adverse reactions were monitored over 14 days,Citation14 after which serum alanine transaminase, blood urea nitrogen, and creatinine clearance were determined.
Statistical analysis
Data were analyzed using the Statistical Package for Social Science (version 13.0; SPSS Inc., Chicago, IL). The statistical significance of differences in mean values between the groups was analyzed using one-way analysis of variance (ANOVA). P values <0.05 were considered statistically significant.
Results
Hemolysis testing
OD values at 545 nm are listed in for each experimental group. Hemolysis rates at the different concentrations of Fe3O4-MNPs/DNR were 2.908%, 2.530%, and 2.415%, respectively. The hemolysis rates for all three concentrations of Fe3O4-MNPs/DNR were less than 5%, which is considered to be the threshold for a hemolytic reaction.
Table 1 Hemolysis test of compound nanoparticles Fe3O4-MNPs/DNR (n = 3, Mean ± SD)
Morphology from micronucleus assay
Femoral bone marrow morphology and micronucleus formation using Giemsa and DAPI staining were observed under an optical microscope and a fluorescence microscope, respectively (). Micronucleus formation rates in femoral bone marrow at different doses of Fe3O4-MNPs/ DNR were not statistically different compared with either the negative control group (P >0.05) or isodose DNR group (P > 0.05), but micronucleus formation rates at different doses of Fe3O4-MNPs/DNR, in the isodose DNR group, and in the negative control group, showed a significant difference compared with the positive control group (; P < 0.05), suggesting that Fe3O4-MNPs/DNR had no inherent toxicity to bone marrow cells in mice.
Figure 1 The result of the micronucleus test of magnetic nanoparticles Fe3O4- MNPs/DNR.
Notes: A1) normal bone marrow of mice observed by optical microscope (Giemsa staining, 10 × 100); B1) normal bone marrow of mice observed by optical microscope (DAPI staining, 10 × 100); A2) formation of micronucleus observed through fluorescence microscope (Giemsa staining, 10 × 100); B2) formation of micronucleus observed through fluorescence microscope (DAPI staining, 10 × 100) (Formation of micronucleus are indicated by “→”).
Abbreviations: Fe3O4-MNPs, magnetic Fe3O4 nanoparticles; DNR, daunorubicin; DAPI, 4,6-diamidino-2-phenylindole.
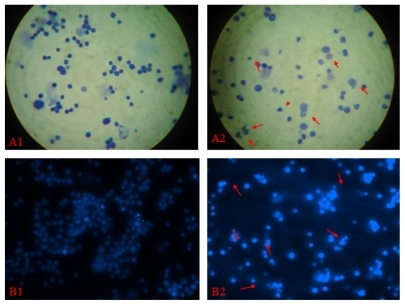
Table 2 Micronucleus test of magnetic nanoparticles Fe3O4-MNPs/DNR
Determination of LD50
Mortality rates in the treatment groups were used to calculate the LD50 of Fe3O4-MNPs/DNR, which was 1009.71 mg/kg (relative concentration of DNR was 10 mg/kg according to the drug-loading rate), and its 95% confidence interval (CI) was 769.11–1262.40 mg/kg, while that of the DNR group was 8.51 mg/kg (95% CI: 6.48–10.37 mg/kg), indicating that Fe3O4-MNPs/DNR have a wide margin of safety.
Acute toxicity in mice
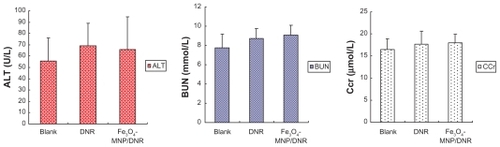
The body weights of mice treated with Fe3O4-MNPs/DNR, isodose DNR, or saline showed no significant difference at 24, 48, and 72 hours (P > 0.05; ). During the 14-day observation period, all the mice remained in good condition, with normal consumption of water and food, and their stools were firm and yellowish-brown. Interestingly, toxic reactions, including instability of gait, convulsion, paralysis, and respiratory depression, were not observed during the study. Alanine transaminase, blood urea nitrogen, and creatinine clearance in the Fe3O4-MNPs/DNR groups were 66.0 ± 28.55 U/L, 9.06 ± 1.05 mmol/L, and 18.03 ± 1.84 μmol/L, respectively (). There was no significant difference compared with the negative control group and isodose DNR group (P > 0.05), suggesting that Fe3O4-MNPs/DNR did not have any acute general toxicity.
Figure 2 The mice weight after being intraperitoneally injected with magnetic nanoparticles Fe3O4-MNPs/DNR at three different time points.
Notes: There was no significant difference in mice weight of the magnetic nanoparticles Fe3O4-MNPs/DNR group, isodose DNR group, and negative group at 24, 48, and 72 hours (P > 0.05).
Abbreviations: Fe3O4-MNPs, magnetic Fe3O4 nanoparticles; DNR, daunorubicin.
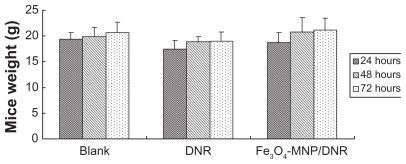
Figure 3 The result of hepatic and renal function of mice in acute toxicity testing.
Notes: ALT, BUN, and Ccr of the compound nanoparticles Fe3O4-MNPs/DNR group or isodose DNR group showed no significant difference compared with the negative group (P > 0.05).
Abbreviations: Fe3O4-MNPs, magnetic Fe3O4 nanoparticles; DNR, daunorubicin; ALT, alanine transarninase; BUN, blood urea nitrogen; Ccr, creatinine clearance rate.
Discussion
Nanomaterials interact directly with tissues and cells after entering the body. Therefore, the most basic criteria for its clinical application are safety and good biocompatibility, which is also important to the industrialization of nanomedicine. At present, evaluation of the biocompatibility of biomaterials is mainly carried out using in vitro and in vivo tests. The biocompatibility of magnetic nanoparticles is determined by their size and surface modifications. Some researchers have reported that Fe3O4-MNPs could be prepared into particles with satisfactory dispersion and biocompatibility.Citation15 Our earlier research showed that the concentrations of these nanoparticles in cells affected by hematologic malignancy were much higher than those in normal somatocytes, indicating that this material has no overt toxicity to normal cells.Citation5 However, findings from in vitro and vivo testing may be quite different because of differences in the number of cells used in such experiments. Thus, toxicologic testing and metabolic processes in vivo need further research. This work investigated the toxicity of self-assembled Fe3O4-MNPs/DNR through hemolysis testing, micronucleus assay, detection of LD50, and acute toxicity testing.
Hemolysis testing, which is a type of acute toxicity screen assay, was mainly used to evaluate the hemocompatibility of the materials when in contact with blood, and the aim was to detect hemolyzation of erythrocytes.Citation16 This trial could sensitively reflect the influence of the nanoparticles on the erythrocyte membrane. Fe3O4-MNPs/DNR are mainly used for chemotherapy and thermotherapy, and may be in contact with blood directly or indirectly for a long time, so evaluation of their hemocompatibility is very important. Both mechanical injury induced by the material surface and the chemical action of soluble remnant molecules could cause abnormal quantity and quality of erythrocyte membrane proteins and lipids, resulting in destruction of erythrocyte membrane integrity and hemolyzation.Citation17–Citation19 The present paper shows that absorbance of free hemoglobin, released by materials in contact with hemocytes in vitro, could be detected using ultraviolet spectrophotometryCitation20 in order to calculate the extent of hemolysis. The hemolyzation rate of these nanoparticles at different concentrations was less than 5%, indicating that Fe3O4-MNPs/DNR do not cause hemolysis and have good hemocompatibility. Further evaluation of the inherent toxicity and carcinogenicity of such biomaterials has been recommended,Citation21 so we chose a micronucleus assay to evaluate the inherent toxicity of Fe3O4-MNPs/DNR. We found no statistically significant difference in micronucleus formation rates at different doses of Fe3O4-MNPs/DNR compared with the negative control group, but there was a significant difference compared with the positive control group. Thus we can infer that these nanoparticles do not induce abnormalities or mutations.
The acute general toxicity test is used to evaluate shortterm toxicity after intraperitoneal administration. In cancer therapy, optimized dosing is crucial not only for inhibiting tumor growth or even promoting apoptosis of tumor cells, but also for preventing the tumor from developing drug resistance and contributing to relapse.Citation22 The development of highly biocompatible, nontoxic nanoparticles is necessary. The LD50 of Fe3O4-MNPs/DNR was 1009.71 mg/kg, and the relative concentration of DNR was 10 mg/kg according to the drug-loading rate, which was much higher than that of therapeutic doses of DNR in mice, suggesting that these nanoparticles have a wide safety margin. Furthermore, it was investigated whether nanoparticles could cause long-term changes in systemic activity, liver function, or renal function. Certain characteristics of nanoparticles, including surface features, size, and shape, can affect particle–cell interactions and interactions with serum proteins.Citation23 Previous research has reported that uptake of the major fraction of injected dextrancoated iron oxide MNPs is by Küpffer cells.Citation24 Other studies have revealed that more than 75% uptake of MNPs occurs in the reticuloendothelial system, particularly in the liver.Citation25 It has also been suggested that Küpffer cells in the liver can degrade iron oxide MNPs and can incorporate most of the iron into ferritin.Citation26 In our study, we monitored for systemic toxic reactions after intraperitoneal administration of Fe3O4-MNPs/DNR for 14 days. None of the experimental animals showed any signs of instability, and indices of liver and renal function, such as alanine transaminase, blood urea nitrogen, and creatinine clearance, were within the normal range. Thus, we can assume that these nanoparticles did not cause any short-term systemic changes.
Conclusion
Fe3O4-MNPs/DNR had good biocompatibility in mice according to this short-term evaluation of toxicity and may be useful for targeted tumor therapy and could replace congener chemotherapeutics in clinical therapy in the future.
Acknowledgments
This work was supported by the 973 (No 2010CB732404) and 863 (No 2007AA0222007) Projects of the People’s Republic of China, the National Nature Science Foundation of the People’s Republic of China (No 3074006230872970), the Special- purpose Science Research Foundation for High Schools (20070286042), and the National Innovation Experiment Program for University Students (No G2007060).
Disclosure
The authors report no conflicts of interest in this work.
References
- AlexiouCArnoldWKleinRJLocoregional cancer treatment with magnetic drug targetingCancer Res2000606641664811118047
- AlexiouCJurgonsRSchmidRJMagnetic drug targeting – biodistribution of the magnetic carrier and the chemotherapeutic agent mitoxantrone after locoregional cancer treatmentJ Drug Target20031113914913129824
- ItoATanakaKKondoKTumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanomaCancer Sci20039430831312824927
- ArtemovDMolecular magnetic resonance imaging with targeted contrast agentsJ Cell Biochem20039051852414523986
- ChengJWuWWChenBAEffect of magnetic nanoparticles of Fe3O4 and 5-bromotetrandrine on reversal of multidrug resistance in K562/A02 leukemic cellsInt J Nanomedicine2009420921619918367
- ChenBAChengJShenMFMagnetic nanoparticles of Fe3O4 and 5-bromotetrandrin interact synergistically to induce apoptosis by daunorubicin in leukemia cellsInt J Nanomedicine20094657119421371
- ChenBAChengJWuYNReversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and 5-bromotetrandrine in xenograft nude-miceInt J Nanomedicine20094737819421372
- MohamedFMarchettiniPStuartOAUtanoMSugarbakerPHThermal enhancement of new chemotherapeutic agents at moderate hyperthermiaAnn Surg Oncol20031046346812734097
- JainTKReddyMKMoralesMALeslie-PeleckyDLLabhasetwarVBiodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in ratsMol Pharm2008531632718217714
- MaMZhuYZhangYPreparation of magnetic nanoparticles and interaction with cancer cellJ Southeast Univ (Natural Science Edition)200333205207
- CuiSShenXDLinBLSynthesis and characterization of magnetic adriamycin HAS nanoparticleChin J Biomed Eng200625114116
- Martino-RothMGViegasJRothDMOccupational genotoxicity risk evaluation through the Comet assay and the micronucleus testGenet Mol Res2003241041715011144
- WuDZhangGDSunLWToxicological study on phenol and o-methyphenol – two kinds of environmental endocrine disrupting chemicalJ Nanjing Univ (Natural Sciences)200137719723
- WangHWuXZhangSExperimental study on the biocompatibility and security of a rhBMP-2 loaded amorphous calcium phosphate delayed release nano-sized materialOrthop J China20081617201723
- ChengFYSuCHYangYSCharacterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applicationsBiomaterials20052672973815350777
- XiTFBiological evaluation of biology based on medical devicesChina Medical Device Information19995914
- SunJGuGZQianYFInfluence of different contact ways and extracting conditions on the hemolytic effect of biomaterialsJ Biomed Eng200320810
- ZhangWYShenYLiNChenJHJiaAQEvaluation of biocompatibility of fiber-reinforced dental compositesMed J Chin PLA200429345347
- LiuWWWangTZhanDSHaemolysis test for evaluating the biocompatibility of SiC implant materialsCRTER20081218731875
- DomkeJDannohlSParakWJSubstrate dependent differences in morphology and elasticity of living osteoblasts investigated by atomic force microscopyColloids Surf B200019367379
- ZhaoXWLinJHWangZRStudy on biocompatibility and security of homemade calcium phosphate cementJournal of Fujian Medical University200236388392
- BezwodaWRHigh-dose chemotherapy with hematopoietic rescue in breast cancer: From theory to practiceCancer Chemother Pharmacol199740S79879272140
- OwensDEPeppasNAOpsonization, biodistribution, and pharmacokinetics of polymeric nanoparticlesInt J Pharm20063079310216303268
- OkonEPouliquenDOkonPBiodegradation of magnetite dextran nanoparticles in the rat. A histologic and biophysical studyLab Invest1994718959037807971
- ChoulyCPouliquenDLucetIJeuneJJJalletPDevelopment of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistributionJ Microencapsul1996132452558860681
- Briley-SaeboKBjomerudAGrantDAhlstromHBergTKindbergGMHepatic cellular distribution and degradation of irom oxide nanoparticles following single intravenous injection in rats: Implications for magnetic resonance imagingCell Tissue Res200431631532315103550