?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Thermoresponsive nanoparticles have become an attractive candidate for designing combined multimodal therapy strategies because of the onset of hyperthermia and their advantages in synergistic cancer treatment. In this paper, novel cetuximab (C225)-encapsulated core-shell Fe3O4@Au magnetic nanoparticles (Fe3O4@Au-C225 composite-targeted MNPs) were created and applied as a therapeutic nanocarrier to conduct targeted magneto-photothermal therapy against glioma cells.
Methods
The core-shell Fe3O4@Au magnetic nanoparticles (MNPs) were prepared, and then C225 was further absorbed to synthesize Fe3O4@Au-C225 composite-targeted MNPs. Their morphology, mean particle size, zeta potential, optical property, magnetic property and thermal dynamic profiles were characterized. After that, the glioma-destructive effect of magnetic fluid hyperthermia (MFH) combined with near-infrared (NIR) hyperthermia mediated by Fe3O4@Au-C225 composite-targeted MNPs was evaluated through in vitro and in vivo experiments.
Results
The inhibitory and apoptotic rates of Fe3O4@Au-C225 composite-targeted MNPs-mediated combined hyperthermia (MFH+NIR) group were significantly higher than other groups in vitro and the marked upregulation of caspase-3, caspase-8, and caspase-9 expression indicated excellent antitumor effect by inducing intrinsic apoptosis. Furthermore, Fe3O4@Au-C225 composite-targeted MNPs-mediated combined hyperthermia (MFH+NIR) group exhibited significant tumor growth suppression compared with other groups in vivo.
Conclusion
Our studies illustrated that Fe3O4@Au-C225 composite-targeted MNPs have great potential as a promising nanoplatform for human glioma therapy and could be of great value in medical use in the future.
Introduction
Gliomas are primary brain tumors arising from the supporting cells of the brain or spinal cord. Accounting for >30% of all primary brain and central nervous system (CNS) malignant tumors, gliomas are the most common tumors of the CNS. They involve almost 80% of primary malignant type of brain tumors and are responsible for a higher rate of mortality than other forms.Citation1 Overall incidence rate for primary malignant brain and CNS tumors is estimated to be 7.27/100,000 per year for all age groups.Citation2 The International Agency for Research on Cancer data estimate 256,213 worldwide cases of brain and CNS tumors, which is 1.8% of all estimated cancers, and 189,394 deaths, which is 2.3% of all cancer-related deaths.Citation3 An estimated 16,700 deaths were attributed to high-grade gliomas in the brain and CNS in the USA in 2017.Citation4 These aggressive brain tumors grow invasively and cause discernible neurologic symptoms within months with an extremely poor prognosis. Even treated with aggressive open surgery combined with adjuvant chemo/radiotherapy, the median survival time is still <15 months,Citation5–Citation8 and current treatment paradigms appear to have reached their maximum benefit. Therefore, for decades, considerable efforts have been devoted to developing more effective antitumor agents and better strategies for targeting human glioma.Citation9,Citation10
Recently, nanostructures with combined diagnostic and therapeutic functions represent a potential application for tumor therapy.Citation11–Citation14 Many inorganic nanoparticles with various compositions, physical features, and functionalities have been widely synthesized and used as drug vehicles, for instance, polymer Prussian blue, zeolitic imidazolate framework, alginate, and calcium phosphate.Citation15–Citation18 Likewise, metal nanoparticles and their oxides with special shapes (sphere, tadpole, and pearl chain) have been successfully generated. Magnetic nanoparticles (MNPs), such as iron oxide, have been widely studied because of their combination of properties such as superparamagnetism, biocompatibility, and ease of synthesis. The most common form of iron oxide used is magnetite (Fe3O4) with a tendency to oxidize, which alters its magnetic properties.Citation19 That is why iron oxide nanoparticles are generally coated with a biocompatible layer such as polymers, silica, or gold (Au).Citation20–Citation27
In particular, core-shell-structured Fe3O4@Au composite MNPs have been the focus of study owing to their intriguing bifunctional properties.Citation28,Citation29 As a form of multifunctional magnetoplasmonic nanomaterials, the nanoparticles combining Au and magnetic materials inherit from the two components excellent surface chemistry, special optical properties, and superparamagnetic properties, all of which would greatly enhance the potential and broaden the practical applications of such nanomaterials.Citation30
Advances in the area of nanotechnology have contributed to the development of magnetic fluid hyperthermia (MFH). In vivo MFH is expected to be one of the best solutions for destroying tumor cells that are deeply seated and localized inside the human body for unlimited tissue penetration and the possibility of multiple hyperthermia cycles. Additionally, high selectivity and heating homogeneity can be expected in the use of MFH. Nevertheless, the practical limitation with MFH is that heating efficiency declines markedly when nanoparticles are taken up by cancer cells, because their endocytosis and aggregation inhibit their Brownian motion. Similarly, the diffusion (thereby local dilution) or enhanced distribution of nanoparticles also decreases nanoparticle heating.Citation31 The drawbacks of MFH could be overcome by combination with near-infrared (NIR) hyperthermia. With local plasmonic heating caused by NIR laser excitation, its efficiency is not impacted by intracellular confinement.Citation31 As a form of multifunctional magnetoplasmonic nanomaterials, Fe3O4@Au composite MNPs are the designs of choice to implement a magneto-photothermal strategy to optimize nanoparticles’ heating efficiency.
We previously reported self-prepared Fe3O4@Au composite MNPs, which are intrinsically magnetic, and exhibit high biocompatibility and safety according to the evaluation of toxicity in vivo and in vitro.Citation32 Moreover, these MNPs have a potential to be used as safe optical and thermal agents, allowing the combination of cancer detection and cancer-specific hyperthermic treatment.Citation32 The present study is aimed at the functionalization of Fe3O4@Au composite MNPs with cetuximab (C225), a monoclonal antibody (McAb) targeting the epidermal growth factor receptor (EGFR) overexpressed in cancer cells. Additionally, the antiglioma effects of MFH combined with NIR hyperthermia mediated by Fe3O4@Au-C225 composite-targeted MNPs were also investigated with in vitro and in vivo experiments.
Experimental
Materials
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid and trypsin were purchased from AMRESCO. Bovine serum albumin (BSA) was purchased from Sijiqing Hangzhou Bioengineering Company. Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from GIBCO BD. MTT, diethylpyrocarbonate, and ethidium bromide were purchased from Sigma. Dimethyl sulfoxide was purchased from Shanghai Ling Feng Chemical Co., Ltd. RNAiso Reagent, AMV reverse transcriptase, deoxyribonucleoside triphosphate (dNTP), Oligo(dT)18, Taq DNA polymerase, 100 bp DNA Marker, RNasin, and RNase free DNase I were purchased from Takara. From Shanghai Shenneng Gaming Biotechnology Co. Ltd. (Whitehouse Station, NJ, USA), 20×TBE, agarose, caspase-3 primer, caspase-8 primer, caspase-9 primer, and β-actin primer were purchased. C225 solution for infusion was purchased from Merck & Co, Germany. All other chemicals were commercially available and of analytical grade.
Cells and animals
U251 cells (human glioma cells) were purchased from Shanghai Cell Research Institute of Chinese Academy of Sciences. Balb/c nu/nu nude mice (5–7 weeks old, male and female, SPF grade) were purchased from Slaccas (Shanghai, China). License number: SCXK (Shanghai) 2002-0010. All of them were raised in Experimental Animal Center of Southeast University, Nanjing, China. All experiments involving animals were performed in compliance with the guidelines of the Animal Care Committee of the Southeast University (Nanjing, China; Approval ID: SYXK-20140310358). All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No 86-23, revised 1996).
Preparation of Fe3O4@Au-C225 MNPs
Fe3O4 MNPs were prepared by chemical co-precipitation method as previously published.Citation33 Then Fe3O4 MNP solution was stirred with sodium citrate (1 mmol/L, 20 mL) for 10 min. The mixture was diluted with deionized water and NH2OH3HCl (1 mol/L, 1 mL) was added. After that, HAuCl4 (1%, 10 mL) was incrementally added dropwise with stirring for 1 h. Fe3O4@Au MNPs were separated by means of a permanent magnet and stored for further use.Citation32 Next, C225 was loaded onto Fe3O4@Au MNPs via physical adsorption. C225 (5 mg/mL, 20 μL) was added into solution of Fe3O4@Au MNPs (0.5 mg Fe/mL, 2.5 mL) whose value of pH was adjusted to 9.0 by HCl (0.1 mol/L), and the mixture was placed in a temperature-controlled shaker at 200 rpm for 2 h at a given temperature of 37°C, followed by BSA (1%, 100 μL) added for reaction for 30 min. At the end of the adsorption, the supernatant and Fe3O4@Au-C225 MNPs were separated by means of a permanent magnet, and the obtained precipitate was dissolved in BSA (1%, 1 mL) and stored at 4°C for further use.
Characterization of Fe3O4@Au-C225 MNPs
The morphology of Fe3O4@Au-C225 MNPs was characterized using transmission electron microscopy (TEM; JEM-1011; JEOL, Tokyo, Japan) and scanning electron microscopy (SEM; JSM-6360LV; JEOL, Tokyo, Japan). The mean particle size and zeta potential were measured using Malvern Instruments (Malvern, UK). The optical properties and magnetic property were measured using 752 Prismatic UV−vis spectroscopy (Shanghai Precision Scientific Instrument Co., China) and vibrating sample magnetometer (PPMS-9; Quantum Design, USA).
Determination of C225 on the surface of Fe3O4@Au-C225 MNPs
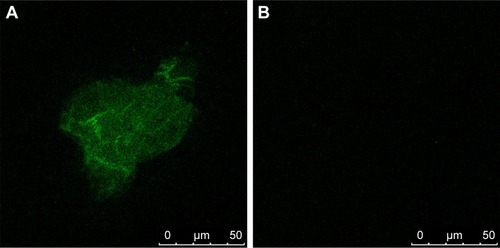
To confirm whether the C225 was linked to Fe3O4@Au-C225 MNPs, the fluorescein isothiocyanate (FITC)-conjugated AffiniPure Goat Anti-Human Immunoglobulin G (IgG) was added to 2 μL of Fe3O4@Au-C225 MNPs with 1% BSA and allowed to react for 1 h at 37°C. After separated by means of a permanent magnet, the precipitate was washed three times with PBS, redispersed in 20 μL of PBS, and observed under a confocal laser scanning microscope (Leica TCS SP8 STED 3×; Leica Microsystems, Wetzlar, Germany). Fe3O4@Au MNPs were used as control.
Adsorption efficiency measurements
To determine the antibody absorption efficiency, the FITC-conjugated AffiniPure Goat Anti-Human IgG was added to 2 μL of Fe3O4@Au-C225 MNPs with 1% BSA and allowed to react for 1 h at 37°C in the dark. After separated by means of a permanent magnet, the precipitate was washed three times with PBS, redispersed in 20 μL of PBS, and observed under a confocal laser scanning microscope (Leica TCS SP8 STED 3×). Fe3O4@Au-C225 MNPs that were not incubated with FITC-conjugated AffiniPure Goat Anti-Human IgG were used as control. The image data were processed with ImageJ software 1.8.
The effective Fc binding of C225 toward the surface of the Fe3O4@Au MNPs was also investigated by enzyme-linked immunosorbent assay according to the literature procedure.Citation34 Goat Anti-Human IgG (Fc)-horseradish peroxidase (HRP) was, respectively, added into an aqueous solution of Fe3O4@Au-C225 MNPs (100 μL) at dilutions of 1:500, 1:1,000, 1:2,000, 1:4,000, 1:8,000, and 1:16,000, and incubated for 1 h at 37°C. After separated by means of a permanent magnet (60 min, 4°C), supernatant (100 μL) was then collected, and ortho-phenylenediamine substrate solution was added at room temperature protected from light. After 10 min, the reaction was stopped by adding H2SO4 (2 mol/L, 50 μL). Part of the dye solution was transferred and the OD was measured at 492 nm with a plate reader. Served as control, treatment of Fe3O4@Au-BSA and C225 mixture followed the same procedure described above.
Heating test of the Fe3O4@Au-C225 MNPs
The Fe3O4@Au-C225 MNPs’ magnetothermal and photothermal effects were tested in aqueous suspensions as a function of [Fe] concentrations, ranging from 0.25 to 1.25 mg Fe/mL. The [Fe] concentrations of the samples dispersed in deionized water were analyzed using Leeman Prodigy inductively coupled plasma-optical emission spectroscopy (USA). Three heating protocols were selected: 1) alternating magnetic field (AMF) alone, at an output frequency of 230 kHz and an output current of 30 A; 2) NIR laser alone at 635 nm laser with 0.3 W/cm2; and 3) combined hyperthermia (simultaneous application of both AMF and NIR).
Cell culture
U251 cell line was maintained in DMEM, supplemented with 10% fetal bovine serum in a standard humidified 5% CO2 and 37°C incubator. The cells were passaged every 2–3 days and harvested on exponential growth stage.
Study of Fe3O4@Au-C225 MNPs on human glioma U251 cells in vitro
Observation of U251 cells labeled by Fe3O4@Au-C225 MNPs
U251 cells were seeded into four holes of a six-well plate with fresh growth medium and incubated (37°C, 5% CO2) overnight to bring the cells to confluence (1×106 cells). The growth medium was then discarded and replaced with fresh medium containing Fe3O4 MNPs, Fe3O4@Au MNPs, and Fe3O4@Au-C225 MNPs at the same [Fe] concentration and PBS as control. The cells were incubated (37°C, 5% CO2) for an additional 24 h and observed with an inverted microscope (Nikon, Tokyo, Japan).
MTT assay of antiproliferative effect
U251 cells (4×104/mL) were cultured in four 96-well plates (100 μL/well) and incubated in an incubator (37°C, 5% CO2). After incubation for 24 h, the growth medium containing different reagents (Fe3O4 MNPs [0.5 mg Fe/mL], C225 [50 μg/mL], Fe3O4@Au MNPs [0.5 mg Fe/mL], Fe3O4@Au-C225 MNPs [0.5 mg Fe/mL]) was added into the wells of experimental groups (eight wells/group) and DMEM as negative control. Four plates were exposed to nonhyperthermia or AMF alone or NIR alone or AMF+NIR, respectively. Nonhyperthermia plate was exposed to no heat treatment. AMF plate was placed into AMF (f=230 kHz, I=30 A) for 30 min. NIR plate was treated with NIR laser (λ=635 nm, 0.3 W/cm2) for 30 min. AMF+NIR plate was possessed with simultaneous application of both AMF and NIR. After incubation for an additional 24 h, the cell viability was measured by a standard MTT method and the cell absorbance was measured at an OD of 492 nm. The cell relative growth rate (RGR) was calculated as (OD of experimental group/OD of blank control group)×100%.
Flow cytometry evaluation of cell apoptosis
U251 cells were seeded in culture flasks (6×105 cells/flask) and divided into eight groups as follows: 1) blank; 2) Fe3O4@Au MNPs+MFH; 3) Fe3O4@Au MNPs+NIR; 4) Fe3O4@Au MNPs+MFH+NIR; 5) Fe3O4@Au-C225 MNPs; 6) Fe3O4@Au-C225 MNPs+MFH; 7) Fe3O4@Au-C225 MNPs+NIR; and 8) Fe3O4@Au-C225 MNPs+MFH+NIR. Cells were incubated with Fe3O4@Au-C225 MNPs (0.5 mg Fe/mL, 5 mL), Fe3O4@Au MNPs (0.5 mg Fe/mL, 5 mL), and medium solution (5 mL) in a humidified incubator (37°C, 5% CO2) for 24 h, respectively. For MFH, cells were placed into AMF (f=230 kHz, I=30 A) for 30 min. For photothermotherapy, cells were treated with NIR laser light (λ=635 nm, 0.3 W/cm2) for 30 min. For combined hyperthermia, cells were possessed with simultaneous application of both MFH and NIR. After incubation for an additional 48 h, cells were collected, washed twice with PBS, and fixed overnight in 70% ethanol at 4°C. Cells were than washed with PBS and stained with propidium iodide for 30 min at room temperature in the dark. Cell cycle analysis was performed with an FACS Vantage SE flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
U251 cells were divided and treated according to the protocol described in the study using flow cytometer assay. After treatment, total RNA was extracted from groups of cells using RNAiso Reagent from Takara and dissolved in DEPC-treated water. Reverse transcription was performed with a 20 μL total volume comprising 2 μL of extracted RNA, 1 μL of Takara Oligo dTl8, 2 μL of Takara dNTP (10 mM), 0.5 μL of Takara RNasin, 1 μL of Takara AMV, 4 μL of 5× AMV Buffer, and 9.5 μL of DEPC-treated water. The qRT-PCR was performed in a 25 μL total volume comprising 2 μL of 10× PCR buffer, 2 μL of Takara dNTP (2.5 mM), 1 μL of 5′ primer (20 pmol/μL), 1 μL of 3′ primer (20 pmol/μL), 0.5 μL of Takara TaqE (5 U/μL), 2.5 μL of MgCl2 (25 mM), 11 μL of DEPC-treated water, and 5 μL of cDNA on the PTC-100 (MJ Research, Inc., Watertown, MA, USA). The sequences of the specific primers used in the research were as follows: caspase-3 – forward: 5′-AAACAGTATGCCGACAAG-3′ and reverse: 5′-GAGGGAAATACAGTACCAAA-3′; caspase-8 – forward: 5′-AAAGGGAACTTCAGACACC-3′ and reverse: 5′-CAGCAGGCTCTTGTTGAT-3′; caspase-9 – forward: 5′-CGAACTAACAGGCAAGCA-3′ and reverse: 5′-TTCACCTCCACCATGAAAT-3′; β-actin – forward: 5′-CGTCTGGACCTGGCTGGCCGGGACC-3′ and reverse: 5′-CATGAAGCATTTGCGGTGGACGATG-3′. The melting curve and threshold cycle data for each PCR product were determined and analyzed.
Western blotting
U251 cells were divided and treated according to the protocol described in the study using flow cytometry assay. Cells under different treatments were lysed, and the protein concentrations were assessed using Bradford assay. Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred from gel to polyvinylidene difluoride membranes. The membranes were blocked with nonfat milk and probed with primary antibodies followed by HRP-conjugated secondary antibodies and the signals were detected.
Study of Fe3O4@Au-C225 MNPs on human glioma U251 cells in vivo
Establishment and treatments of a nude mouse xenograft model of human glioma
All the animal experiments were approved by the animal protection and care Committee of Southeast University. Balb/c nu/nu nude mice (Slaccas Shanghai, China) aged 5–7 weeks were used.
U251 cells (2×106 cell/site) were implanted subcutaneously into nude mice and allowed to grow for 20–25 days after inoculation. As the tumors grew to approximately a diameter of 0.5 cm, the mice were randomly divided into eight groups with six mice in each group as follows: 1) negative control; 2) Fe3O4@Au MNPs+MFH; 3) Fe3O4@Au MNPs+NIR; 4) Fe3O4@Au MNPs+MFH+NIR; 5) Fe3O4@Au-C225 MNPs; 6) Fe3O4@Au-C225 MNPs+MFH; 7) Fe3O4@Au-C225 MNPs+NIR; and 8) Fe3O4@Au-C225 MNPs+MFH+NIR.
Tumor volumes (V, mm3) were calculated using the formula V=(a×b2)/2, where a and b refer to the length and width of the tumor, respectively. After that, Fe3O4@Au MNPs (0.5 mg Fe/mL) and Fe3O4@Au-C225 MNPs (0.5 mg Fe/mL) were peritumorally injected at a dose of one-third of tumor volume, respectively. For MFH, the tumors were exposed to AMF (f=230 kHz, I=30 A) three times for 30 min each and a time interval of 24 h. For photothermotherapy, the tumors were irradiated with NIR laser light (λ=635 nm, 0.3 W/cm2) three times for 30 min each and a time interval of 24 h. For combined hyperthermia, tumors were possessed with simultaneous application of both MFH and NIR.
Tumor growth evaluation
The mice were killed on day 42 after the initial treatment. The quality and volume of stripped tumors were measured. Tumors and major organs (heart, liver, spleen, kidneys, lungs, and tumor) were surgically removed at the end of the treatment after killing. The tissues were fixed in 4% formaldehyde and embedded in paraffin, sectioned into thick slices, and stained with hematoxylin/eosin. Ultrastructural characteristics of the tumor were observed by TEM.
Statistical analysis
All statistical analyses were performed using SPSS version 19.0 software (SPSS Inc., Chicago, IL, USA). Results were expressed as mean ± SD. Statistically significant differences were assessed via analysis of variance. Statistical significance was set at P<0.05.
Results
Preparation and characterization of Fe3O4@Au-C225 MNPs
Fe3O4@Au MNPs were synthesized as previously reported, and the C225 loading was achieved via physical adsorption.Citation32 TEM and SEM micrographs () show that prepared Fe3O4@Au-C225 MNPs were approximately spherical and uniform with good dispersibility.
Figure 1 Transmission electron microscopy and scanning electron microscopy (top) of Fe3O4@Au-C225 magnetic nanoparticles.
Abbreviation: C225, cetuximab.

The Fe3O4@Au-C225 MNPs had an average hydrodynamic diameter of 46 nm with single peak shape and narrow particle size distribution () compared to Fe3O4@Au MNPs (35 nm).Citation32 The zeta potential of Fe3O4@Au-C225 MNPs was positive at 11.1±1.8 mV in the neutral environment of pH 7.4 (), as a result of the presence of the C225 as part of the overall MNP structure. The clusters of Fe3O4@Au-C225 MNPs were observed in TEM and SEM. It may be attributable to slightly decreased electrostatic force between the positively charged Fe3O4@Au-C225 MNPs according to the decreased zeta potential (11.1±1.8 mV) compared to negatively charged Fe3O4@Au MNPs (−23.2±1.8 mV).Citation32
Figure 2 Characterization of Fe3O4@Au-C225 MNPs.
Notes: (A) Hydrodynamic diameter distribution of Fe3O4@Au-C225 MNPs; (B) Zeta potential of Fe3O4@Au-C225 MNPs; (C) UV–vis absorption spectra of Fe3O4@Au-C225 MNPs (curve b) and Fe3O4@Au MNPs (curve a). (D) The hysteresis loops of Fe3O4@Au-C225 MNPs.
Abbreviations: C225, cetuximab; MNPs, magnetic nanoparticles.
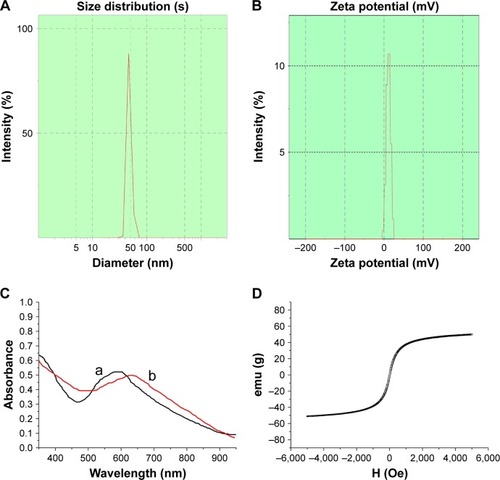
Differences in the chemical structure between Fe3O4@Au-C225 MNPs and Fe3O4@Au MNPs also resulted in differences in the peak absorption wavelengths. Specifically, the absorption peak of Fe3O4@Au-C225 MNPs (630 nm) was red-shifted compared to that of Fe3O4@Au MNPs (612 nm; ), which indicated excellent adsorption of C225.
Magnetic hysteresis measurements revealed that the magnetization of Fe3O4@Au-C225 MNPs shortly became saturated and exhibited zero remanence or coercive force, with the hysteresis loop close to the coincident “S”-shaped curve (). It provided a good demonstration of the superparamagnetic properties with Fe3O4@Au-C225 MNPs. The saturation magnetization value obtained from the hysteresis loops at 300 K is 51.2 emu/g Fe3O4@Au-C225 MNPs, which was close to Fe3O4@Au MNPs (51.8 emu/g).Citation32
Determination of C225 adsorption
After being incubated with FITC-conjugated AffiniPure Goat Anti-Human IgG, green fluorescence from Fe3O4@Au-C225 MNPs was observed by confocal laser scanning microscope. In contrast, no fluorescence was detected in the Fe3O4@Au MNPs ().
Absorption efficiency
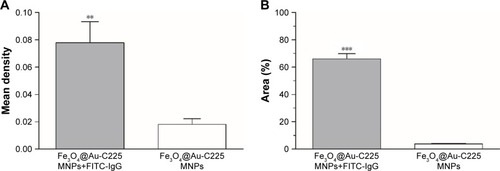
C225 absorption efficiency was determined by measuring the fluorescence percentage. The mean density and fluorescence percentage of Fe3O4@Au-C225 MNPs after incubation with FITC-conjugated AffiniPure Goat Anti-Human IgG were much higher than Fe3O4@Au-C225 MNPs without incubation with FITC-conjugated AffiniPure Goat Anti-Human IgG (), showing an effective antibody absorption.
Figure 4 Determination of the C225 absorption efficiency by measuring mean density and fluorescence percentage.
Notes: (A) Mean density of Fe3O4@Au-C225 MNPs after incubation with FITC-labeled IgG and Fe3O4@Au-C225 MNPs without incubation with FITC-labeled IgG. (B) Fluorescence percentage of Fe3O4@Au-C225 MNPs after incubation with FITC-labeled IgG and Fe3O4@Au-C225 MNPs without incubation with FITC-labeled IgG. **P<0.01; ***P<0.001.
Abbreviations: C225, cetuximab; FITC, fluorescein isothiocyanate; IgG, immunoglobulin G; MNPs, magnetic nanoparticles.
The binding of Goat Anti-Human IgG (Fc)-HRP at different dilutions to adsorbed Fe3O4@Au-C225 MNPs solution prepared was indicated by OD, and Fe3O4@Au-BSA and C225 mixture was used as control. To obtain the Fc binding efficiency of Fe3O4@Au MNPs toward C225, a percentage of Fe3O4@Au-C225 MNPs solution OD versus Fe3O4@Au-BSA and C225 mixture OD was calculated as shown in . It was found that the Fc binding of C225 was significant.
Table 1 Adsorption effect of Fe3O4@Au-C225 to antibody Fc
Heat generation by Fe3O4@Au-C225 MNPs in suspension
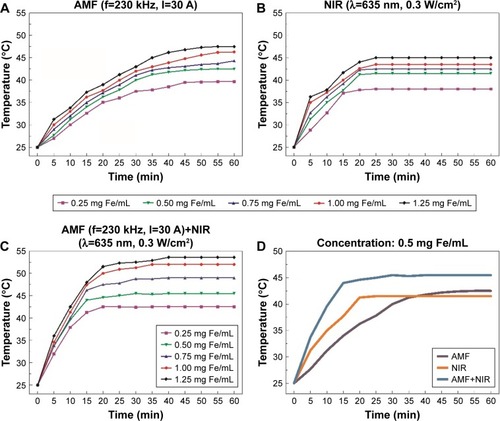
The iron concentrations of the Fe3O4@Au-C225 MNPs were ranging from 0.25 to 1.25 mg Fe/mL. When varying concentrations of Fe3O4@Au-C225 MNPs were exposed to AMF, the temperature rose rapidly within the first 30 min, then rose gently, and stabilized at a certain level after 50 min (). When exposed to NIR, the temperature rose rapidly within the first 15 min and stabilized at a certain level after 30 min (). When exposed to AMF+NIR, the temperature rose rapidly within the first 15 min and stabilized at a certain level after 20 min (). In three heating protocols, the rise in temperature increased with the iron concentration. Among all of the tested concentrations, 0.5 mg Fe/mL was the optimum. This hyperthermic behavior contributes to the killing of tumor cells while not harming normal tissues. In addition, Fe3O4@Au-C225 MNPs with the concentration of 0.5 mg Fe/mL exhibited a sufficient heating capacity (specific absorption rate [SAR]=84.56, intrinsic loss power [ILP]=0.018) to achieve satisfactory temperatures, which were selected in the following experiments. Temporal response curves for iron concentration of 0.5 mg Fe/mL in different heating protocols are also shown in .
Figure 5 Heat generation by Fe3O4@Au-C225 MNPs in suspension (0.25–1.25 mg Fe/mL).
Notes: (A) AMF (f=230 kHz, I=30 A), (B) NIR (λ=635 nm, 0.3 W/cm2), and (C) AMF (f=230 kHz, I=30 A) combined with NIR (λ=635 nm, 0.3 W/cm2). (D) Temporal response curves for concentration of 0.5 mg Fe/mL.
Abbreviations: AMF, alternating magnetic field; MNPs, magnetic nanoparticles; C225, cetuximab; NIR, near-infrared hyperthermia.
Tumor cell proliferation and cellular apoptosis study in vitro
Fe3O4 MNPs, Fe3O4@Au MNPs, and Fe3O4@Au-C225 MNPs were incubated with U251 cells for 24 h. The cellular internalization of different nanoparticles was observed by reverse microscopy ().
Figure 6 Proliferation of U251 cells after different treatments.
Notes: (A) Fe3O4 MNPs, Fe3O4@Au MNPs, and Fe3O4@Au-C225 MNPs were incubated with U251 cells. (B) The cell RGR of U251 glioma cells with different treatments . (C) U251 cells were treated with combined hyperthermia (MFH+NIR) mediated by different nanoparticles. *Compared with the negative control group, P<0.05; #AMF+NIR group compared with AMF or NIR group, P<0.05; ∆Fe3O4@Au-C225 MNPs compared with Fe3O4@Au MNPs when exposed to AMF combined with NIR, P<0.05.
Abbreviations: AMF, alternating magnetic field; C225, cetuximab; DMEM, Dulbecco’s Modified Eagle’s Medium; MFH, magnetic fluid hyperthermia; MNPs, magnetic nanoparticles; NIR, near-infrared hyperthermia; RGR, relative growth rate.
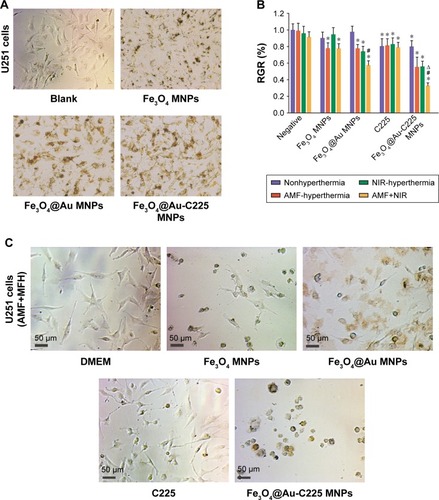
MTT assay was performed to measure viability of U251 glioma cells after hyperthermia (nonhyperthermia or MFH alone or NIR alone or MFH+NIR) mediated by Fe3O4 MNPs, Fe3O4@Au MNPs, C225, Fe3O4@Au-C225 MNPs, and untreated cells as control. The cell RGR was calculated as (OD of experimental group/OD of blank control group)×100%.
When comparing the antiproliferation results (), it was found that the combined hyperthermia (MFH+NIR) mediated by Fe3O4@Au-C225 MNPs or Fe3O4@Au MNPs significantly decreased the cell viability (P<0.05), compared to sole hyperthermia (MFH or NIR). Moreover, significant loss in cell viability in U251 cells was observed after combined hyperthermia (MFH+NIR) mediated by Fe3O4@Au-C225 MNPs, and superior to that mediated by Fe3O4@Au MNPs (P<0.05; ), indicating the highest antiproliferative activity against U251 cells in this study.
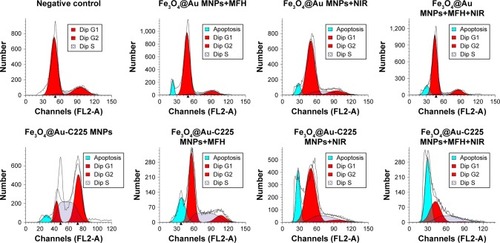
For further investigation of the antiproliferative effect, the cell cycle progression was measured using flow cytometry. Results showed that S arrest was prominent in U251 cells exposed to Fe3O4@Au-C225 MNPs with or without hyperthermia (). We also observed an increase in the percentage of apoptotic cells in Fe3O4@Au MNPs+MFH group (7.92%), Fe3O4@Au MNPs+NIR group (8.15%), Fe3O4@Au MNPs+MFH+NIR group (12.37%), Fe3O4@Au-C225 MNPs group (5.77%), Fe3O4@Au-C225 MNPs+MFH group (21.97%), Fe3O4@Au-C225 MNPs+NIR group (21.89%), and Fe3O4@Au-C225 MNPs+MFH+NIR group (46.21%), compared to negative control without a clear hypodiploid peak. The highest hypodiploid peak indicated an excellent therapeutic efficacy of combined hyperthermia (MFH+NIR) mediated by Fe3O4@Au-C225 MNPs toward U251 cells by induction of apoptosis.
Figure 7 The flow cytometry analysis of U251 cells after different treatments.
Note: Apoptosis peak were observed in Fe3O4@Au MNPs+MFH group (7.92%), Fe3O4@Au MNPs+NIR group (8.15%), Fe3O4@Au MNPs+MFH+NIR group (12.37%), Fe3O4@Au-C225 MNPs group (5.77%), Fe3O4@Au-C225 MNPs+MFH group (21.97%), Fe3O4@Au-C225 MNPs+NIR group (21.89%), and Fe3O4@Au-C225 MNPs+MFH+NIR group (46.21%), comparing to negative control group without obvious apoptosis peak.
Abbreviations: C225, cetuximab; MFH, magnetic fluid hyperthermia; MNPs, magnetic nanoparticles; NIR, near-infrared hyperthermia.
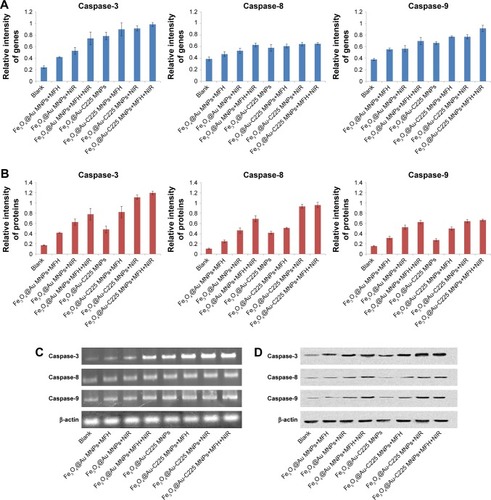
To evaluate the molecular mechanisms of Fe3O4@Au-C225 MNPs-mediated hyperthermia-induced apoptosis, we performed qRT-PCR and Western blot analyses. The results demonstrated that when compared to Fe3O4@Au MNPs-mediated hyperthermia, Fe3O4@Au-C225 MNPs-mediated hyperthermia successfully upregulated the mRNA and protein levels of caspase-3, caspase-8, and caspase-9 in U251 cells (). In addition, a marked upregulation of caspase-3, caspase-8, and caspase-9 expression, particularly caspase-3 and caspase-9, was evaluated using the cells treated with Fe3O4@Au-C225 MNPs-mediated combined hyperthermia (MFH+NIR), indicating the excellent antitumor effect of Fe3O4@Au-C225 MNPs-mediated combined hyperthermia by inducing intrinsic apoptosis in this study.
Figure 8 qRT-PCR and Western blot analyses of U251 cells after different treatments.
Notes: (A) The quantitative real-time polymerase chain reaction analyses to evaluate the relative mRNA levels of caspase-3, caspase-8, and caspase-9 in U251 cells with various treatments and (C) β-actin was used as an internal control to normalize the data. (B) Western blot analyses to evaluate the protein levels of caspase-3, caspase-8, and caspase-9 in U251 cells of different treatment groups, and (D) β-actin was used as an internal control to normalize the data.
Abbreviations: MFH, magnetic fluid hyperthermia; MNPs, magnetic nanoparticles; NIR, near-infrared hyperthermia.
Study of Fe3O4@Au-C225 MNPs on human glioma U251 cells in vivo
A subcutaneous tumor model of U251 glioma cells was used to evaluate the in vivo therapeutic efficacy of Fe3O4@Au-C225 MNPs-mediated combined hyperthermia (MFH+NIR) in comparison with that mediated by Fe3O4@Au MNPs. At 42 days post-treatment, mice in groups were killed and the stripped tumor volume and weight were measured (). Compared to the negative control, the tumor growth was suppressed to various degrees in the tumor weight and volume among experimental groups (P<0.05; , left and right). Furthermore, treatments with Fe3O4@Au-C225 MNPs-mediated combined hyperthermia had more efficiency in reduction of tumor growth than that of the Fe3O4@Au MNPs-mediated combined hyperthermia (P<0.05; , left and right).
Figure 9 Efficacy of various therapeutic strategies in treating nude mice bearing U251 glioma tumors.
Notes: (A) The quality and volume changes of stripped tumors in various treatment groups. (B) Tumors rejected from mice in various treatment groups. (C) Histopathologic examination of human glioma transplanted in nude mice (hematoxylin/eosin staining, ×100 magnification). Transmission electron microscopy of the tumors exposed to Fe3O4@Au-C225 MNPs-mediated combined hyperthermia (MFH+NIR). (D) Numerous vesicles containing the aggregative Fe3O4@Au-C225 MNPs. (E) The denatured collagen zones in the close vicinity of Fe3O4@Au-C225 MNPs localization. *Compared with the negative control group, P<0.05; #Fe3O4@Au-C225 MNPs compared with Fe3O4@Au MNPs when exposed to AMF combined with NIR, P<0.05.
Abbreviations: AMF, alternating magnetic field; C225, cetuximab; MFH, magnetic fluid hyperthermia; MNPs, magnetic nanoparticles; NIR, near-infrared hyperthermia.
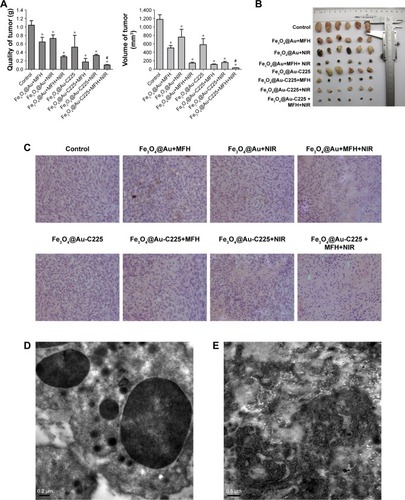
Subsequently, we performed histologic examinations of tumor tissues post-treatment via hematoxylin/eosin staining (). The tumor cells grew densely in the groups of control, Fe3O4@Au MNPs+MFH, Fe3O4@Au MNPs+NIR, and Fe3O4@Au-C225 MNPs. In the Fe3O4@Au MNPs+MFH+NIR, Fe3O4@Au-C225 MNPs+MFH, Fe3O4@Au-C225 MNPs+NIR, and Fe3O4@Au-C225 MNPs+MFH+NIR groups, the tumor cells atrophied and obvious zones of necrosis were observed in Fe3O4@Au-C225 MNPs+MFH+NIR group.
Ultrastructural characteristics of the tumor in Fe3O4@Au-C225 MNPs+MFH+NIR group were observed. TEM revealed intracellular localization of Fe3O4@Au-C225 MNPs. Numerous vesicles containing the aggregative Fe3O4@Au-C225 MNPs were visible intracellularly and were dispersed randomly in the cytoplasm (). Scattered and chaotic collagen fibers () were also shown on the TEM micrographs.
Discussion
The combination of chemotherapy and thermotherapy has been the focus of cancer therapy in recent years. However, one of the challenges encountered was to achieve the optimal effects of the combination therapy. Thus, a robust therapeutic platform is urgently needed to achieve the most effective synergic effect for tumor therapy.
The importance of EGFR in the malignant progression of glioblastoma and the effect of EGFR on patient survival has been extensively documented. Overexpressed EGFR is present in up to 40% of primary glioblastoma and the common overexpression makes EGFR a theoretically attractive target, and consequently, EGFR inhibitors have been among the most studied in clinical trials.Citation35–Citation38 C225, an IgG1 human–murine chimeric of McAb M225 from murine, has been approved for use in patients with head and neck cancer, which is the only EGFR inhibitor that has conferred a survival advantage when combined with platinum-based combination chemotherapy.Citation39
In this study, we decorated Fe3O4@Au composite MNPs with C225 via physical adsorption in order to maintain the biologic activities of C225. The green fluorescence observed by confocal laser scanning microscope () and the UV–vis absorption spectra of Fe3O4@Au-C225 MNPs demonstrated the presence of C225 on the surface of Fe3O4@Au-C225 MNPs. The absorption peak of Fe3O4@Au-C225 MNPs (630 nm) was red-shifted compared to Fe3O4@Au MNPs (612 nm; ), suggesting the excellent adsorption of C225. In addition, zeta potential and particle size are two important factors. The size of the nanoparticle may have an effect on uptake efficiency. The Fe3O4@Au-C225 MNPs that we synthesized revealed a diameter of 46 nm with a zeta potential of 11.1±1.8 mV. It is known that a nanoparticle with a positive charge has more efficient cellular internalization than a nanoparticle with a neutral or negative charge.Citation40,Citation41 Cell surface usually has a negative charge; therefore, Fe3O4@Au MNPs (zeta potential=−23.2±1.8 mV) have less affinity to cancer cells because of negatively charged Au shell on its surface. Hence, C225 adsorption changed the zeta potential of the Fe3O4@Au-C225 MNPs to a positive charge, which possibly enhances the interaction between the nanoparticles and glioma cells. Perhaps more importantly, Fe3O4@Au-C225 MNPs may enter glioma cells through receptor-dependent C225-mediated endocytosis.
In our cell studies, MTT assay showed that the combined hyperthermia (MFH+NIR) mediated by Fe3O4@Au-C225 MNPs significantly decreased the U251 cell viability compared to sole hyperthermia (MFH or NIR), suggesting the optimizing heating efficiency. In addition, the highly anti-proliferative effect of combined hyperthermia (MFH+NIR) mediated by Fe3O4@Au-C225 MNPs against U251 cells was also observed compared to that mediated by Fe3O4@Au MNPs groups, confirming the significant role of C225 in the targeting to U251 cells with overexpressed EGFR. For further investigation of the antiglioma effect, the cell cycle progression was measured using flow cytometry, and then qRT-PCR and Western blot analyses were performed to evaluate the molecular mechanisms of induced apoptosis. The combined hyperthermia (MFH+NIR) mediated by Fe3O4@Au-C225 MNPs exhibited excellent therapeutic efficacy for U251 cells by induction of apoptosis (). Furthermore, a marked upregulation of caspase-3, caspase-8, and caspase-9 expression, particularly caspase-3 and caspase-9, was evaluated using the cells treated with Fe3O4@Au-C225 MNPs-mediated combined hyperthermia (MFH+NIR), indicating its excellent antitumor effect by inducing intrinsic apoptosis ().
Evaluation of animal models displayed that therapeutic efficacy of Fe3O4@Au-C225 MNPs-mediated combined hyperthermia (MFH+NIR) was superior compared to sole hyperthermia (MFH or NIR) and Fe3O4@Au MNPs-mediated combined hyperthermia in mice with tumor model of human glioma. Representative sections of tumors are shown in . Control tumors, as well as NIR or MFH-treated tumors, exhibited a relatively viable and homogenous tumor tissue. The Fe3O4@Au-C225 MNPs did not affect the viability of U251 cells when hyperthermia was not applied, as neither zones of necrosis nor immune cell infiltrates were observed. In contrast, combined hyperthermia led to diffusion and subsequent dilution of Fe3O4@Au-C225 MNPs throughout the tumors, and enlarging zones of necrosis were also observed. TEM revealed intracellular localization of Fe3O4@Au-C225 MNPs. Numerous vesicles containing the aggregative Fe3O4@Au-C225 MNPs were visible intracellularly and were dispersed randomly in the cytoplasm (). The denatured collagen zones in the close vicinity of Fe3O4@Au-C225 MNPs localization were shown on the TEM micrographs ().
Structural modification of nanoparticles with targeting ligands like aptamers, short peptides, and other small molecules has been found to increase the accumulation of nanoparticles at the tumor site just because the ligands can recognize special and unique biophysical markers on the surface of tumor cells.Citation42–Citation44 Therefore, in our study, designed Fe3O4@Au-C225 composite-targeted MNPs with C225, with high affinity for EGFR, caused to enhanced efficacy of cellular MNPs uptake through receptor-dependent C225-mediated endocytosis and antiglioma effect of C225 itself. Subsequently, the simultaneous application of both MFH and NIR hyperthermia contributes to intrinsic apoptosis of U251 glioma cells and enhanced efficacy in tumor size reduction. We developed a robust therapeutic platform for glioma therapy based on Fe3O4@Au-C225 composite-targeted MNPs. On the basis of the experimental results of this study, MFH combined with NIR hyperthermia mediated by Fe3O4@Au-C225 composite-targeted MNPs will show promise in future human clinical trial for patients with glioma.
Conclusion
We developed a robust therapeutic platform for tumor therapy based on Fe3O4@Au-C225 composite-targeted MNPs. C225 was physically adsorbed to the surface of Fe3O4@Au MNPs. Furthermore, the dual magnetic and photothermal action mediated by Fe3O4@Au-C225 composite-targeted MNPs resulted in intrinsic apoptosis of U251 cells in vitro and growth suppression of tumor in vivo, which showed remarkable improvement in synergistic therapeutic effects for glioma treatment.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number: 81301313; 81600159), the Natural Science Foundation of Jiangsu Province (grant number: BK20131015; BK20141015), Jiangsu Provincial College Students’ Practical Innovation Training Program (grant number: 201410312015Z), and Nanjing Developing Project of Medical Science (grant number: YKK13174).
Disclosure
The authors declare no conflicts of interest in this work.
References
- AnjumKShaguftaBIAbbasSQCurrent status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: a reviewBiomed Pharmacother20179268168928582760
- ParasramkaSTalariGRosenfeldMGuoJVillanoJLProcarbazine, lomustine and vincristine for recurrent high-grade gliomaCochrane Database Syst Rev201771CD01177328744879
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- Cancer Facts & Figures 2017Atlanta, GAAmerican Cancer Society2017
- BrandesAATosoniAFranceschiEReniMGattaGVechtCGlioblastoma in adultsCrit Rev Oncol Hematol200867213915218394916
- GlaserTHanIWuLZengXTargeted nanotechnology in glioblastoma multiformeFront Pharmacol2017816628408882
- TosiUMarnellCSChangRAdvances in molecular imaging of locally delivered targeted therapeutics for central nervous system tumorsIn J Mol Sci2017182E351
- ReniMMazzaEZanonSGattaGVechtCJCentral nervous system gliomasCrit Rev Oncol Hematol201711321323428427510
- SeystahlKPapachristodoulouABurghardtIBiological role and therapeutic targeting of TGF-β3 in glioblastomaMol Cancer Ther20171661177118628377490
- ZhouJTienACAlbertaJAA sequentially priming phosphorylation cascade activates the gliomagenic transcription factor olig2Cell Rep201718133167317728355568
- LiYLinTLuoYA smart and versatile theranostic nanomedicine platform based on nanoporphyrinNat Commun20145471225158161
- Di CoratoRBéalleGKolosnjaj-TabiJCombining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomesACS Nano2015932904291625695371
- PasparakisGManourasTVamvakakiMArgitisPHarnessing photochemical internalization with dual degradable nanoparticles for combinatorial photo-chemotherapyNat Commun20145362324710504
- MuXZhangFKongCEGFR-targeted delivery of DOX-loaded Fe3O4@polydopamine multifunctional nanocomposites for MRI and antitumor chemo-photothermal therapyInt J Nanomedicine2017122899291128435266
- LianHYHuMLiuCHYamauchiYWuKCWHighly biocompatible, hollow coordination polymer nanoparticles as cisplatin carriers for efficient intracellular drug deliveryChem Commun (Camb)201248425151515322514015
- ShiehFKWangSCYenCLImparting functionality to biocatalysts via embedding enzymes into nanoporous materials by a de novo approach: size-selective sheltering of catalase in metal−organic framework microcrystalsJ Am Chem Soc2015137134276427925781479
- LiangYHLiuCHLiaoSHCosynthesis of cargo-loaded hydroxyapatite/alginate core-shell nanoparticles (HAP@Alg) as pH-responsive nanovehicles by a pre-gel methodACS Appl Mater Interfaces20124126720672723151216
- BastakotiBPHsuYCLiaoSHInorganic-organic hybrid nanoparticles with biocompatible calcium phosphate thin shells for fluorescence enhancementChem Asian J2013861301130523526653
- McnamaraKTofailSANanosystems: the use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applicationsPhys Chem Chem Phys20151742279812799526024211
- TassaCShawSYWeisslederRDextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapyAcc Chem Res2011441084285221661727
- LutzJFStillerSHothAKaufnerLPisonUCartierROne-pot synthesis of pegylated ultrasmall iron-oxide nanoparticles and their in vivo evaluation as magnetic resonance imaging contrast agentsBiomacromolecules20067113132313817096542
- KohlerNFryxellGEZhangMA bifunctional poly(ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agentsJ Am Chem Soc2004126237206721115186157
- MasoudiAMadaah HosseiniHRShokrgozarMAAhmadiROghabianMAThe effect of poly(ethylene glycol) coating on colloidal stability of superparamagnetic iron oxide nanoparticles as potential MRI contrast agentInt J Pharm20124331–212914122579990
- MaDGuanJDénomméeSEnrightGVeresTSimardBMultifunctional nano-architecture for biomedical applicationsChem Mater200618719201927
- BanZBarnakovYALiFGolubaVOO’ConnoCJThe synthesis of core–shell iron@gold nanoparticles and their characterizationJ Mater Chem2005154346604662
- ChenMYamamuroSFarrellDMajetichSAGold-coated iron nanoparticles for biomedical applicationsJ Appl Phys2003931075517553
- ZhouTWuBXingDBio-modified Fe3O4 core/Au shell nanoparticles for targeting and multimodal imaging of cancer cellsJ Mater Chem2011222470477
- BrullotWValevVKVerbiestTMagnetic-plasmonic nanoparticles for the life sciences: calculated optical properties of hybrid structuresNanomedicine20128555956821945901
- ZhouHChoiSIZouFCytotoxicity and gene expression in sarcoma 180 cells in response to spiky magnetoplasmonic supraparticlesACS Appl Mater Interfaces2014622196801968925369231
- ZhouHZouFKohKLeeJMultifunctional magnetoplasmonic nanomaterials and their biomedical applicationsJ Biomed Nanotechnol201410102921294925992424
- EspinosaADiCRKolosnjaj-TabiJFlaudPPellegrinoTWilhelmCDuality of iron oxide nanoparticles in cancer therapy: amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatmentACS Nano20161022436244626766814
- LiYuntaoLiuJingZhongYuejiaoBiocompatibility of Fe3O4@Au composite magnetic nanoparticles in vitro and in vivoInt J Nanomedicine201162805281922131827
- DuYZhangDLiuHLaiRThermochemotherapy effect of nanosized As2O3/Fe3O4 complex on experimental mouse tumors and its influence on the expression of CD44v6, VEGF-C and MMP-9BMC Biotechnol200998419804631
- TzonevaRFaucheuxNGrothTWettability of substrata controls cell-substrate and cell-cell adhesionsBiochim Biophys Acta20071770111538154717804166
- WongAJBignerSHBignerDDKinzlerKWHamiltonSRVogelsteinBIncreased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplificationProc Natl Acad Sci U S A19878419689969033477813
- van den BentMJBrandesAARamplingRRandomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034J Clin Oncol20092781268127419204207
- LassmanABRossiMRRaizerJJMolecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01Clin Cancer Res200511217841785016278407
- DokalaAThakurSSExtracellular region of epidermal growth factor receptor: a potential target for anti-EGFR drug discoveryOncogene201736172337234427775071
- HerbstRSLangerCJEpidermal growth factor receptors as a target for cancer treatment: the emerging role of IMC-C225 in the treatment of lung and head and neck cancersSemin Oncol200229292736
- ChenXZhuXLiLInvestigation on novel chitosan nanoparticle-aptamer complexes targeting TGF-β receptor IIInt J Pharm2013456249950723999222
- JoDHKimJHLeeTGKimJHSize, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseasesNanomedicine20151171603161125989200
- WangHZhaoXGuoCAptamer-dendrimer bioconjugates for targeted delivery of miR-34a expressing plasmid and antitumor effects in non-small cell lung cancer cellsPLoS One2015109e013913626406332
- GuoJGaoXSuLAptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug deliveryBiomaterials201132318010802021788069
- TaghaviSNiaAHAbnousKRamezaniMPolyethylenimine-functionalized carbon nanotubes tagged with AS1411 aptamer for combination gene and drug delivery into human gastric cancer cellsInt J Pharm20175161–230131227840158