?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
In the present research, we report a quick and green synthesis of magnetite nanoparticles (Fe3O4-NPs) in aqueous solution using ferric and ferrous chloride, with different percentages of natural honey (0.5%, 1.0%, 3.0% and 5.0% w/v) as the precursors, stabilizer, reducing and capping agent, respectively. The effect of the stabilizer on the magnetic properties and size of Fe3O4-NPs was also studied.
Methods
The nanoparticles were characterized by X-ray diffraction (XRD) analysis, field emission scanning electron microscopy, energy dispersive X-ray fluorescence, transmission electron microscopy (TEM), vibrating sample magnetometry (VSM) and Fourier transform infrared spectroscopy.
Results
The XRD analysis indicated the presence of pure Fe3O4-NPs while the TEM images indicated that the Fe3O4-NPs are spherical with a diameter range between 3.21 and 2.22 nm. The VSM study demonstrated that the magnetic properties were enhanced with the decrease in the percentage of honey. In vitro viability evaluation of Fe3O4-NPs performed by using the MTT assay on the WEHI164 cells demonstrated no significant toxicity in higher concentration up to 140.0 ppm, which allows them to be used in some biological applications such as drug delivery.
Conclusion
The presented synthesis method can be used for the controlled synthesis of Fe3O4-NPs, which could be found to be important in applications in biotechnology, biosensor and biomedicine, magnetic resonance imaging and catalysis.
Introduction
Recently, rapid advancement in nanotechnology has made synthesis, characterization and improvement of nanoparticles in terms of functional properties possible for various applications. The inspiration for the synthesis of artificial materials for the better quality and quantity comes from nature. The extensive application of metal nanoparticles in different fields, especially in biotechnology, has motivated the metal nanoparticles synthesis.Citation1 Nanoparticles has attracted incredible attention because of their smaller size and larger surface area compared with bulk materials, in addition to having novel biological and mechanical properties, optical absorption, electrical conductivity and catalytic activity.Citation2–Citation4
Among the different types of nanoparticles, magnetic nanoparticles, especially magnetite and maghemite nanoparticles with proven biological properties and good biocompatibility, have been the subject of various studies, due to the wide range of important biomedical and environmental applications such as cancer hyperthermia therapy, drug delivery, cell labeling, enhanced magnetic resonance imaging and magnetic separation. So far, the synthesis and preparation of iron oxide nanoparticle has been an important research priority, and it has been extensively studied and characterized.Citation5–Citation7
Over the past decade, numerous methods have been proposed for the synthesis of magnetite nanoparticles (Fe3O4-NPs), including physical, chemical and biological methods. These include methods such as microemulsions, coprecipitation of ferrous and ferric ions aqueous solution using a base,Citation8–Citation10 sol–gel method,Citation11 sonochemistry,Citation12 colloidal method,Citation13 nonaqueous route,Citation14 pyrolysis reaction,Citation15 thermal decomposition of organic iron precursor in organic solvents,Citation16–Citation18 solvothermal synthesis,Citation19 hydrothermal synthesis,Citation20,Citation21 mechano-chemical processingCitation22 and emulsion techniques.Citation23,Citation24 Although, bulk chemical synthesis of large amount of nanoparticles is fast and simple, to obtain the effective size stabilization of the nanoparticles, it is important to use capping agents. Furthermore, some of the chemicals used in the synthesis and stabilization are harmful and could produce noneco-friendly, unsafe and hazardous products, and therefore there is a growing demand for the use of green technology in the synthesis of nanoparticles. Thus, new and advanced methods for the growth of nanoparticles must draw inspiration from biological systems.Citation25–Citation27
Honey is one of the most beneficial foods accessible, which largely consists of fructose and glucose. Also, it is rich in amino acids, essential minerals, vitamin C and enzymes. Honey has been subjected to broad study throughout the world.Citation5–Citation9 It contains antioxidants, which are important in cancer prevention.Citation20 Recently, aqueous synthesis of silver nanoparticles utilizing natural honey has been reported.Citation21
In this work, Fe3O4-NPs are synthesized using iron (III) chloride, iron (II) chloride, sodium hydroxide and natural honey at room temperature through a fast precipitation method. The reaction was completed under optimal conditions, and low cost and energy using friendly environment and fresh, nontoxic materials, solvents and also inert residue materials. The synthesis method is based on green chemistry, which is a main step toward safer synthesis and preparation of nanoparticles. To the best of our knowledge, there is no report on the synthesis and characterization of Fe3O4-NPs using natural honey as a stabilizer and co-reducing agent.
Materials and methods
Materials and reagents
All chemicals used in this study were utilized without further purification and were of analytical grade. Chemicals used for the synthesis of Fe3O4-NPs, such as FeCl3⋅6H2O and FeCl2⋅4H2O (99.89%), were obtained from Merck (Frankfurt, Germany), natural honey was collected from the unpolluted cold highlands of Northeastern China, and NaOH (99.0%) was provided by Merck (Frankfurt, Germany). Deionized water was used to prepare all solutions. Glassware was cleaned in HNO3/HCl (3:1, v/v) solution, washed with deionized water and dried before use.
Preparation of Fe3O4 nanoparticles
In the synthesis of Fe3O4-NPs, different amounts of natural honey 0.5, 1.0, 3.0 and 5.0 g were added to 100 mL of deionized water, as the coprecipitation agent and stabilizer to control the particle size. Then FeCl3⋅6H2O and FeCl2⋅4H2O (with a 2:1 molar ratio) were added under continuous stirring and nitrogen gas bubbling to inhibit oxidation. The mixture was titrated against a NaOH (2.0 M) solution under continuous stirring until the pH reached 10. The Fe3O4-NPs were formed instantly from the reduction procedure. The resulting black suspension was centrifuged, washed three times with the deionized water and ethanol mixure and then dried in an oven at 60°C.
Characterization methods and instruments
The phase structure of the nanoparticles was obtained by X-ray diffraction (Cu Kα 1.5406 Å radiation), using a Bruker D8 Advance diffractometer (Bruker AXS, Billerica, MA, USA) at room temperature between 20°–25°, in the 2q scale, with a scanning speed of 0.02°/s and a step time of 3 seconds. Transmission electron microscopy (TEM) was done using a Tecnai G2 F20 transmission electron microscope from FEI (USA), with an acceleration voltage of 200 kV. The size distributions of particles were identified using the ImageJ version 1.46 r program. The morphology of the Fe3O4-NPs was determined through Field emission scanning electron microscopy (FESEM). FESEM and energy-dispersive X-ray (EDX) spectroscopy were performed using Quanta™ 450 FEG, Oxford instrument (USA). The dried samples were coated with gold using a sputter coater. A vibrating sample magnetometer (VSM) was used to study the magnetic properties of the samples utilizing a VSM, Lake Shore Model 7400, Tokyo, Japan, with magnetic fields up to 8 kOe. Fourier transform infrared spectroscopy (FT-IR) was performed between 400 and 4,000 cm−1 to characterize possible biomolecules that are responsible for the capping and efficient stabilization of the Fe3O4-NPs. The FT-IR spectra were observed using a Spectrum 400 FT-IR/FT-FIR spectrometer (Perkin Elmer, Waltham, MA, USA).
Results and discussion
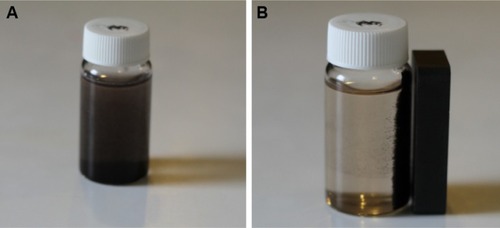
As shown in , once NaOH solution was added as a reducing agent to the honey aqueous mixture with Fe3+ and Fe2+ chloride (2:1 molar ratio), the color of suspension turned black and the Fe3O4-NPs were easily separated by a magnet.
Figure 1 The 0.5% (w/v) honey/magnetite nanoparticles suspension without (A) and with (B) a magnetic field.
The chemical reaction for the precipitation of Fe3O4 is as follows:
Phase structure of nanoparticles
The Fe3O4-NPs were characterized through X-ray powder diffraction (XRD) and all the peaks were analyzed and indexed using the ICDD database, by comparing with the magnetite standards ().Citation28 The peaks were indexed to the (220, 311, 400, 422, 511, 440) and (533) planes, which is attributed to the 2θ of 30.46°, 35.76°, 43.51°, 53.24°, 56.88°, 63.32° and 71.41°, respectively, with the standard diffraction spectrum (ref. code Fe3O4:01-088-0315).Citation29
Figure 2 Powder X-ray diffraction patterns of Fe3O4-NPs without (a) and with different concentrations of honey (0.5, 1, 3, and 5% [w/v]) (b–e), respectively.
Abbreviations: Fe3O4-NPs, magnetite nanoparticles; ref, reference.
![Figure 2 Powder X-ray diffraction patterns of Fe3O4-NPs without (a) and with different concentrations of honey (0.5, 1, 3, and 5% [w/v]) (b–e), respectively.Abbreviations: Fe3O4-NPs, magnetite nanoparticles; ref, reference.](/cms/asset/19d703e8-117c-4baf-9cd8-45a403dd64aa/dijn_a_12193996_f0002_c.jpg)
Generally, the diffraction peaks with less intensity in the XRD pattern show the small size of the Fe3O4-NPs. In this case, there is a decreased intensity of the peaks with increasing honey concentration, which also indicates the decrease in the particle size.
FT-IR spectra analysis
Honey contains proteins, minerals, vitamins and natural sugars (mostly fructose, sucrose and glucose).Citation30–Citation34 Fourier transform infrared spectroscopy (FT-IR) was performed to determine the potential biomolecules that are responsible for efficient stabilization and also capping of Fe3O4-NPs synthesized using honey. The FT-IR spectrum in shows a strong absorption band at 564.99 cm−1, which is assigned to the Fe−O bond, demonstrates a high grade of crystallinity of the Fe3O4-NPs.Citation35 The absorption band at 609.45 cm−1 in the FT-IR spectrum of sample () indicates the presence of some amount of oxidized maghemite on the surface of magnetite. The characteristic band around 3,200 cm−1 is due to the existence of the O–H group.Citation36
Figure 3 Fourier-transform infrared spectra of honey (a); magnetite nanoparticles with 0.5, 1, 3, and 5% (w/v) honey (b–e, respectively).
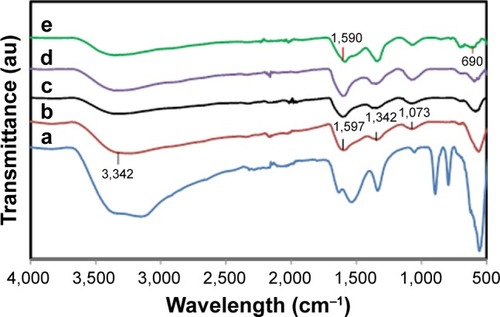
The C–O stretching mode merges band of protein in honey arise from the C–O–C symmetric stretching and C–O–H bending vibrations, expected to occur around 1,073 cm−1.Citation37 The amide I and II bands of proteins are expected to occur around 1,660 and 1,535 cm−1, respectively,Citation38–Citation43 in the current study, these bands occurred around 1,600 and 1,300 cm−1. These bands occur as a result of the carboxyl stretching and N−H deformation vibrations in the amide linkages of protein. Proteins can attach to the Fe3O4-NPs via the free amine group or carboxylate ion of amino acid residues.Citation41,Citation44,Citation45 The lack of C=O band because of the stretching mode, the existence of the C−O stretch and amide I and II bands in the FT-IR spectrum () of Fe3O4-NPs represent the stabilization of the system by the –COO– (carboxylate ion) groups of amino acid remains with free carboxylate groups in the proteins.
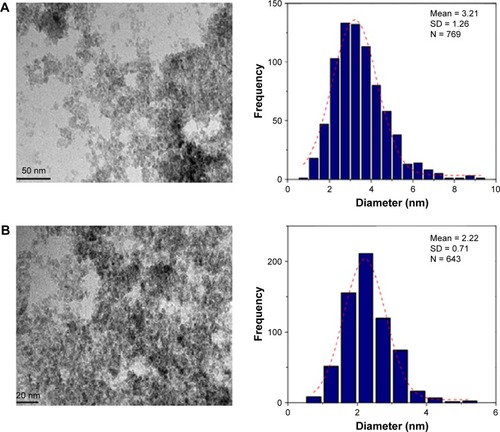
TEM micrographs of the particle size and distribution of the Fe3O4-NPs are presented in . The images reveal significantly smaller nanomagnetite particles with identical particle sizes which have similar shape and are uniformly dispersed. Also, the Fe3O4-NPs have spherical morphology and a uniform distribution. From the TEM images, it is obvious that the particle size decreases from 3.21 to 2.22 nm for 0.5% and 3.0% (w/v) with the increase in the amount of honey. It is significant to mention that sucrose, glucose (decomposition product of sucrose) and gluconic acid have multiple hydroxyl groups, apart from the carboxylic groups in gluconic acid, in the magnetite synthesis. These functional groups can be absorbed into define crystal planes or chelated with the Fe atoms as a covering material to create steric block, like the common stabilizers and surfactants.Citation46
Figure 4 TEM images and histograms of particle size distribution for 0.5% and 3% (w/v) honey/Fe3O4-NPs. (A) and (B): 0.5% and 3% (w/v), respectively.
Abbreviations: Fe3O4-NPs, magnetite nanoparticles; TEM, transmission electron microscopy.
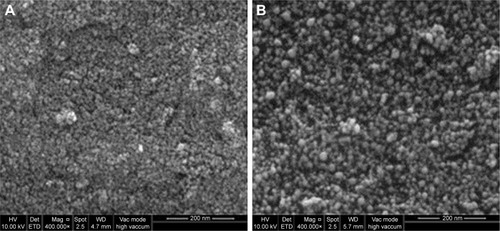
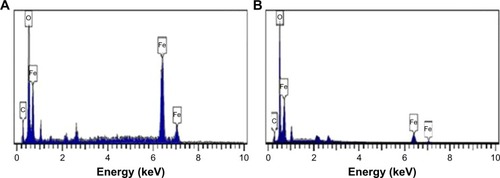
The FESEM images show that the Fe3O4-NPs synthesized using honey have spherical structure (). Significantly, no morphological differences were detected with the increase in the honey concentration and confirm that the structure of the nanoparticles remained unchanged. shows the chemical composition of the prepared nanoparticles. From the EDX spectrum, the oxygen and iron peaks reveal the existence of Fe3O4-NPs; the peaks that arise around 0.7, 6.4 and 7 keV are corresponding to the Fe element.Citation47 Besides, the EDX spectra of the Fe3O4-NPs confirm the presence of elemental Fe without any impurity peaks.
Magnetic properties of nanoparticles
The magnetic characterization of the Fe3O4-NPs was performed with VSM. presents the hysteresis loop of all samples measured with a magnetic field of −8,000 to 8,000 Oe at room temperature. Samples (a), (b), (c), (d) and (e) display almost immensurable coercivity and remanence, indicating that the magnetic nanoparticles prepared are superparamagnetic.Citation48
Figure 7 VSM of pure Fe3O4 (a) and Fe3O4-NPs with 0.5, 1, 3 and 5% (w/v) honey (b–e, respectively).
Abbreviations: Fe3O4-NPs, magnetite nanoparticles; VSM, vibrating sample magnetometry.
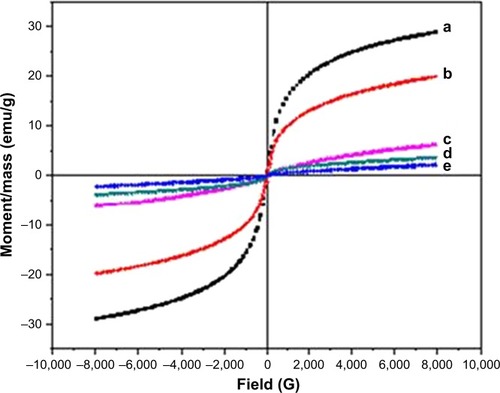
The saturation magnetization (Ms) of Fe3O4-NPs with 5.0, 3.0, 1.0 and 0.5% (w/v) honey are 2.19, 3.75, 6.25 and 19.91 emu g−1, respectively, which increases with the decrease in the honey concentration. This might be referring to the decrease of the surface adsorbed species and growth of the particle size.Citation49 The VSM shows that the Ms of the Fe3O4-NPs prepared using NaOH (without honey) as the reducing agent is 28.98 emu g−1 for sample (a). The presence of organic coating agents on sample (b), (c), (d) and (e) decreased the saturation magnetization value compared with sample (a), which decreases the homogeneity caused by the reducing of the surface moments.Citation50 It is widely identified that the size of magnetic particles has an effect on the energy of that particle in an exterior field through the quantity of magnetic molecules in a single magnetic domain. When the energy is converted into the thermal energy, thermal variations around the prepared Fe3O4-NPs magnetically disturbed surface, which will considerably decrease the overall magnetic moments of a given field.Citation51,Citation52 Thus, this circumstance is more critical for smaller nanoparticles because of the more available surface area; consequently the decrease in the saturation magnetization is reasonable. gives a comparison between the synthetic methods, particle sizes and saturation magnetization of Fe3O4 NPs from the results of other researchers and this work.
Table 1 Comparison between the synthetic methods, particle sizes and saturation magnetization of Fe3O4 NPs from the results of other researchers and this work
Cytotoxicity assay
In vitro cytotoxicity evaluation of Fe3O4-NPs was performed by using 3-(4, 5-dimethylthiazol-2-yl)–2,5-diphenyltetrazolium bromide (MTT) assay. The results are shown in . Briefly, WEHI164 fibro sarcoma cells (Institute Pastor, Iran) at the density of 1×104 cells per well were seeded in 96-well plates and incubated (37°C in 5% CO2) for 24 hours. Then, various concentrations of Fe3O4-NPs were added and incubated for 24 hours. After 24 hours, 20 µL of 5 mg/mL MTT was added and cells were incubated for another 4 hours. Then the medium was removed and 100 µL of DMSO added in each well to dissolve formazan crystals that produced by living cells. In the following, optical absorbance was measured at 570 (630 used as a reference wavelength) by plate reader (Epoch, BioTech instrument, USA). All experiments were performed in quadruplicate, and % cell viability was shown as a percent relative of untreated control cells.Citation62–Citation64
Figure 8 Cell viability of WEHI164 cells measured by the MTT assay (cells were incubated for 24 hours with the indicated concentrations of the magnetite nanoparticles).
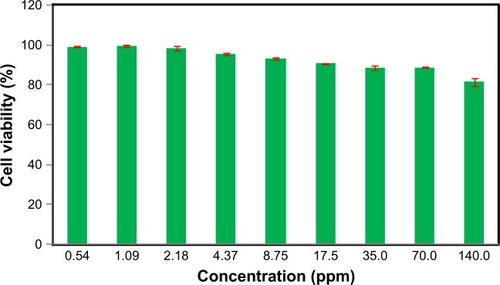
As illustrated in , the toxicity of the synthesized Fe3O4 Au-NPs to the cells was found to be nonsignificant in higher concentrations up to 140.0 ppm and they were well tolerated by WEHI164 cells in the MTT assay.
Conclusion
This study reports the facile and green synthesis of super-paramagnetic Fe3O4-NPs using natural honey as the reducing and stabilizing agent. A sharp band at 564 cm−1 in the FT-IR spectra supplementary confirmed the presence of Fe3O4-NPs. The particle size of the synthesized material can be certainly controlled with the change in the concentration of the natural honey. The TEM images showed that the particle size decreased from 3.21 to 2.22 nm with the increase in the amount of honey from 0.5% to 3.0% (w/v), respectively. The VSM analysis shown a super-paramagnetic behavior of the Fe3O4-NPs, with a small magnetization of 19.91 emu⋅g−1 as compared to the Fe3O4-NPs prepared in the absence of honey (28.98 emu⋅g−1), which is related to the nanoparticle size. From the results of these studies, we believe that the present method can be used for the controlled synthesis of Fe3O4-NPs, which can find important applications in biotechnology, biosensor and biomedicine, magnetic resonance imaging and catalysis. The most possible co-reducing agent is fructose and the existence proteins in the natural honey as the capping agent is responsible for stabilization.
Acknowledgments
We would like to acknowledge the financial support provided by University of Malaya under the Equitable Society Research Cluster (ESRC) research grant GC001C-14SBS.
Disclosure
The authors report no conflicts of interest in this work.
References
- KhandanlouRAhmadMBFard MasoumiHRShameliKBasriMKalantariKRapid adsorption of copper(II) and lead(II) by rice straw/ Fe3O4 nanocomposite: optimization, equilibrium isotherms, and adsorption kinetics studyPLoS One2015103e012026425815470
- Bogunia-KubikKSugisakaMFrom molecular biology to nanotechnology and nanomedicineBiosystems2002652–312313812069723
- DanielMCAstrucDGold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnologyChem Rev2004104129334614719978
- ZharovVPSelf-assembling nanoclusters in living systems: application for integrated photothermal nanodiagnostics and nanotherapyNanomedicine20051432634517292107
- QiaoRYangCGaoMSuperparamagnetic iron oxide nanoparticles: from preparations to in vivo MRI applicationsJ Mater Chem2009193562746293
- SilvaCRFonsecaMGBaroneJSAiroldiCLayered Inorganic– Organic Talc-like NanocompositesChem Mater2002141175179
- ParkJAnKHwangYUltra-large-scale syntheses of monodisperse nanocrystalsNat Mater200431289189515568032
- YuWZhangTZhangJQiaoXYangLLiuYThe synthesis of octahedral nanoparticles of magnetiteMater Lett2006602429983001
- ZhuYWuQSynthesis of magnetite nanoparticles by precipitation with forced mixingJ Nanopart Res199913393396
- MaityDChandrasekharanPYangCTFacile synthesis of water-stable magnetite nanoparticles for clinical MRI and magnetic hyperthermia applicationsNanomedicine20105101571158421143034
- CaiWWanJFacile synthesis of superparamagnetic magnetite nanoparticles in liquid polyolsJ Colloid Interface Sci2007305236637017084856
- VijayaákumarRPreparation of amorphous magnetite nanoparticles embedded in polyvinyl alcohol using ultrasound radiationJ Mater Chem200010511251129
- Martínez-MeraIEspinosa-PesqueiraMEPérez-HernándezRArenas-AlatorreJSynthesis of magnetite (Fe3O4) nanoparticles without surfactants at room temperatureMater Lett20076123–2444474451
- PinnaNGrancharovSBeatoPBonvillePAntoniettiMNiederbergerMMagnetite Nanocrystals: Nonaqueous Synthesis, Characterization, and SolubilityChem Mater2005171130443049
- ChiuWSRadimanSAbdullahMHKhiewPSHuangNMAbd-ShukorROne pot synthesis of monodisperse Fe3O4 nanocrystals by pyrolysis reaction of organometallic compoundMater Chem Phys20071062–3231235
- El GhandoorHSynthesis and some physical properties of magnetite (Fe3O4) nanoparticlesInt J Electrochem Sci20127657345745
- MaityDKaleSNKaul-GhanekarRXueJ-MDingJStudies of magnetite nanoparticles synthesized by thermal decomposition of iron (III) acetylacetonate in tri(ethylene glycol)J Magn Magn Mater20093211930933098
- WooKHongJChoiSEasy synthesis and magnetic properties of iron oxide nanoparticlesChem Mater2004161428142818
- PeiWKumadaHNatusmeTSaitoHIshioSStudy on magnetite nanoparticles synthesized by chemical methodJ Magn Magn Mater2007310223752377
- MizutaniNIwasakiTWatanoSYanagidaTTanakaHKawaiTEffect of ferrous/ferric ions molar ratio on reaction mechanism for hydrothermal synthesis of magnetite nanoparticlesBull Mater Sci2008315713717
- ZhangWShenFHongRSolvothermal synthesis of magnetic Fe3O4 microparticles via self-assembly of Fe3O4 nanoparticlesParticuology201192179186
- HanCCaiWTangWWangGLiangCProtein assisted hydrothermal synthesis of ultrafine magnetite nanoparticle built-porous oriented fibers and their structurally enhanced adsorption to toxic chemicals in solutionJ Mater Chem201121301118811196
- BretcanuOVernéECöissonMTibertoPAlliaPMagnetic properties of the ferrimagnetic glass-ceramics for hyperthermiaJ Magn Magn Mater20063052529533
- JunYWHuhYMChoiJSNanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imagingJ Am Chem Soc2005127165732573315839639
- OsakaTMatsunagaTNakanishiTArakakiANiwaDIidaHSynthesis of magnetic nanoparticles and their application to bioassaysAnal Bioanal Chem2006384359360016402174
- PengSWangCXieJSunSSynthesis and stabilization of monodisperse Fe nanoparticlesJ Am Chem Soc200612833106761067716910651
- SunSZengHRobinsonDBMonodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticlesJ Am Chem Soc2004126127327914709092
- Ayala-ValenzuelaOMatutes-AquinoJBetancourt-GalindoRMagnetite-cobalt ferrite nanoparticles for kerosene-based magnetic fluidsJ Magn Magn Mater20052942e37e41
- KalantariKBin AhmadMShameliKKhandanlouRSynthesis of talc/Fe3O4 magnetic nanocomposites using chemical co-precipitation methodInt J Nanomedicine201381817182323696700
- WhiteJWJrHoneyAdvances in Food Research24Cambridge, MAAcademic Press1978287374367113
- SinghNBathPKQuality evaluation of different types of Indian honeyFood Chem1997581–2129133
- TerrabADíezMJHerediaFJCharacterisation of Moroccan unifloral honeys by their physicochemical characteristicsFood Chem2002793373379
- NandaVSarkarBCSharmaHKBawaASPhysico-chemical properties and estimation of mineral content in honey produced from different plants in Northern IndiaJ Food Compost Anal2003165613619
- DevillersJMorlotMPham-DelègueMHDoréJCClassification of monofloral honeys based on their quality control dataFood Chem2004862305312
- KaraoğluEBaykalAErdemiHAlpsoyLSozeriHSynthesis and characterization of dl-thioctic acid (DLTA)–Fe3O4 nanocompositeJ Alloys Compd20115093792189225
- ChenXYuJZhangZLuCStudy on structure and thermal stability properties of cellulose fibers from rice strawCarbohydr Polym2011851245250
- LiSShenYXieARapid, room-temperature synthesis of amorphous selenium/protein composites using Capsicum annuum L extractNanotechnology20071840405101
- PhilipDBiosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extractSpectrochim Acta A Mol Biomol Spectrosc200973237438119324587
- PhilipDHoney mediated green synthesis of gold nanoparticlesSpectrochim Acta A Mol Biomol Spectrosc200973465065319376740
- BasavarajaSBalajiSDLagashettyARajasabAHVenkataramanAExtracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectumMater Res Bull200843511641170
- AhmadASenapatiSKhanMIKumarRSastryMExtracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora spLangmuir200319835503553
- ShankarSSAhmadAPasrichaRKhanMIKumarRSastryMImmobilization of biogenic gold nanoparticles in thermally evaporated fatty acid and amine thin filmsJ Colloid Interface Sci20042741697515120279
- SolomunTSchimanskiASturmHIllenbergerEReactions of amide group with fluorine as revealed with surface analyticsChem Phys Lett20043874–6312316
- HuangJLiQSunDBiosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leafNanotechnology20071810105104
- AnkamwarBChaudharyMSastryMGold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensingSynth React Inorg Met Org Nano Metal Chem20053511926
- CushingBLKolesnichenkoVLO’ConnorCJRecent advances in the liquid-phase syntheses of inorganic nanoparticlesChem Rev200410493893394615352782
- ChenJWangFHuangKLiuYLiuSPreparation of Fe3O4 nanoparticles with adjustable morphologyJ Alloys Compd20094751–2898902
- LiZSunQGaoMPreparation of water-soluble magnetite nanocrystals from hydrated ferric salts in 2-pyrrolidone: mechanism leading to Fe3O4Angew Chem Int Ed Engl2005441123126
- GoyaGFBerquóTSFonsecaFCMoralesMPStatic and dynamic magnetic properties of spherical magnetite nanoparticlesJ Appl Phys200394535203528
- KimDKMikhaylovaMZhangYProtective coating of super-paramagnetic iron oxide nanoparticlesChem Mater200315816171627
- ChantrellRPopplewellJCharlesSMeasurements of particle size distribution parameters in ferrofluidsIEEE Trans Magn1978145975977
- ShafiKVPMGedankenAProzorovRBaloghJSonochemical Preparation and Size-Dependent Properties of Nanostructured CoFe2O4 ParticlesChem Mater1998101134453450
- DungDTKHaiTHPhucLHLongBDVinhLKTrucPNPreparation and characterization of magnetic nanoparticles with chitosan coatingJ Phys: Conf Ser20091871012036
- KhandanlouRBin AhmadMShameliKKalantariKSynthesis and characterization of rice straw/Fe3O4 nanocomposites by a quick precipitation methodMolecules20131866597660723739066
- KalantariKAhmadMBShameliKHusseinMZBKhandanlouRKhanehzaeiHSize-Controlled synthesis of Fe3O4 magnetic nanoparticles in the layers of montmorilloniteJ Nanomater2014739485
- SunXZhengCZhangFSize-Controlled Synthesis of Magnetite (Fe3O4) Nanoparticles Coated with Glucose and Gluconic Acid from a Single Fe(III) Precursor by a Sucrose Bifunctional Hydrothermal MethodJ Phys Chem C2009113361600216008
- KalantariKAhmadMBShameliKSize-controlled synthesis of Fe3O4 magnetite nanoparticles on the exterior of talc layersRes Chem Intermed201541421392151
- AhmadSRiazUKaushikAAlamJSoft template synthesis of super paramagnetic Fe3O4 nanoparticles a novel techniqueJ Inorg Organomet Polym Mater2009193355360
- AwwadAMSalemNMA Green and Facile Approach for Synthesis of Magnetite NanoparticlesNanosci Nanotechnol201226208213
- MahdaviMNamvarFAhmadMBMohamadRGreen biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extractMolecules20131855954596423698048
- PalanisamyKSynthesis and characterization of olive oil mediated iron oxide nanoparticlesDig J Nanomater Biostruct201382607612
- MokhodoevaOVlkMMálkováEStudy of 223Ra uptake mechanism by Fe3O4 nanoparticles: towards new prospective theranostic SPIONsJ Nanopart Res20161810301
- LahootiASarkarSSaligheh RadHPEGylated superparamagnetic iron oxide nanoparticles labeled with 68Ga as a PET/MRI contrast agent: a biodistribution studyJ Radioanal Nucl Chem20173111769774
- SharkeyJStarkey LewisPJBarrowMFunctionalized superparamagnetic iron oxide nanoparticles provide highly efficient iron-labeling in macrophages for magnetic resonance-based detection in vivoCytotherapy201719455556928214127