Abstract
The purpose of this study was to develop a novel formulation of paclitaxel (PTX) that would improve its therapeutic index. Here, we combined a concept of polymer–PTX drug conjugate with a concept of polymeric micelle drug delivery to form novel free PTX-loaded poly(L-γ-glutamylglutamine) (PGG)–PTX conjugate nanoparticles. The significance of this drug formulation emphasizes the simplicity, novelty, and flexibility of the method of forming nanoparticles that contain free PTX and conjugated PTX in the same drug delivery system. The results of effectively inhibiting tumor growth in mouse models demonstrated the feasibility of the nanoparticle formulation. The versatility and potential of this dual PTX drug delivery system can be explored with different drugs for different indications. Novel and simple formulations of PTX-loaded PGG–PTX nanoparticles could have important implications in translational medicines.
Introduction
Paclitaxel (PTX; Bristol-Myers Squibb, New York, NY, USA), which is extracted from the bark of the Pacific Yew tree,Citation1 is known as Taxol® (Cremophor–ethanol formulation of PTX) and is approved by the US Food and Drug Administration (FDA) for the treatment of ovarian cancer, breast cancer, and lung cancer. However, the formulation of PTX has a low therapeutic index due to its inability to selectively target tumor tissues and to the toxic side effects of the Cremophor–ethanol diluent.Citation2,Citation3 Recently, other formulations have been explored for improving the therapeutic response of PTX. Polymeric micelle formulation of PTX is one of the recent formulations and clinical trials are now underway. NK105 is one of the polymeric micelle formulations that contains amphiphilic block copolymers of poly(ethylene glycol)-modified poly(aspartate) with 4-phenyl-1-butanol modification and entrapped PTX.Citation4 Hamaguchi et al reported that NK105 had higher antitumor activity than PTX and reduced neurotoxicity in mouse models.Citation4 Results of clinical trial studies of NK105 showed that it was well toleratedCitation5 and efficacious in patients with gastric cancers.Citation6 Another polymeric micelle formulation of PTX is Genexol-PM® (Samyang Pharmaceuticals, Daejeon City, Korea),Citation7 amphiphilic micelle-forming diblock copolymers of poly(D, L-lactide) and poly(ethylene glycol), and PTX incorporated within the core. A Phase I clinical trial study showed that Genexol-PM delivered higher PTX drug doses without additional toxicity,Citation8 and it also had a better response rate in patients with metastatic breast cancerCitation9 compared with Taxol.Citation10–Citation12 In addition to polymeric micelles, polymer–PTX conjugate is another formulation of PTX. Several polymer conjugates have been actively pursued and extensively reviewed.Citation13,Citation14 Poly(L-glutamic acid)–PTX conjugate (CT-2103) was considered one of the most successful polymer–PTX conjugates to date.Citation14 CT-2103 was investigated in multitrial Phase III studies,Citation15,Citation16 yet it has not been approved by the FDA. CT-2103 is currently undergoing a Phase III clinical trial in combination with carboplatin against chemotherapy-naïve advanced non-small cell lung cancer in women with estradiol >25 pg/mL.Citation17 Recent formulations of PTX have shown encouraging results.
Previously, we developed a new poly-(L-γ-glutamylglutamine)–paclitaxel nanoconjugate (PGG–PTX).Citation18 PGG–PTX was demonstrated to be efficacious in antitumor activity in vivo and outperformed Abraxane® (Abraxis Bioscience, Summit, NJ, USA) (albumin-bound PTX nanoparticle) in some mouse models.Citation19 Pharmacokinetics and tissue distribution of PGG–PTX resulted in prolonged half-life of total taxane, extractable taxanes, and active free PTX in both the plasma and tumor compartments compared with the Cremophor–ethanol formulation of PTX in BABL/c nude mice bearing lung cancer NCI-H460 xenografts.Citation20 We speculated that hybridizing polymer–PTX conjugate with free PTX would create a novel class of PTX nanoparticle formulation and improve the therapeutic index of PTX.
Materials and methods
Materials
PTX was obtained from NuBlocks (Vista, CA, USA). Ethanol was purchased from Sigma-Aldrich, St Louis, MO, USA. PGG–PTX was synthesized in our laboratory, as described previously.Citation18 1H-nuclear magnetic resonance (1H-NMR) spectra were recorded at 400 MHz with a JEOL spectrometer (JEOL USA Inc., Peabody, MA, USA) at room temperature. 1H chemical shifts were reported in parts per million (ppm). Purified deionized water was prepared by the Milli-Q plus system from Millipore (Billerica, MA).
BABL/c nude mice were purchased from Sino-British SIPPR/BK Lab Animal Ltd (Shanghai, China). All the mice were acclimated for at least 1 week before experimentation. All the studies were performed in accordance with the approved animal protocols.
Preparation of free PTX-loaded PGG–PTX nanoparticles
The free PTX-loaded PGG–PTX nanoparticles were prepared as follows. A solution of free PTX (75 mg) in ethanol (5 mg/mL) was added dropwise into a solution of PGG–PTX (1 g) in distilled water at a concentration of 25 mg/mL, while gently stirring at room temperature. The solution was then opened to air overnight (~15 hours) to allow slow evaporation of ethanol and formation of the nanoparticles. The residual ethanol was removed by vacuum distillation with a rotary evaporator. The colorless nanoparticle solution was filtered with a 0.45 μm pore-sized microfiltration membrane before dynamic light scattering (DLS) studies and transmission electron microscopy (TEM) studies of particle sizing and before injecting to maximum tolerated dose (MTD) and efficacy studies.
1H-NMR characterization
To show the PTX-loading characteristics of the PTX-loaded nanoparticles, the nanoparticle solution was freeze-dried, and the nanoparticles were reconstituted in deuterated water (D2O) or deuterated methanol (CD3OD) at room temperature. 1H-NMR spectra of the free PTX in CD3OD, PGG–PTX nanoconjugate in D2O, and PTX-loaded PGG–PTX nanoparticles D2O and CD3OD were recorded on a Varian 400 MHz spectrometer (Varian, Palo Alto, CA, USA).
Transmission electron microscopy
The morphologies PGG–PTX nanoconjugate and PTX-loaded PGG–PTX nanoparticles were observed by using a TEM H-7000 (Hitachi, Tokyo, Japan), an electron microscope operating at an accelerating voltage of 75 kV. Negative staining was performed as follows: 1) a drop of sample solution was placed onto a copper grid coated with carbon, 2) the sample drop was taped with a filter paper to remove surface water and air-dried for 5 min followed by the application of 0.01% phosphotungstic acid to deposit the micelles on the grid, and 3) the samples were air-dried before observation.
Dynamic light scattering measurements
The particle size of the free PTX-loaded PGG–PTX nanoparticles obtained directly from the nanoparticle formulation was determined by DLS using a Zetasizer Nano-ZS (Malvern Instruments Inc, Malvern, UK) equipped with an He–Ne laser (4 mW, 633 nm) light source and 90° angle scattered light collection configuration. The solution of the free PTX-loaded PGG–PTX nanoparticles was further diluted to the final concentration of approximately 2 mg/mL and was allowed to equilibrate for 2 min at 25°C before the measurements. The hydrodynamic diameter of the free PTX-loaded PGG–PTX nanoparticles was calculated based on the Stokes–Einstein equation. Correlation function was curve fitted by cumulant method to calculate mean size and polydispersity index. All measurements were repeated three times, and mean particle size was presented as the average diameter with standard deviation.
Maximum tolerated dose studies
MTD was defined as the dose that produced 15% weight loss. MTD for PTX-loaded PGG–PTX nanoparticles administered via tail vein injection was investigated in healthy BABL/c nude mice (20 ± 2 g). The nude mice were divided into four groups (n = 5), which were injected with: 1) saline as a control, 2) 210 mg/kg of total PTX of PTX-loaded PGG–PTX nanoparticles, 3) 230 mg/kg of total PTX of PTX-loaded PGG–PTX nanoparticles, and 4) 250 mg/kg of total PTX of PTX-loaded PGG–PTX nanoparticles. Mice survival and variation in body weight were observed and recorded daily over 14 days.
Therapeutic efficacy experiments
Female athymic nude mice were randomly divided into four groups (n = 6). Each group was injected subcutaneously in their shoulders with 0.1 mL of suspension cells containing 4 × 106 viable NCI-H460 human lung cancer cells. Tumors were allowed to grow until they reached an average volume of about 100 mm3. Tumor size in mm3 was estimated from the formula (w2 × l)/2, where “l” is the longest diameter of the tumor and “w” is the diameter perpendicular to the longest diameter measured in millimeters. A single dose of 1) saline, 2) 50 mg/kg PTX formulated in Cremophor–ethanol, 3) 180 mg/kg total PTX equivalent of PTX-loaded PGG–PTX nanoparticles, or 4) 230 mg/kg total PTX equivalent of PTX-loaded PGG–PTX nanoparticles in saline was administered intravenously(IV) via tail vein injection. Body weight change and tumor volume of the mice were measured daily for the duration of the study until total tumor burden of the saline injection as a control reached 3000 mm3, at which time all the mice were terminated from the study.
Statistics
The tumor volume data were shown as mean ± standard error of the mean. The statistical significance of differences in the data between two groups was calculated by means of repeated measures. The slope of the regression of log (tumor volume) on time was determined for each individual tumor and the mean of the slopes of all tumors within a group was compared using Student’s t-test. A value of P < 0.05 was considered statistically significant.
Results and discussion
Polymers have been extensively explored for drug delivery of PTX.Citation4,Citation7,Citation13,Citation14 Polymers can be used either as polymeric PTX micelles or polymer–PTX conjugates. Polymeric micelles employing di-block copolymers are an effective formulation to solubilize PTX. The formulation results in the formation of micellar nanoparticles, which are intended for tumor passive targeting, known as enhanced permeability and retention effects.Citation21,Citation22 PTX conjugated onto PGG is another formulation. Previously, we reported the synthesis, characterization, and in vitro evaluation of PGG–PTX conjugate.Citation18 The PGG–PTX conjugate intriguingly self-assembled into nanoparticles whose size remained in the range of 12–15 nm (volume) over the concentration range from 25 to 2000 μg/mL in saline.Citation18 PGG–PTX conjugate was further demonstrated to possess superior in vivo antitumor efficacyCitation19 and to exhibit prolonged half-life pharmacokinetics.Citation20 Polymeric PTX micelles and PGG–PTX conjugate have their own unique properties.
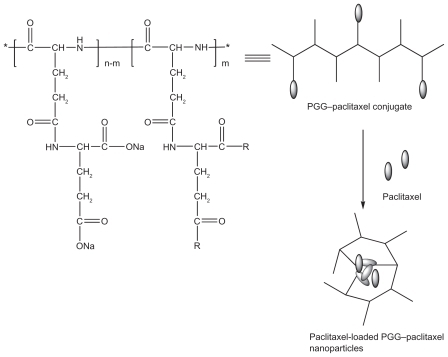
In this study, we presented the feasibility of combining polymeric micelles and polymer conjugates into one nanoparticle delivery system of PTX. The novel nanoparticle delivery system featured entrapped PTX and conjugated PTX. A schematic formulation of PTX-loaded PGG–PTX nanoparticles is shown in . The design of the nanoparticle formation was simple. A solution of free PTX in ethanol (5 mg/mL) was added to a solution of polymer–PTX conjugate in water (25/mL) while being stirred, and the nanoparticles were formed after the ethanol was slowly evaporated overnight. The percentage of PTX relative to PGG–PTX conjugate was 7.5% weight by weight. To our knowledge, this is the first polymeric nanoparticulate containing entrapped free PTX drug and conjugated PTX drug united into one delivery system.
Figure 1 Schematic of formation of free paclitaxel-loaded PGG–PTX nanoparticles.
Abbreviations: PGG, poly(L-γ-glutamylglutamine); PTX, paclitaxel.
Encapsulation of free PTX with PGG–PTX conjugate was demonstrated by comparing 1H-NMR spectra of free PTX in CD3OD, PGG–PTX conjugate in D2O, and PTX-loaded PGG–PTX nanoparticles in CD3OD and D2O. 1H-NMR spectra of PTX in CD3OD (A), PGG–PTX conjugate in D2O (B), PTX-loaded nanoparticles in D2O (C), and CD3OD (D), respectively, are shown in . When PTX was encapsulated inside the nanoparticles, the characteristic peaks of PTX were hardly seen in D2O due to their insufficient mobility in D2O. However, in CD3OD, resonance peaks corresponding to the PTX were clearly observed. This observation was consistent with 1H-NMR studies of the other polymer micelles in D2O reported.Citation23,Citation24 The 1H-NMR results indicated that PTX was successfully entrapped into the hydrophobic inner core of the polymeric nanoparticles.
Figure 2 A) 1H-NMR spectra of paclitaxel in CD3OD; B) PGG–PTX nanoconjugate in D2O; C) paclitaxel-loaded PGG–PTX nanoparticles in D2O; and D) paclitaxel-loaded PGG–PTX nanoparticles in CD3OD.
Note: *Free paclitaxel protons.
Abbreviations: PGG, poly(L-γ-glutamylglutamine); ppm, parts per million; PTX, paclitaxel.

Further DLS and TEM studies confirmed the formation of PTX-loaded PGG–PTX nanoparticles. The average particle size and the unimodal size distribution of both PGG–PTX conjugate and PTX-loaded PGG–PTX were examined by DLS, and the results are illustrated in . The Z-mean diameter of PGG–PTX conjugate in water was approximately 20 nm, with a narrow polydispersity index (PDI) of 0.27. The particle size of PGG–PTX conjugate in water was consistent with the previous study of the conjugate in saline, which was about 12–15 nm.Citation18 The particle size and distribution of free PTX-loaded nanoparticles in water were 270 nm and 0.08 PDI, respectively. Furthermore, TEM images of PGG–PTX conjugate and PTX-loaded nanoparticle exhibited a spherical shape of moderate uniform particle size, as shown in . The particle size of PGG– PTX conjugate observed by TEM was about 20 nm, which was consistent with the DLS data. However, particle size of PTX-loaded PGG–PTX nanoparticle observed by TEM was about 50 nm, which was smaller than that determined by DLS. We speculated that the particle size determined by DLS represents their hydrodynamic diameter, whereas that obtained by TEM is related to the collapsed micelles after water evaporation. The TEM diameter of the PTX-loaded nanoparticles was smaller than the DLS diameter.
Figure 3 The particle size distribution of PGG–PTX nano-conjugate A) and paclitaxel-loaded PGG–PTX nanoparticles B) measured by DLS.
Abbreviations: d.nm; diameter in nanometers; DLS, dynamic light scattering; PGG, poly(L-γ-glutamylglutamine); PTX, paclitaxel.
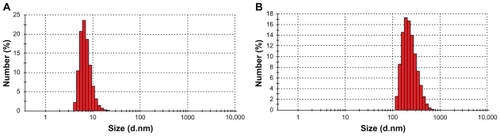
Figure 4 TEM micrograph of the PGG–PTX nanoconjugates a) and paclitaxel–loaded PGG–PTX nanoparticles b).
Abbreviations: PGG, poly(L-γ-glutamylglutamine); PTX, paclitaxel; TEM, transmission electron microscopy.

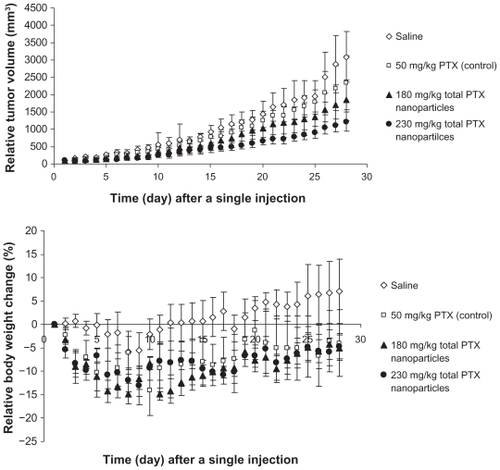
To confirm the applicability of the free PTX-loaded PGG–PTX nanoparticles, the nanoparticles were investigated for antitumor activity in mouse-bearing human lung NCI-H460 cancer models. Prior to antitumor efficacy study, MTD of the nanoparticles needed to be determined. With respect to PTX-loaded PGG–PTX nanoparticles, MTD was defined as the dose that yielded 15% weight loss. The MTD studies for the PTX-loaded nanoparticles were carried out in healthy BABL/c nude mice. The mice were divided into three experimental groups and one saline control group (n = 5 for each group). The mice of experimental groups were administered with a single IV tail vein injection of 1) 210 mg/kg, 2) 230 mg/kg, and 3) 250 mg/kg of total PTX of PTX-loaded PGG–PTX nanoparticles. Mice survival was observed, and their body weight change was measured and recorded daily for the duration of the study. No mice died during the MTD study period. The details of their body weight change are shown in . When the doses of the PTX-loaded nanoparticles were administered, 5%–15% body weight loss was observed. The body weight of the healthy BABL/c nude mice was consistent with the escalation of the doses. On the basis of the results, MTD of the PTX-loaded nanoparticles was decided at 230 mg/kg of total PTX. With the MTD of the PTX-loaded nanoparticles, a study of antitumor efficacy was carried out on BABL/c nude mice bearing NCI-H460 human lung cancer xenografts. The mice were randomly divided into two experimental groups (180 and 230 mg/kg of the total PTX equivalence of PTX-loaded nanoparticles), one positive control group (50 mg/kg of PTX), and one saline negative control group (n = 6 for each group). The mice received a single IV tail vein injection after 12 days of subcutaneous implantation with the NCI-H460 human lung cancer cells when the mean tumor volume reached 100 mm3. The antitumor activity of the nanoparticles was compared with that of PTX and saline by measuring tumor volume and survival rates of the mice. Results of the efficacy study are shown in . The results indicated that the PTX-loaded nanoparticles inhibited tumor growth. At the dose of 230 mg/kg of the total PTX equivalents, PTX-loaded nanoparticles showed stronger tumor regression with statistically significant differences compared with that of 50 mg/kg of PTX (P = 0.036), 180 mg/kg of the total PTX of the PTX-loaded nanoparticles (P = 0.019), and saline (P = 0.006) after administration. However, it seemed that the PTX-loaded PGG–PTX nanoparticles exhibited higher toxicity in mice-bearing human lung NCI-H460 cancer (, bottom) compared with that of healthy mice () based on their body weight loss. Surprisingly, the dose of 180 mg/kg, PTX equivalents, of the nanoparticles appeared to lead to more weight loss than that of 230 mg/kg (, bottom). No statistical significance of tumor inhibition was observed between 180 mg/kg of the total PTX of the PTX-loaded nanoparticles and 50 mg/kg of PTX (P = 0.934). The antitumor efficacy of 230 mg/kg of the total PTX of the novel PTX-loaded nanoparticles confirmed the feasibility of a dual PTX drug delivery system to be applicably useful for chemotherapy.
Figure 5 Maximum tolerated dose of paclitaxel-loaded PGG–PTX nanoparticles. Body weight change as a function of time in the normal nu/nu mice (n = 5) with a single IV tail vein injection of either saline (⋄), 210 mg PTX/kg (■), 230 mg PTX/kg (▴), or 250 mg PTX/kg (●) paclitaxel-loaded PGG–PTX nanoparticles. Vertical bars ±SEM.
Abbreviations: IV, intravenous; PGG, poly(L-γ-glutamylglutamine); PTX, paclitaxel; SEM, standard error of the mean.
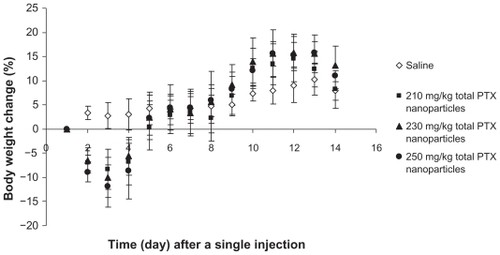
Figure 6 Relative tumor volume (top) and body weight change (bottom) as a function of time in nude mice (n = 6) bearing NCI-H460 tumors treated with a single IV tail vein injection of either saline (⋄), 50 mg PTX/kg (□) (paclitaxel Cremophor–ethanol formulation), 180 mg PTX/kg (▴) (paclitaxel-loaded PGG–PTX nanoparticles), or 230 mg PTX/kg (●) (paclitaxel-loaded PGG–PTX nanoparticles). Vertical bars ±SEM.
Abbreviations: IV, intravenous; PGG, poly(L-γ-glutamylglutamine); PTX, paclitaxel; SEM, standard error of the mean.
Conclusion
The results of this study contribute two important scientific issues. First, we provide the first and most simple method of forming nanoparticles featuring free PTX and conjugated PTX in the same drug delivery system. Second, these nanoparticles can effectively inhibit tumor growth. The versatility and potential of this dual drug system can be explored with different drugs for different indications. Developing novel and simple PTX-loaded PGG–PTX nanoparticles could have important implications in translational medicines.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, 2007CB935802) and Nitto Denko Technical Corporation.
Disclosure
No conflicts of interest were declared in relation to this paper.
References
- WaniMCTaylorHLWallMECoggonPMcPhailATPlant antitumor agents. VI. The isolation and structure of taxol, a novel anti-leukemic and antitumor agent from Taxus brevifoliaJ Am Chem Soc197193232523275553076
- WeissRDonehowerRCWiernikPHHypersensitivity reactions from TaxolJ Clin Oncol19908126312681972736
- Meerum TerwogtJMNuijenBHuininkTenBokkelWWBeijnenJHAlternative formulations of paclitaxelCancer Treat Rev19972387959225960
- HamaguchiTMatsumuraYSuzikiMNK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxelBr J Cancer2005921240124615785749
- KatoKHamaguchiTYasuiHPhase I study of NK105, a paclitaxel-incorporating micellar nanoparticle, in patients with advanced cancerJ Clin Oncol20062418S2018
- ChinKKatoKYoshikawaTPhase II study of NK105, a paclitaxel-incorporating micellar nanoparticle as second-line treatment for advanced or recurrent gastric cancerJ Clin Oncol20102815S4041
- KimSCKimDWShimYHIn vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacyJ Control Release20017219120211389998
- KimTYKimDWChungJYPhase I and pharmacokinetic study of Genexol-PM, a Cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignanciesClin Cancer Res2004103708371615173077
- LeeKSChungHCImSAMulticenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancerBreast Cancer Res Treat200810824125017476588
- Di LeoAGomezHLAzizZPhase II, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancerJ Clin Oncol2008265544555218955454
- JonesSEErbanJOvermoyerBRandomized phase III study of decetaxel compared with paclitaxel in metastatic breast cancerJ Clin Oncol2005235542555116110015
- ParidaensRBiganzoliLBruningPPaclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European organization for research and treatment of cancer randomized study with cross-overJ Clin Oncol20001872473310673513
- DuncanRThe dawning era of polymer therapeuticsNat Rev Drug Discov2003234736012750738
- HaagRKratzFPolymer therapeutics: concepts and applicationsAngew Chem Int Ed20064511981215
- O’BrienMERSocinskiMAPopovichAYRandomized phase III trial comparing sing-agent paclitaxel poliglumex (CT-2103, PPX) with single-agent gemcitabine or vinorelbine for the treatment of PS 2 patients with chemotherapy-naïve advanced non-small cell lung cancerJ Thoracic Oncol20083728734
- Paz-AresLRossHO’BrienMPhase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancerBr J Cancer2008981608161318475293
- US National Institute of HealthClincalTrails.gov Available from: http://clinicaltrials.gov/Accessed 2010 Dec 02
- VanSDasSKWangXSynthesis, characterization, and biological evaluation of poly(L-γ-glutamylglutamine)-paclitaxel nanoconjugateInt J Nanomedicine2010582583721042550
- FengZZhaoGYuLGoughDHowellSBPreclinical efficacy studies of a novel nano-particle-based formulation of paclitaxel that out-performs AbraxaneCancer Chemother Pharmacol20106592393019685054
- WangXZhaoGVanSPharmacokinetics and tissue distribution of PGG-paclitaxel, a novel macromolecular formulation of paclitaxel, in nu/nu mice bearing NCI-460 lung cancer xenograftsCancer Chemother Pharmacol20106551552619593566
- MatsumuraYMaedaHA new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumouritropic accumulation of proteins and the antitumor agent smancCancer Res198646638763922946403
- MaedaHWuJSawaTMatsumuraYHoriKTumor vascular permeability and the EPR effect in macromolecular therapeutics: a reviewJ Control Release2000291723
- HealdCRStolnikSKujawinskiKSPoly(lactic acid)–poly(ethylene oxide) (PLA−PEG) nanoparticles: NMR studies of the central solidlike PLA core and the liquid PEG coronaLangmuir20021836693675
- HrkachJSPeracchiaMTDombALotanNLangerRNanotechnology for biomaterials engineering: structural characterization of amphiphilic polymeric nanoparticles by 1H NMR spectroscopyBiomaterials19971827309003893