Abstract
The main goal of this investigation was to develop and test a new method of treatment for human hepatocellular carcinoma (HCC). We present a method of carbon nanotube-enhanced laser thermal ablation of HepG2 cells (human hepatocellular liver carcinoma cell line) based on a simple multiwalled carbon nanotube (MWCNT) carrier system, such as human serum albumin (HSA), and demonstrate its selective therapeutic efficacy compared with normal hepatocyte cells. Both HepG2 cells and hepatocytes were treated with HSA–MWCNTs at various concentrations and at various incubation times and further irradiated using a 2 W, 808 nm laser beam. Transmission electron, phase contrast, and confocal microscopy combined with immunochemical staining were used to demonstrate the selective internalization of HSA–MWCNTs via Gp60 receptors and the caveolin-mediated endocytosis inside HepG2 cells. The postirradiation apoptotic rate of HepG2 cells treated with HSA–MWCNTs ranged from 88.24% (for 50 mg/L) at 60 sec to 92.34% (for 50 mg/L) at 30 min. Significantly lower necrotic rates were obtained when human hepatocytes were treated with HSA–MWCNTs in a similar manner. Our results clearly show that HSA–MWCNTs selectively attach on the albondin (aka Gp60) receptor located on the HepG2 membrane, followed by an uptake through a caveolin-dependent endocytosis process. These unique results may represent a major step in liver cancer treatment using nanolocalized thermal ablation by laser heating.
Introduction
Hepatocellular carcinoma (HCC) represents a leading cause of cancer deaths worldwide.Citation1–Citation3 Despite recent discoveries in screening and early detection, HCC exhibits a rapid clinical course with an average survival of 6 months and an overall 5-year survival rate of 5%.Citation4 As chemotherapy and radiotherapy show modest resultsCitation5 and surgery is possible in 10%–30% of patients,Citation6,Citation7 new therapeutic methods offer hope for a better outcome.
Most data suggest that nanotechnologies could play a major role in the development of new anticancer therapies. A thermal approach using nanoparticles, nanoemulsion, pH-responsive nanoparticles, nanoparticles combined with radiation, and nanovectors for drug delivery are the most explored nanoparticle-based cancer treatment methods.Citation8
The ability of carbon nanotubes (CNTs) to convert near-infrared (NIR) laser radiation into heat, due to the photon–phonon and electron interactions,Citation9 provides the opportunity to create a new generation of immunoconjugates for cancer phototherapy, with good performance and efficacy in selective cancer thermal ablation, as well as in the application of nanotechnology in molecular diagnostics (nanodiagnostics).Citation8,Citation10
Nanotechnology has already shown promising results in HCC research and treatment. MicrowaveCitation11 ablation and radiofrequencyCitation12 ablation were proposed for the treatment of HCC. Intratumorally administered CNTs combined with laser irradiation proved to be efficient in the treatment of HCC on animal models.Citation13 However, a major challenge in treating HCC is represented by therapies strictly directed toward the tumor cells inside the liver parenchyma. Generally, the use of targeting molecules such as antibodies, folates, and growth factors has been specifically proposed for carrying nanomaterials to the cancer cells and tumors.Citation14–Citation16 However, 100% selective internalization of nanobioconjugates in the cancer cells remains problematic.Citation17 This can be explained by the presence of the receptors used for the specific binding of the targeting molecules on the membranes of the noncancerous cells, although in smaller concentrations compared with the cancer cells.Citation18
The use of CNTs as bioactive molecules is still at an early research stage, but their unique physical and chemical properties hold great hope for cancer treatment.Citation8,Citation14,Citation16–Citation18 Nevertheless, there are many toxicity concerns to be addressed. It has been stated that a proficient method needed to minimize toxic effects and also to increase the level of therapeutic response for CNTs is represented by their conjugation to a carrier molecule.Citation19–Citation23 The use of these biological carriers for the development of specific and sensitive site-targeted bionanosystems also allows the selective internalization of CNTs into cancer cells.
Research data have shown that highly proliferative tumors have the capacity to create albumin deposits.Citation24 The reports have demonstrated the liver cancer cells’ overexpression of specific human serum albumin (HSA) receptors and their ability to internalize large amounts of albumin through the mechanism of caveolae-mediated endocytosis.Citation25 The resulting amino acids are further used for the synthesis of various substrates needed for tumor growth.Citation26,Citation27 Considering all these data together, we propose a method for the functionalization of multiwalled carbon nanotubes (MWCNTs) with HSA for the selective targeting and laser-mediated necrosis of liver cancer cells. To our knowledge, this is the first demonstration of selective targeting via Gp60 receptors located on the membrane of malignant liver cancer cells using a conjugate of HSA and CNTs.
Material and methods
Antibodies and reagents
For the experiments involving the noncovalent functionalization of CNTs, MWCNTs (>90% carbon basis, OD × ID ×L 10–15 nm ×2–6 nm ×0.1–10 μm, product number 677248), HSA, and Sephacryl 100-HR were purchased from Sigma-Aldrich (Steinheim, Germany), and all the other chemicals were purchased from Merck (Darmstadt, Germany). HepG2 cells and immortalized hepatocyte epithelial cells (CRL-4020) were purchased from ATCC (Rockville, MD, USA), and all the other reagents needed for cell culture were purchased from Sigma-Aldrich. For the experiments involving cell apoptosis, Cell Death Detection ELISAPLUS was purchased from Roche Applied Science (Mannheim, Germany). For immunostaining procedures, Draq5, 4′-6-diamidino-2-phenylindole (DAPI), and anti-caveolin-1–Cy3 antibody (Ab) produced in rabbit were purchased from Sigma-Aldrich. Polyclonal Gp60 Ab was prepared as previously described,Citation28 and for use as a fluorescent probe a cy3 derivative of anti-Gp60 was prepared according to the existing protocol.Citation29
Noncovalent functionalization of CNTs with HSA
A total of 60 mg MWCNTs were dispersed in a 3:1 (v/v) mixture of concentrated sulfuric and nitric acid and sonicated for 3 ×10 s with a tip sonicator. Subsequently, the mixture was refluxed at 120°C for 30 min. The oxidized MWCNTs treated in water solution were then centrifuged at 8000 rpm to remove any large unreacted CNTs from the solution and metallic impurities. Finally, the oxidized MWCNTs were vacuum filtered through a 0.2-μm polycarbonate filter (Whatman) until the elution was clear and at neutral pH. The filter cake was dried overnight at room temperature. After filtration, the solution concentration was re-estimated using UV–Vis–NIR spectroscopy (JASCO V530, Gross-Umstadt, Germany). A total of 1 mg of fluorescein isothiocyanate (FITC) (10 mg/mL in dimethyl sulfoxide) was mixed with 50 mg HSA in sodium buffer (20 mM, pH 8.5), followed by incubation for 2 h in darkness, at room temperature, with continuous stirring. The HSA–FITC conjugate was purified by gel chromatography using a Sephacryl 100-HR column eluted with 10 mM phosphate buffered saline (PBS).Citation30
Oxidized MWCNTs and HSA–FITC were mixed with deionized water at a concentration of 0.25 and 1.25 mg/mL, respectively. The mixture was sonicated for 1 h with a tip sonicator in an ice bath and was then centrifuged for 5 min at 12,000 rpm. The solid was settled at the bottom of the centrifuge tube and consisted of unbound nanotubes, impurities, metals, and bundles of oxidized nanotubes. The resulting supernatant was collected and subjected to a second centrifugation round. The supernatant collected contained the desired MWCNT–HSA conjugate.
For further purification, the supernatant was subjected to a gel chromatography purification process. Sephacryl 100-HR that was presoaked and deaerated using a vacuum pump was packed up to 15 cm in a 2.5 cm diameter ×24 cm long glass column. The oxidized MWCNT–HSA supernatant recovered after centrifugation was layered on the top of the gel and eluted using water flowing under gravity. Volume fractions were collected for periods of 1 min duration and analyzed for the presence of MWCNTs and HSA by measuring the absorbance at 500 and 280 nm, respectively, using the spectrophotometer (JASCO V530). Fractions showing protein content were pooled for further use.
Preparation of MWCNTs
The nonconjugated highly purified MWCNT control solution was prepared as previously described.Citation31 The solution was diluted in minimum essential medium at a 1:10 (v/v) ratio.
Characterization of MWCNTs bioconjugates
The morphology of MWCNTs functionalized with HSA was examined using a WITEC alpha 300 Atomic Force Microscope (Ulm, Germany), operating under ambient conditions. The images were collected in tapping mode using a silicon nitride cantilever.
The optical properties of oxidized MWCNTs functionalized with HSA–FITC were monitored using a UV–Vis spectrophotometer (JASCO V570).
Fourier transform infrared (FTIR) measurements were performed with a JASCO 6100 spectrometer in the 4000–500 cm−1 spectral region, with a resolution of 4 cm−1 using the KBr pellet technique.
Cell culture
HepG2 and CRL-4020 cells, purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA), were grown in 25 cm3 Corning plastic plates in minimum essential medium, supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. The cells were maintained in a humidified 5% CO2 incubator at 37°C. The cells were kept in the logarithmic growth phase by routine passage every 3–4 days. When reaching confluence, the cells were split after rinsing with PBS and detached with trypsin.
For the experiments, the cells were cultivated to confluence on 60 mm plates. The MWCNTs functionalized with HSA were further administered to the cell cultures by adding to the culture medium and incubating for various periods of time (1 min; 30 min; 1 h; 5 h; 24 h) at increased concentrations: 1, 5, 20, 50 mg/L. For each concentration, all the experiments were performed in triplicate.
Cell characterization
For the microscopy analysis, the cells were trypsinated and transferred to 35 mm plates, at a density of 25 ×104 cells/dish. After administration and irradiation, the cells were thoroughly washed with 1×PBS three times, fixed with 10% formaldehyde solution for 10 min, washed three times with PBS, and stained with methyl green dye for 10 min. Cells in culture were examined using an Olympus CKX 31 (Munich, Germany) inverted microscope with phase contrast.
Cell viability
The extent of apoptosis was evaluated using a Cell Death Detection ELISAPLUS assay kit from Roche Applied Science. The assay is a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) that uses the fact that, due to cellular death, nucleosomes are released from the nucleus into the cytosol. These nucleosomes can be detected by antihistone biotin-labeled Abs. The nucleosome–Abs complex will bind to streptavidin-coated well plate and give a signal at 405 nm on the addition of substrate. After irradiating the cells that were previously treated with various doses of HSA–MWCNTs, the culture media were removed and briefly centrifuged in order to collect the floating cells. The culture dish was rinsed with PBS, and then 0.25% trypsin was added to detach the cells. Once detached, the cell suspension was combined with the cells collected from the media. The resulting mixture of the cells was briefly spun to collect the cells. The supernatant was discarded, and the pelleted cells were resuspended in ice-cold PBS. The cell suspension was subjected to the final centrifugation, and the pellet was resuspended in the Roche lysis buffer. After 30 min incubation at room temperature, the reaction mixture was centrifuged at 200 g (4°C) for 10 min. The pellet, which contains the nucleus, was removed, and the supernatant, which represents the cytoplasmic fraction, was aliquoted into new tubes and kept frozen at −80°C until use.
This supernatant solution would contain the fragmented nucleosomes if the cells underwent apoptosis. After measuring the protein concentration of the resulting supernatant using bicinchoninic acid (BCA) assay, 20 g of total protein in 20 μL were added to the streptavidin-coated 96-well plate. Twenty microliters of each incubation buffer and DNA–histone complex was used as a background control and positive control, respectively. Then, 80 μL of immunoreagent was added to each well and incubated for 2 h at room temperature, with gentle and continuous stirring. After the incubation, the solution in the wells was thoroughly removed using gentle suction and rinsed three times in incubation buffer. Finally, 100 μL of 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) substrate solution was added to each well and incubated until the desired strength of color was achieved, which took about 7 min.
The multiwell plates were then placed into a Labsystem Multiskan Plus Spectrophotometer (Helsinki, Finland). The absorbance was measured at 405 nm, with 495 nm as the reference wavelength. The absorbance at 495 nm was deducted from the absorbance at 405 nm for all samples and controls. Then, the OD405–OD495 value of the background control, which is composed of the incubation buffer and ABTS solution, was subtracted from all OD405–OD495 values of the samples. The intensity of apoptosis can be expressed as enrichment factor = (mU of the sample)/(mU of the corresponding negative control), where mU is the absorbance ×10−3 after subtracting the reference absorbance and the OD405–OD495 value of the background control. The enrichment factor exhibits the specific enrichment of mono-nucleosomes and oligonucleosomes released into the cytoplasm of the cells that are dying and dead due to apoptosis. Finally, the values were normalized so that the untreated sample could have an enrichment factor equal to 1.Citation32
Laser treatment
We used 2 W of power laser (Apel Laser, Bucharest, Romania) operating at 808 nm for a 2 minutes irradiation of a monolayer of cells placed on a glass substrate, after being incubated with HSA–MWCNTs for various periods of time. The laser diode was placed 3 cm away from the surface of the glass, at a vertical angle, and the beam had a Gaussian distribution with a 1/e2 value of 2 mm.
Laser confocal microscopy of cells
Fluorescent images were acquired using a Zeiss LSM 710 confocal laser scanning unit (Oberkochen, Germany) equipped with argon and an HeNe laser mounted on an Axio Observer Z1 Inverted Microscope. Hep2G cells or human hepatocytes from the suspension were briefly rinsed with PBS and fixed in 4% formaldehyde (pH 7) for 15 min. After three washing procedures in PBS for 15 min, the slides were covered for 60 min with a serum-free blocking buffer (Dako Cytomation, Glostrup, Denmark). The dying procedures were made in accordance with manufacturers’ protocols. Specific visualization of cell structures was performed using 364, 488, and 568 nm excitation laser lines to detect Draq5 (BP 590–650 nm emission), DAPI (BP 385–470 nm emission), FITC (BP505–550 emission), and cy3 fluorescence (LP585 emission), respectively.
Transmission electron microscopy analysis
The internalization of the functionalized nanotubes was investigated using transmission electron microscopy (TEM) in conventional electron beam conditions. Live cells were incubated in an HSA–MWCNT solution as described previously. After the final PBS rinsing, the cells were fixed using 2.5% glutaraldehyde in 0.1 M cacodylate buffer and embedded in agarose. After three rinses with sodium phosphate buffer, the monolayers were sectioned into small pieces, postfixed with 1% osmium tetroxide, en bloc stained with 1% uranyl acetate, dehydrated in graded ethanol series (30%, 50%, 75%, 100%, 10 min each), and embedded in EMbed 812 resin. Ultrathin (<100 nm) sections were cut on an LEICA EM UC6 Ultramicrotome (Leica Microsystems, Wetzlar, Germany), poststained with 4% uranyl acetate and lead citrate, and viewed using a Jeol JEM 1010 TEM (Jeol, Tokyo, Japan). The images were captured using a Mega VIEW III camera (Olympus, Soft Imaging System, Münster, Germany).
Statistical data analysis
All data were expressed as mean ± standard error of the mean. Nonparametric tests were selected due to data nonnormality (Kolmogorov–Smirnov test). Between-group comparisons for the same concentration were tested using the Wilcoxon test. Alpha error level of <0.05 was selected for all tests. SPSS Statistics Version 17.0 (Chicago, IL, USA) packages, as well as the Microsoft Office Excel application, were used for data analysis.
Results
Functionalization of MWCNTs with HSA
In order to obtain a directly targeted delivery of MWCNTs into the cancer cells and to visualize and detect the localization of the nanotubes inside the cell, the FITC–HSA system was preformed and noncovalently labeled on the oxidized surface of MWCNTs.
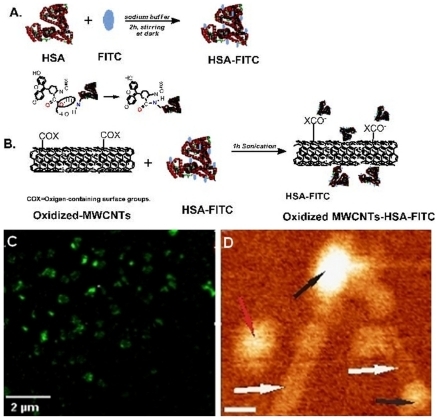
To provide clues regarding the success of noncovalent HSA–MWCNT functionalization, confocal microscopy was proposed for the identification of FITC-labeled CNTs in solution. As shown in , globular green CNTs corresponding to large molecules of fluorescent albumin were observed.
Figure 1 A) Illustration of the covalent labeling of HSA with FITC. B) The formation of oxidized MWCNTs–HSA–FITC. C) A typical fluorescent image of HSA–MWCNTs (100 mg/L): globular fluorescent CNTs corresponding to attached large molecules of fluorescent albumin are being observed. D) 140 ×120 nm AFM topographic image of HSA (black arrows) conjugated with MWCNTs (white arrows). The red arrow indicates the presence of an unconjugated HSA molecule. The scale bar represents 20 nm (bottom-right panel).
Abbreviations: AFM, atomic force microscopy; FITC, fluorescein isothiocyanate; HSA, human serum albumin; MWCNTs, multiwalled carbon nanotubes.
The oxidation of the nanotubes using a 3:1 (v/v) mixture of concentrated sulfuric and nitric acid gave them hydrophilicity and stability in aqueous systems due to the formation of –COOH, OH groups at the end and along the sidewalls of the tubes.Citation21
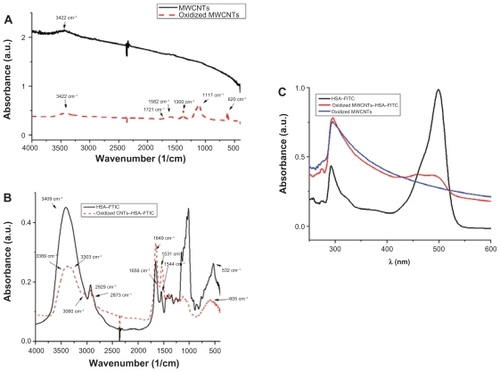
FTIR spectra from confirm successful oxidation. Comparing the FTIR spectra of pristine MWCNTs (black) with those of oxidized MWCNTs (red), the characteristic bands of the oxygen-containing groups appear at 3422 cm−1, corresponding to the stretching vibration of O–H and water,Citation33 a band at 1721 cm−1, corresponding to the carbonyl and carboxyl C=O stretching vibration, at 1582 and 1380 cm−1, corresponding to the O–H deformation vibration, and the band at 1117 cm−1, corresponding to the C–O stretching vibration. The band at 620 cm−1 corresponds to the CO out-of-plane deformation.Citation34
Figure 2 FTIR spectra of A) pristine MWCNTs (black) and oxidized MWCNTs (red); B) HSA–FITC (black) and HSA–FITC-coated oxidized MWCNTs (red); C) UV–Vis adsorption spectra of HSA–FITC (black), oxidized MWCNTs (blue), oxidized MWCNTs–HSA–FITC (red).
Abbreviations: FITC, fluorescein isothiocyanate; FTIR, Fourier transform infrared; HSA, human serum albumin; MWCNTs, multiwalled carbon nanotubes.
Further, we conjugated the HSA–FITC system noncovalently on the surface of oxidized MWCNTs. First, we covalently labeled HSA with FITC at an increased pH (above pH = 9), as shown schematically in .Citation35 FITC covalently attached to the protein through the alpha-amino group. Second, HSA– FITC complex was adsorbed on the nanotubes, presumptively, through electrostatic interactions between the functional groups of MWCNTs and the protein-positive domains (). Considering the fact that not all the surface of the nanotubes is oxidized, hydrophobic interactions can also occur.Citation36
UV–Vis spectroscopy is a simple but efficacious method that confirms the formation of the oxidized MWCNT–HSA– FITC complex. The nanotubes solutions give an adsorption band at 295.7 cm−1, which corresponds to the +-plasmon transition of MWCNT.Citation37
The yellowish HSA–FITC solution has the characteristic adsorption band at 489 cm−1 and a second adsorption band at 292 cm−1, suggesting the existence of aromatic amino acids from HSA. Comparing the aforementioned spectra, the formation of the MWCNTs–HSA–FITC complex becomes obvious due to the appearance of the oxidized MWNT band and the HSA–FITC band at 475.6 cm−1, which is shifted and has low intensity ().
The conjugation of HSA–FITC onto the surface of the nanotubes is also confirmed by FTIR spectroscopy as seen in . No similarity can be observed when comparing the spectra of HSA–FITC with those of the nanotube-conjugated HSA–FITC. All the corresponding peaks had shifted their position, and some even disappeared. In the higher region, the stretching vibration band of the N–H groups at 3409 cm−1 changed their shape in a broad band that included two peaks: one at 3389 cm−1 (N–H groups stretching vibration) and the second at 3303 cm−1, which is the pyridine aromatic C–H vibrations band. The aliphatic C–H stretching vibration at 2929 and 2873 cm−1 moved at 2922 and 2865 cm−1, such that these groups were involved in electrostatic bonds. In addition, the amide I and II are shifted to low frequency: amide I, from 1656 to 1649 cm−1; amide II, from 1544 to 1532 cm−1. The asymmetric and symmetric deformations of CH3 have changed their bands from 1459 to 1447 cm−1 and 1416–1389 cm−1, respectively. The region in between has dramatically changed their intensity. This is due to the spontaneous adsorption of the crystalline HSA–FITC complex on the MWCNTs and the formation of a well-organized oxidized MWCNT–HSA–FITC.
To that end, atomic force microscopy (AFM) analysis of the HSA–MWCNTs solution was performed. Representative AFM evidence of the successful attachment of HSA molecules onto the surface of the nanotubes is shown in . By AFM, analysis at the nanometric scale of the two HSA molecules (black arrows in ) attached at the end of the nanotubes (white arrows) was carried out. A single HSA molecule (red arrow) has also been observed in the topographic image shown here. The length of the CNTs was estimated as being <200 nm. The lateral resolution of an AFM image is determined by the tip of the object that is imaged. In the presented image, the width of the nanotube appears to be >2 nm, as we used an AFM tip with a ~15 nm radius of curvature.
HSA–MWCNT internalization
The ability of an FITC-labeled bioconjugate of HSA– MWCNTs to internalize inside an HepG2 cell was evaluated by confocal fluorescence microscopy imaging. The results presented in show that at low concentration and short exposure time, HSA–MWCNT accumulates inside HepG2 cells. Thus, we provided imaging evidence that HSA can act as a carrier for MWCNTs, and because we were unable to identify any fluorescence in the epithelial cells in similar conditions () we reasoned that HSA–MWCNT bioconjugates exhibit specific affinity for liver cancer cells.
Figure 3 Selective nanophotothermolysis of HepG2 cells. A) Confocal image of human hepatocytes incubated for 30 min with 5 mg/L FITC–HSA–MWCNTs. (The nucleus was stained with DRAQ5-red.) B) Confocal detection of MWCNT–HSA–FITC (green) selectively internalized into HepG2 cells (exposed for 30 min to 5 mg/L of FITC–HSA– MWCNTs). C) HepG2 cells were irradiated for 2 min using a 2-W, 808-nm laser beam. Image of cell lysate and aggregated cells after internalization of MWCNT–HSA–FITC and laser radiation. D) CRL-4020 cells incubated for 30 min with 5 mg/L FITC–HSA–MWCNTs visualized by phase contrast microscopy (×400 magnification). E) HepG2 cells incubated for 30 min with 5 mg/L FITC–HSA–MWCNTs visualized by phase contrast microscopy (×400 magnification). F) Transmission electron microphotograph showing clusters of MWCNTs surrounded by plasmalemmal vesicles, confirming the presence of nanomaterial inside the cell (×24,000 magnification).
Abbreviations: FITC, fluorescein isothiocyanate; HSA, human serum albumin; MWCNTs, multiwalled carbon nanotubes.
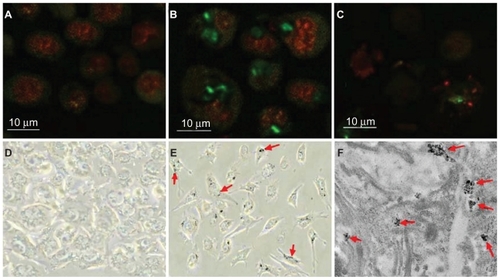
Furthermore, phase contrast microscopy was used to demonstrate the presence of CNTs inside HepG2 cells following HSA–MWCNT administration. As seen in (red arrows), intracellular aggregates of MWCNTs appear as dark, optically dense signals that associate with a refringent signal under phase contrast. Once more, we were unable to identify any aggregates inside the epithelial cells that have been similarly treated. () Moreover, the cellular areas that appeared to contain MWCNTs were further subjected to TEM analysis. When these regions were observed under TEM, MWCNTs could be clearly identified in the form of intracellular aggregates, as shown by the red arrows in .
The mechanism of selective internalization of HSA–MWCNTs inside the malignant liver cells
In order to shed light on the molecular mechanisms involved in the specific uptake of HSA–MWCNTs in HepG2 cells, we investigated the possibility that a 60 kDa glycoprotein, Gp60, which is known to function in albumin transcytosis in malignant cells,Citation38 was involved in the selective uptake of albumin bound to CNTs. To accomplish this, we allowed the cells treated with 5 mg/L HSA–MWCNTs for 1 h to incorporate cy3–anti-Gp60 Ab for 30 min at 37°C. To that end, we obtained fluorescent images demonstrating the internalized cy3 fluorescence (, first panel).
Figure 4 HSA–MWCNTs in vitro endocytosis mechanism in human liver cancer cells. A) Colocalization of Cy-Gp60 antibody and FITC–HSA–MWCNTs in HepG2 cells. B) Colocalization of Cy-Gp60 antibody and FITC–HSA–MWCNTs in hepatocyte epithelial cells. C) Colocalization of caveolin-1-Cy antibody and FITC–HSA–MWCNTs in HepG2 cells. Results are representative of three experiments. Scale bar: 20 μm in all panels. Abbreviations: DAPI, 4′-6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; HSA, human serum albumin; MWCNTs, multiwalled carbon nanotubes.
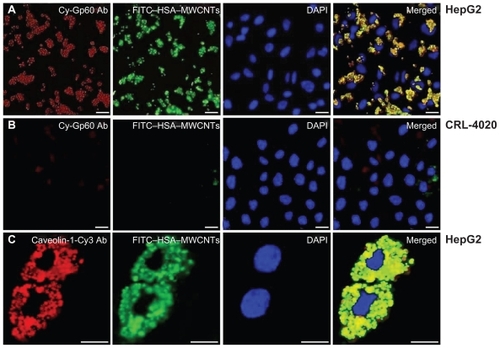
Also, we showed that HepG2 cells internalized with albumin-bound MWCNTs (fluorescently labeled with FITC) were distributed into the punctate structure inside the cells (, 2nd panel). DAPI, which is known to form fluorescent complexes with natural double-stranded DNA, was used for nuclei staining. In , fourth panel, nearly complete colocalization of the FITC fluorescence (green image) and cy3 fluorescence (red image) was evident by yellow in the merged image. This finding suggests that albumin bound to MWCNTs was incorporated into plasmalemmal vesicles containing Gp60 as a membrane protein, further validating HSA–MWCNT specificity for Gp60 receptors. Importantly, as seen in , no significant colocalization in the hepatocyte cells (CRL-4020) was observed for cy3–Gp60 Ab and HSA–FITC–MWCNTs incubated under same circumstances.
Therefore, based on these data, we showed that HSA– MWCNTs can act as specific and sensitive site-targeted nanosystems against Gp60 receptor located on the liver cancer cell membrane.
Association of caveolin-1 with FITC–HSA–MWCNTs-containing vesicles
Most data indicate that caveolae-mediated endocytosis in cells is stimulated by the binding of albumin to Gp60, a receptor located in the caveolae.Citation38
Given these data and the described role of caveolin in albumin endocytosis, we reasoned that the mechanism of HSA–MWCNT internalization in HepG2 cells was similar. To test this hypothesis, we immunostained the HepG2 cells with Cy3–anti-caveolin-1 Ab. As shown in , confocal imaging revealed that the majority of FITC–HSA–MWCNT-containing plasmalemmal vesicles stained for caveolin-1 used this fluorescent anti-caveolin-1 monoclonal Ab. Taken together, all these data demonstrate that HSA–MWCNTs selectively internalize in human hepatocellular cancer cells via caveolae-mediated endocytosis by the binding of the albumin carrier to Gp60, a specific albumin-binding protein.
Cytotoxicity induced by laser irradiation or by the administration of HSA–MWCNTs
Before testing the in vitro response of HSA–MWCNT-treated cells to laser irradiation, we investigated the possible effect of cytotoxicity induced by the administration of CNTs in the cells. HepG2 cells and the epithelial cells were treated with various concentrations of HSA–MWCNT at various incubation periods. Cell Death Detection ELISAPLUS was used to evaluate the effect of MWCNT bioconjugates on cell viability.
After 24 h of incubation, HepG2 exposed to 50 mg/L of HSA–MWCNT showed a 5.71% decrease in viability compared with 1.6% (P < 0.02) ().
Table 1 Cytotoxic-induced effects on HepG2 and CRL-4020 cells by various concentrations of bionanomaterial at various incubation times
The next step in order to eliminate any potential errors was represented by a 2 minutes irradiation of a sample of cells without nanoparticles, using a 2 W, 808 nm laser beam. There was no lysis among the cells after irradiation. The process demonstrates the transparency of HepG2 for NIR beam.
Assessment of cellular necrosis after laser treatment and administration of HSA–MWCNTs
The postirradiation lysis rate of HepG2 cells treated with HSA–MWCNTs ranged from 35.45% (for 1 mg/L) to 88.24% (for 50 mg/L) at 60 sec (P < 0.001), whereas at 30 min the necrotic rate increased from 59.34% (1 mg/L) to 92.34% (50 mg/L), P value <0.001. Significantly lower apoptotic rates were obtained in irradiated epithelial cells treated for 60 sec and 30 min at concentrations ranging from 1 mg/L to 50 mg/L (6.78%–64.32% for 60 sec; 9.89%–70.78% for 30 min). As can be observed, the optimal apoptotic effect of malignant cells after incubation with HSA–MWCNT was obtained at a concentration of 5 mg/L (HepG2/CRL-4020: 65.79%/11.34% at 60 sec, and 75.34%/14.67% at 30 min) (). After 60 min of incubation, the difference among the apoptotic rates was also statistically significant among the two cell lines for low/medium concentrations of HSA– MWCNT (78.92%: 1 mg/L, 88.34%: 5 mg/L, 87.88%: 20 mg/L, for HepG2; 15.56%: 1 mg/L, 21.34%: 5 mg/L, 52.14%: 20 mg/L, for CRL-4020). P values were <0.001 for comparisons between various forms of nanomaterials. No significant differences (P = 0.143) among the apoptotic rates of HepG2 and CRL-4020 treated with HSA–MWCNT could be observed (100%: HepG2; 84.13%: CRL-4020) for a high concentration of nanomaterials (50 mg/L).
Figure 5 Results of experimental seriate exposure to nanomaterials (control vs MWCNTs–HSA) in different concentrations, followed by laser irradiation. Bars represent the average percentage of dead cells (%).
Abbreviations: HSA, human serum albumin; MWCNTs, multiwalled carbon nanotubes.
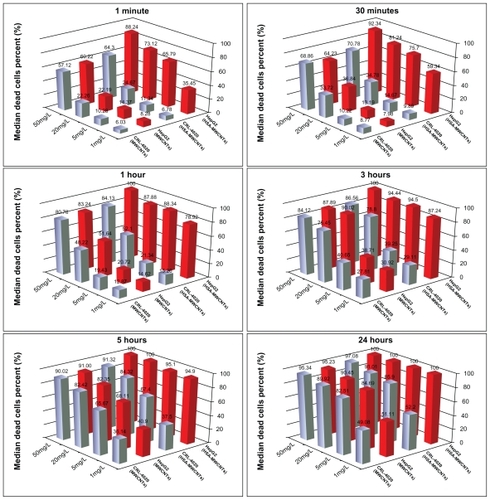
After 3–5 h of incubation, a significant apoptotic rate of the two cell lines was obtained only when the cells were treated with low concentrations of nanomaterials (<20 mg/L). Elevated concentrations recorded a nonsignificant difference in the cell lysis effect of the two cell lines (P = 0.256–20 mg/L; P = 0.296–50 mg/L).
After 24 h of incubation, the HepG2 cells treated with 1 mg/L HSA–MWCNT were 100% necrotic after laser irradiation, as compared with 52.2% of the CRL-4020 cells similarly treated. For very low concentrations of HSA–MWCNTs, we could observe a difference among the percentage of dead cells of the two cell lines. However, the difference reached only a marginal significance (P = 0.07). The lysis rate of the irradiated cells incubated with more than 5 mg/L nanomaterials for 24 h was almost similar for the two cell lines (100% vs 85.94%).
In contrast, no significant differences in the percentage of nonviable cells were obtained between the two cell lines when the nonfunctionalized MWCNT solution was used for treatment (P > 0.05 for all comparison and each exposure interval). Moreover, for HepG2 cells, the results showed a significant difference between MWCNTs and MWCNT– HSA-exposed groups for low concentrations (1, 5, and 20 mg/L) and short exposures (60 sec, 30 min, 1 h, 3 h, and 5 h) ().
Discussion
The main goal of this investigation was to develop and test a new method of treatment of human HCC. Preliminary data from literature support the involvement of albumin in tumor growth. The implication is supported by the fact that albumin enhances tumor expansion, as it is used for synthesis in various cellular compartments.Citation38
In order to investigate the toxicity effects of the nano-conjugates, HepG2 cells and CRL-4020 epithelial cells were exposed and incubated with HSA–MWCNTs at various concentrations and incubation times. Consistent with other findings, we demonstrate that only high concentrations of MWCNT bioconjugates exhibit cytotoxic effects.Citation31 Nevertheless, the toxicity, which represents a major obstacle in using CNTs in clinical applications, may be minimized by administration of low doses of nanoconjugates.Citation9,Citation13,Citation23
Further, we used HSA–MWCNTs as heat-inducing agents under laser radiation during the process of nanophotothermolysis. This method is based on the presence and clustering of HSA–MWCNTs inside the cells and their highly optical absorption capabilities responsible for inducing thermal effects, especially under NIR irradiation, where the biological systems have low absorption and high transparency.Citation10,Citation19–Citation22,Citation39,Citation40 The optoelectronic transitions in the graphitic structures of the MWCNTs clusters generate thermal energyCitation41 that rapidly diffuses into the subcellular compartments, where the nanoconjugates are present.
Laser-induced thermal ablation of cancer cells labeled with HSA–MWCNTs may be used in two main modes: pulsed and continuous. The pulsed mode produces localized (few micrometers) damage of individual cancer cells by laser-induced micro- and nanobubbles around overheated nanoparticles without harmful effects on the surrounding healthy cells.Citation42 It particularly favors in vivo killing of single circulating tumor cells using just 1 ns laser pulses. The second mode is more time consuming (a few minutes of exposure) and results in the effects of thermal denaturation and coagulation as main mechanisms of cell damage. It is more appropriate for the treatment of primary tumors measuring a few millimeters or more.Citation42
The use of continuous laser irradiation proved significant differences in HepG2 postirradiation apoptotic percentage (P < 0.05) for concentrations of <20 mg/L, at 60 sec and 30 min, compared with the apoptotic rate of CRL-4020. This finding may be particularly relevant for low concentrations of HSA–MWCNTs (eg, plasma levels after intra-arterial administration).Citation43 It has been previously stated that the mechanism of HepG2 uptake for albumin is a caveolae-dependent endocytosis similar to that for other types of ligands such as cholesterol or folic acid.Citation44 The mechanism represents a distinct form of transport and elicits features different from independent or clathrin-mediated endocytosis. After internalization of caveolae, the biomaterials are accumulated in caveosomes, a specific type of organelles.Citation45 Folic acid has been intensely studied for its potential in targeted therapies. Significant results were obtained after binding folate-functionalized poly (ethylene glycol)-coated nanoparticles to the targeted receptor (folate receptor).Citation46 Within the field of chemotherapy, caveolae-mediated transport mechanisms have been largely used for targeted drug delivery. The pathway has been preferred as it was demonstrated to be a nondegradative mechanism using pH-dependent chemotherapy release. For instance, a combination of cytostatic drugs and albumin called Trexall® (Duramed Pharmaceuticals, New York, NY, USA) is currently prescribed for the treatment of metastatic liver cancer in humans.Citation47 The literature has already suggested new ideas of targeted therapies that could elude lysosomal harmful transit and will therefore offer a higher protection level for drug compounds.Citation48 A specific endothelin receptor associated with the described uptake mechanism is the Gp60 receptor (albondin).Citation49 Using phase contrast, confocal, and TEM, we demonstrated in this study, without precedent, that the mechanism of HSA– MWCNT uptake in HepG2 cells occurs through caveolaedependent endocytosis initiated by the albumin-binding Gp60 receptor (albondin) ().
In the present study, we observed that in the treatment of HepG2 cells with high concentrations of HSA–MWCNTs for more than 5 h, the percentage of necrotic HepG2 cells is not significantly different from that of epithelial cells. This finding suggests a nonselective, passive intracellular diffusion of nanomaterial inside the cells when the cells are exposed to high concentrations of nanomaterials for long periods of time.
In contrast, we obtained a selective lysis of HepG2 cells treated with HSA–MWCNTs for incubation periods shorter than 30 min, regardless of the concentration. In cellular systems, the molecular membrane association/dissociation processes are very short, ranging from seconds to minutes.Citation50 Therefore, our finding could be of decisive importance when using HSA–MWCNTs for the in vivo targeting of liver cancer cells.
Conclusion
We have developed a method of functionalization of CNT with human albumin for the selective targeting of liver cancer cells. Moreover, to our knowledge, this is the first evidence of improved selective thermal ablation of liver cancer cells using HSA–MWCNTs compared with the normal epithelial cells. Based on the results presented here, we believe that HSA–MWCNTs selectively attach to albondin (aka Gp60) receptor located on HepG2 cell membrane, followed by uptake through a caveolin-dependent endocytosis process.
These results may represent a first step in the process of complete in vivo elimination of liver cancer cells using nanolocalized thermal ablation by means of laser heating.
However, further research is required in order to fully understand the mechanisms of selective binding of HSA– MWCNTs in malignant cells.
Nevertheless, further investigations are also required for the careful assessment of unexpected toxicities and biological interactions of HSA–MWCNTs inside the living organism.
Acknowledgments
The authors acknowledge grant support from the Romanian Ministry of Research (CNMP-PNCDI II: NANOPAN 41-009 and NANOHEP 42-115). This research was also supported by Romanian Society of Nanomedicine.
Disclosure
The authors report no conflicts of interest in this work.
References
- WongRJCorleyDASurvival differences by race/ethnicity and treatment for localized hepatocellular carcinoma within the United StatesDig Dis Sci20095492031203919117131
- VarelaMBruixJHepatocellular carcinoma in the United States. Lessons from a population-based study in Medicare recipientsJ Hepatol200644181016297492
- BoschFXRibesJDíazMClériesRPrimary liver cancer: worldwide incidence and trendsGastroenterology20041275 Suppl 1S51615508102
- KiyosawaKUmemuraTIchijoTHepatocellular carcinoma: recent trends in JapanGastroenterology20041275 Suppl 1S172615508082
- RamponeBSchiavoneBMartinoAVivianoCConfuortoGCurrent management strategy of hepatocellular carcinomaWorld J Gastroenterol200915263210321619598295
- LaiECFanSTLoCMChuKMLiuCLWongJHepatic resection for hepatocellular carcinoma. An audit of 343 patientsAnn Surg199522132912987717783
- CanceWGStewartAKMenckHRThe National Cancer Data Base Report on treatment patterns for hepatocellular carcinomas: improved survival of surgically resected patients, 1985–1996Cancer200088491292010679662
- FerrariMCancer nanotechnology: opportunities and challengesNat Rev Cancer20055316117115738981
- ChakravartyPMarchesRZimmermanNSThermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubesProc Natl Acad Sci U S A2008105258697870218559847
- LiuZTabakmanSWelsherKDaiHCarbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug deliveryNano Res2009228512020174481
- LiangPWangYMicrowave ablation of hepatocellular carcinomaOncology200772Suppl 1S124131
- WangZYSongJZhangDSNanosized As2O3/Fe2O3 complexes combined with magnetic fluid hyperthermia selectively target liver cancer cellsWorld J Gastroenterol200915242995300219554652
- CardinalJKluneJRChoryENoninvasive radiofrequency ablation of cancer targeted by gold nanoparticlesSurgery2008144212513218656617
- LapotkoDLukianovaEPotapnevMAleinikovaOOraevskyAMethod of laser activated nano-thermolysis for elimination of tumor cellsCancer Lett20062391364516202512
- WelsherKLiuZDaranciangDDaiHSelective probing and imaging of cells with single walled carbon nanotubes as near-infrared fluorescent moleculesNano Lett20088258659018197719
- BiancoAKostarelosKPartidosCDPratoMBiomedical applications of functionalised carbon nanotubesChem Commun (Camb)2005557157715672140
- SrinivasanCCarbon nanotubes in cancer therapyCurr Sci2008943300301
- PeerDKarpJMHongSFarokhzadOCMargalitRLangerRNanocarriers as an emerging platform for cancer therapyNat Nanotechnol200721275176018654426
- BhirdeAAPatelVGavardJTargeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug deliveryACS Nano20093230731619236065
- DumortierHLacotteSPastorinGFunctionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cellsNano Lett2006671522152816834443
- Shi KamNWJessopTCWenderPADaiHNanotube molecular transporters: internalization of carbon nanotube-protein conjugates into Mammalian cellsJ Am Chem Soc2004126226850685115174838
- GhoshSDuttaSGomesEIncreased heating efficiency and selective thermal ablation of malignant tissue with DNA-encased multiwalled carbon nanotubesACS Nano2009392667267319655728
- SchipperMLNakayama-RatchfordNDavisCRA pilot toxicology study of single-walled carbon nanotubes in a small sample of miceNat Nanotechnol20083421622118654506
- Di StefanoGFiumeLBolondiLLanzaMParialiMChiecoPEnhanced uptake of lactosaminated human albumin by rat hepatocarcinomas: implications for an improved chemotherapy of primary liver tumorsLiver Int200525485486015998437
- KratzFAlbumin, a versatile carrier in oncologyInt J Clin Pharmacol Ther201048745345520557842
- KratzFAlbumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticlesJ Control Release2008132317118318582981
- DennisMSJinHDuggerDImaging tumors with an albumin-binding Fab, a novel tumor-targeting agentCancer Res200767125426117210705
- TiruppathiCFinneganAMalikABIsolation and characterization of a cell surface albumin-binding protein from vascular endothelial cellsProc Natl Acad Sci U S A19969312502548552615
- TiruppathiCSongWBergenfeldtMSassPMalikABGp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathwayJ Biol Chem19972724125968259759325331
- HeisterENevesVTilmaciuCTriple functionalisation of single-walled carbon nanotubes with doxorubicin, a monoclonal antibody, and a fluorescent marker for targeted cancer therapyCarbon200947921522160
- RaffaVCiofaniGNitodasSCan the properties of carbon nanotubes influence their internalization by living cells?Carbon2008461216001610
- SuhYAfaqFKhanNJohnsonJJKhusroFHMukhtarHFisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cellsCarcinogenesis20103181424143320530556
- KovtyukhovaNIMalloukTEPanLDickeyECIndividual single-walled nanotubes and hydrogels made by oxidative exfoliation of carbon nanotube ropesJ Am Chem Soc2003125329761976912904042
- SocratesGInfrared and Raman Characteristic Group Frequencies Tables and Charts3rd edChichester (UK)John Wiley & Sons2001
- MaedaHIshidaNKawauchiHTsujimuraKReaction of fluorescein- isothiocyanate with proteins and amino acids. I. Covalent and non-covalent binding of fluorescein-isothiocyanate and fluorescein to proteinsJ Biochem19696557777835806968
- AzamianBRDavisJJColemanKSBagshawCBGreenMLBioelectrochemical single-walled carbon nanotubesJ Am Chem Soc200212443126641266512392405
- FengYFengWNodaHPhotoinduced anisotropic response of azobenzene chromophore functionalized multiwalled carbon nanotubesJ Appl Phys20071025053102053105
- BotosEKlumpermanJOorschotVCaveolin-1 is transported to multi-vesicular bodies after albumin-induced endocytosis of caveolae in HepG2 cellsJ Cell Mol Med2008125A1632163918053095
- XiaoYGaoXTaratulaOAnti-HER2 IgY antibody- functionalized single-walled carbon nanotubes for detection and selective destruction of breast cancer cellsBMC Cancer2009935119799784
- KamNWO’ConnellMWisdomJADaiHCarbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destructionProc Natl Acad Sci U S A200510233116001160516087878
- DresselhausMSDaiHCarbon nanotubes: continued innovations and challengesMRS Bull2004294237243
- ZharovVPGalitovskayaENJohnsonCKellyTSynergistic enhancement of selective nanophotothermolysis with gold nanoclusters: potential for cancer therapyLasers Surg Med200537321922616175635
- CherukuriPGannonCJLeeuwTKMammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescenceProc Natl Acad Sci U S A200610350188821888617135351
- ChangWJRothbergKGKamenBAAndersonRGLowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folateJ Cell Biol1992118163691618907
- TiruppathiCNaqviTWuYVogelSMMinshallRDMalikABAlbumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cellsProc Natl Acad Sci U S A2004101207699770415136724
- DautyERemyJSZuberGBehrJPIntracellular delivery of nano-metric DNA particles via the folate receptorBioconjug Chem200213483183912121139
- GarberKStromal depletion goes on trial in pancreatic cancerJ Natl Cancer Inst2010102744845020339135
- BathoriGCervenakLKaradiICaveolae – an alternative endocytotic pathway for targeted drug deliveryCrit Rev Ther Drug Carrier Syst2004212679515202927
- BarefordLMSwaanPWEndocytic mechanisms for targeted drug deliveryAdv Drug Deliv Rev200759874875817659804
- FesceRMeldolesiJPeeping at the vesicle kissNat Cell Biol199911E3410559869