Abstract
Purpose
Studies have shown that strontium-doped medical applications benefit bone metabolism leading to improved bone healing and osseointegration. Based on this knowledge, the aim of the study was to evaluate the performance of an implant surface, functionalized by a physical vapor deposition (PVD) coating (Ti-Sr-O), designed to yield predictable release of strontium. The Ti-Sr-O functionalized surface is compared to a routinely used, commercially available surface (SLActive™) with respect to bone-to-implant contact (BIC%) and new bone formation (BF%) in two defined regions of interest (ROI-I and ROI-II, respectively).
Materials and methods
Ti-Sr-O functionalized, SLActive, and Grade 4 titanium implants were inserted in the femoral condyle of adult male New Zealand White rabbits. The PVD magnetron-sputtered Ti-Sr-O surface coating was characterized using scanning electron microscopy (SEM) for morphology and coating thickness. Strontium release and mechanical stability of the coating, under simulated insertion conditions, were evaluated. Furthermore, histomorphometrical BIC and BF were carried out 2 weeks after insertion.
Results
Histomorphometry revealed increased bone formation of Ti-Sr-O with significant differences compared to SLActive and Grade 4 titanium in both regions of interest, ROI-I and ROI-II, at 0–250 µm and 250–500 µm distance from the implant surfaces. Analogous results of bone-to-implant contact were observed for the two modified surfaces.
Conclusion
The results show that a nanopatterned Ti-Sr-O functionalized titanium surface, with sustained release of strontium, increases peri-implant bone volume and could potentially contribute to enhancement of bone anchorage of osseointegrated implants.
Introduction
Implant insertion and subsequent prosthetic treatment has become a reliable method with predictable results in modern dentistry. Artificial root insertion is regarded as an effective treatment for several prosthetic clinical setups considering survival rates between 75% and 98% in defined observation periods and study groups.Citation1,Citation2 Nevertheless, in industrialized countries with influence of demographic changes in particular, different challenges remain. Elderly patients with compromised bone conditionsCitation3 due to, eg, osteoporosis, antiresorptive therapy, or patients having undergone irradiation with a need for oral rehabilitation represent a patient population with requisitions for further enhancement of endosseous implant devices.
Following the discovery of the phenomenon of osseointegration by Brånemark et al,Citation4 research initially focused on implant geometry.Citation5 This focus has now shifted toward biofunctionalization of surfaces aiming for acceleration of the biological process of osseointegration, allowing for early implant loading. Nowadays, different approaches for enhancement of surface properties are used, such as generation of defined geometries in the macro-, micro-, and nanometer rangeCitation6 (eg, sandblasting and etching),Citation7 implementation of osteoinductive ions,Citation8 laser ablation,Citation9 anodic oxidation,Citation10 or preparation under N2 protection/storage in liquid resulting in ultrahydrophilic surfaces.Citation11 The latter process with increased wettability was introduced in the last decade and evolved to a commonly used surface within dental implantology due to advanced surface free energy resulting in extended woven bone formation.Citation11,Citation12 Preclinical studies showed that SLActive™ implants achieved up to 60% more bone-to-implant contact 2 weeks post-insertionCitation13 and demonstrated accelerated and more developed bone formation when compared to a conventional sandblasted and acid-etched surface.Citation14 Incorporation and subsequent release of bioactive ions, eg, calcium (Ca), magnesium (Mg), or strontium (Sr), has been investigated in various applications,Citation15–Citation17 constituting beneficial effects on bone metabolism. Based on the documented effects in vitro, in vivo, as well as in clinical trials,Citation17–Citation19 strontium, an essential trace element in the human body, represents a promising route for enhancement of osseointegration with effects on osteogenic gene expression, cell differentiation, and increased bone apposition when introduced into, eg, bioabsorbable alloys, cements, bioglasses, composites, or surface coatings.Citation20–Citation24 The mechanisms through which strontium affects bone remodeling, with its dual effect on bone-forming osteoblasts and bone-resorbing osteoclasts, are still elusive. In vitro studies have shown that clinically used strontium ranelate (SrRan) affects the RANK/RANKL/OPG pathwayCitation25 as well as osteogenic differentiation of mesenchymal stem cells (MSCs), which is mediated by activation of canonical and noncanonical Wnt signaling.Citation26 Moreover, SrRan was able to reduce the adherence of osteoclasts to bone by disrupting the actin sealing zone,Citation27 while it also influences bone forming cells by osteoblast differentiation via increasing alkaline phosphate activity and collagen synthesis.Citation28,Citation29
With respect to functionalization of endosseous medical devices, it is known that strontium-enriched biomaterials benefit the process of osteoinduction and osseointegration.Citation17,Citation30–Citation32 Concerning dental implantology and surface topography, it is commonly accepted that moderately rough surfaces have favourable effects on osseointegration, while biofilm formation is facilitated with roughness parameter Ra of 0.2 µm and aboveCitation33,Citation34 with potential subsequent inflammatory reactions at the implant interface and possible implant failure. Smoother surfaces could diminish bacterial adhesionCitation35 while promoting protein absorption, thus allowing for attachment of different cell types relevant for the osseointegration process.Citation30,Citation36 Those topographic properties, with supplementary incorporation of strontium in particular, could influence the mechanical anchorage of titanium implants.
With research currently evaluating not only improved topographic settings for accelerated bone growth but also biofunctionalization toward osteogenic properties, the aim of the present study was to investigate the effect of a Ti-Sr-O physical vapor deposition (PVD) coating, a novel functionalized surface with continuous release of strontium in comparison to a clinically used SLActive implant surface in vivo.
Materials and methods
Sample preparation and characterization
Surface preparation and characterization were thoroughly investigated earlier in detail for both SLActive,Citation37 and Ti-Sr-O coating.Citation17,Citation38 Briefly, test implants, measuring 8 mm in length and having a maximum outer diameter of 3.75 mm, were manufactured from titanium Grade 4 (Elos Medtech Pinol A/S, Gørløse, Denmark). The implants were self-tapering and had a turned surface finish. Part of the manufactured items was retained to be used as reference implants, while the Ti-Sr-O coating was applied to the other part, using an industrial-scale magnetron sputtering system (CemeCon AG, Wuerselen, Germany).Citation38 Subsequently, the thickness and morphology of the Ti-Sr-O coating was assessed using scanning electron microscopy (SEM) (Nova 600; FEI Company, Eindhoven, the Netherlands). Strontium release from Ti-Sr-O implants was evaluated by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (AMETEK Spectro Arcos, AMETEK, Berwyn, PA, USA), over a period spanning 7 days, as previously described.Citation17 Briefly, five implants were submerged in PBS. At the relevant time points (0, 1, 3, 5, and 7 days), the PBS was removed and subsequently pooled. Three ICP-AES measurements for each time point were performed. The deviation between the individual measurements was typically less than 1%. To allow for evaluation of the coating thickness, implants were embedded in a conducting resin (PolyFast, Struers, Denmark) and subsequently cut, ground, and polished to allow for a cross-sectional view of the Ti-Sr-O coating. Benchmark implants, carrying the SLActive surface (diameter 3.3 mm and 8 mm in length), were acquired through standard commercial trade routes (Straumann AG, Basel, Switzerland).
Mechanical stability
To evaluate the mechanical stability of the developed Ti-Sr-O coating, in relation to the forces experienced during implant insertion, the implantation procedure was simulated using solid blocks of the polymer polyoxymethylene (POM; Ensinger Denmark A/S, Ringsted, Denmark). Following insertion, the implants were retrieved from the POM material and the block was split along the center axis of the hole, to see if the material from the Ti-Sr-O coating had been transferred to the POM. Additionally, the surface of the inserted implants was examined using SEM and energy-dispersive X-ray spectroscopy (EDX). In relation to this, focus was on the cutting edges of the implant, as these areas will experience the largest forces.
Surgery
With permission of the Austrian government (BMWF-66.011/0143-II/3b/2013) and the ethics committee of the Medical University of Innsbruck, twelve 9-month-old male New Zealand White rabbits (average body weight 4,500 g) underwent surgical procedure following a 2-week observation period for evaluation of early osseointegration stagesCitation24,Citation39 in accordance with the ARRIVE guidelines.Citation40 In addition, Austrian guidelines for welfare of animals was followed (Federal Act on the Protection of Animals 2014; ERV_2004_1_118). After a 2-week settling-in period, animals were randomly chosen with insertion of either SLActive, Grade 4 titanium (Ti), or the strontium-modified surface (Ti-Sr-O); with insertion in both femoral condyles, n=8 per group was ensured. Thus, animals were carrying two implants, one in each femur.
In detail, animals were anesthetized with Medetomidine (Domitor® 1 mg/mL; 0.2 mL; Orion Corporation, Espoo, Finland) and Ketamine (Ketavet® 100 mg/mL; 0.2 mL; Zoetis, Zurich, Switzerland) intravenously with subsequent surgery. Briefly, under aseptic conditions, a 20 mm incision was made medial to the patella with following soft and hard tissue preparation and implant insertion according to defined protocols. All steps were performed while cooling the operation site with isotonic saline solution (Sigma-Aldrich Co., St Louis, MO, USA). Multilayer soft tissue closure was performed with Vicryl 4-0 (Ethicon; Johnson & Johnson Medical GmbH, Norderstedt, Germany). Postsurgical treatment included analgesic as well as antibiotic subcutaneous injections with Carprofen (Rimadyl® 4 mg/kg body weight [BW]; Zoetis) and Enrofloxacin (Baytril® 7.5 mg/kg BW; Bayer AG, Leverkusen, Germany). The rabbits were held in a room with a 12 h:12 h light:dark cycle with room temperature of 18°C–20°C. They were individually housed and fed ad libitum with rabbit pellets (V2333, ssniff® K-H; Ssniff GmbH, Soest, Germany), while tap water was available ad libitum by an automatic drinking system. Sacrification was conducted with pentobarbital (Narcoren® 400 mg/kg BW; Boehringer Ingelheim, Ingelheim am Rhein, Germany) intravenously after 2 weeks, and peri-implant tissue was embedded in Technovit 9100 new® (Kulzer Austria GmbH, Vienna, Austria) according to the manufacturer’s manual.
Histomorphometry
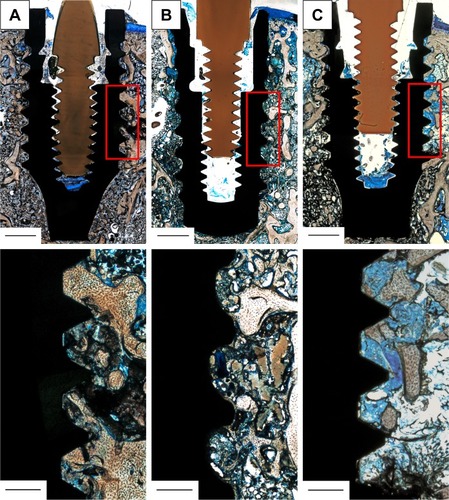
Subsequent to being sacrificed, samples were put in formalin, followed by embedding in Technovit 9100 new and further processing with the method of Donath and Breuner,Citation41 as previously described.Citation17,Citation42 In brief, Technovit blocks were cut along the centrosymmetric axis of the implants, which was facilitated by insertion of individual polyetheretherketone guide pins into the internal screw geometry before initiation of the embedding process, allowing visualization of the implant position and orientation. Samples were then glued to microscope slides and, after a grinding process for parallelization, underwent secondary cutting with a blade width of 0.1 mm (Exakt 300; Exakt, Norderstedt, Germany) and finally ending up in slide thickness of ~100 µm. Sanding was performed (grit size 1,000, 2,500 and 4,000, respectively) to achieve a final sample thickness of 50 µm, and slides were subsequently polished with Micropolish II, 1.0 micron (Buehler, Braunschweig, Germany) and stained with toluidine blue. Analysis of bone apposition (magnification 40×) was conducted using NIS Elements BR 3.10 software (Nikon GmbH, Vienna, Austria) evaluating bone-to-implant contact directly at the implant interface and new bone formation in defined areas 0–250 µm and 250–500 µm distance from the implant interface (two defined regions of interest [ROI-I] and [ROI-II], respectively). Results were expressed as bone-to-implant contact (BIC%) and new bone formation (BF%). Two slides per implant were analyzed with all measurements taken on the spongy part of the femur as shown in .
Figure 1 Histological samples, stained with toluidine blue, including reference boxes (red squares) (top) and zoom-in of these (bottom).
Notes: Reference box marks the standard area employed to evaluate new bone formation, which was used to calculate the percentage of de novo bone synthesis in defined distances from the implant interface with regions of interest I (0–250 µm) and II (250–500 µm) with respect to the total reference area. The part of the implant surface defined by the reference was also used to evaluate the percentage of direct bone-to-implant contact with respect to the total length of the reference box: (A) Ti-Sr-O; (B) SLActive; and (C) Ti. The PEEK guiding pin is clearly identified as the brown, screw-shaped structure in the internal geometry of the implant. Scale bar is 1,000 µm in the upper total section and 250 µm in the lower zoomed-in segment.
Abbreviations: Ti-Sr-O, strontium-functionalized surface; PEEK, polyetheretherketone.
Statistical analysis
Analysis was performed using IBM SPSS Statistics 24 (IBM, Armonk, NY, USA) and R 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria). The data of BIC% and BF% are presented as mean ± standard deviation. The data were plotted in box plots and inspected for outliers. The normal distribution of the data was assessed by inspection of QQ-plots and Shapiro–Wilk’s test for each group. To explore if there were statistical differences between the study groups concerning the data of BIC% and BF%, one-way ANOVA was performed together with Games–Howell post hoc analysis. Levene’s test was used for assessment of homogeneity of variances, for all groups. The significance level for statistical tests was set at α = 0.05. Statistical significance was indicated by *P < 0.05, **P < 0.01, and ***P < 0.001, respectively.
Results
All 12 animals completed the study as planned in a 2-week observation period. With exception of the anticipated swelling in the knee joint area, healing was uneventful in all animals; no complications such as infections, allergic reactions, or implant loss were observed. Furthermore, no premature exposure of inserted implants was detected.
Sample preparation and characterization
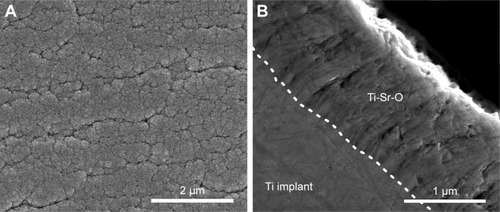
The surface morphology was investigated, as previously described.Citation42 presents representative images of the top-view surface structure and cross-sectional view of the Ti-Sr-O coating, applied to the surface of the turned Ti Grade 4 implants. As is evident from the images, a nanopatterned and granular structure is observed for the top-view images. The cross-sectional view reveals a columnar structure of the coating.
Figure 2 SEM images of Ti-Sr-O.
Notes: (A) Top-view SEM of the Ti-Sr-O coating. The coating morphology is characterized by a granular nanostructure. (B) Cross-sectional SEM view of the Ti-Sr-O coating. The boundary between the machined titanium implant and the Ti-Sr-O coating is highlighted by the white line. The Sr-comprising top layer is characterized by a columnar structure. Scale bar is 2 µm and 1 µm for (A and B), respectively.
Abbreviations: Ti-Sr-O, strontium-functionalized surface; SEM, scanning electron microscopy.
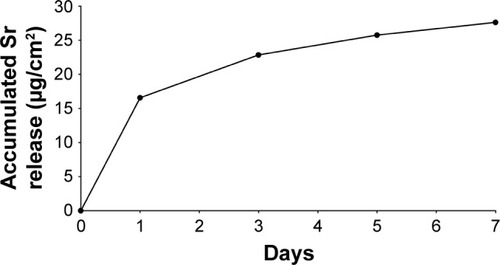
Strontium release from the Ti-Sr-O coating was evaluated over a 7-day period and the accumulated release is presented in .
Figure 3 Accumulated Sr release, as determined by ICP-AES.
Notes: The highest release of Sr is observed within the first 24 hours. However, the release persists for the entire duration of the evaluated period. Each point is the mean of three consecutive measurements having a variation of less than 1%.
Abbreviation: ICP-AES, inductively coupled plasma atomic emission spectroscopy.
Mechanical stability
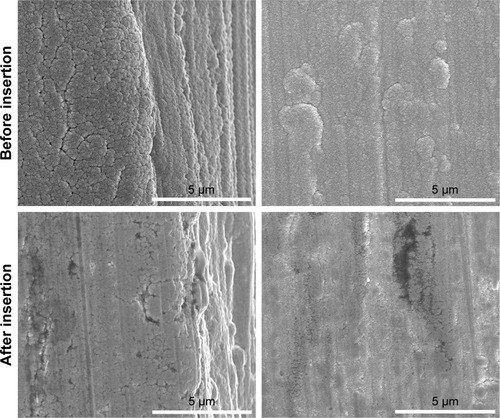
After insertion into POM, the surface of the Ti-Sr-O coated implants, as well as the internal surface of the POM insertion hole (data not shown), was examined using SEM and EDX. shows representative images of the cutting edges and approximate areas of the implant screw, before and after insertion. It is evident that the coating is affected by the insertion as the structure appears to be compressed. In addition to compression, some degree of fracturing is also observed; however, no signs of delamination were found. To further investigate if the insertion into POM would cause delamination of the coating, the internal structure of the vacant hole in the POM block was also examined using SEM and EDX. No signs of material transfer from the implant to the POM were found (data not shown). Additionally, images acquired at areas of the implant subjected to lower forces, than the cutting edges, did not show any signs of changes to the coating structure (data not shown).
Histomorphometry
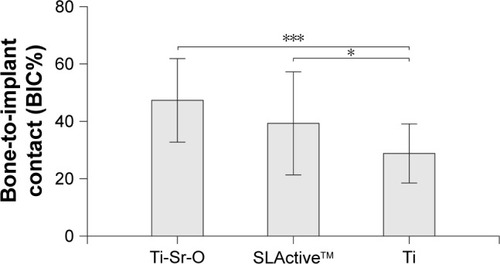
provides exemplary histological slides for all examined surfaces. With respect to the evaluated BIC%, as seen in , Ti-Sr-O showed a higher mean as compared to the SLActive surface, however, with no statistical significance. Ti was inferior to both investigated surfaces (P < 0.05 and P < 0.001). Results are presented as mean ± standard deviation.
Figure 5 BIC% 2 weeks postoperatively. Significant differences of Ti as compared to SLActive™ and Ti-Sr-O are marked with *P < 0.05 and ***P < 0.001, respectively. Data are presented as mean ± standard deviation.
Abbreviations: BIC%, bone-to-implant contact; Ti-Sr-O, strontium-functionalized surface.
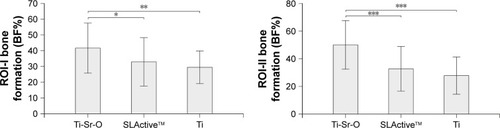
Data on new bone formation (BF%) are presented in . Analysis in ROI-I (0–250 µm from the implant interface) revealed significantly more bone formation for Ti-Sr-O as compared to SLActive (P < 0.05) and Ti (P < 0.01). This was also the case for bone apposition in ROI-II (250–500 µm) (P < 0.001).
Figure 6 Comparison of BF% in two defined regions of interest (ROI-I and ROI-II) 2 weeks postoperatively. Significant differences for Ti-Sr-O, compared to SLActive™ and Ti, are marked with *P < 0.05, **P < 0.01, and ***P < 0.001. Data are presented as mean ± standard deviation.
Abbreviations: BF%, new bone formation; Ti-Sr-O, strontium-functionalized surface.
Discussion
The aim of this study was to evaluate a functionalized titanium surface with sustained release characteristics of strontium and compare it to SLActive, a clinically used well-established surface, with respect to bone-to-implant contact and bone formation in a rabbit femur model.
With demographic changes and an increasing incidence of diminished bone conditions, dental implantology is facing various challenges for implant success due to, eg, medical interventions in bone metabolism such as antiresorptive therapy or irradiation.Citation43,Citation44 Investigations on osseointegration published over the last decade focused not only on osseointegration per se but particularly on biofunctionalizationCitation8,Citation9,Citation11,Citation16,Citation45 and surface characterization.Citation6 With different approaches toward incorporation of active ions, strontium-doped implants have gained increased attention for enhancement of osseointegration. This is based on a variety of data from in vitro investigations, which show the effect of strontium on bone-related gene expression,Citation46 MSC differentiation, interference in the RANK/RANKL/OPG pathway, and Wnt signaling.Citation18 In vivo experiments showed beneficial effects on bone-to-implant contact and bone apposition,Citation21,Citation47 while clinical trials with oral administration of SrRan in osteoporotic patients revealed risk reduction of vertebral fractures up to 49%.Citation27
With respect to dental implantology, the presence of strontium on the implant interface promotes osteoinduction with reports on significant increase in bone-to-implant contact for hydrothermally prepared titanium implants, surpassing the effect of super-hydrophilicity in promoting early bone appositionCitation24 or beneficial effects of a strontium-substituted hydroxyapatite coating manufactured via a sol-gel dipping method and tested in osteopenic bone conditions.Citation47
In the present study, the advantageous effect of strontium on bone metabolism was investigated. Tailored release profiles of the osteogenic element were obtained by a Ti-Sr-O coating prepared by an industrial-scale magnetron sputtering process, resulting in a nanostructured surface topography.Citation17,Citation42 It is well known that nanotopographical features may influence osteogenic differentiation and mineralization,Citation48,Citation49 while in vivo effects were observed as well.Citation30,Citation50 However, in the present case, it is clearly the chemical cue which is the stronger determinant. This conclusion is supported by several earlier studies,Citation17,Citation42 where similar nanotopographies to the present resulted in significantly different amounts of bone ingrowth. Specific nanostructures supporting mineralizationCitation51 might lead to synergistic effects.Citation52 However, this still has to be investigated. Based on our previous investigations,Citation17,Citation42,Citation53 the current in vivo experiments were conducted to test the performance of the Ti-Sr-O technology when implanted into the femoral condyle of rabbits. The study was designed to evaluate early osseointegration in comparison to a leading, commercially available implant surface, namely SLActive. This surface modification with increased surface free energy was introduced to decrease the hydrophobicity of sandblasted and acid-etched implants.Citation11 The high surface free energy functionalization facilitates primary protein interaction at the implant interface with subsequent shortening of healing periods and early implant loadingCitation54 by altering biological events, resulting in higher bone-to-implant contact.Citation13
Incorporation of strontium as an osteoinductive component could promote the osteogenic capacity, facilitated by releasing defined amounts of strontium from implant surfaces. With respect to histomorphometrical analysis, bone-to-implant contact (BIC%) revealed similar results with slight advantages for Ti-Sr-O as compared to the two other groups. As listed in numerous publicationsCitation55–Citation58 over the years, BIC% is regarded as golden standard for evaluation of osseointegration. Resembling BIC% values of Ti-Sr-O and SLActive proved an adequate osteogenic response to both surfaces with different topographical and chemical features. Additionally, significant differences with respect to BF% within 0–250 µm (ROI-I) and 250–500 µm (ROI-II) distance from the implant interface were observed, contributing to conceivable increased mechanical anchorage. These results were interpreted as a consequence of the sustained release of strontium from the functionalized interface in the near-surface environment.
With insertion of unloaded implants in the limbs of New Zealand White rabbits, an established animal model was used in this studyCitation24,Citation30 mimicking submerged healing conditions. Loading protocols could be assessed with further in vivo experiments in large animal models but were not part of this study as a consecutive investigation of previously unloaded implants. The aforementioned increase in new bone formation, up to a distance of 500 µm, should be directed to future pull out or torque-testing as a secondary measure for assessing the potentially enhanced implant stability. The results are notable, as the Ti-Sr-O functionalized implant represents a machined smooth surface with subsequent modification and was compared to a different surface topography of SLActive. Although this was not the focus of the current investigation, this fact emphasizes the positive osteoinductive effect of strontium. To our knowledge, this is the first time an implant with a turned surface finish has been found to surpass a state-of-the-art dental implant surface with respect to bone apposition in defined regions of interest.
Histomorphometric results showed that strontium-modified titanium implants can achieve commensurable bone healing parameters, presumably due to controlled release of strontium, in comparison to SLActive implants. Thus, implants with sustained release of strontium constitute a potential candidate for altering biological processes of osteogenic cells toward accelerated bone growth and could thereby contribute to an early enhancement of osseointegration.
Conclusion
This in vivo study is the first to report on a strontium-functionalized surface (Ti-Sr-O) manufactured by a PVD magnetron sputtering process with sustained release profiles of strontium in comparison to SLActive, a clinically used, well-established surface. Improved findings with respect to new bone formation and similar results for bone-to-implant contact were observed. Moreover, no sign of coating disintegration was detected. The controlled release of strontium as osteoinductive element from functionalized titanium implants could hold potential as a future surface modification candidate, thus representing a considerable candidate for further improvement of early osseointegration.
Acknowledgments
We like to acknowledge the guidance of Prof Hermann Dietrich and Mag vet Anja Beierfuß for assistance in fulfilling the ARRIVE guidelines. Additionally, we thank Dr Nora Fink for the contribution with respect to the performed surgeries and Mag Thomas Waldner for his support in the laboratory process. The project has been supported by the Innovation Fund Denmark through the project “Strontium functionalized titanium implants” and by the Danish Agency for Science, Technology and Innovation through a mobility scholarship.
Disclosure
The authors report no conflicts of interest in this work.
References
- BuserDJannerSFWittnebenJGBraggerURamseierCASalviGE10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: a retrospective study in 303 partially edentulous patientsClin Implant Dent Relat Res201214683985122897683
- ChappuisVBuserRBraggerUBornsteinMMSalviGEBuserDLong-term outcomes of dental implants with a titanium plasma-sprayed surface: a 20-year prospective case series study in partially edentulous patientsClin Implant Dent Relat Res201315678079023506385
- SrinivasanMMeyerSMombelliAMullerFDental implants in the elderly population: a systematic review and meta-analysisClin Oral Implants Res201728892093027273468
- BrånemarkPIAdellRBreineUHanssonBOLindströmJOhlssonAIntra-osseous anchorage of dental prostheses. I. Experimental studiesScand J Plast Reconstr Surg196932811004924041
- ShalabiMMGortemakerAVan’t HofMAJansenJACreugersNHImplant surface roughness and bone healing: a systematic reviewJ Dent Res200685649650016723643
- SmeetsRStadlingerBSchwarzFImpact of dental implant surface modifications on osseointegrationBiomed Res Int201662856201011
- CochranDLJacksonJMBernardJPA 5-year prospective multicenter study of early loaded titanium implants with a sandblasted and acid-etched surfaceInt J Oral Maxillofac Implants20112661324133222167440
- CollaertBWijnenLDe BruynHA 2-year prospective study on immediate loading with fluoride-modified implants in the edentulous mandibleClin Oral Implants Res201122101111111621244503
- GuarnieriRSerraMBavaLGrandeMFarronatoDIorio-SicilianoVThe impact of a laser-microtextured collar on crestal bone level and clinical parameters under various placement and loading protocolsInt J Oral Maxillofac Implants201429235436324683561
- BonfanteEAGranatoRMarinCBiomechanical testing of microblasted, acid-etched/microblasted, anodized, and discrete crystalline deposition surfaces: an experimental study in beagle dogsInt J Oral Maxillofac Implants201328113614223377058
- RuppFScheidelerLOlshanskaNde WildMWielandMGeis-GerstorferJEnhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfacesJ Biomed Mater Res A200676232333416270344
- SchwarzFHertenMSagerMWielandMDardMBeckerJHistological and immunohistochemical analysis of initial and early osseous integration at chemically modified and conventional SLA titanium implants: preliminary results of a pilot study in dogsClin Oral Implants Res200718448148817484737
- BuserDBrogginiNWielandMEnhanced bone apposition to a chemically modified SLA titanium surfaceJ Dent Res200483752953315218041
- SchwarzFWielandMSchwartzZPotential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implantsJ Biomed Mater Res B Appl Biomater200988254455718837448
- FontanaFRocchiettaIAddisASchupbachPZanottiGSimionMEffects of a calcium phosphate coating on the osseointegration of endosseous implants in a rabbit modelClin Oral Implants Res201122776076621198895
- GalliSNaitoYKarlssonJLocal release of magnesium from mesoporous TiO2 coatings stimulates the peri-implant expression of osteogenic markers and improves osteoconductivity in vivoActa Biomater201410125193520125153781
- AndersenOZOffermannsVSillassenMAccelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implantsBiomaterials201334245883589023672822
- MariePJStrontium ranelate in osteoporosis and beyond: identifying molecular targets in bone cell biologyMol Interv201010530531221045244
- MeunierPJRouxCSeemanEThe effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosisNew Engl J Med2004350545946814749454
- TieDGuanRLiuHAn in vivo study on the metabolism and osteogenic activity of bioabsorbable Mg-1Sr alloyActa Biomater20162945546726577986
- ThormannURaySSommerUBone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized ratsBiomaterials201334348589859823906515
- GentlemanEFredholmYCJellGThe effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitroBiomaterials201031143949395620170952
- GuZHuangBLiYTianMLiLYuXStrontium-doped calcium polyphosphate/ultrahigh molecular weight polyethylene composites: a new class of artificial joint components with enhanced biological efficacy to aseptic looseningMater Sci Eng C Mater Biol Appl20166152653326838880
- ParkJWKwonTGSuhJYThe relative effect of surface strontium chemistry and super-hydrophilicity on the early osseointegration of moderately rough titanium surface in the rabbit femurClin Oral Impl Res2013246706709
- PengSLiuXSHuangSThe cross-talk between osteoclasts and osteoblasts in response to strontium treatment: involvement of osteoprotegerinBone20114961290129821925296
- YangFYangDTuJZhengQCaiLWangLStrontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signalingStem Cells201129698199121563277
- BonnelyeEChabadelASaltelFJurdicPDual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitroBone200842112913817945546
- FromigueOHayEBarbaraAMariePJEssential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelateJ Biol Chem201028533252512525820554534
- SaidakZMariePJStrontium signaling: molecular mechanisms and therapeutic implications in osteoporosisPharmacol Ther2012136221622622820094
- AdamMGanzCXuWSarajianHRGotzWGerberTIn vivo and in vitro investigations of a nanostructured coating material – a preclinical studyInt J Nanomedicine2014997598424627631
- Santocildes-RomeroMECrawfordAHattonPVGoodchildRLReaneyIMMillerCAThe osteogenic response of mesenchymal stromal cells to strontium-substituted bioactive glassesJ Tissue Eng Regen Med20159561963125757935
- LiYQiYGaoQEffects of a micro/nano rough strontium-loaded surface on osseointegrationInt J Nanomedicine2015104549456326213468
- QuirynenMvan der MeiHCBollenCMAn in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaqueJ Dent Res1993729130413098395545
- TeughelsWVan AsscheNSliepenIQuirynenMEffect of material characteristics and/or surface topography on biofilm developmentClin Oral Implants Res200617Suppl 2688116968383
- Izquierdo-BarbaIGarcia-MartinJMAlvarezRNanocolumnar coatings with selective behavior towards osteoblast and staphylococcus aureus proliferationActa Biomater201515202825573448
- MendoncaGMendoncaDBAragaoFJCooperLFAdvancing dental implant surface technology – from micron- to nanotopographyBiomaterials200829283822383518617258
- WennerbergAAlbrektssonTOn implant surfaces: a review of current knowledge and opinionsInt J Oral Maxillofac Implants2010251637420209188
- SillassenMJeppesenCSAndersenOZControlled Sr release from Ti–Sr–O films deposited by non-reactive magnetron sputtering in an industrial setupSurface and Coatings Technology20142525663
- LiJPLiPHuJEarly healing of hydroxyapatite-coated implants in grafted bone of zoledronic acid-treated osteoporotic rabbitsJ Periodontol201485230831623688100
- KilkennyCBrowneWJCuthillICEmersonMAltmanDGImproving bioscience research reporting: the ARRIVE guidelines for reporting animal researchPLoS Biology201086e100041220613859
- DonathKBreunerGA method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) techniqueJ Oral Pathol19821143183266809919
- OffermannsVAndersenOZRiedeGBone regenerating effect of surface-functionalized titanium implants with sustained-release characteristics of strontium in ovariectomized ratsInt J Nanomedicine2016112431244227313456
- YeritKCPoschMSeemannMImplant survival in mandibles of irradiated oral cancer patientsClin Oral Implants Res200617333734416672031
- GuazzoRSbricoliLRicciSBressanEPiattelliAIaculliFMedication-related osteonecrosis of the jaw and dental implants failures: a systematic reviewJ Oral Implantol2017431515728231038
- DangYZhangLSongWIn vivo osseointegration of Ti implants with a strontium-containing nanotubular coatingInt J Nanomedicine2016111003101127042055
- ParkJWKimYJJangJHSongHPositive modulation of osteogenesis- and osteoclastogenesis-related gene expression with strontium-containing microstructured Ti implants in rabbit cancellous boneJ Biomed Mater Res A2013101129830623065737
- LiYLiQZhuSThe effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized ratsBiomaterials201031349006901420800275
- KolindKLeongKWBesenbacherFFossMGuidance of stem cell fate on 2D patterned surfacesBiomaterials201233286626663322748769
- LordMSFossMBesenbacherFInfluence of nanoscale surface topography on protein adsorption and cellular responseNano Today2010516678
- BjurstenLMRasmussonLOhSSmithGCBrammerKSJinSTitanium dioxide nanotubes enhance bone bonding in vivoJ Biomed Mater Res A20109231218122419343780
- DalbyMJGadegaardNTareRThe control of human mesenchymal cell differentiation using nanoscale symmetry and disorderNature Materials2007612997100317891143
- YoungPSGreerAIMTsimbouriMPMeekRMDGadegaardNDalbyMJStrontium-eluting nanotopographical surfaces to control bone homeostasisBone & Joint Journal Orthopaedic Proceedings Supplement201799-BSuppl 1822
- OffermannsVAndersenOZFalkensammerGEnhanced osseointegration of endosseous implants by predictable sustained release properties of strontiumJ Biomed Mater Res B Appl Biomater2014251033279
- MortonDBornsteinMMWittnebenJGEarly loading after 21 days of healing of nonsubmerged titanium implants with a chemically modified sandblasted and acid-etched surface: two-year results of a prospective two-center studyClin Implant Dent Relat Res201012191719744195
- BuserDBrogginiNWielandMEnhanced bone apposition to a chemically modified SLA titanium surfaceJ Dent Res200483752953315218041
- SchwarzFHertenMSagerMWielandMDardMBeckerJHistological and immunohistochemical analysis of initial and early osseous integration at chemically modified and conventional SLA titanium implants: preliminary results of a pilot study in dogsClin Oral Implants Res200718448148817484737
- ParkJWKwonTGSuhJYThe relative effect of surface strontium chemistry and super-hydrophilicity on the early osseointegration of moderately rough titanium surface in the rabbit femurClin Oral Implants Res201324670670922409778
- JannerSFMGahlertMBosshardtDDBone response to functionally loaded, two-piece zirconia implants: a preclinical histometric studyClin Oral Implants Res201829327728929288505