?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The purpose of this study was to develop a method for targeted delivery of 10-hydroxycamptothecin (HCPT)-loaded nanoparticles (NPs) to cancer cells.
Methods
We first used a supercritical antisolvent process to prepare micronized HCPT (nHCPT), and then folate-conjugated human serum albumin (HSA) nHCPT-loaded NPs (FA-HSA-nHCPT-NPs) were prepared using a NP-coated method combined with a desolvation technique. The amount of folate conjugation was 16 μg · mg−1 HSA.
Results
The particle size of the spherical nHCPT microparticles obtained was 118.5 ± 6.6 nm. The particle size and zeta potential of the FA-HSA-nHCPT-NPs were 233.9 ± 1.2 nm and −25.23 ± 2.98 mV, respectively. The FA-HSA-nHCPT-NPs exhibited a smooth surface and a distinct spherical shape, and the results of differential scanning calorimetry and X-ray diffraction indicated that the FA-HSA-nHCPT-NPs presented in a nanostructured amorphous state. The FA-HSA-nHCPT-NPs showed sustained-release characteristics for 120 hours in vitro, with a drug-loading content of 7.3% and an encapsulating efficiency of 79.1%.
Conclusion
The FA-NPs were effective delivery systems for uptake by SGC7901 cells compared with folate-free NPs. These results suggest that a NP-coated method combined with a desolvation technique is effective for preparing NPs with drugs having poor solubility in water and most organic solvents, using albumin as the wall material. FA-HSA-NPs are a stable delivery system and have the potential for targeted delivery of anticancer drugs.
Introduction
Camptothecin and related analogs have shown promise as anticancer agents, which could lead to the death of tumor cells by targeting the nuclear enzyme, topoisomerase I, and inhibiting the relegation of the cleaved DNA strand.Citation1 One of the camptothecin analogs, hydroxycamptothecin (HCPT), a plant alkaloid derived from Camptotheca acuminata, has demonstrated strong antitumor activity against gastric, lung, ovarian, breast, and pancreatic carcinomas.Citation2 Unfortunately, HCPT has poor solubility in water and other physiologically acceptable solvents, which limits its clinical application. The earlier clinical trials used its highly water-soluble sodium salt, but the opening of the active lactone ring at physiological pH limited the tumor response, was much less active, and was highly toxic.Citation3–Citation5 To overcome these limitations, many attempts had been made to develop high-performance delivery systems. Niosomes,Citation6 micelles,Citation7,Citation8 and nanoparticlesCitation9 had been explored with some success. Among these delivery systems, albumin has been used widely in recent years because of its biodegradability, stability, and lower toxicity, which significantly retard the lactone ring opening.Citation10,Citation11
Many targeting agents have been coupled with drug delivery systems to improve their site-specific targeting. Folic acid is a low- molecular- weight (441 Da) vitamin, the receptor for which is frequently overexpressed in human cancer cells, but is restricted in most normal tissues.Citation12,Citation13 Folate conjugates of radiopharmaceutical agents, chemotherapeutic agents, antisense oligodeoxynucleotides, immunotherapeutic agents, and plasmids had all been delivered to cancer cells overexpressing the folate receptor.Citation14
The supercritical antisolvent process (SAS) is a new micronization technology developed in recent years, which has been adapted for preparation of drug nanocrystals because of its inertia and low temperature.Citation15 In this study, we prepared micronized hydroxycamptothecin (nHCPT) using SAS, and folate-conjugated (FA) human serum albumin (HSA) nanoparticles (NPs) were considered to be promising carriers. FA-HSA-nHCPT-NPs were prepared by a NP-coated method combined with a desolvation technique. The preparation and related characteristics of the NPs obtained were investigated, and an in vitro release test and measurement of uptake of NPs by cancer cells were also conducted.
Materials and methods
Materials
10-HCPT (98%) and HSA were provided by Hisun Pharmaceutical Co Ltd (Zhejiang, China). Folate-free RPMI 1640, folic acid, fluorescein isothiocyanate (FITC), and phosphate-buffered solution (pH = 7.4) were purchased from Sigma-Aldrich (St. Louis, MO). RPMI 1640 and trypsin-ethylenediaminetetraacetic acid were purchased from Gibco (Invitrogen, Carlsbad, CA). Acetonitrile was of high-pressure liquid chromatography grade and all the other reagents were of analytical grade.
Preparation of nHCPT by SAS
nHCPT was prepared by SAS, based on the method used in the early research.Citation15 Three important parameters in the SAS process, ie, the pressure of the precipitation chamber, the concentration of HCPT in dimethyl sulfoxide solution, and the speed of the liquid pump, were 25 mPa, 6 mg·mL−1, and 13.3 mL·min−1, respectively.
Preparation of FA-HSA
The method used for preparing an N-hydroxysuccinimide ester of folate (NHS folate) had been reported.Citation16 The carboxylic groups of the NHS folate were conjugated with the amino groups of the HSA under alkaline conditions. NHS folate 2.5 mg was dissolved in 1 mL carbonate/bicarbonate buffer (0.1 M, pH = 10), and added slowly to the stirring HSA solution, and the reaction was maintained for 45 minutes at ambient temperature. The unreacted NHS folate was precipitated by adjusting the pH to the 4–5 range using 0.1 M HCl. The mixture was treated in an ultrasonicator for 20 minutes, and centrifuged at 12,000 rpm for 10 minutes three times. The supernatant, FA-HSA, was lyophilized to give a yellow powder.
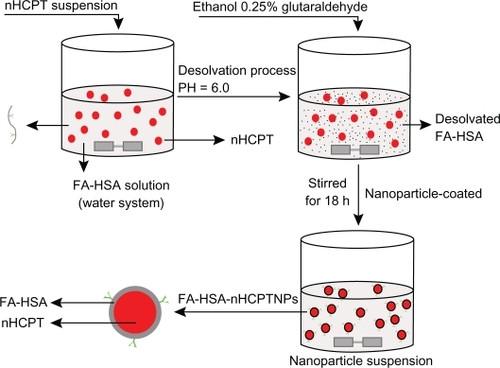
Preparation of FA-HSA-nHCPT-NPs
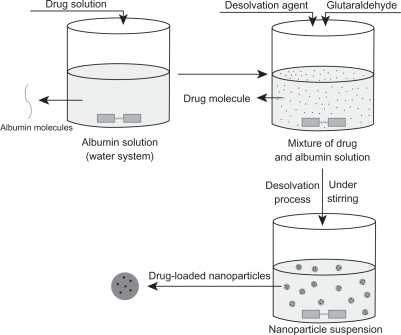
FA-HSA-nHCPT-NPs were prepared using a NP-coated method combined with a desolvation technique. nHCPT 2 mg was added to 3.0 mL of purified water, and sonicated until the nHCPT was completely dispersed. The nHCPT suspension was added slowly to the stirring 3.0 mL FA-HSA purified water solution (6.7 mg·mL−1), and the pH of the mixture was adjusted to 6.0 to protect the potent lactone form of HCPT. Ethanol 6.6 mL was added dropwise under constant stirring, and 0.2 mL of 0.25% glutaraldehyde solution was added to the reaction mixture as a cross-linking agent. The mixture was continuously stirred for 18 hours to obtain the NPs (). The mixture was then centrifuged at 40,000 rpm for 10 minutes to separate the unloaded nHCPT, and was washed with purified water twice. The supernatant was collected to detect the drug-loading and encapsulating efficiency, the precipitation was redispersed with purified water, 1 mL was withdrawn for particle size measurement and zeta potential analysis, and the rest was lyophilized. Mannitol was added as a lyoprotectant if needed. The control FA-HSA-NPs were prepared according to the aforementioned process, but did not contain nHCPT.
Figure 1 Schematic process for preparation of micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles.
Abbreviations: nHPCT, micronized 10-hydroxycamptothecin; FA-HSA-nHCPT-NPs, micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles.
Determination of amount of FA-HSA
The lyophilized FA-HSA-NPs were redispersed with purified water and digested by trypsin (0.1 mg·mg−1 HSA) overnight at room temperature, then the resulting tryptic hydrolysis of FA-HSA-NPs was detected using an ultraviolet spectrophotometer (Shimadzu, Tokyo, Japan) in the 270–450 nm range. The degree of folate conjugated with HSA was calculated relative to a NHS folate reference of y = 0.016x + 0.002, R2 = 0.9999 (y, NHS folate concentration in μg·mL−1; x, absorption).
Characterization of FA-HSA-nHCPT-NPs
The mean particle size and zeta potential of the FA-HSA-nHCPT-NPs were determined by laser light scattering and a zeta potential analyzer (Zetaplus, Brookhaven Instruments, Holtsville, NY), respectively. The MSD analysis method was used to obtain laser light scattering measurements. The samples were diluted with purified water and measured according to the average number of particles at room temperature. Each experiment was determined in triplicate. The morphology of the nanoparticles was observed by scanning electron microscopy (FEI Co, Eindhoven, The Netherlands).
The X-ray diffraction patterns were collected in transmission using an X-ray diffractometer with a rotating anode (Philips, Xpert-Pro, The Netherlands). The scanning rate was 5°C·min−1, and the diffraction angle (2θ) was recorded at 5–80°C. Cu Kα1 radiation was generated at 30 mA and 50 kV as the X-ray source.
Differential scanning calorimetry analysis was performed using a TA-60WS Thermal Analyzer (Shimadzu, Japan). The curves of the samples were recorded at a heating rate of 10°C·min−1 from 40°C to 300°C.
Drug-loading and encapsulating efficiency
To evaluate drug-loading and encapsulating efficiency, the unloaded nHCPT in the supernatant after centrifugation was determined by reversed-phase high-pressure liquid chromatography. A high-pressure liquid chromatography system equipped with a Jasco 975 ultraviolet detector and HIQ SIL C18 column (250 mm × 4.6 mm, 5 μm; Jasco,Tokyo, Japan) was used. The mobile phase comprised 35% (v/v) acetonitrile in water (pH = 5.5) at 1 mL·min−1. Detection was performed at 370 nm. All samples were conducted in triplicate. Drug-loading and encapsulating efficiency were determined by Equationequations (1)(1) and Equation(2)
(2) .
In vitro release of nHCPT from FA-HSA-nHCPT-NPs
Release of nHCPT from the nanoparticles was carried out in phosphate-buffered solution (pH = 7.4) as follows. FA-HSA-nHCPT-NPs were dispersed in 1 mL phosphate-buffered solution, and placed in a dialysis bag (molecular weight cutoff 3500 Da). The end-sealed dialysis bag was incubated in 100 mL phosphate-buffered solution at 37°C with gentle agitation. At predetermined time intervals, 10 mL of dialysis solution was withdrawn and replaced with an equal volume of fresh phosphate-buffered solution. The concentration of HCPT was determined by high-pressure liquid chromatography as described earlier. Each sample was determined in triplicate.
Cell culture
SGC7901 and A549 cells were used in the NP cell uptake studies, and were purchased from the Shanghai Cell Line Bank (Chinese Academy of Sciences, Shanghai, China). The cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 U·mL−1 penicillin, and 100 μg·mL−1 streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. In the cell uptake studies, folate-free RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum was used to minimize the effect of folate.Citation17
Cell uptake
The FA-HSA-NPs and HSA-NPs were labeled with FITC to observe the extent of uptake by SGC7901 and A549 cells. The cells were seeded at 3 × 105 cells per well and cultured in 12-well plates for one day. Each well was incubated with FITC-labeled FA-HSA-NPs or HSA-NPs at indicated concentrations for four hours at 37°C. The cells were washed three times with phosphate-buffered solution, and observed by laser scanning confocal microscopy (Nikon, Tokyo, Japan). The excitation wavelength was 488 nm.
Results and discussion
Characterization of FA-HSA-nHCPT-NPs
In this research, FA-HSA-nHCPT-NPs were prepared based on SAS micronized 10-HCPT using a NP-coated method combined with desolvation technology. The method included two steps. As showed in , the first step was the desolvation process. We used ethanol as the desolvating agent to decrease the solubility of the coating material (FA-HSA) gradually in water, and 0.25% glutaraldehyde solution was added to crosslink the desolvated FA-HSA. The second step was the NP-coated process, whereby the nHCPT was loaded through electrostatic interaction between the drug and FA-HSA. As the core, nHCPT was coated gradually under stirring for the indicated time interval, and FA-HSA-nHCPT-NPs were formed.
The amount of folate conjugated with HSA was determined using an ultraviolet spectrophotometer. As show in , the absorption peaks of tryptic hydrolysis for the FA-HSA-NPs at 280 nm and 365 nm were consistent with the absorption peak of NHS folate, which confirmed that the folate was successfully conjugated with HSA. The amount of folate associated with HSA was 16 μg·mg−1, which was evaluated according to the standard curve of NHS folate. Folate could enable binding, promote cell uptake, and thus increase toxicity when 2–3 folate groups were inserted per HSA molecule.Citation18 Our findings were as follows.
Figure 2 A) Determination of folate content conjugated with human serum albumin, a) N-hydroxysuccinimide ester of folate, b) tryptic hydrolysis of folate-conjugated human serum albumin nanoparticles, c) tryptic hydrolysis of human serum albumin nanoparticles; the particle size distribution of B) micronized 10-hydroxycamptothecin, C) micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles, D) HSA-nHCPT-NPs; the morphology observation of E) raw hydroxycamptothecin, F) micronized hydroxycamptothecin, G) micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles, and H) human serum albumin-loaded 10-hydroxycamptothecin nanoparticles, by scanning electron microscopy.
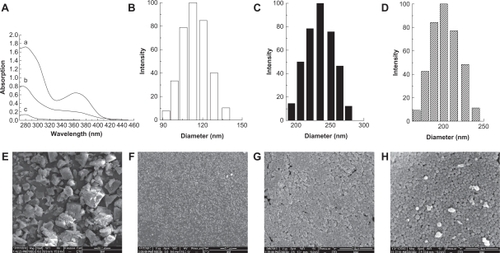
Mean diameter and size distribution of the prepared NPs were determined by laser light scattering. nHCPT had an average diameter of 118.5 ± 6.6 nm (), the mean diameters of FA-HSA-nHCPT-NPs and HSA-nHCPT-NPs were 233.9 ± 1.2 nm and 192.5 ± 7.4 nm, respectively. HSA-nHCPT-NPs were of smaller size compared with FA-HSA-nHCPT-NPs, both of which had a narrow size distribution (). The mean diameters of control FA-HSA and HSA nanoparticles (unloaded nHCPT) were 159.0 ± 3.3 nm and 140.1 ± 2.6 nm, respectively.
The surface charge has a significant effect on the stability of a colloidal nanoparticle system in medium.Citation19 The zeta potential of FA-HSA-nHCPT-NPs and HSA-nHCPT-NPs were −25.23 ± 2.98 mV and −25.40 ± 2.55 mV, respectively, which indicates stability.
The morphology of the NPs was investigated by scanning electron microscopy. In , the unprocessed HCPT particles were in irregular bulk form or seen as lamelliform crystals, with diameters in the 1–30 μm range. In contrast, as shown in , processed HCPT were seen as spherical microparticles and were of uniform distribution, with no drug crystals visible. FA-HSA-nHCPT-NPs and HSA-nHCPT-NPs exhibited a smooth surface and a distinct spherical shape without conglutination ().
The X-ray diffraction results for raw HCPT, nHCPT, and FA-HSA-nHCPT-NPs are shown in . The peaks of raw HCPT at 2θ = 9.9°, 18.7°, 20.1°, and 27.6° show the crystalline structure of raw HCPT. Nevertheless, nHCPT only presented a weak peak at 2θ = 9.9°, indicating that most of the processed HCPT might be present in an amorphous state. The characteristic peaks of HCPT disappeared in the pattern of FA-HSA-nHCPT-NPs. The results show that nHCPT had been coated as the central core by the hydrophilic outer shell of FA-HSA.
Figure 3 A) X-ray diffraction patterns, B) differential scanning calorimetry (DSC) curves, a) raw 10-hydroxycamptothecin, b) micronized 10-hydroxycamptothecin, and c) micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles.
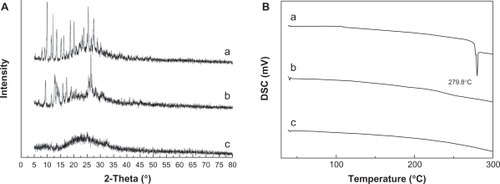
shows the results of differential scanning calorimetry. The raw HCPT exhibited an obvious melting process, with a peak of 279.8°C, which implied the crystalline form of HCPT. No melting process was observed for nHCPT and FA-HSA-nHCPT-NPs, indicating that both were nanostructured and noncrystalline. The results were consistent with the results of the X-ray diffraction analysis.
In vitro release of nHCPT from NPs
FA-HSA-nHCPT-NPs used in the in vitro release study had a drug-loading content of 7.3% and a high encapsulating efficiency of 79.1%. shows the release profiles of nHCPT and FA-HSA-nHCPT-NPs in vitro over 120 hours. nHCPT was released very slowly from the FA-HSA-nHCPT-NPs. Less than 25% of the loaded nHCPT was released from the FA-HSA-nHCPT-NPs after 16 hours, and the release profile exhibited a very steady sustained-release pattern, with a negligible initial burst release. However, nHCPT had fast release properties. Over 90% of the nHCPT had been released within 16 hours. The release characteristics of the FA-HSA-nHCPT-NPs were in accordance with the Higuchi kinetics model, with an equation of y = −111.627e(−x/67.70) + 113.228 (R2 = 0.996).
Figure 4 The release profiles of micronized 10-hydroxycamptothecin and micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles in vitro. (▴) Micronized 10-hydroxycamptothecin. (▵) Micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles.
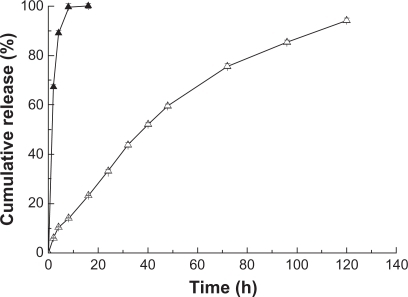
These data suggest that FA-HSA-nHCPT-NPs have a significant effect on sustained release. Being the main constituent of the hydrophilic outer part, HSA could increase the solubility and absorption rate of the insoluble drug. Combining drugs with albumin could prevent release at the injection site and enable the drug to be released slowly.Citation20,Citation21
Uptake of FITC-labeled NPs by tumor cells
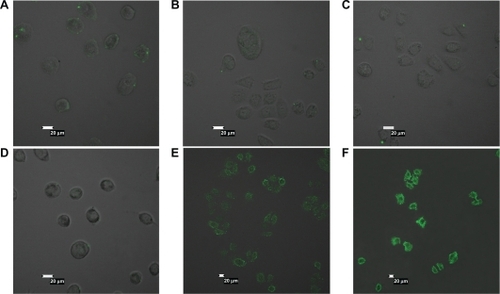
FITC-labeled albumin nanoparticles with or without folic acid ligand was used in a previously reported uptake study.Citation22 Tumor cells were treated with different concentrations of FITC-labeled nanoparticles for four hours. A significant amount of FA-HSA-NPs was attached to the surfaces of SGC7901 cells, and numerous green fluorescent spots were observed around the SGC7901 cell surface (). The intensity of fluorescence around the cells was enhanced in a concentration-dependent pattern as shown in . However, the intensity of fluorescence around the SGC7901 cell surface was very weak when the cells were incubated with HSA-NPs which lacked folic acid ligand (). When folate receptor-deficient A549 cells were incubated with FITC-labeled FA-HSA-NPs and HSA-NPs (0.5 mg·mL−1), there were no obvious fluorescence spots on the cell surfaces because of the lack of folate receptors (). This implied that SGC7901 cells were more sensitive to FA-HSA-NPs, and higher internalization of FA-HSA-NPs could be observed in comparison with HSA-NPs, which is consistent with the report by Han et al.Citation23 FA-HSA-NPs were effective delivery systems for uptake by tumor cells.
Figure 5 Confocal microscopy results. A549 cells (A and D) and SGC7901 cells (B, C, E, and F) were incubated with fluorescein isothiocyanate-labeled folate-conjugated human serum albumin nanoparticles or human serum albumin nanoparticles at indicated concentrations for four hours at 37°C. A) and B) 0.5 mg·mL−1 human serum albumin nanoparticles. C) 1 mg·mL−1 human serum albumin nanoparticles. D) and E) 0.5 mg·mL−1 folate-conjugated human serum albumin nanoparticles. F) 1 mg·mL−1 folateconjugated human serum albumin nanoparticles.
The search for effective drug target delivery systems is a hot topic in tumor therapy. NPs represent promising drug carriers, especially for specific transport of anticancer drugs to the tumor site, because of less drug leakage, high drug-loading efficiency, good storage stability, and an ability to circumvent multidrug resistance in cancer cells.Citation24 NPs made of HSA have several specific advantages, such as being well tolerated without any serious side effects, and are biodegradable. Importantly, HSA offers the benefits of being able to carry functional groups (ie, amino and carboxylic groups) that could be used for surface modifications.Citation25 Therefore, folate as a drug targeting ligand has often been used in the recent years for cancer-targeted drug delivery because of its high affinity (10−10 M)Citation26 through association with albumin.
Generally, there were two different methods applied for albumin NP preparation, ie, desolvation technology and emulsion formation. The desolvation of albumin with organic solvents followed by crosslinking with glutaraldehyde is commonly used to assemble protein NPs.Citation27 The classical desolvation process is illustrated in . Drugs were dissolved in water together with albumin or added to an albumin solution, and the desolvation agent was subsequently added dropwise into the mixture solution under stirring. When adding the desolvation agent to the protein solution, the albumin was phase-separated as its water solubility diminished, and nanoparticles were then formed from the albumin solution. Simultaneously, the drug molecules in the solution were encapsulated or diffused into the NPs.Citation28–Citation30 In our early research, we dissolved drug in the desolvation agent which was subsequently added to the BSA solution and crosslinked with glutaraldehyde to prepare NPs. The NPs obtained were then decorated with folic acid under alkaline conditions. Unfortunately, the lactone form of HCPT was insoluble in both water and conventional desolvating agents, eg, ethanol and acetone. Moreover, most HCPT molecules existed in the inactive carboxylated form at physiological pH values. Therefore, traditional desolvation technology was not suitable for the preparation of FA-HSA-nHCPT-NPs.
In the early research, 10-HCPT-loaded bovine serum albumin NPs were prepared by Yang et alCitation31 using a reformative emulsion-heat stabilization technique. HCPT and bovine serum albumin were dissolved together in NaOH solution, and added to castor oil containing 2% Span-80 to form a water/oil emulsion. After stirring and high-speed emulsification, HCPT-loaded NPs were formed. During the process, HCPT transformed from lactone to carboxylate and finally back to the lactone form. Although the investigators obtained HCPT-loaded bovine serum albumin NPs successfully, much more castor oil was used (aqueous to nonaqueous phase ratio 1:5), which could induce unpredictable toxicity. Removal of organic residues was needed. The NPs obtained had a particle size of about 600 nm. According to Müller,Citation32 albumin particles below 500 nm were difficult to obtain by the emulsion method. Moreover, perhaps because of the transformation between the lactone and carboxylate forms in the preparation process, these NPs had a lower drug-loading content and encapsulating efficiency (2.21% and 57.5%, respectively).
In our study, we first conjugated folate with HSA, and used a NP-coated method combined with a desolvation technique to prepare FA-HSA-nHCPT-NPs without changing the lactone of HCPT into the carboxylate form. The preparation process is described earlier. The pH of the reaction system was adjusted to 6.0; on the one hand, it was used to protect the potent lactone form of nHCPT and, on the other hand, according to the drug-loading mechanism reported by Huang et al.Citation33 The lactone form of nHCPT was loaded by electrostatic interaction between the negative charges of the FA-HSA and the positive charges of the drug when the pH of the solution was adjusted to higher than the isoelectric point of HSA. NPs obtained by this method had a smaller average diameter (less than 240 nm), and higher drug-loading content and encapsulating efficiency (7.3% and 79.1%, respectively). Used as the desolvation agent, ethanol was removed, followed by centrifugation and, finally, lyophilization. Many different NP-coated methods have been used to prepare microparticles, emulsions, and magnetic NPs, and special construction could modify drug release from the particles.Citation34–Citation36
The mechanism of drug release from albumin NPs has been shown to be dependent on the location of the drug in the carrier, as well as on the properties of the NP matrix.Citation37 There were two main forms of drug associated with albumin NPs; one is adsorption onto the particle surface and the other is inclusion in the NP matrix. If the albumin matrix is stable enough, the release of HCPT from NPs appears to be difficult.Citation31 The crosslinking process (ie, time of crosslinking and glutaraldehyde concentration) plays an important role in the stability and drug release from albumin NPs.Citation38,Citation39 In the process of FA-HSA-nHCPT-NP preparation, we used 0.25% glutaraldehyde as a crosslinking agent, which provided sustained-release of NPs for more than 120 hours.
In comparison with folate-free HSA nanoparticles, the FA-HSA-NPs showed higher uptake by SGC7901cells which were sensitive to folic acid ligand. This suggests that if cancer cells only overexpressed folate receptors on their surface, FA-HSA-NPs could have a better uptake effect. FA-HSA-NPs show multivalent interactions with folate receptors, which generally exist in large clusters.Citation40 Therefore, a FA-HSA-NP might occupy many receptors because of its special structure, and in this way may have high affinity for tumor cells.
Conclusion
We used SAS technology to prepare nHCPT, and our results indicate that nHCPT has a smaller diameter and narrow size distribution compared with raw HCPT. Moreover, nHCPT presented in an amorphous state. We prepared FA-HSA-nHCPT-NPs using a NP-coated method combined with a desolvation technique. The method used was advanced and simple. The spherically shaped HCPT-loaded NPs obtained had a small average diameter, and their drug-loading content and encapsulating efficiency were considerable. The sustained-release characteristics of the FA-HSA-nHCPT-NPs indicated that HCPT was well protected in a physiological environment (pH = 7.4). There was marked uptake of FA-HSA-nHCPT-NPs by cancer cells in vitro. FA-NPs constitute a promising drug delivery system, and we will focus our research energy on their antitumor effects. Their body distribution and tumor-targeting potential in vivo should also be evaluated in further research.
Acknowledgements
This work was supported by the Program for New Century Excellent Talents in University, the Young Science Foundation of Heilongjiang Province, the Scientific Research Foundation for Returned Scholars, Heilongjiang Province, the Fundamental Research Funds for the Central Universities, and the Talents Foundation of Harbin City.
Disclosure
The authors report no conflicts of interest in this work.
References
- WangJWangRLiLBPreparation and properties of hydroxycamptothecin-loaded nanoparticles made of amphiphilic copolymer and normal polymerJ Colloid Interface Sci2009336280881319520376
- PuXSunJWangYDevelopment of a chemically stable 10-hydroxycamptothecin nanosuspensionInt J Pharm2009379116717319505545
- ErtlBPlatzerPWirthMGaborFPoly (D,L-lactic-co-glycolic acid) microspheres for sustained delivery and stabilization of camptothecinJ Control Release199961330531710477803
- ShenderovaABurkeTGSchwendemanSPStabilization of 10-hydroxycamptothecin in poly (lactide-co-glycolide) microsphere delivery vehiclesPharm Res19971410140614149358554
- O’LearyJMuggiaFMCamptothecins: a review of their development and schedules of administrationEur J Cancer19983410150015089893620
- HongMZhuSJiangYYTangGTPeiYYEfficient tumor targeting of hydroxycamptothecin loaded PEGylated niosomes modified with transferringJ Control Release200913329610218840485
- YangXLiLWangYTanYPreparation, pharmacokinetics and tissue distribution of micelles made of reverse thermo-responsive polymersInt J Pharm20093701–221021519114094
- ZhangCDingYYuLLPingQPolymeric micelle systems of hydroxycamptothecin based on amphiphilic N-alkyl-N-trimethyl chitosan derivativesColloids Surf B Biointerfaces200755219219917223019
- MinKHParkKKimYSHydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapyJ Control Release2008127320821818336946
- RubinoOPKowalskyRSwarbrickJAlbumin microspheres as a drug delivery system: relation among turbidity ratio, degree of cross-linking, and drug releasePharm Res1993107105910658378248
- OpanasopitPYokoyamaMWatanabeMKawanoKMaitaniYOkanoTInfluence of serum and albumins from different species on stability of camptothecin-loaded micellesJ Control Release2005104231332115907582
- FranklinWAWaintrubMEdwardsDNew anti-lung-cancer antibody cluster 12 reacts with human folate receptors present on adenocarcinomaInt J Cancer Suppl199457889958194901
- WeitmanSDLarkRHConeyLRDistribution of the folate receptor GP38 in normal and malignant cell lines and tissuesCancer Res19925212339634011596899
- LeamonCPCooperSRHardeeGEFolate-liposome-mediated antisense oligonucleotide targeting to cancer cells: evaluation in vitro and in vivoBioconjug Chem200314473874712862426
- ZhaoXHZuYGLiQYPreparation and characterization of camptothecin powder micronized by a supercritical antisolvent (SAS) processJ Supercrit Fluids2010513412419
- LeamonCPLowPSDelivery of macromolecules into living cells: a method that exploits folate receptor endocytosisProc Natl Acad Sci U S A19918813557255762062838
- KimSHKimJKLimSJParkJSLeeMKKimCKFolate-tethered emulsion for the target delivery of retinoids to cancer cellsEur J Pharm Biopharm200868361862517949957
- DosioFArpiccoSStellaBBrusaPCattelLFolate-mediated targeting of albumin conjugates of paclitaxel obtained through a heterogeneous phase systemInt J Pharm20093821–211712319712735
- ZhaoDMZhaoXHZuYGPreparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticlesInt J Nanomedicine2010566967720957218
- ZhangYDGongLSPreparation of amycin loading magnetic albumin nanoparticlesJ China Modern Med20011113
- SuHHuJHLiFQPreparation process and target progress of albumin nanoparticlesJ Chin Pharm200540641644
- ZhangLHouSMaoSWeiDSongXLuYUptake of folate-conjugated albumin nanoparticles to the SKOV3 cellsInt J Pharm20042871–215516215541922
- HanXLiuMXieC9-NC-loaded folate-conjugated polymer micelles as tumor targeted drug delivery system: preparation and evaluation in vitroInt J Pharm20093721–212513119166923
- ChoKWangXNieSChenZGShinDMTherapeutic nanoparticles for drug delivery in cancerClin Cancer Res20081451310131618316549
- WartlickHMichaelisKBalthasarSStrebhardtKKreuterJLangerKHighly specific HER2-mediated cellular uptake of antibody-modified nanoparticles in tumour cellsJ Drug Target200412746147115621671
- AtkinsonSFBettingerTSeymourLWBehrJPWardCMConjugation of folate via gelonin carbohydrate residues retains ribosomal-inactivating properties of the toxin and permits targeting to folate receptor positive cellsJ Biol Chem200127630279302793511359781
- MartyJJOppenheimRCSpeiserPNanoparticles – a new colloidal drug delivery systemPharm Acta Helv19785311723643885
- LiFQSuHWangJPreparation and characterization of sodium ferulate entrapped bovine serum albumin nanoparticles for liver targetingInt J Pharm20083491–227428217870261
- MerodioMArnedoARenedoMJIracheJMGanciclovir-loaded albumin nanoparticles: characterization and in vitro release propertiesEur J Pharm Sci200112325125911113644
- ZuYZhangYZhaoXZhangQLiuYJiangROptimization of the preparation process of vinblastine sulfate (VBLS)-loaded folate-conjugated bovine serum albumin (BSA) nanoparticles for tumor-targeted drug delivery using response surface methodology (RSM)Int J Nanomedicine2009432133320054435
- YangLCuiFCunDTaoAShiKLinWPreparation, characterization and biodistribution of the lactone form of 10-hydroxycamptothecin (HCPT)-loaded bovine serum albumin (BSA) nanoparticlesInt J Pharm20073401–216317217482779
- MüllerBGLeuenbergerHKisselTAlbumin nanospheres as carriers for passive drug targeting: an optimized manufacturing techniquePharm Res199613132378668675
- HuangSJSunSLFengTHSungKHLuiWLWangLFFolate-mediated chondroitin sulfate-Pluronic 127 nanogels as a drug carrierEur J Pharm Sci2009381647319540339
- BeckRCPohlmannARHoffmeisterCDexamethasone-loaded nanoparticle-coated microparticles: correlation between in vitro drug release and drug transport across Caco-2 cell monolayersEue J Pharm Biopharm20076711830
- EskandarNGSimovicSPrestidgeCAChemical stability and phase distribution of all-trans-retinol in nanoparticle-coated emulsionsInt J Pharm20093761–218619419422895
- GillCSPriceBAJonesCWSulfonic acid-functionalized silica-coated magnetic nanoparticle catalystsJournal of Catalysis20072511145152
- GuptaPKHungCTPerrierDGAlbumin microspheres. I. Release characteristics of adriamycinInt J Pharm1986331–3137146
- RubinoOPKowalskyRSwarbrickJAlbumin microspheres as a drug delivery system: relation among turbidity ratio, degree of cross-linking, and drug releasePharm Res1993107105910658378248
- WeberCCoesterCKreuterJLangerKDesolvation process and surface characterisation of protein nanoparticlesInt J Pharm200019419110210601688
- KamenBAWangMTStreckfussAJPeryeaXAndersonRGDelivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recyclesJ Biol Chem19882632713602136093417674