Abstract
The aim of this article is to study the changes inhibited T lymphocytes and cytokines related to the cellular immunity in ICR (imprinting control region) mice fed with Fe3O4 magnetic nanoparticles (Fe3O4-MNPs). The Fe3O4-MNPs were synthesized, and their characteristics such as particle size, zeta potential, and X-ray diffraction patterns were measured and determined. All ICR mice were sacrificed after being exposed to 0, 300, 600, and 1200 mg/kg of Fe3O4-MNPs by single gastric administration for 14 days. Splenocytes proliferation was indicated with stimulate index by MTT assay; release of cytokines in the serum of ICR mice was detected by enzyme-linked immunosorbent assay, and the phenotypic analyses of T-lymphocyte subsets were performed using flow cytometry. Our results indicated that there were no significant differences in splenocyte proliferation and release of cytokines between exposed and control groups. Furthermore, there was no significant difference in the proportions of T-lymphocyte subsets in the low-dose Fe3O4-MNPs group when compared to the control group, but the proportions of CD3+CD4+ and CD3+CD8+ T-lymphocyte subsets both in the medium- and high-dose Fe3O4-MNPs groups were higher than those in the control group. It is concluded that a high dose of Fe3O4-MNPs, to some extent, could influence in vivo immune function of normal ICR mice.
Introduction
Recently, nanometer-sized magnetic particles have been intensively investigated due to their particularly large surface:volume ratio, quantum-size effect, magnetic character as well as their potential application in the areas of bioscience and medicine. Very promising nanoparticles are magnetic iron oxide (Fe3O4) nanoparticles with appropriate surface modification, which have been widely used experimentally for numerous in vivo applications, such as magnetic resonance imaging, tissue repair, immunoassay, detoxification of biological fluids, drug delivery, hyperthermia, cell separation, and so on.Citation1,Citation2
In our previous studies,Citation3,Citation4 we demonstrated that Fe3O4 magnetic nanoparticles (Fe3O4-MNPs) combined with chemotherapy drugs could inhibit tumor proliferation and induce apoptosis of tumor cells in a dose- and time-dependent manner, which may be related to the change in drug formulations caused by magnetic nanomaterials. As a targeted therapeutic drug agent for malignant tumor due to its high specificity, Fe3O4-MNPs may become the mainstay for tumor-targeted therapy.
So far, clinical application of magnetic nanoparticles has focused mainly on the areas of magnetic ferrite. Compared with other magnetic materials, magnetic iron oxide nanoparticles (mainly Fe3O4 and gamma-Fe2O3), which have good chemical stability, magnetic responsiveness, biocompatibility, easy preparation, and significant features in the diagnosis and treatment of diseases, have attracted the attention of researchers worldwide.
The prospects of extensive application of magnetic nanoparticles, account for their impact on human health, living environment, and even life in all aspects of the process are the focus of researchers,Citation5,Citation6 and there are high expectations for their magnetic characteristics and lower cytotoxicity. However, the risk assessment of the biological safety of magnetic nanoparticles has just started and remains in the initial stage of accumulation of controversial data. As a result of their short period of use in human activities, there are few reports on the toxicity of nanomaterials.Citation7–Citation9 One experiment reported that nanosilver at a high concentration had a significant cytotoxic effect on the healthy human peripheral blood mononuclear cells and significantly inhibited the phytohemagglutinin-induced cytokine production.Citation10 Therefore, a safety evaluation, especially of the in vitro and in vivo immunological effects of Fe3O4-MNPs is urgently needed.
According to the requirements of the State Drug Administration in 2002 as stated in ‘Guiding Principles for the Chemical and Therapeutic Biological Products Study’,Citation11 clinical treatment with Fe3O4-MNPs is <5 days, and the experimental period is 14 days. In our study, Fe3O4-MNPs were orally administered once as a single dose imprinting control region (ICR) mice, and in their general performance was observed. Furthermore, all mice were sacrificed separately and used to assess splenocyte proliferation, release of cytokines, and T-lymphocyte subsets and also to understand whether Fe3O4-MNPs are toxic to ICR mice, thus providing a basis for its safe use in clinical settings.
Material and method
Animals and animal care
Female and male ICR mice, which were age-matched (8 weeks of age) and weight-matched (18–22 g), were purchased from Shanghai National Center for Laboratory Animals. The mice were kept in a 12-hour dark/light cycle and received water and food ad libitum in a semibarrier system.
Preparation of Fe3O4-MNPs
As previously described,Citation12 Fe3O4-MNPs were produced by electrochemical deposition under oxidizing conditions. Briefly, a quantity of ferric chloride hexahydrate (0.02 mol/L) and ferrous sulfate heptahydrate (0.012 mol/L) was dissolved in 200 mL deionized water under nitrogen protection, and then about 50 mL of 25% ammonium hydroxide was added slowly with violent magnetic force stirring until the pH was 9. The procedure took 30 minutes to generate a dark precipitate. Next, the resultant preciptate was separated using a permanent magnet and then washed repeatedly by deionized water 5–7 times until the pH of the supernatant was 7. Finally, the characteristics of Fe3O4-MNPs were analyzed.
Determination of Fe3O4-MNPs size and zeta potential
To measure the Fe3O4-MNPs particle size, the sample was dispersed in deionized water and then the particle size was measured using a glass cuvette by the ZetaPlus particle seizer (Brookhaven Instruments Corporation, Holtsville, NY). The same suspension was used for measuring the zeta potential of particles. The average zeta potential and the standard deviation of the three analyses were reported.
Analysis of X-ray diffraction patterns
X-ray diffraction (XRD) pattern of lyophilized sample of Fe3O4-MNPs was carried out using an X-ray diffractometer (ARL X’TRA; Thermo Electron Co, Ecublens, Switzerland). The equipment used a copper target X-ray tube with Cu Kα radiation at a scan speed of 5°/minute. The acceleration voltage and the current flux chosen for the measurement were 45 kV and 35 mA, respectively.
Experimental groups
Forty ICR mice, half male and half female, were individually and randomly divided into four groups: control group (0.5 mL of sterile physiologic saline), low-dose group (300 mg/kg Fe3O4-MNPs), medium-dose group (600 mg/kg Fe3O4-MNPs), and high-dose group (1200 mg/kg Fe3O4-MNPs). The mice in the exposed group were given different concentrations of Fe3O4-MNPs, and those in the control group were given RPMI-1640 (Gibco Chemical Co, Carlsbad, CA) medium by single gastric perfusion and observed for the poisoning symptoms after administration of the drug; finally, they were all sacrificed under Avertin® anesthesia by cervical dislocation after 14 days and used to measure various immune indicators.
Preparation of blood samples
Blood samples were obtained from ICR mice by extirpated eyeballs after being exposed for 14 days. Blood was centrifuged (1500 rpm) for 5 minutes at 4°C to separate plasma from blood cells. The blood mononuclear cells were used for analyzing surface markers of T lymphocytes, and the plasma was prepared for quantitative determination of cytokines.
MTT assays for splenocyte proliferation
Single-cell suspensions were prepared aseptically from ICR mice spleens in RPMI-1640 medium. Briefly, a single-cell suspension was prepared by puncturing the spleen with a 20-gauge needle and gently flushing the organ with ice-cooled (4°C) RPMI-1640. The remaining splenocyte suspension, which was freed from debris by centrifugation at 1000 rmp for 20 minutes at 4°C, was washed twice and adjusted to 2 × 106 cells/mL with RPMI-1640. The adjusted cells were placed in a 96-well microtiter plate in 200 μL aliquots and cultured in the presence of T-lymphocytes mitogen (5 μg/mL Concanavalin A [ConA]) (Sigma Chemical Co, St. Louis, MO) for 68 hours at 37°C in a 5% humidified CO2 atmosphere. The cells that received complete RPMI-1640 medium in the place of mitogen were regarded as the control group. Following this, 10 μL 3-2 (4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide (MTT) (0.5 mg/mL) (Pharmingen, San Diego, CA) was added to each well and cultured for another 4 hours. The formazan was dissolved with 150 μL dimethyl sulfoxide after blotting the culture medium. The plates were shaken lightly for 10 minutes, and then the reduction of methyl thiazol tetrazolium (MTT) was quantified by absorbance at a wavelength of 570 nm using a microplate reader (Model-550; Bio-Rad Laboratories, Hercules, CA). The results were expressed as a mean differential stimulate index (SI) (SI = optical density with ConA/optical density without ConA) of each animal.
ELISA assays for quantitative determination of cytokines
The release of interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 10 (IL-10), and interferon-γ (INF-γ) in serum were measured in duplicate using enzyme-linked immunosorbent assay (ELISA) kit (Gibco, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, 50 μL samples or standard control was added to 50 μL assay diluents in each well, incubated at room temperature for 2 hours, and after thorough washing, 100 μL conjugate was added to each well for incubation for 2 hours. A 100 μL substrate solution was then added to each well and incubated for a further 30 minutes. Finally, a 100 μL stop solution was added to each well, and the optical density was measured using an ELISA reader (Bio-Rad Laboratories) with dual wavelength of 450 nm.
FCM assays for T-lymphocyte subsets
Phenotypic analyses of blood lymphocytes were performed using flow cytometry. Briefly, blood mononuclear cells were separated on Ficoll-Hypaque, washed twice in phosphate-buffered saline (PBS), and adjusted to a density of 1 × 107 cells/mL. The cells were incubated with PE or FITC-conjugated monoclonal antibodies (antimouse CD3 [PE-Cy5], antimouse CD4 [FITC], or antimouse CD8 [PE]) (all sourced from Pharmingen) for 10 minutes, washed three times, and resuspended in PBS immediately before determination. At least 10,000 cells in fluorescent channel 3 for CD3, fluorescent channel 1 for CD4, and fluorescent channel 2 for CD8 were detected using FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and analyzed with CELLQuest®. Results were expressed as mean fluorescence intensity for a given molecule per cell.
Statistical analysis
Data were expressed as means ± standard deviation (x̄ ± S) Statistical analyses were done using SAS software (version 9.1; SAS Institute, Cary, NC). One-way analysis of variance (ANOVA) and rank sum test were carried out to compare the normal data distribution between the exposed and control groups. Wilcoxon rank test was carried out to compare the nonnormal data distribution between the exposed and control groups. Statistical significance for all tests was P = 0.05.
Results
Physical characteristics of magnetic nanoparticles
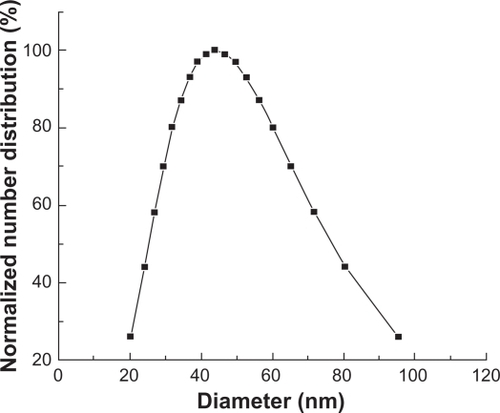
The particle size of the analyzer showed that the size of Fe3O4-MNPs ranged from 20.3 to 95.5 nm and the median particle size was 44.0 nm (). The zeta potential of Fe3O4-MNPs in water was −24.71 mV.
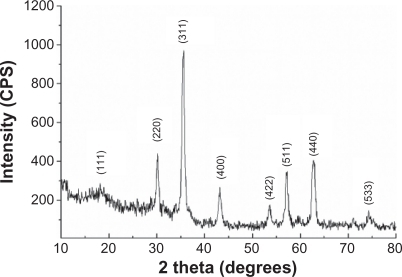
XRD pattern of prepared Fe3O4-MNPs reveal diffraction peaks at (111), (220), (311), (400), (422), (511), (440), and (533), which are the characteristic peaks of the Fe3O4 crystal with a cubic spinel structure ().
Splenocytes proliferation
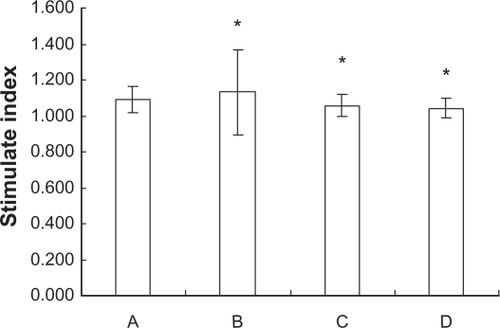
Wilcoxon rank test was carried out to compare the data of splenocyte proliferation from the ICR mice in the groups fed with a different dose of Fe3O4-MNPs, which were not subject to the normal distribution. Our results showed that there was no significant difference in SI between the exposed and control groups (P > 0.05) ().
Figure 3 Splenocytes proliferation of ICR mice fed with different doses of Fe3O4-MNPs.
Notes: A Control group, B low-dose group (300 mg/kg Fe3O4-MNPs), C medium-dose group (600 mg/kg Fe3O4-MNPs), D high-dose group (1200 mg/kg Fe3O4-MNPs), *P > 0.05, there were no difference between exposed groups and control group.
Abbreviations: Fe3O4-MNPs, magnetic Fe3O4 nanoparticles; ICR, imprinting control region.
The level of serum cytokine in ICR mice
With the exception of INF-γ, the data on cytokine production from the ICR mice in these four groups fed with a different doses of Fe3O4-MNPs showed a normal distribution. One-way ANOVA and rank sum test were carried out to compare the data on IL-2, IL-4, and IL-10 production between the exposed and control groups. Wilcoxon rank test was carried out to compare the data on INF-γ production between the exposed and control groups. We found that the various differences of cytokine production from the ICR mice were not statistically significant in the four groups fed with a different dose of Fe3O4-MNPs ().
Table 1 The level of serum cytokine in ICR mice fed with Fe3O4-MNPs
T-lymphocyte subsets
A Wilcoxon rank test was carried out to compare the data of T-lymphocyte subsets from the ICR mice in the four groups fed with a different dose of Fe3O4-MNPs, which were not subject to a normal distribution. We found that there was no difference between the low-dose group and the control group in the proportion of CD3+CD4+ and CD3+CD8+ T lymphocytes in peripheral blood of ICR mice (P > 0.05), but a greater proportion of CD3+CD4+ and CD3+CD8+ T lymphocytes in peripheral blood of ICR mice was detected in both the medium- and high-dose groups than the control group (P < 0.05) ().
Table 2 The proportion of T-lymphocyte subsets of ICR mice fed with different doses of Fe3O4-MNPs (n = 10, x̄ ± S)
Discussion
Fe3O4-MNPs with excellent properties have been successfully prepared by electrochemical deposition under oxidizing conditions and confirmed by XRD. The positions of the characteristic peaks agreed well with Joint Committee on Powder Diffraction Standards (JCPDS) card (No. 19-0629), which showed that the product is in the pure Fe3O4 phase and that there are no other detectable phases. Based on the characteristics of prepared Fe3O4-MNPs, they can be used to study its effect on the immune function in vivo.
Splenic lymphocytes contain T lymphocytes and B lymphocytes, and both contents are approximate. ConA is a mitogen of T lymphocytes, which promotes the proliferation of only T lymphocytes, and lipopolysaccharides is a mitogen of B lymphocytes, which promotes the proliferation of only B lymphocytes. Lymphocytes proliferation is the most direct reflection of the cellular immunity. T lymphocytes and B lymphocytes stimulated by antigens can split and proliferate, resulting in a specific immune response and producing antibodies or cytokines. Therefore, we have investigated splenocyte proliferation of ICR mice fed with a different dose of Fe3O4-MNPs, following exposure to ConA.
T lymphocytes include three major functional subsets: T-helper lymphocytes (Th cells), T-suppresser lymphocytes (Ts cells), and T-cytotoxic lymphocytes (Tc cells). Th cells express CD3+CD4+, but Ts cells and Tc cells express CD3+CD8+. CD3+CD4+ cells can secrete various cytokines and play an important role in regulating the body’s immune response. Th1 cells secrete interleukins such as IL-2 and INF-γ, whose main function is to promote cell-mediated immune response; they are involved in proliferation, differentiation, and maturity in late hypersensitivity reacting T cells and cytotoxic T lymphocytes. They can also activate macrophages and natural killer cells. Th2 cells secrete interleukins such as IL-4 and IL-10, which can induce not only the humoral immune mechanism but also the specific cellular immune responses through other ways. Under normal circumstances, Th1/Th2 maintain a balance in the human body, and their imbalance will lead to many diseases.Citation13
We chose IL-2 and IL-4, which reflect Th1 cells’ function, and IL-10 and INF-γ, which reflect Th2 cells’ functions, as our study subjects. With the exception of INF-γ, the quantitative determination of serum cytokines from the ICR mice fed with a different dose of Fe3O4-MNPs showed a normal distribution.
Our results showed that there were no significant changes in the level of IL-2, IL-4, IL-10, and INF-γ in ICR mice fed with Fe3O4-MNPs when compared with the control group (). However, a recent study of Liu et alCitation14 reported that 100 μL Fe3O4 nanoparticles absorbed autovaccine suspension (2 mg/mL) were subcutaneously injected into H22 live cancer mice, the mass IL-4 inhibitory rate of mice H22 live cancer was raised, the activity of cytotoxicity was boosted, the level of INF-γ cytokine was enhanced, and the amount of IL-4 was downregulated, which indicates that Fe3O4 nanoparticles as adjuvants had great potential to enhance antitumor immune response. Our different results may be related to the different manner and dosage of drug administration.
Previous studies on the toxicity of iron nanoparticles reported that high doses of iron nanoparticles not only cause tissue damage, but also elevate the levels of white blood cell count.Citation15 A possible explanation for the mechanism of tissue damage could be that large doses of iron nanoparticles cause tissue damage, which then induces cytotoxicity, leading to an inflammatory reaction, eventually causing the change of T-lymphocyte subsets.
Our recent article indicates that the proportion of CD4+ and CD8+ T-lymphocytes subset in the peripheral blood of the low-dose group was more than that of the control group in ICR mice, though there was no difference in the number of CD4+ T cells between the medium-dose group and the low-dose group. Interestingly, the Fe3O4-MNPs altered the production of IL-2, INF-γ, and IL-10, but no significant changes of IL-4 were observed in peripheral blood. Therefore, it is concluded that Fe3O4-MNPs could influence immune functions of normal ICR mice in a dose-dependent manner.Citation16 The results presented in this article are different from these of other recent reports, because we have employed the method of offering oral Fe3O4-MNPs instead of intravenous Fe3O4-MNPs. Oral Fe3O4-MNPs must be affected by the acidic environment in the mice’s stomach and absorbed in gastrointestinal organs. How to affect the metabolism of Fe3O4-MNPs at the molecular level by the actions of stomach acidity and gastrointestinal absorption will be investigated in our future research.
Conclusions
Based on the characteristics and the effects of Fe3O4-MNPs on splenocyte proliferation, cytokine production, and T-lymphocyte subsets in ICR mice, our studies conclude that a high dose of Fe3O4-MNPs could influence in vivo immune function of normal ICR mice. However, the reasons why there are changes in splenocyte proliferation, cytokine production, and T-lymphocyte subsets in ICR mice fed with high dose of Fe3O4-MNPs need to be further explored. Moreover, subacute and chronic toxicity of Fe3O4-MNPs in vivo still need to be further explored in the future.
Acknowledgements
This work was supported by 973 National Key Fundamental Research Project of China (No. 2006CB933205), 863 Project of People’s Republic of China (No. 2007AA022007), National Nature Science Foundation of People’s Republic of China (No. 30740062, 30872970), and Special-Purpose Science Research Foundation for High School (No. 20070286042).
Disclosure
The authors report no conflicts of interest in this work.
References
- SinghRLillardJWJrNanoparticle-based targeted drug deliveryExp Mol Pathol20098621522319186176
- HongRYFengBChenLLSynthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticlesBiochem Eng J200842290300
- ChenBAChengJWuYReversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and 5-bromotetrandrine in xenograft nude-miceInt J Nanomedicine20094737819421372
- ChenBAChengJShenMFMagnetic nanoparticle of Fe3O4 and 5-bromotetrandrin interact synergistically to induce apoptosis by daunorubicin in leukemia cellsInt J Nanomedicine20094657119421371
- SanhaiWRSakamotoJHCanadyRFerrariMSeven challenges for nanomedicineNat Nanotechnol2008324224418654511
- LinkovISatterstromFKCoreyLMNanotoxicology and nanomedicine: making hard decisionsNanomedicine2008416717118329962
- HanYYinLPLongLDistribution of nano-Fe3O4 and nano-TiO2 in tissues of miceChin J Public Health200925835836
- LiuLTangMLiuLThe pharmacokinetics study of nanoparticles of Fe2O3 coated with glutamic acidJ Environ Occup Med20062313
- WenMSongLBoWLiSLLiBBPreparation of superparamagnetic iron oxide nanoparticles and its acute toxicity to miceAcad J Sec Mil Med Univ20072811041108
- ShinSHYeMKKimHSKangHSThe effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cellsInt Immunopharmacol200771813181817996693
- ZhengSGuiding Principles for the Chemical and Therapeutic Biological Products Study (Trial)Beijing, ChinaChina Medical Science and Technology Press2002197
- WangJChenYChenBAPharmacokinetic parameters and tissue distribution of magnetic Fe3O4 nanoparticles in miceInt J Nanomedicine2010586186621042548
- GaoYJLiHWDongNCD4+ T helper lymphocytes and cytokines participates in the immune tolerance following liver transplantationJ Clin Rehabilitative Tissue Eng Res2009131052110524
- LiuHZhangDSDuYThe immunotherapeutic effect of Fe3O4 nanoparticles as adjuvants on mice H22 live cancerJ Nanosci Nanotechnol20101051451920352885
- LiuLTangMGuNStudy on acute and chronic toxicity to rats of nanopartkks of Fe2O3 coated with glutamk addJ Environ Occup Med200421430433
- ChenBAJinNWangJThe effect of magnetic nanoparticles of Fe3O4 on immune function in normal ICR miceInt J Nanomedicine2010559359920856834