Abstract
Background
Although elemental selenium has been found to be effective against Eimeria, no study has yet investigated the effects of selenium nanoparticles (SeNPs) on the Eimeria parasite. The aim of this study, therefore, was to evaluate the ameliorative effect of SeNPs compared with elemental selenium on mice jejunum infected with sporulated oocysts of Eimeria papillata.
Methods
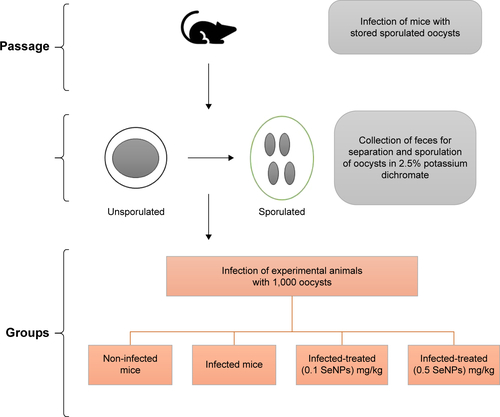
The mice were divided into 4 groups, with the first being the non-infected, control group, and the second, third, and fourth groups being orally inoculated with 1,000 sporulated oocysts of E. papillata. The third and fourth groups also received, respectively, an oral dose of 0.1 mg/kg sodium selenite and 0.5 mg/kg SeNPs daily for 5 consecutive days.
Results
The infection induced severe histopathological jejunal damage, reflected in the form of destroyed jejunal mucosa, increased jejunal oxidative damage, a reduction in the number of jejunal goblet cells, and increased production of pro-inflammatory cytokines, quantified by real-time polymerase chain reaction. Treatment of mice with SeNPs significantly decreased the oocyst output in the feces by ~80%. Furthermore, the number of parasitic stages counted in stained jejunal paraffin sections was significantly decreased after the mice were treated with SeNPs. In addition, the number of goblet cells increased from 42.6±7.3 to 95.3±8.5 cells/10 villus-crypt units after treatment. By day 5 post-infection with E. papillata, SeNPs could be seen to have significantly increased the activity of glutathione peroxidase from 263±10 to 402.4±9 mU/mL. Finally, SeNPs were able to regulate the gene expression of mucin 2, interleukin 1β, interleukin 6, interferon-γ, and tumor necrosis factor α in the jejunum of mice infected with E. papillata.
Conclusion
The results collectively showed that SeNPs are more effective than sodium selenite with regard to their anti-coccidial, anti-oxidant, and anti-inflammatory role against eimeriosis induced in the jejunum of mice.
Introduction
Coccidiosis is a severe condition affecting a large number of vertebrates. It is caused by the coccidian parasite of the genus Eimeria and affects the digestive tract of its hosts, causing diarrhea and weight loss. Eimerian parasites are both species and site specific, are able to reproduce rapidly, and can easily infect young animals. The parasite spreads from one animal to the next by contact with infected feces,Citation1 and this direct fecal–oral life cycle is ideal for spreading rapidly through susceptible hosts.Citation2
Domestic animals suffering from coccidiosis exhibit low meat and milk production due to malabsorption from the intestinal tract, and coccidiosis is, therefore, a significant issue in the meat and dairy industry, internationally. Attempts to tackle coccidiosis, along with other infectious diseases of animals, have resulted in a tremendous increase in the value of veterinary drugs bought worldwide, rising from $8.65 billion in 1992 to $20.1 billion in 2010. By 2018, this figure is expected to increase further to $42.9 billion.Citation3–Citation5 Despite these large sums, the side effects of synthetic anticoccidial drugs cause serious problems in animals, and therefore natural products that can serve as promising sources of novel anti-eimerial agents are increasingly being sought.Citation1 These products should not only target the parasite, but also play a role in protecting the organs of the host from the consequences of the infection.Citation1
Nanotechnologies are now offering new opportunities for innovative medical treatments to fight parasitic infection. In particular, nanoparticles are considered to be promising agents for the treatment of several parasitic diseases, since particles with nanometer dimension have novel properties that are different from those of both isolated atoms and bulk material. For example, nano-sized selenium exhibits an important biological activity by scavenging free radicals in a size-dependent fashion.Citation6 It has also shown promise as a therapeutic regenerative material against several diseases, including cancer, by preventing cellular damage.Citation7 Rayman reported that selenium nanoparticles (SeNPs) have antioxidant and anti-inflammatory effects,Citation8 and, recently, Dkhil et al showed that nanoselenium could act against murine schistosomiasis.Citation9 Furthermore, SeNPs are also known to exhibit anti-leishmanial effects.Citation10
Although Dkhil et al reported the anti-eimerial role of zinc oxide nanoparticles,Citation11 to our knowledge, no study has yet investigated the effects of SeNPs on the Eimeria parasite, although elemental selenium has been found to be effective against Eimeria.Citation12 Since the potential effect of a substance depends upon its size,Citation13 the aim of this study was to evaluate the ameliorative effect of SeNPs compared to elemental selenium on mice jejunum infected with sporulated oocysts of E. papillata.
Materials and methods
SeNPs and characterization
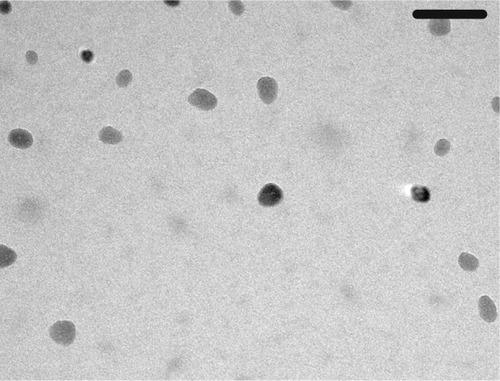
SeNPs with particle sizes of 5–50 nm were purchased from Nanocs Inc. (Boston, MA, USA). The SeNPs were separated using a high-speed centrifuge and re-dispersed in aqueous medium using a sonicator.Citation13 Transmission electron microscopy (TEM) was used to characterize the shape and size of the SeNPs, using a JEOL JEM-2100 (JEOL Ltd., Tokyo, Japan) high-resolution TEM at an accelerating voltage of 200 kV.
Animals
Thirty-two male C57BL/6 mice, weighing 20–25 g and aged 10–12 weeks, were obtained from the animal facilities of the Zoology Department, College of Science, King Saud University, Riyadh, Saudi Arabia. The mice were fed a standard diet and water ad libitum. All animal procedures and experiments in this study were approved by the ethical committee at the Department of Zoology, King Saud University (DGS 1438/6/20) and were conducted according to the guidelines for animal experimentation issued by King Saud University.
Mice infection and experiment design
To prepare the parasite infective stage (sporulated oocysts) of E. papillata, fecal pellets of infected mice were suspended in 2.5% (w/v) potassium dichromate and diluted in saturated sodium chloride for oocyst flotation. Using a McMaster counting chamber, the oocysts were counted and expressed as number of oocysts per gram of wet feces.Citation14
The animals were divided into 4 groups, with 10 mice per group. The first group was the non-infected, control group, which was gavaged with 100 μL of distilled water. The second, third, and fourth groups were orally inoculated with 1,000 sporulated oocysts of E. papillata suspended in 100 μL of distilled water.Citation15 The third and fourth groups received, respectively, an oral dose of 0.1 mg/kg sodium selenite (NaSe)Citation12 and 0.5 mg/kg SeNPs suspended in 100 μL of distilled water daily for 5 consecutive days.Citation7 The dose was chosen according to the results of our previous experiments (). For clarification, a scheme presented in shows the steps, duration, and dose administration.
Evaluation of the histopathological changes and goblet cell responses
Pieces of jejuna were freshly cut and fixed in 10% neutral buffered formalin for 24 hours. Specimens were then dehydrated with ethanol and embedded in paraffin. After this, 5 μm paraffin-embedded sections were stained with H&E. The number of E. papillata parasitic stages in 10 well-orientated villus-crypt units (VCU) was counted. Scoring for jejunum inflammatory lesions was carried out according to Dommels et al.Citation16 The histological score was indicated for infiltrations of mononuclear cells, neutrophils, eosinophils, and plasma cells, as well as for tissue destruction and tissue repair. A rating score between 0 (no change from normal tissue) and 3 (for lesions present in most areas and all jejunal layers) was given for each aspect of inflammatory lesions, tissue destruction, and tissue repair. In order to better reflect the histopathological changes associated with eimeriosis, the sum of the inflammatory lesions was doubled to give more weight to this parameter.
Paraffin sections were stained with Alcian blue to help identify goblet cells and the number of goblet cells in 10 VCU was counted for the jejunum of each animal.Citation17
Evaluation of glutathione peroxidase (GPx) activity
Mice jejuna were weighed and homogenized in an ice-cold medium that contained 50 mM Tris–HCl (Sigma, St Louis, MO, USA) at pH 7.4. The jejunum homogenate was centrifuged at 2,000×g for 10 min at 4°C. The supernatants were used for this assay.
GPx was measured according to the method described by Paglia and Valentine.Citation18 This is an indirect measure of the activity of GPx. In brief, GPx stimulates the reduction of glutathione (GSH). Oxidized GSH changes back to the reduced state (GSH) by means of the nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reductase. The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm, providing a spectrophotometric means for monitoring GPx enzyme activity. To assay GPx, a jejunum tissue homogenate is added to a solution containing GSH, GSH reductase, and NADPH, and the enzymatic reaction is initiated by adding the substrate. This reaction results in a decreased absorbance at 340 nm, which is directly related to GPx activity.
Gene expression analysis
The total RNA was isolated from pieces of mice jejuna using Trizol (Thermo Fisher Scientific, Waltham, MA, USA). Contaminating genomic DNA was digested with DNase, using the DNA-free™ kit (Applied Biosystems, Darmstadt, Germany). Then, cDNA was synthesized using the QuantiTect® Reverse Transcription kit (Qiagen, Hilden, Germany). Real-time polymerase chain reaction (PCR) was performed using the QuantiTect™ SYBR® Green PCR kit (Qiagen) using a TaqMan 7500 (Applied Biosystems) and the gene-specific QuantiTect™ primer assay (Qiagen), according to the manufacturer’s instructions and with the following primers purchased from Qiagen: MUC2 (Mm_Muc2_2_SG, Cat. No. Mm_Muc2_2_SG), IL-1β (Mm_Il1β_2_SG, Cat. No. QT01048355), IL-6 (Mm_Muc4_2_SG, Cat. No. QT00138663), IFN-γ (Mm_ Ifng_1_SG, Cat. No. QT01038821), TNF-α (Mm_Tnf_1_SG, Cat. No. QT00104006), iNOS (Mm_Nos2_1_SG, Cat. No. QT00100275), CASP3 (Mm_Casp3_1_SG, QT00260169), BCL2 (Mm_Bcl2_1_SG, Cat. No. QT00156282), BAX (Mm_Bax_1_SG, Cat. No. QT00102536), and GAPDH (Mm_Gapdh_3_SG, Cat. No. QT01658692). Following an initial incubation at 50°C for 2 min, Taq polymerase was activated at 95°C for 10 min, followed by 45 cycles of 95°C for 15 s, 60°C for 35 s, and 72°C for 30 s. The PCR product was measured as SYBR green fluorescence intensity at the end of the extension phase. Relative quantitative evaluation of the amplification data was performed using the TaqMan 7500 system software v.1.2.3f2 (Applied Biosystems) and following the 2−ΔΔc T method.Citation19 Expression of the genes was compared to that of glyceraldehyde 3-phosphate dehydrogenase.Citation20
Statistical analysis
Significance was evaluated by one-way analysis of variance, and statistical comparisons between the groups were performed by Duncan’s test, using a statistical package program (SPSS version 17.0). All values are expressed as the mean and the standard error of the mean (SEM). All p-values are two-tailed and p≤0.05 is considered as significant for all statistical analyses in this study.
Results
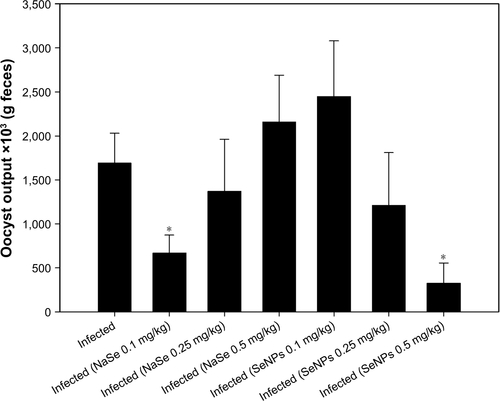
shows that while SeNPs exert a dose-dependent inhibitory effect on oocyst output, NaSe reduces the number of oocysts only at a dose of 0.1 mg/kg. Based on this initial assessment, mice infected with E. papillata were administered doses of 0.1 mg/kg of NaSe or 0.5 mg/kg of SeNPs. In the latter case, TEM was first used to confirm the size (10–30 nm) and spherical shape of the SeNPs ().
Figure 1 Size characterization of SeNPs by TEM. Scale bar=100 nm.
Abbreviations: SeNPs, selenium nanoparticles; TEM, transmission electron microscopy.
The infection induced a high number of oocysts to be output in the feces (1,693×103±339 oocysts/g feces) on day 5 post-infection (p.i.). Treatment of the infected mice with 0.5 mg/kg SeNPs significantly (p≤0.01) reduced the oocyst count in the expelled feces by ~80% when compared with that in the infected group (). The results of treatment with nano-sized selenium were better than those with 0.1 mg/kg NaSe ().
Table 1 Selenium treatment induced changes in the number of oocysts output and parasitic stages of Eimeria papillata
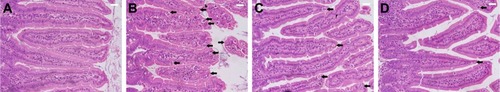
Examination of the H&E-stained jejunal sections revealed that there were ~69.4±7 E. papillata development stages per 10 VCU in the infected mice villi (). Treatment of the infected mice with NaSe and SeNPs significantly decreased the number of development stages by 25±5 and 16±4 stages/10 VCU, respectively ().
Figure 2 Histology of the jejunum showing the parasitic stages.
Notes: (A) Non-infected jejunum. (B) Infected jejunum. (C) Jejunum treated with NaSe. (D) Jejunum treated with SeNPs. Different developmental stages (arrows) appearing inside the jejunal epithelium. Sections stained with H&E. Scale bar=100 μm. N=5.
Abbreviations: NaSe, sodium selenite; SeNPs, selenium nanoparticles.
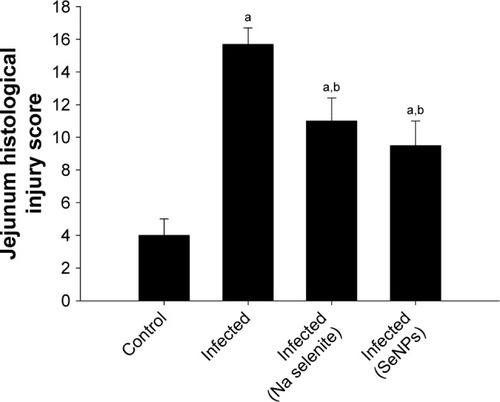
Histopathological alterations were evident in the infected jejuna, including vacuolated injured jejunal mucosa, disintegration of submucosa with the presence of lymphatic infiltrations and some damaged muscle fibers. In addition to the apparent destruction and shedding of the epithelia of the jejunum due to the parasitic invasion, the inflammatory status of the jejunum was assessed using a semi-quantitative scoring system devised by Dommels et al.Citation16 Based on this scoring, while the infected group was found to have suffered moderate inflammatory injury (), it was evident that both NaSe and SeNPs could significantly improve the inflammatory lesions induced in mice by E. Papillata infection ().
Figure 3 Jejunum histological injury scores on day 5 post-infection for control, non-infected, and infected mice, and for infected mice treated with either NaSe or SeNPs.
Notes: The histology scores were calculated according to Dommels et al.Citation16 Values are mean±standard error of the mean. ap<0.05, significant change with respect to control group; bp<0.05, significant change with respect to infected group.
Abbreviations: NaSe, sodium selenite; SeNPs, selenium nanoparticles.
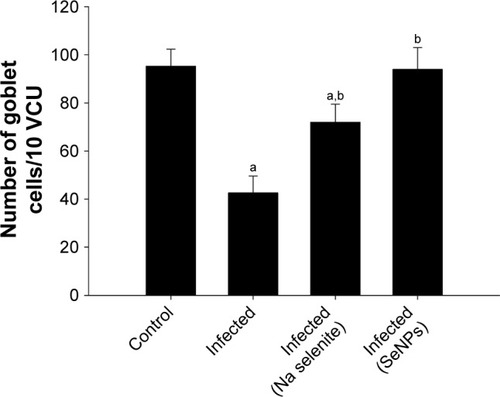
Examination of sections stained with Alcian blue () showed that on day 5 p.i. with E. papillata, the number of goblet cells per 10 VCU was decreased compared with that in the control non-infected mice. Nonetheless, the number of goblet cells was significantly higher in the group treated with SeNPs than in either the untreated infected group or the NaSe-treated group ().
Figure 4 Alcian blue staining of jejunum sections showing goblet cells in (A) control mice, (B) infected mice, and infected mice treated with (C) NaSe and (D) SeNPs. N=5. Scale bar=100 μm.
Abbreviations: NaSe, sodium selenite; SeNPs, selenium nanoparticles.
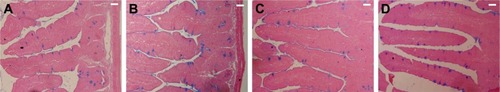
Figure 5 Changes in the number of jejunal goblet cells in control mice, infected mice, and infected mice treated with NaSe and SeNPs. N=5.
Notes: Values are mean±standard error of the mean. ap<0.05, significant change with respect to control group; bp<0.05, significant change with respect to infected group.
Abbreviations: NaSe, sodium selenite; SeNPs, selenium nanoparticles.
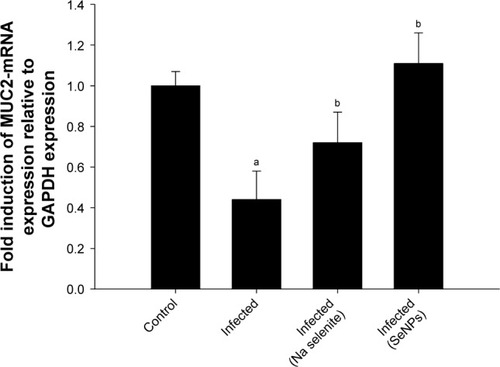
shows the downregulation of muc2 mRNA in the jejuna of mice infected with E. papillata. The gene was significantly upregulated (by ~2-folds); however, after the mice were treated with SeNPs, the NaSe-treated group also showed increased expression of muc2 but to a lesser degree than that shown by the SeNP-treated group ().
Figure 6 Mucin gene (muc2) expression in the jejunum on day 5 post-infection in mice infected with Eimeria papillata and after treatment with NaSe or SeNPs.
Notes: Results (mean±standard error of the mean of 3 assays) were normalized to the GAPDH mRNA level and are shown as fold induction (in log 2 scale) relative to the mRNA level in the control, by RT-PCR. ap<0.05, significant change with respect to control group; bp<0.05, significant change with respect to infected group using Duncan’s post-hoc test.
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NaSe, sodium selenite; RT-PCR, real-time polymerase chain reaction; SeNPs, selenium nanoparticles.
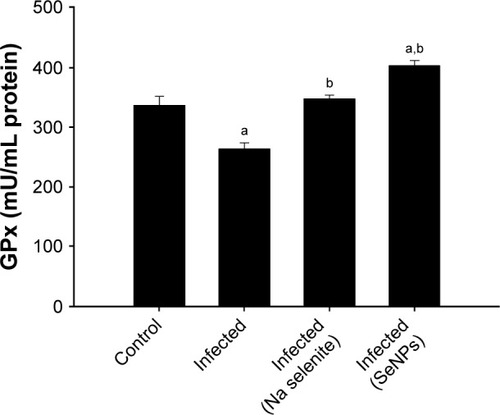
The level of GPx was significantly reduced in the jejunal homogenate taken from the E. papillata infected group, compared with the control. The GPx level was significantly restored, however, in both the NaSe- and SeNPs-treated groups, compared with the untreated infected group ().
Figure 7 Changes in the level of jejunal GPx in control mice, infected mice, and infected mice treated with NaSe and SeNPs.
Notes: Values are mean±standard error of the mean. ap<0.05, significant change with respect to control group; bp<0.05, significant change with respect to infected group. N=5.
Abbreviations: GPx, glutathione peroxidase; NaSe, sodium selenite; SeNPs, selenium nanoparticles.
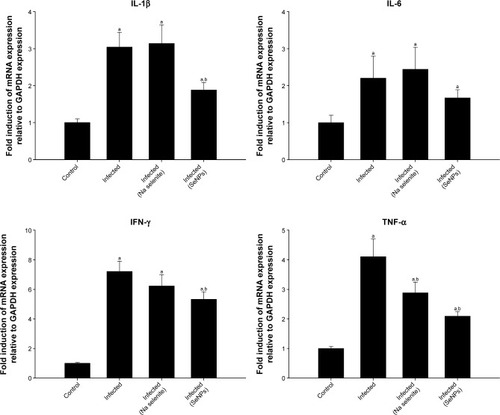
Infection-induced inflammation was recorded in the mice jejuna on day 5 p.i. through the increased mRNA expression of interleukin 1β, interferon-γ, and tumor necrosis factor α. However, treatment with NaSe or SeNPs significantly ameliorated the expression of these inflammation-linked genes ().
Figure 8 Effect of NaSe and SeNPs on the mRNA expression of jejunal IL-1β, IL-6, IFN-γ, and TNF-α in Eimeria papillata-infected mice.
Notes: Results (mean±standard error of the mean of 3 assays) were normalized to GAPDH mRNA level and are shown as fold induction (in log 2 scale) relative to the mRNA level in the control, by RT-PCR. ap<0.05, significant change with respect to control group; bp<0.05, significant change with respect to infected group according to Duncan’s post-hoc test.
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFN-γ, interferon-γ; IL, interleukin; NaSe, sodium selenite; RT-PCR, real-time polymerase chain reaction; SeNPs, selenium nanoparticles; TNF-α, tumor necrosis factor-α.
Discussion
More attention is needed on the use of nanoselenium as an anti-parasitic agent, due to its low toxicity and high bioavailability.Citation21,Citation22 In the present study, we used SeNPs for the first time as an anti-eimerial agent.
E. papillata infection begins with the oral uptake of sporulated oocysts, resulting in the release of sporozoites that, in turn, invade the site-specific jejunal epithelial cells.Citation23 The intracellular stages of the parasites rapidly multiply in asexual schizogonic cycles before oocysts are formed.Citation24 Finally, the oocysts are released with the feces and can be highly infectious. The treatment for eimeriosis currently depends on anti-coccidial drugs such as toltrazuril.Citation25 Such drugs, however, induce problematic residual storage of these compounds or their metabolites in animal tissues and/or in their products, such as eggs.Citation11 As a result, natural products and nanoparticles are now being considered as promising sources of novel anti-eimerial agents.Citation11,Citation26–Citation28 Compared to many of the extracts from natural products that have been used against eimeriosis, like extracts from neem,Citation26 pomegranate,Citation27 and garlic,Citation28 SeNPs were found to be more effective as they significantly reduce the oocyst output by about 80% ().
Selenium has already been shown to be able to reduce parasitic infection in the intestine. Smith et al revealed that mice were able to expel the nematode worm Heligmosomoides bakeri more efficiently following selenium treatment.Citation29 Our previous research, meanwhile, has indicated that selenium can significantly lower oocyst output in the feces of mice infected with E. papillata.Citation12 The results of this new study reinforce the earlier work by revealing that a dose of 0.1 mg/kg of NaSe caused a substantial lowering of oocyst output (which may be attributed to the increased mice immune response to the parasite),Citation30,Citation31 but that SeNPs were an even more effective inhibitor of E. papillata infection (; and ). As reported above, these doses were chosen on the basis of our previous research,Citation12 but also on the basis of the intention to use a supranutritional dose, since it has been confirmed that supranutritional levels of Se or nano-Se cause no toxicity when given to mice (0.5–1.5 mg Se/kg, daily, for 7 days).Citation32 Moreover, Hassnin et alCitation7 reported that, SeNPs are highly bioactive agents, with low toxicity and good absorptive ability due to the interaction between SeNPs and −NH2, C=O, −COO, and −C−N− groups of proteins. In general, our results are in agreement with other investigations reporting that SeNPs are much more efficient than NaSe.Citation33,Citation34
SeNPs could improve the infection-induced histopathological changes in mice jejunum. This was evidenced by the significant reduction of the jejunum histological injury score () and the improvement in the hypoplasia of goblet cells induced by infection. Such ameliorative effects on the histopathological changes may be due to the previously proved anticoccidial effects of selenium and the associated reduction in the number of intracellular parasitic stages, and hence the parasitic load and consequent damage to the infected jejunal tissue.Citation12 Comparing our results concerning SeNPs with that of Dkhil et alCitation11 using zinc oxide nanoparticles as an anti-coccidial agent, it is evident that SeNPs offer the potential for a more pronounced improvement in the histopathological alterations induced in the jejunum by E. papillata infection.
Speckmann and Steinbrenner have reported on the role of selenium and selenoproteins during inflammation; showing that intestinal epithelial cells use dietary selenium compounds for the synthesis of selenoprotein P, which might support the intestinal immune system by providing selenium to antibody-producing plasma cells.Citation35 Some studies have also provided evidence that the mucus released by goblet cells can function as a defensive barrier,Citation36,Citation37 bearing in mind that, during eimeriosis, the Eimeria parasite invades and destroys goblet cells.Citation26,Citation38 In this context, it is interesting that the present results indicate the upregulation of the goblet cell-specific gene muc2 in mice treated with SeNPs. Several diseases are associated with altered mucin gene expression, including colon cancer.Citation38–Citation40 Jaspers et al reported that selenium controls mucus secretion during respiratory diseases.Citation41 Moreover, Lee et al reported on the protective role of selenium against mucosal injury.Citation42
In the jejunum, toxic effects due to the accumulation of reactive oxygen species (ROS) cause destructive changes in the epithelia.Citation26 In this regard, feed additives are one of the tools used by nutritionists to combat cellular oxidative stress. Nève et al and Yan et al reported that selenium, as a component of selenoproteins, has a metabolic role in preventing oxidative damage to body tissues and Huang et al have reported that SeNPs are able to suppress inflammation by restraining nitric oxide production and enhancing antioxidant enzyme activity.Citation43–Citation45 In this context, GPx is one of the family of antioxidant enzymes that uses GSH to reduce peroxides.Citation46 Generally, GPx has an important role in the reduction of ROS levels and in mitigating inflammatory pathways in the intestinal mucosa. The GPx enzymes are able to use selenium at their active sites to detoxify ROS.Citation43 In our study, SeNPs are shown significantly to increase the activity of GPx in mice infected with E. papillata ().
Eimeriosis also stimulates the production of pro- inflammatory cytokines that play an important role in the jejunal inflammation that is a feature of the disease.Citation28 The inflammation caused by eimeriosis is associated with migration of blood leukocytes to the jejunal mucosa, and these activated leukocytes then guide epithelial cells to cascades of biochemical and cellular events, leading to the production of superoxide radical anions (O2−).Citation28 It is well known that the increased levels of pro-inflammatory cytokines observed in this study on day 5 p.i. with E. papillata () amplify the inflammatory cascade and result in damage to the intestinal tissues of mice (). In this context, Lee et al have reported on the anti-inflammatory role of selenium during intestinal injury,Citation42 and selenium has also been shown to significantly downregulate levels of pro-inflammatory cytokines and affect gene expression, signaling pathways and cellular functions involved in inflammation.Citation47 Comparing our results when using SeNPs against eimeriosis and both our previous work using zinc oxide nanoparticles,Citation11 and some natural extracts,Citation28 it is evident that SeNPs have a more potent anti-inflammatory effect.
Our study has built on this work to reveal the anti-inflammatory effect of both NaSe and SeNPs in the context of inflammation induced by Eimeria infection. In this regard, we showed that treatment with selenium is effective in ameliorating the upregulation of genes associated with inflammation () and this was further reflected in the histological results (). Furthermore, we showed that SeNPs had a more marked beneficial effect than NaSe across all the investigated pro-inflammatory genes, and in terms of the histological outcomes. Generally, when comparing the current results to those using natural products as anti-eimerial agents, it is evident that SeNPs can more effectively improve eimeriosis-induced injuries.Citation26–Citation28
Conclusion
Our results collectively show that SeNPs are more effective than NaSe, at least at a parasitological level, at reducing both the number of E. papillata parasitic stages in the jejunum and in decreasing the number of oocysts expelled in mice feces, and therefore could be used as an anti-eimerial, anti-oxidant, and anti-inflammatory agent involved in immunoregulation.Citation43 Further studies are required to understand the mechanism of action of SeNPs on both the host and the parasite.
Acknowledgments
This project was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Supplementary materials
Figure S1 Selenium treatment induced changes in the number of Eimeria papillata oocysts.
Notes: Different doses (0.1, 0.25, and 0.5 mg/kg) of NaSe and SeNPs indicated changes in oocyst output in mice feces. N=10. Values are expressed as mean±standard error of the mean. *p<0.05 with respect to the infected group.
Abbreviations: NaSe, sodium selenite; SeNPs, selenium nanoparticles.
Disclosure
The authors report no conflicts of interest in this work.
References
- PakandlMSelection of a precocious line of the rabbit coccidium Eimeria flavescens Marotel and Guilhon (1941) and characterisation of its endogenous cycleParasitol Res200597215015515986244
- MehlhornHEncyclopedic Reference of Parasitology4th ed2014
- Animal health market to hit $43 billion in five yearsWestern Farm-Press2012 Available from: http://westernfarmpress.com/management/animal-health-market-hit-43-billion-five-yearsAccessed December 3, 2017
- ReportsQChina veterinary drug industry 2012 market research report2012 Available from: http://www.qyresearchreports.com/report/china-veterinary-drug-industry-2012-market-research-report.htmAccessed December 3, 2017
- HaoHChengGIqbalZBenefits and risks of antimicrobial use in food-producing animalsFront Microbiol2014528824971079
- PengDZhangJLiuQTaylorESize effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activityJ Inorg Biochem2007101101457146317664013
- HassninKHashemKAbdel-KawiSThe prospective protective effect of selenium nanoparticles against chromium-induced oxidative and cellular damage in rat thyroidInt J Nanomed2013817131720
- RaymanMSelenium and human healthLancet201237998221256126822381456
- DkhilMAl-QuraishySBauomyADiabMProtective role of selenium nanoparticles against Schistosoma mansoni induced hepatic injury in miceBiomed Res2016271214219
- BeheshtiNSoflaeiSShakibaieMEfficacy of biogenic selenium nanoparticles against Leishmania major: in vitro and in vivo studiesJ Trace Elem Med Biol201327320320723219368
- DkhilMAl-QuraishySWahabRAnticoccidial and antioxidant activities of zinc oxide nanoparticles on Eimeria papillata-induced infection in the jejunumInt J Nanomed20151019611968
- DkhilMAbdel-BakiAWunderlichFSiesHAl-QuraishySDietary selenium affects intestinal development of Eimeria papillata in miceParasitol Res2013113126727424221886
- JiangJOberdörsterGElderAGeleinRMercerPBiswasPDoes nanoparticle activity depend upon size and crystal phase?Nanotoxicology200821334220827377
- SchitoMBartaJChobotarBComparison of four murine Eimeria species in immunocompetent and immunodeficient miceJ Parasitol19968222552628604093
- SchitoMBartaJNonspecific immune responses and mechanisms of resistance to Eimeria papillata infections in miceInfect Immun199765316531709234770
- DommelsYButtsCZhuSCharacterization of intestinal inflammation and identification of related gene expression changes in mdr1a(−/−) miceGenes Nutr20072220922318850176
- AllenAHuttonDLeonardAPearsonJSellersLThe role of mucus in the protection of the gastroduodenal mucosaScand J Gastroenterol Suppl198612571783103205
- PagliaDValentineWStudies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidaseJ Lab Clin Med19677011581696066618
- LivakKSchmittgenTAnalysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT methodMethods200125440240811846609
- KrückenJDelićDPauenHAugmented particle trapping and attenuated inflammation in the liver by protective vaccination against Plasmodium chabaudi malariaMalaria J20098154
- HuCLiYXiongLZhangHSongJXiaMComparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickensAnim Feed Sci Technol20121773–4204210
- GangadooSDinevIChapmanJSelenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitziiAppl Microbiol Biotechnol201810231455146629250719
- SeemannEKurthTEntzerothRInsight into the ultrastructural organisation of sporulated oocysts of Eimeria nieschulzi (Coccidia, Apicomplexa)Parasitol Res201211152143214722955498
- FrölichSJohnsonMRobinsonMEntzerothRWallachMThe spatial organization and extraction of the wall-forming bodies of Eimeria maximaParasitology2013140787688723622252
- AlnassanAShehataAKotschMEfficacy of early treatment with toltrazuril in prevention of coccidiosis and necrotic enteritis in chickensAvian Pathol201342548249023941631
- DkhilMAl-QuraishySAbdel MoneimADelicDProtective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosisParasitol Res2012112110110622972359
- AmerOSDkhilMAHikalWMAl-QuraishySAnti-oxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in miceBiomed Res Int201520157
- Al-QuraishySDelicDSiesHWunderlichFAbdel-BakiADkhilMDifferential miRNA expression in the mouse jejunum during garlic treatment of Eimeria papillata infectionsParasitol Res2011109238739421301871
- SmithACheungLBeshahEShea-DonohueTUrbanJSelenium status alters the immune response and expulsion of adult Heligmosomoides bakeri worms in miceInfect Immun20138172546255323649095
- HawkesWKelleyDTaylorPThe effects of dietary selenium on the immune system in healthy menBiol Trace Elem Res200181318921311575678
- FrölichSEntzerothRWallachMComparison of protective immune responses to apicomplexan parasitesJ Parasitol Res20122012111
- ZhangJWangXXuTElemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in miceToxicol Sci20071011223117728283
- ArnérEHolmgrenAPhysiological functions of thioredoxin and thioredoxin reductaseEur J Biochem2000267206102610911012661
- CopelandPRegulation of gene expression by stop codon recoding: selenocysteineGene2003312172512909337
- SpeckmannBSteinbrennerHSelenium and selenoproteins in inflammatory bowel diseases and experimental colitisInflamm Bowel Dis2014201110119
- DeplanckeBGaskinsHMicrobial modulation of innate defense: goblet cells and the intestinal mucus layerAm J Clin Nutr2001731131S1141S11393191
- ChengHOrigin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine II. Mucous cellsAm J Anatomy19741414481501
- KimYGumJBrockhausenIMucin glycoproteins in neoplasiaGlycoconj J19961356937078909996
- HanskiCRiedeEGratchevAMUC2 gene suppression in human colorectal carcinomas and their metastases: in vitro evidence of the modulatory role of DNA methylationLab Invest19977766856959426407
- JefferyPLiDAirway mucosa: secretory cells, mucus and mucin genesEurop Resp J199710716551662
- JaspersIZhangWBrightonLCarsonJStybloMBeckMSelenium deficiency alters epithelial cell morphology and responses to influenzaFree Rad Biol Med200742121826183717512462
- LeeJChunHChoiHSelenium administration attenuates 5-flurouracil-induced intestinal mucositisNutr Cancer201769461662228353366
- NèveJSelenium as a “nutraceutical”: how to conciliate physiological and supra-nutritional effects for an essential trace elementCurr Opin Clin Nutr Metab Care20025665966312394641
- YanLJohnsonLSelenium bioavailability from naturally produced high-selenium peas and oats in selenium-deficient ratsJ Agricult Food Chem2011591163056311
- HuangZRoseAHoffmannPThe role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunitiesAntioxid Redox Sign2012167705743
- Al-QuraishySOthmanMDkhilMAbdel MoneimAOlive (Olea europaea) leaf methanolic extract prevents HCl/ethanol-induced gastritis in rats by attenuating inflammation and augmenting antioxidant enzyme activitiesBiomed Pharmacother20179133834928463797
- ZhuCZhangSSongCSelenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammationJ Nanobiotechnol201715120