?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Resveratrol (RES) is a natural anti-inflammatory and antioxidant compound with poor water solubility and oral bioavailability. The present study takes the advantages of nanocarriers combined with a ligand (galactose) anchoring to orally deliver RES in an attempt to improve its bioavailability and pharmacological activity.
Methods
RES-loaded galactosylated nanoparticles (RES-GNPs) were prepared by solvent diffusion technique using poly(lactic-co-glycolic acid), synthesized N-oleoyl-d-galactosamine and Tween 80. RES-GNPs were characterized by particle size, morphology, entrapment efficiency (EE) and in vitro release. Oral bioavailability and in vitro anti-inflammatory activity were investigated in rats and lipopolysaccharides-induced RAW 264.7 cells, respectively.
Results
The resulting RES-GNPs were 108.4 nm around in particle size with a polydispersity index of 0.217. Furthermore, RES-GNPs possessed a high EE and a slow drug release in water. After oral administration, RES-GNPs significantly enhanced the oral bioavailability of RES, up to 335.7% relative to RES suspensions. In situ single-pass intestinal perfusion and cellular uptake experiments showed that GNPs could improve the intestinal permeability and transcellular transport of RES. Moreover, the anti-inflammatory efficacy of RES-GNPs in RAW 264.7 cells model was superior to free RES and RES-GNPs.
Conclusion
The results indicate that RES-GNPs can effectively promote the intestinal absorption of RES and strengthen its bioactivity, which may be a promising system for the treatment of inflammatory diseases.
Introduction
Resveratrol (RES) is a natural polyphenol which is rich in a variety of plants and fruits, such as grapes, peanut and mulberry. This compound has been shown of possessing multiple pharmacological and healthy benefits, including anti-inflammation/oxidation,Citation1 antitumor,Citation2 and immunoregulation.Citation3 Currently, the French Paradox, a low incidence of cardiovascular diseases relative to a high saturated fats diet, makes this compound become popular. It is assumed to be linked with the consumption of red wine that contains a wealth of RES. However, pharmacokinetic studies revealed that the oral bioavailability of RES was extremely inadequate,Citation4–Citation6 due to poor solubility, chemical instability and intestinal metabolism.Citation7–Citation9
Encapsulation in nanoparticles (NPs) can effectively raise the intestinal supersaturated concentration, enhance the physiological stability and facilitate the transepithelial transport of poorly bioavailable molecules.Citation10 According to the gastrointestinal degradation nature, NPs can be generally classified into rapidly degradable nanocarriers (eg, lipid NPs and nanoemulsions), slowly degradable nanocarriers (eg, polylactide-based NPs and polyester-based NPs) and nondegradable nanocarriers (eg, mesoporous carbons and inorganic NPs). Generally, rapidly degradable nanocarriers have great difficulty to provide a satisfactory protective effect for labile drugs in the gastrointestinal tract, whereas nondegradable nanocarriers have a poor absorption-enhancing effect. Slowly degradable nanocarriers possess unique advantages as oral delivery vehicles of poorly soluble and instable drugs, over the other two types. The degradation rate and content of slowly degradable nanocarriers, such as poly(lactic-co-glycolic acid) (PLGA) NPs, are negligible relative to their gastrointestinal transport time. Thus, such NPs can maintain a good structural integrity during the gastrointestinal transport and can provide a protection for labile drugs. Furthermore, polymeric NPs can facilitate drug transport across the intestinal epithelia through endocytosis or pinocytosis of enterocytes,Citation11–Citation13 thus enhancing the oral bioavailability. Nevertheless, the promotive effect of polymeric NPs on drug absorption is limited due to insufficient bioadhesion and rapid mucus turnover.Citation14,Citation15 If a ligand able to trigger the cytosis gets anchored, the absorption-enhancing effect of NPs will be considerably enlarged.
In the gastrointestinal tract, there are many kinds of transporters that mediate the transmembrane transport of a variety of nutrients,Citation16 such as peptides transporters, glucose transporters and sodium-dependent vitamins cotransporters. Among all sorts of monosaccharides, galactose exhibits the fastest absorption rate in the intestine, which is mediated by sodium glucose–linked transporter 1 (SGLT1), an intestinal Na+-glucose cotransporter.Citation17 It is indicative that the galactose moiety may provide compounds with an improved route of intestinal transport by the way of SGLT1.Citation18 Recently, nanostructured lipid carriers (NLCs) have been employed for RES encapsulation.Citation19 The constructed NLCs based on PEG-40 stearate demonstrated enhanced tyrosinase inhibitory activity. Pujara et al developed RES-encapsulated soy protein isolate nanocomplexes and tested the performance for solubility and dissolution improvement.Citation20 Mesoporous silica was also utilized to encapsulate RES in an attempt to ameliorate its dissolution, permeability and anti-inflammatory response.Citation21 These delivery systems, to some extent, improved the physicochemical properties and pharmacological activities of RES. Nonetheless, a more efficient and smart drug delivery system is still desired to potentiate the potency of RES.
In this study, we developed a galactosylated PLGA NPs (GNPs) to orally deliver RES, aiming to enhance the oral bioavailability and in vitro anti-inflammatory effect. Galactosylation was achieved through surface engineering of NPs by introducing N-oleoyl-d-galactosamine into the formulation. RES-loaded GNPs (RES-GNPs) were prepared by solvent diffusion method and characterized by particle size, entrapment efficiency (EE) and in vitro release. The formulation performances of RES-GNPs were evaluated with in situ single-pass intestinal perfusion, cellular uptake, in vivo pharmacokinetics and in vitro anti-inflammation in lipopolysaccharide (LPS)-induced RAW264.7 cells.
Materials and methods
Materials
RES (transisomer) was purchased from Shaanxi Huike Botanical Development Co., Ltd. (Xi’an, People’s Republic of China). PLGA (DL-lactide:glycolide = 75:25, Mw = ~10,000) was obtained from RESENBio Technology Co., Ltd (Xi’an, People’s Republic of China). Galactosamine hydrochloride, galactose, LPS, chlorpromazine and simvastatin were purchased from Sigma-Aldrich (St Louis, MO, USA). Oleoyl chloride, triethylamine and Tween 80 were provided by Sinopharm Chemical Reagent (Shanghai Shi, People’s Republic of China). Filipin and 3-amino-6-chloro-N-(diaminomethylene)-5-(ethyl(isopropyl)amino)pyrazine-2-carboxamide (EIPA) were purchased from APExBIO (Shanghai, China). Purified water was produced by a Milli-Q water purifier (Merck Millipore, MA, USA). All other chemicals were of analytical grade and used as provided.
Synthesis of N-oleoyl-d-galactosamine
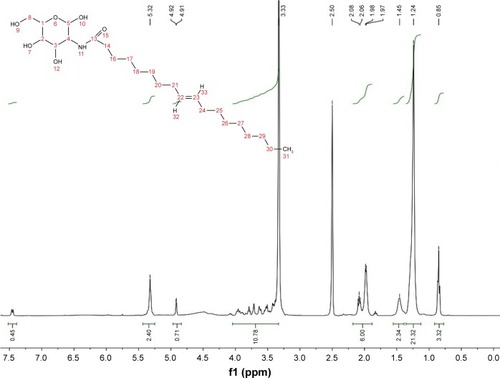
The synthesis of amphiphilic N-oleoyl-d-galactosamine was based on galactosamine hydrochloride and oleoyl chloride in the presence of triethylamine. Briefly, galactosamine hydrochloride was dissolved in deionized water followed by addition of triethylamine. Then, a slight excess of oleoyl chloride was slowly added to start the acylation reaction. The reaction was maintained for 24 h at room temperature. The crude products were precipitated against acetone and then washed with methanol several times. The purified product was characterized by 1HNMR using deuterated dimethyl sulfoxide (DMSO-d6) as a solvent.
Preparation of RES-GNPs
RES-GNPs were prepared by a solvent diffusion method.Citation22 Briefly, RES and PLGA were dissolved in acetone to form an organic phase; N-oleoyl-d-galactosamine and Tween 80 were dissolved in purified water. Then, the organic phase was rapidly injected into the aqueous phase under stirring at 8,000 rpm. Upon acetone diffusion into the water, the materials self-assembled into RES-GNPs. Afterward, the residual solvent was removed under reduced pressure using a rotatory evaporator. The factors influencing the formulation properties were screened by changing one variable and keeping others unchanged, including the amount of PLGA, the amount of N-oleoyl-d-galactosamine and the concentration of Tween 80 in the system.
Characterization of RES-GNPs
The particle size and ζ potential of RES-GNPs were measured at 25°C based on the dynamic light scattering and electrophoretic mobility principles using Zetasizer Nano ZS analyzer (Malvern Instruments Ltd, Malvern, UK). The samples were diluted 25 times with purified water before determination. After equilibrating for 120 s, the samples were subjected to laser diffraction or Doppler velocimetry for the output of particles size and ζ potential. The morphology of RES-GNPs was inspected by transmission electron microscope (TEM) (Tecnai 10; Philips, Amsterdam, the Netherlands).
The EE of RES-GNPs was determined by the centrifugal ultrafiltration technique with a minor modification.Citation23 The unentrapped RES was first removed by centrifugation at 15,000 rpm for 5 min. Then, the upper layer of RES-GNPs was pipetted and placed in a centrifugal filter device (Amicon® Ultra-0.5, MWCO 5,000; Merck Millipore). Afterward, the procedure of centrifugal ultrafiltration was performed to separate free RES from the system. The concentration of free RES (Cfre) in the filtrate was determined by high performance liquid chromatography (HPLC) at 306 nm (Dionex Ultimate 3000 HPLC; Acclaim™120 C18 column (5 μm, 4.6 × 150 mm); Thermo Fisher Scientific, MA, USA). The mobile phase consisted of 30% acetonitrile and 70% phosphoric acid solution (0.05%, v/v) pumped at a flow rate of 1.0 mL/min. EE was calculated by the following equation: EE (%) = (1 − Cfre/Ctot) × 100%, where Ctot denoted the total concentration of RES in the system.
In vitro release study
The in vitro release of RES-GNPs was studied using the dialysis bag method with RES solution and RES-loaded NPs (RES-NPs) as references. RES solution was prepared by dissolving RES in the cosolvent of water/ethanol/PEG400 (20/40/40, v/v/v). RES-NPs were prepared following the same procedure of RES-GNPs. Aliquots of samples (equivalent to 10 mg of RES) were placed into the dialysis bags (MWCO ~14,000) and sealed. Then, the bags loading RES preparations were dialyzed against 100 mL of purified water at 37°C, in which 1% Tween 80 added as the solubilizing agent. At predetermined time points, 200 μL of release medium were withdrawn and immediately replenished with the same volume of fresh medium. The release medium was then diluted with 200 μL of methanol and subjected to HPLC analysis for RES quantification. The release curves were plotted based on the accumulative release percentage vs time.
Oral bioavailability study
The animal experiments were conducted with the Guidelines on the Care and Use of Animals for Scientific Purposes (2004). The experimental protocols were reviewed and approved by the Experimental Animal Welfare Ethics Committee of South China Agricultural University. Sprague-Dawley (SD) rats (220 ± 20 g) were fasted overnight before administration but freely accessible to water. The rats were randomly divided into four groups (n = 5), ie, RES suspensions (dispersed in 0.3% CMC-Na solution) (introgastric [ig]), RES-NPs (ig), RES-GNPs (ig) and RES solution (intravenous). Rats were given RES preparations by gavage (40 mg/kg) or intravenous injection (8 mg/kg). Approximately 0.25 mL of blood was collected into the heparinized tube from the tail vein of rats at 0.133, 0.25, 0.5, 1, 2, 3, 5, 8 and 12 h following administration. The blood samples were subsequently centrifuged at 4,500 rpm for 5 min to prepare the plasma.
To quantify the plasma RES, a liquid–liquid extraction procedure was used to retrieve RES from the plasma. In brief, 100 μL of plasma was mingled with 500 μL of acetonitrile. After vortex for 5 min, the mixtures were centrifuged at 13,000g for 15 min. The supernatants were transferred to new tubes followed by evaporation at 30°C under vacuum using a concentrator (Eppendorf, NY, USA). The residues were reconstituted in 150 μL of 50% acetonitrile for UPLC-QTOF/MS analysis (Xevo G2 QTOF, Waters). Elution was performed using a gradient procedure at a flow rate of 0.45 mL/min using 0.1% formic acid in water (mobile phase A) and 0.1% formic acid in acetonitrile (mobile phase B). The gradient elution was 10% B at 0–1 min, 10%–95% B at 1–3 min, 95% B at 3–4.5 min and 95%–10% B at 4.5–5 min. The plasma RES was quantified based on extracted ion chromatograms using MassLynx. PKSolver 2.0, a freely available add-in Excel program, was used to process the data and extract the pharmacokinetic parameters.
In situ single-pass intestinal perfusion
To appreciate the effect of unmodified NPs and GNPs on the intestinal permeability of RES, in situ single-pass intestinal perfusion was carried out following the reported procedure.Citation23,Citation24 SD rats were fasted overnight prior to the experiment but allowed free access to water. The rats were anesthetized with 20% urethane (1.0 g/kg) by intraperitoneal injection. The intestine was exposed by making an incision in the midline of the abdomen. The proximal and distal ends of duodenum, jejunum, ileum and colon were identified and cannulated with silicone tubes (Φ2.5 × 4 mm). A pH 7.4 Krebs Ringer’s buffer (KRB) was used to rinse the intestinal contents. Perfusion solutions were prepared by diluting RES solution, RES-NPs or RES-GNPs with KRB to 25 μg/mL. After preperfusion for 30 min, the perfusates were collected per 15 min up to 120 min. Finally, the diameter and length of the intestinal segments were measured. The net water flux in the perfusion experiment was calibrated by the gravimetric method based on the sham-operated group. The effective permeability coefficient (Peff) was calculated according to the inlet and outlet concentrations of RES using the following equation:
Cellular uptake and internalization
Caco-2 cells from American Type Culture Collection were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) at 37°C under 5% CO2 atmosphere, in which fetal bovine serum (FBS, 10%) and penicillin-streptomycin solution (100 IU/mL) were supplemented. For cellular uptake study, Caco-2 cells were seeded in 12-well plate at a density of 5 × 104 cells/well and cultured for 24 h. After that, the culture medium was removed and replaced with DMEM-diluted RES, RES-NPs or RES-GNPs (20 μg/mL). The cells were treated for 1, 2 and 4 h at 37°C, respectively, then washed twice with cold PBS (pH 7.4) and followed by lysis with 60% acetonitrile. After vortex for 2 min, the lysates were centrifuged at 13,000 rpm for 5 min under 4°C to obtain the supernatant. RES in the supernatant was then quantified by HPLC.
To ascertain the cellular trafficking mechanism of RES-GNPs, the cellular uptake of RES-GNPs was analyzed in the presence of various transport inhibitors or competitors.Citation25 Caco-2 cells were pretreated with hypertonic sucrose (0.4 M), chlorpromazine (20 μM), simvastatin (50 μM), Filipin (1.5 μM), EIPA (20 μM) or galactose (50 μM) at 37°C for 0.5 h. Then, RES-GNPs (20 μg/mL) were added and continued to incubate for 2 h. Another batch of cells were used to investigate the cellular uptake of RES-GNPs under 4°C. Thereafter, the cells were washed, lysed and centrifuged at 13,000 rpm. RES concentration in the supernatants was determined by HPLC. The cellular trafficking mechanism of RES-GNPs was interpreted based on the changes in cellular uptake.
To investigate the cellular internalization of RES-GNPs, Caco-2 cells were cultured in a 6-well plate containing round microslides for 24 h. Subsequently, the cells were incubated with coumarin 6-labeled RES-NPs and RES-GNPs at 37°C for 30 min. After that, the cells were washed twice with cold PBS and fixed with 4% paraformaldehyde for 30 min. The internalized RES-GNPs was visualized on Caco-2 cells by confocal laser scanning microscopy after nucleus staining with DAPI.
In vitro anti-inflammatory effect
The anti-inflammatory effect of RES-GNPs was evaluated in vitro with LPS-induced inflammatory model.Citation26 Mouse macrophage cell line RAW 264.7 was purchased from the Type Culture Collection of Chinese Academy of Sciences (Beijing, People’s Republic of China). Cells were cultured in RPMI-1640 medium supplemented with 10% FBS (Thermo Fisher Scientific) at 37°C in 5% CO2. RAW 264.7 cells were seeded in 24-well plates at 2 × 105 cells/well and then treated with LPS (1 μg/mL) in the absence or presence of tested samples at indicated concentration for 24 h. The cells treated with culture medium and LPS alone were designated as the control and model group, respectively. The supernatants of cells were isolated by centrifugation at 10,000 rpm under 4°C after incubation with RES solution, RES-NPs and RES-GNPs. The inflammatory mediators of tumor necrosis factor α (TNF-α), interleukin-6 (IL-6) and nitric oxide (NO) were measured using their ELISA kits (ExCell Bio, Shanghai, China), according to the manufacturers’ instructions.
Results
Synthesis and identification of N-oleoyl-d-galactosamine
The synthesis of N-oleoyl-d-galactosamine was accomplished via the acylation between galactosamine and oleoyl chloride. The resultant product exhibited a distinct thin layer chromatographic signal from the reactants (data not shown). 1HNMR was utilized to verify the chemical structure of the product. shows the 1HNMR spectrum of the product. It clearly displayed the proton signals of galactosamine (δ 3.42, 3.64, 3.85 and 7.47) and oleoyl (δ 1.25, 1.49, 2.18 and 5.44), indicating successful synthesis of N-oleoyl-d-galactosamine. The galactosamine moiety of N-oleoyl-d-galactosamine possesses a hydrophilic property, whereas the oleoyl moiety is hydrophobic. This amphiphilic nature imparts this compound an excellent surface activity that can be used to modify NPs and facilitate drug loading.
Preparation and characterization of RES-GNPs
The solvent diffusion method is a facile and straightforward preparative process of polymeric NPs.Citation27,Citation28 The formulation constitutions influence the formulation performance, such as particle size, drug loading and in vivo transport. The effects of formulation variables on the particle size and EE of RES-GNPs are shown in . The amount of PLGA in the formulation greatly affected the particle size of RES-GNPs, other than EE. The formulation containing a higher level of PLGA resulted in larger NPs. It indicates that less PLGA tends to form smaller RES-GNPs when using a fixed amount of surfactant. N-oleoyl-d-galactosamine had a certain effect on the particle size and EE of RES-GNPs. High ratio of N-oleoyl-d-galactosamine was favorable to reduce the particle size and increase the EE, which may be associated with the surface activity of this compound that reduced the interfacial tension by inserting the interfacial membrane of NPs. On the other hand, instantaneous and prompt formation of NPs was conducive to enabling drug entrapment timely. The effect of Tween 80 on the formulation performances was also significant where high level of Tween 80 resulted in smaller particle size of RES-GNPs. However, there was no difference between 5% level and 7.5% level, indicating that Tween 80 positively facilitated the formation of RES-GNPs under a certain level. Similar to N-oleoyl-D-galactosamine, Tween 80 lent a hand with the entrapment of RES as a surfactant.
Figure 2 Factors influencing the formulation properties (particle size and entrapment efficiency) of RES-GNPs: PLGA vs 10 mg of RES, 10 mg of N-oleoyl-D-galactosamine and 125 μL of Tween 80 (A); N-oleoyl-D-galactosamine vs 10 mg of RES, 10 mg of PLGA and 125 μL of Tween 80 (B); and Tween 80 vs 10 mg of RES, 10 mg of PLGA and 20 mg of N-oleoyl-d-galactosamine (C).
Abbreviations: PLGA, poly(lactic-co-glycolic acid); RES, resveratrol; RES-GNPs, RES-loaded galactosylated nanoparticles.
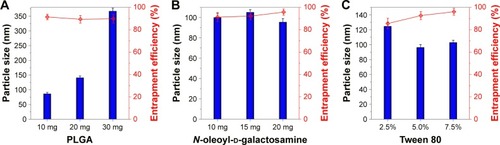
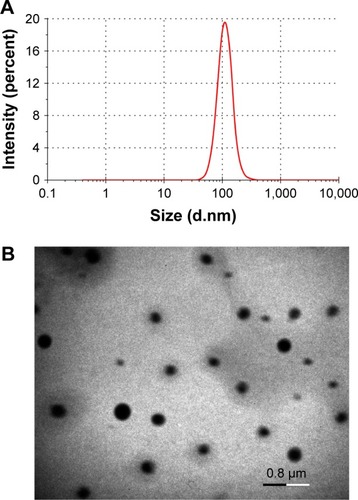
Considering the advantages of small particle size and high EE in drug delivery, the final formulation was typically determined as 10 mg of RES, 10 mg of PLGA, 20 mg of N-oleoyl-D-galactosamine and 125 μL of Tween 80 formulated into 2.5 mL of water. The resulting RES-GNPs were 108.4 nm in particle size with a polydispersity index of 0.217 (). RES-GNPs appeared a spherical morphology as observed by TEM (). The particle size as gauged by the scale bar was ~150 nm, which was a little larger than the hydrodynamic size measured by dynamic light scattering, which may be associated with the flattening of particles upon sampling. The EE of RES-GNPs was determined to be 97.22% ± 2.31%, showing an excellent drug entrapment capacity. Of note, RES-GNPs was negatively charged with a ζ potential of -46.3 mV. The absolute ζ potential >25 mV also suggests a high colloidal stability of RES-GNPs due to the electrostatic repulsion.Citation29 The physical stability of RES-GNPs was shown to be fine for a short-term storage survey. The significant changes of particle size and polydispersity index of nanoparticles did not occur.
In vitro drug release
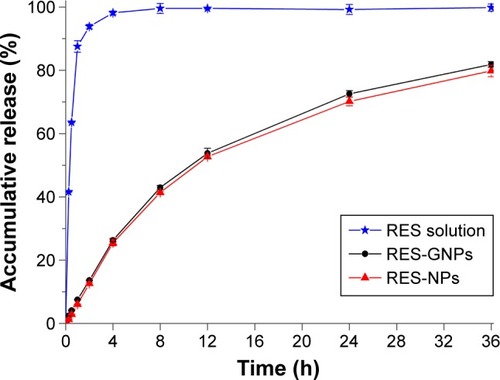
The release profiles of RES from RES-NPs and RES-GNPs are shown in . Free RES (solution formulation) was released completely in a very short time, indicating less release resistance providing with the dialysis bag. Both RES-NPs and RES-GNPs exhibited a slow release process, where the accumulative release percentages of two formulations were less than 43% within 8 h. The result may suggest that slow release allows the majority of drug to be retained in NPs when transporting through the gastrointestinal tract, which is favorable for RES absorption via NPs. It still had 20% of drug that has not been released at the time point of 36 h. However, there was no difference in release between RES-NPs and RES-GNPs. This may be ascribed to the similar matrix structure between RES-NPs and RES-GNPs, in which RES shares an identical crystallinity property. The participation of N-oleoyl-D-galactosamine has less impact on the crystal matrix of nanoparticles. In order to elucidate the release mechanism, the release curves were fitted with various models. It shows that the in vitro releases of RES-NPs and RES-GNPs conform to the first-order kinetic model (R2 = 0.9948), which belongs to the diffusion-based release process.
Enhanced bioavailability
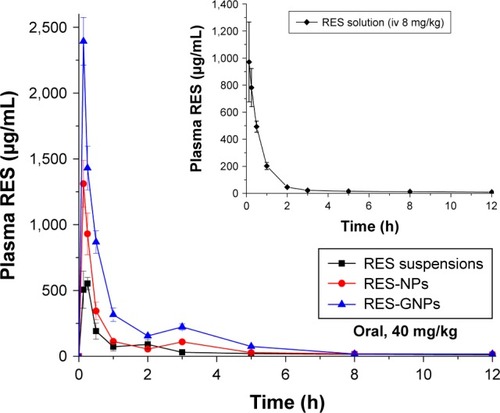
The profiles of plasma RES concentration vs time in rats are shown in . The main pharmacokinetic parameters extracted from noncompartment model are listed in . The blood drug level sharply declined in the case of RES solution (inset), indicating that RES can quickly distribute and be eliminated in vivo once injected into the blood vessel. For three formulations, they all exhibited a rapid absorption after oral administration. The maximum plasma concentration (Cmax) appeared at the first time point, showing that the intestinal absorption of RES is not as poor as expected, although limited. RES-NPs brought about a quicker and higher degree of RES absorption compared with RES suspensions. Nevertheless, RES-GNPs resulted in the best absorption of RES after oral administration. The Cmax of RES-GNPs was almost two times as high as that of RES-NPs. These results indicate that NPs can positively improve the oral absorption of RES, though GNPs can strengthen the absorption-enhancing effect. Of note, the blood drug concentration vs time curves presented a double peak for all RES formulations, suggesting that an enterohepatic circulation may take place on RES.Citation30 The relative bioavailability of RES-NPs was 165.7% compared with RES suspensions, whereas RES-GNPs yielded a higher bioavailability, up to 335.7%. RES-GNPs significantly enhanced the oral bioavail-ability of RES perhaps due to galactosylation.
Table 1 Main pharmacokinetic parameters of RES after oral administration of RES suspensions, RES-NPs and RES-GNPs or intravenous injection of RES solution in rats (n = 5)
Figure 5 Pharmacokinetic profiles of plasma RES concentration vs time after oral administration of RES suspensions, RES-NPs and RES-GNPs at a dose of 40 mg/kg (n = 5).
Note: The inset represents the pharmacokinetics of RES solution dosed by intravenous injection (8 mg/kg).
Abbreviations: RES, resveratrol; RES-NPs, RES-loaded nanoparticles; RES-GNPs, RES-loaded galactosylated nanoparticles.
In general, gastrointestinal absorption of substances can be carried through the passive, carrier-mediated and cytosis (membrane mobile) transport.Citation31 For small molecular compounds, passive- and carrier-mediated transports play the basic role in their gastrointestinal absorption. In terms of macromolecules or NPs, cytosis transport is the principal mechanism of transepithelial absorption. The intestinal absorption of galactose is fulfilled by way of SGLT1,Citation32 a transporter-mediated process. Surface modification with galactose moiety imparts NPs a high affinity and specificity for enterocytes, which can facilitate NPs transport across the absorptive epithelia by cytosis or carrier-mediated transport. Zhang et al developed galactosylated chitosan-based NPs for the oral delivery of a mitogen-activated protein kinase 4 (Map4k4) siRNA, finding that GNPs more efficiently promoted siRNA distribution into the ulcerative colon following oral administration.Citation33 Jain et al also demonstrated that galactosylated solid lipid NPs could significantly enhance the cellular uptake of doxorubicin.Citation34 Our findings accorded with the reported outcomes, showing that galactosylation may be a promising strategy to ameliorate the oral delivery of NPs, hence the bioavailability.
Intestinal permeability
The Peff of free RES, RES-NPs and RES-GNPs is listed in . Free RES exhibited a moderate intestinal permeability, up to the exponential order of 10−4. Aside from the duodenum and colon, the limited step for RES absorption should be its intrinsic solubility and metabolism,Citation35 rather than the permeability. Nevertheless, the intestinal permeability of RES was also significantly improved when encapsulated into NPs, especially for the sites of duodenum and ileum. The improvement in permeability was more noticeable in the case of RES-GNPs. RES-GNPs could enhance the permeability of RES in all investigated intestinal segments compared with RES-NPs. We assume that the permeability optimization can be attributed to galactose attachment of NPs that mediates and facilitates the internalization of NPs into the enterocytes. Galactose-modified NPs have great advantages in heightening the drug’s permeability and absorptivity by virtue of excellent intestinal affinity.
Table 2 The effective intestinal permeability (Peff) of free RES, RES-NPs and RES-GNPs determined by in situ single-pass intestinal perfusion (n = 3)
Cellular uptake and internalization
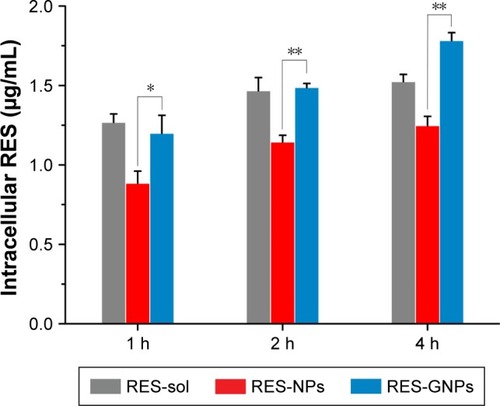
presents the cellular uptake of free RES, RES-NPs and RES-GNPs in Caco-2 cells. The solution formulation (free RES) exhibited a rapid cellular uptake relative to RES-NPs at 1, 2 and 4 h. It may be attributive to the small molecular dimension of RES and effortless approachability to the cells via the transcellular transport. The cellular uptake of RES-NPs can be conducted by the membrane mobile transport, thus the transport efficiency is comparatively slow. However, there was a significant difference in cellular uptake between RES-GNPs and RES-NPs. The cellular uptake level of RES-GNPs was parallel to that of free RES at 1 and 2 h, but significantly higher than RES-NPs. It was noted that the cellular uptake of RES-GNPs incessantly increased over time, which was also different from RES solution. At the time point of 4 h, the cellular uptake of RES-GNPs was larger than that of RES solution. These results demonstrate that there is a different mechanism responsible for the cellular uptake of RES-GNPs.
Figure 6 Cellular uptake at 1, 2 and 4 h with a RES level of 20 μg/mL quantified by intracellular RES concentration.
Notes: Paired t-test, *P<0.05, **P<0.01, compared with RES-NPs. Data expressed as mean ± SD (n = 3).
Abbreviations: RES, resveratrol; RES-NPs, RES-loaded nanoparticles; RES-GNPs, RES-loaded galactosylated nanoparticles.
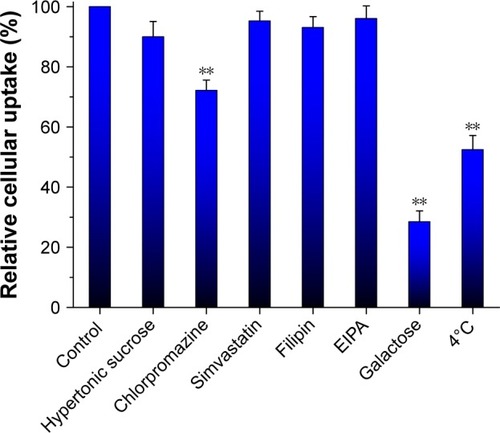
To clarify the cellular trafficking mechanism of RES-GNPs, we investigated the cellular uptake of RES-GNPs in the presence of different physiological inhibitors, competitor (galactose) or under a lower temperature (4°C). shows the relative cellular uptake of RES-GNPs in limited conditions. The cellular uptake of RES-GNPs was significantly inhibited by chlorpromazine and galactose, whereas hypertonic sucrose, simvastatin, Filipin and EIPA almost had no effect on the cellular uptake. Chlorpromazine, a specific inhibitors of clathrin-mediated endocytosis, reduced the cellular uptake by 27.82% relative to the control. This indicates that clathrin-mediated endocytosis is involved in the transport process of RES-GNPs. Likewise, the cellular uptake of RES-GNPs was greatly inhibited under 4°C, resulting in reduction of 47.53%. As known, endocytosis is a kind of cytosis transport, in which ATP energy is required.Citation36 In addition, the cellular uptake of RES-GNPs strikingly declined under the intervention of galactose, suggesting an uptake competition between RES-GNPs and galactose. These results indicate that cytosis plays an important role in the cellular trafficking of RES-GNPs, especially the clathrin-mediated endocytosis by ligand-receptor/transporter interaction.Citation37
Figure 7 Cellular trafficking mechanisms characterized by relative cellular uptake rate in the presence of physiological inhibitors, galactose or under limited condition.
Notes: Paired t-test, **P<0.01, compared with control. Data expressed as mean ± SD (n = 3).
Abbreviation: EIPA, 3-amino-6-chloro-N-(diaminomethylene)-5-(ethyl(isopropyl) amino)pyrazine-2-carboxamide.
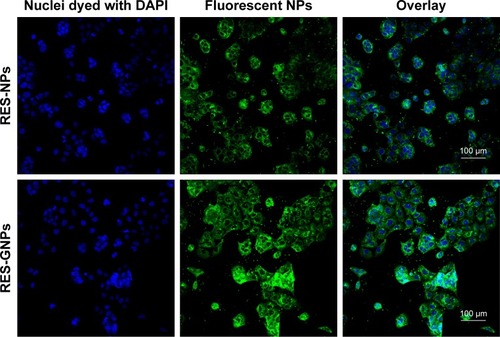
The fluorescent staining of Caco-2 cells treated with coumarin 6-labeled RES-NPs and RES-GNPs is shown in . Both RES-NPs and RES-GNPs could be internalized into the cells. NPs (green) colocalized with the cell nucleus (blue), taking on a light green upon overlay. However, at the same incubation time, the intracellular fluorescence intensity treated with RES-GNPs was apparently stronger than that treated with RES-NPs. This demonstrates that RES-GNPs have better cellular internalization and cytosolic delivery ability than RES-NPs.
Improved anti-inflammatory activity
TNF-α and IL-6 are well-known proinflammatory cytokines, and the level of NO represents the inflammatory degree. They play important roles in the pathological development of many inflammatory diseases. RAW 264.7 macrophages provide a useful platform for the study of anti-inflammatory activity.Citation38,Citation39 LPS can conveniently induce upregulation and secretion of proinflammatory cytokines, such as TNF-α and IL-6, and release of NO. In this study, RAW 264.7 macrophages were coincubated with LPS and RES preparations (25 μg/mL). The levels of proinflammatory mediators in different groups are shown in . It turned out that RES, RES-NPs and RES-GNPs substantially suppressed expression of TNF-α, IL-6 and NO compared to the LPS model. But, there were significant differences in the inhibitory effect among them. RES-GNPs exhibited the highest inhibitory activity on TNF-α release, followed by RES-NPs and then RES. The effects of various RES preparations on IL-6 release were somewhat distinct from TNF-α. RES-NPs did not show an advantage over free RES, but RES-GNPs gave an unexpected performance on IL-6 inhibition. As for NO, the inhibitory effects of three formulations were more conspicuous, especially for RES-GNPs. RES encapsulated in NPs possess a higher inhibitory activity on NO production compared to free RES. Improvement in anti-inflammation of RES through NPs can be attributed to the enhanced cellular internalization level and prolonged pharmacological action due to sustained drug release.
Figure 9 Inhibitory effects of RES, RES-NPs and RES-GNPs on TNF-α (A), IL-6 (B) and NO (C) in LPS-induced RAW264.74 cells.
Notes: Data expressed as mean ± SD (n = 3). Paired t-test, **P<0.01, compared with RES and RES-NPs.
Abbreviations: TNF, tumor necrosis factor; LPS, lipopolysaccharide; RES, resveratrol; RES-NPs, RES-loaded nanoparticles; RES-GNPs, RES-loaded galactosylated nano-particles; IL, interleukin; NO, nitric oxide.
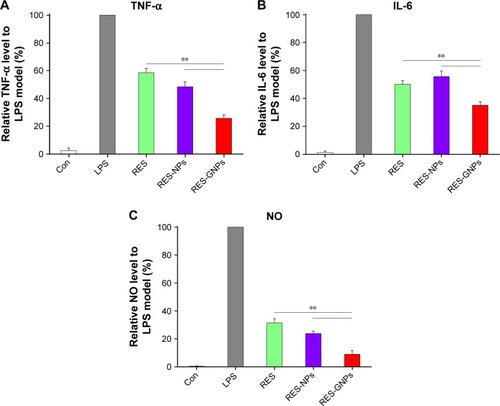
TNF-α and IL-6 are representative inflammatory mediators and the level of NO reflects the severity of inflammatory disease. TNF-α is a cell signaling protein (cytokine) involved in the systemic inflammation, which is one of the cytokines that mediate the acute phase reaction. It is mainly produced by activated macrophages, certainly can be generated by many other cell types, such as lymphocytes and dendritic cells.Citation40 IL-6 is an interleukin that acts as both a proinflammatory cytokine and an anti-inflammatory cytokine.Citation41 It is secreted by T cells and macrophages that can stimulate the immune response, thus causing inflammation if overexpressed. NO is a signaling molecule that plays a key role in the pathogenesis of inflammation. It produces an anti-inflammatory effect under normal physiological conditions. Meanwhile, NO is considered as a proinflammatory mediator that induces inflammation due to overproduction in abnormal situations.Citation42 Inhibiting production of TNF-α, IL-6 and NO will be of important significance for relieving the inflammatory reaction and controlling the inflammatory progress. Our developed RES-GNPs exhibit an excellent in vitro anti-inflammatory effect, showing a promising application for the treatment of inflammatory diseases, such as myocarditis, nephritis and vasculitis.
Conclusion
In this study, GNPs were developed and their suitability as oral delivery vehicle of RES was evaluated. It is found that our developed RES-GNPs possess excellent formulation performances compared with conventional polymeric NPs. RES-GNPs exhibit a higher intestinal permeability and can significantly enhance the oral bioavailability of RES after administration. Cell uptake and trafficking tests reveal that RES-GNPs can be more easily transported into the enterocytes. The in vitro anti-inflammatory efficacy of RES-GNPs was also higher than that of RES-NPs and free RES. Enhanced bioavailability and bioactivity for RES-GNPs is demonstrated to be concerned with galactosylation of NPs that renders them superior transepithelial transport and absorption. The developed formulation not only enhances the oral bioavailability of RES but also strengthen its bioactivity, demonstrating a promising application for inflammatory diseases. This work provides insight into the use of GNPs for oral delivery of poorly water-soluble drugs to potentiate their bioactivity.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (31672563), the National Key Research and Development Program of China (2016YFD0501004) and the Guangdong Provincial Key Laboratory of Prevention and Control for Severe Clinical Animal Diseases (2017B030314142).
Disclosure
The authors report no conflicts of interest in this work.
References
- XuDLiYZhangBResveratrol alleviate hypoxic pulmonary hypertension via anti-inflammation and anti-oxidant pathways in ratsInt J Med Sci2016131294295427994500
- Andrade VolkartPBenedetti GassenRMuhlen NogueiraBNery PortoBEduardo VargasJArigony SoutoAAntitumor activity of resveratrol is independent of Cu(II) complex formation in MCF-7 cell lineBioorg Med Chem Lett201727153238324228647350
- MontesanoALuziLSenesiPMazzocchiNTerruzziIResveratrol promotes myogenesis and hypertrophy in murine myoblastsJ Transl Med20131131024330398
- PenalvaREsparzaILarranetaEGonzalez-NavarroCJGamazoCIracheJMZein-based nanoparticles improve the oral bioavailability of resveratrol and its anti-inflammatory effects in a mouse model of endotoxic shockJ Agric Food Chem201563235603561126027429
- YenCCChangCWHsuMCWuYTSelf-nanoemulsifying drug delivery system for resveratrol: enhanced oral bioavailability and reduced physical fatigue in ratsInt J Mol Sci2017189E185328841149
- AmriAChaumeilJCSfarSCharrueauCAdministration of resveratrol: What formulation solutions to bioavailability limitations?J Control Release2012158218219321978644
- WalleTBioavailability of resveratrolAnn N Y Acad Sci2011121591521261636
- ZhouJZhouMYangFFInvolvement of the inhibition of intestinal glucuronidation in enhancing the oral bioavailability of resveratrol by labrasol containing nanoemulsionsMol Pharm20151241084109525723098
- FranciosoAMastromarinoPMasciAd’ErmeMMoscaLChemistry, stability and bioavailability of resveratrolMed Chem201410323724524329932
- Bernkop-SchnurchANanocarrier systems for oral drug delivery: do we really need them?Eur J Pharm Sci201349227227723537503
- da LuzCMBoylesMSFalagan-LotschPPoly-lactic acid nanoparticles (PLA-NP) promote physiological modifications in lung epithelial cells and are internalized by clathrin-coated pits and lipid raftsJ Nanobiotechnology20171511128143572
- WangJLiLWuLDevelopment of novel self-assembled ES-PLGA hybrid nanoparticles for improving oral absorption of doxorubicin hydrochloride by P-gp inhibition: in vitro and in vivo evaluationEur J Pharm Sci20179918519227989702
- PaulPKNopparatJNuanplubMTreetongASuedeeRImprovement in insulin absorption into gastrointestinal epithelial cells by using molecularly imprinted polymer nanoparticles: microscopic evaluation and ultrastructureInt J Pharm20175301–227929028764982
- ShahnazGEdagwaBJMcMillanJDevelopment of mannose-anchored thiolated amphotericin B nanocarriers for treatment of visceral leishmaniasisNanomedicine (Lond)20171229911527879160
- ZhangXChenGZhangTMaZWuBEffects of PEGylated lipid nanoparticles on the oral absorption of one BCS II drug: a mechanistic investigationInt J Nanomedicine201495503551425473287
- KarasovWHIntegrative physiology of transcellular and paracellular intestinal absorptionJ Exp Biol2017220Pt 142495250128724701
- CuraAJCarruthersARole of monosaccharide transport proteins in carbohydrate assimilation, distribution, metabolism, and homeostasisCompr Physiol20122286391422943001
- MizumaTOhtaKHayashiMAwazuSIntestinal active absorption of sugar-conjugated compounds by glucose transport system: implication of improvement of poorly absorbable drugsBiochem Pharmacol1992439203720391596291
- FachinettiNRigonRBEloyJOSatoMRDos SantosKCChorilliMComparative study of glyceryl behenate or polyoxyethylene 40 stearate-based lipid carriers for trans-resveratrol delivery: development, characterization and evaluation of the in vitro tyrosinase inhibitionAAPS PharmSciTech20181931401140929404955
- PujaraNJambhrunkarSWongKYMcGuckinMPopatAEnhanced colloidal stability, solubility and rapid dissolution of resveratrol by nanocomplexation with soy protein isolateJ Colloid Interface Sci201748830330827838554
- JuereEFlorekJBouchouchaMJambhrunkarSWongKYPopatAKleitzFIn vitro dissolution, cellular membrane permeability, and anti-inflammatory response of resveratrol-encapsulated mesoporous silica nanoparticlesMol Pharm201714124431444129094948
- MohammadiGShakeriAFattahiAMohammadiPMikaeiliAAliabadiAAdibkiaKPreparation, physicochemical characterization and anti-fungal evaluation of nystatin-loaded PLGA-glucosamine nanoparticlesPharm Res201734230130927928646
- LiWZhangTYeYZhangXWuBEnhanced bioavailability of tripterine through lipid nanoparticles using broccoli-derived lipids as a carrier materialInt J Pharm2015495294895526453780
- NozawaTImaiTPrediction of human intestinal absorption of the prodrug temocapril by in situ single-pass perfusion using rat intestine with modified hydrolase activityDrug Metab Dispos20113971263126921474683
- DengWXieQWangHMaZWuBZhangXSelenium nanoparticles as versatile carriers for oral delivery of insulin: Insight into the synergic antidiabetic effect and mechanismNanomedicine20171361965197428539272
- RuanBFGeWWChengHJXuHJLiQSLiuXHResveratrol-based cinnamic ester hybrids: synthesis, characterization, and anti-inflammatory activityJ Enzyme Inhib Med Chem20173211282129029072109
- ZhangXWangHZhangTZhouXWuBExploring the potential of self-assembled mixed micelles in enhancing the stability and oral bioavailability of an acid-labile drugEur J Pharm Sci20146230130824956461
- BalzusBSahleFFHonzkeSFormulation and ex vivo evaluation of polymeric nanoparticles for controlled delivery of corticosteroids to the skin and the corneal epitheliumEur J Pharm Biopharm201711512213028189623
- ChenZTaiZGuFHuCZhuQGaoSAptamer-mediated delivery of docetaxel to prostate cancer through polymeric nanoparticles for enhancement of antitumor efficacyEur J Pharm Biopharm201610713014127393562
- MarierJFVachonPGritsasAZhangJMoreauJPDucharmeMPMetabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat modelJ Pharmacol Exp Ther2002302136937312065739
- PachaJDevelopment of intestinal transport function in mammalsPhysiol Rev20008041633166711015621
- LehmannAHornbyPJIntestinal SGLT1 in metabolic health and diseaseAm J Physiol Gastrointest Liver Physiol201631011G887G89827012770
- ZhangJTangCYinCGalactosylated trimethyl chitosan-cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophagesBiomaterials201334143667367723419643
- JainAKesharwaniPGargNKGalactose engineered solid lipid nanoparticles for targeted delivery of doxorubicinColloids Surf B Biointerfaces2015134475826142628
- KuhnleGSpencerJPChowrimootooGResveratrol is absorbed in the small intestine as resveratrol glucuronideBiochem Biophys Res Commun2000272121221710872829
- SelviRBChatterjeeSJagadeesanDChaturbedyPSumaBSEswaramoorthyMKunduTKATP driven clathrin dependent entry of carbon nanospheres prefer cells with glucose receptorsJ Nanobiotechnology2012103522857258
- JainAJainAParajuliPRecent advances in galactose-engineered nanocarriers for the site-specific delivery of siRNA and anticancer drugsDrug Discov Today Epub2017111010.1016/j.drudis.2017.11.003
- MatsudaYMinagawaTOkuiTYamazakiKResveratrol suppresses the alveolar bone resorption induced by artificial trauma from occlusion in miceOral Dis201824341242128944599
- XiangFFHeJWLiuZXPengQZWeiHTwo new guaiane-type sesquiterpenes from Curcuma kwangsiensis and their inhibitory activity of nitric oxide production in lipopolysaccharide-stimulated macrophages. Nat Prod ResEpub20179201610.1080/14786419.2017.1378203
- FeiMBhatiaSOrissTBTNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infectionProc Natl Acad Sci U S A2011108135360536521402950
- ZhangHChenWInterleukin 6 inhibition by triptolide prevents inflammation in a mouse model of ulcerative colitisExp Ther Med20171432271227628962154
- SharmaJNAl-OmranAParvathySSRole of nitric oxide in inflammatory diseasesInflammopharmacology200715625225918236016