Abstract
Nanoparticles have enormous applications in textiles, cosmetics, electronics, and pharmaceuticals. But due to their exceptional physical and chemical properties, particularly antimicrobial, anticancer, antibacterial, anti-inflammatory properties, nanoparticles have many potential applications in diagnosis as well as in the treatment of various diseases. Over the past few years, nanoparticles have been extensively used to investigate their response on the neuronal cells. These nanoparticles cause stem cells to differentiate into neuronal cells and promote neuronal cell survivability and neuronal cell growth and expansion. The nanoparticles have been tested both in in vitro and in vivo models. The nanoparticles with various shapes, sizes, and chemical compositions mostly produced stimulatory effects on neuronal cells, but there are few that can cause inhibitory effects on the neuronal cells. In this review, we discuss stimulatory and inhibitory effects of various nanoparticles on the neuronal cells. The aim of this review was to summarize different effects of nanoparticles on the neuronal cells and try to understand the differential response of various nanoparticles. This review provides a bird’s eye view approach on the effects of various nanoparticles on neuronal differentiation, neuronal survivability, neuronal growth, neuronal cell adhesion, and functional and behavioral recovery. Finally, this review helps the researchers to understand the different roles of nanoparticles (stimulatory and inhibitory) in neuronal cells to develop effective therapeutic and diagnostic strategies for neurodegenerative diseases.
Introduction of nanoparticles
Nanoparticles or nanomaterials are one millionth of a millimeter, ~100,000 times smaller than the diameter of a human hair. Most nanoparticles are too small to be seen with the naked eye and even with conventional lab microscopes. Nanoparticles can be derived from both natural and synthetic sources. Over the past few years, synthetically derived nanoparticles generated tremendous interests and based on the chemical compositions, nanoparticles can be broadly classified into two major classes such as organic materials, which are liposomes, dendrimers, carbon nanotubes, emulsions, and other polymers, and inorganic materials, which include metals.Citation1–Citation3 Nanoparticles can be synthesized in different sizes (1.0–500 nM) and shapes (cones, cubes, rods, tubes, and shells).Citation4–Citation6
There are various applications of nanoparticles in biotechnology, biosensing, catalysis, magnetic fluids, separation techniques, energy storage, and environmental modificationCitation7–Citation12 and also in biomedical field, especially in diagnostics, and drug or gene delivery.Citation13–Citation19 Interestingly, nanoparticles have been extensively used as drug carrier systems for therapeutic molecules with the primary aim to improve the therapeutic effect and decrease their side effects and drug/gene delivery.Citation20–Citation23 One of the major attributes of nanoparticles is their precise targeting, biocompatibility, bioavailability, and multifunctional capabilities.Citation24–Citation26 In the recent past, several attempts have been made to study the effect of different classes of nanoparticles on cancer cells.Citation27–Citation38 In addition, interests have also been generated to study the effects of nanoparticles on neurons and there are several reports that suggest that nanoparticles promote neuronal differentiation, and neuroprotection studied in both in vitro and in vivo conditions.Citation3,Citation39–Citation43 To get better therapeutic results, various types of nanoparticles have been studied in neurons, and among those, carbon-based nanoparticles are mostly reported,Citation4,Citation44–Citation48 followed by gold and silver nanoparticles (AgNPs).Citation49–Citation51
Despite having many beneficial properties, nanoparticle also raises few health hazard and toxicity issues. To better understand the safety profile of the nanoparticles, several attempts have been made to know whether nanoparticles cause any side effects or toxic effects. It has been shown that nanomaterials possess highly activated surfaces that are capable of inducing carcinogens, mutagens, or health hazard responses.Citation52–Citation54 Furthermore, it has been reported that carbon nanotubes induced fibrogenesis on nanostructured substrates.Citation55 Moreover, nanoparticles are 100 times smaller than normal red blood cells, which increase the potential for interaction, and there is evidence that nanoparticles interact with proteins, DNA,Citation56 lung cells, and viruses. The current assumption is that nanoparticles such as silica featured as hydrophilic, hydrophobic, or even amphiphilic that can be taken up by human membranes may pose serious threats. Hence, understanding nanoparticles’ interaction with living cells and other biologic systems, especially with central nervous system (CNS), is critical. Nanoparticles have potential functionality and toxic effects on human neuronal cells because they can pass through biologic membranes.Citation57 It is known that the biologic half-life of silver in the CNS is longer than that in other organs, suggesting that there may be some significant physiologic functions, consequences, and risks to the brain because of prolonged exposure. In addition, effects of nanoparticles on the blood–brain barrier (BBB) were also evaluated, and it was found that administration of Ag, Cu, or Al/Al2O3 nanoparticles showed disrupted BBB function and induced brain edema formation.Citation58 Moreover, AgNPs induced BBB destruction and astrocyte swelling and caused neuronal degeneration.Citation59 In the present review, we have discussed various nanoparticles and their impacts on the neuron’s biology and tried to evaluate their responses (stimulatory or inhibitory), which were studied in both in vitro and in vivo models, respectively.
Stimulatory effect of nanoparticles on neuronal cells
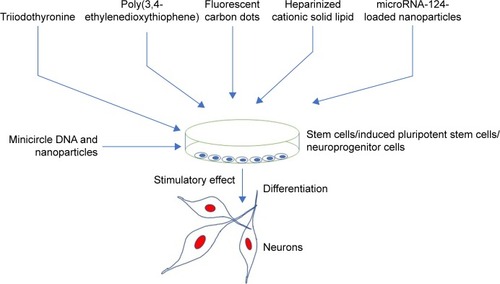
Nanoparticles have tremendous capabilities to stimulate neuronal cells toward neuronal cell proliferation, axonal growth, neuronal cell adhesion, and neuroprotection (). It has been demonstrated that nanoparticles can also differentiate stem cells into neuronal cells. The nanoparticles with different shapes such as nanotubes, nanofibers, nanocone, and nanoemulsion have been used to test their effects on the neuronal cells. For example, nanotubes and nanofibers promoted neuronal regeneration, activated hippocampus neurons activities, neurons growth, and neuronal protection.Citation44,Citation45,Citation49,Citation60–Citation64 In addition, there are few reports about use of nanoscaffold, nanocomplexes, and nanomembrane in neuron regeneration and neural tissue reconstruction.Citation65–Citation67 The stimulatory effects of some of the nanoparticles are diagrammatically depicted in . Like shapes of the nanoparticles, size of the nanoparticles is also important in inducing biologic response.Citation68 For example, nerve growth factor (NGF)-encapsulated chitosan nanoparticles with size 80–90 nM caused differentiation of canine mesenchymal stem cells into neurons,Citation69 whereas calcium phosphate–lipid nanoparticles with size 30 nM caused neuronal differentiation.Citation70 In another report, it has been found that prodrug nanoparticles with 50 nM size improved neuronal survival.Citation71
Nanoparticles are either used alone or in combination or conjugation with other molecules to achieve better response on the neuronal cells. It is not easy to discuss each nanoparticle in detail, so we briefly describe the impact of nanoparticles on neurons. For example, it was reported that the use of the nanoparticle triiodothyronine along with retinoic acid caused neuronal differentiation.Citation72 In addition, treatment of triiodothyronine along with retinoic acid also caused a significant increase in the expression of neural lineage-specific markers. Moreover, treatment of triiodothyronine also caused 10-fold increase in the gene expression of β-III-tubulin, and five-time increase in microtubule-associated protein 2 gene expressions.Citation72 It was reported that three-dimensional poly(3,4-ethylenedioxythiophene) doped with hyaluronic acid nanoparticles conjugated with chitosan or gelatin matrix caused neuronal cell differentiation.Citation73 In another study, it was reported that poly(3,4-ethylenedioxythiophene) coated with microelectrodes have significantly reduced neuronal death and neuronal damage as compared to noncoated controls.Citation74 Carbon dots (C-dots), a class of fluorescent nanoparticles with pure carbon core, have great bioanalytical potential. In addition, the application of multifunctional fluorescent C-dots caused neuronal differentiation in adult stem cells.Citation75 In another study, it was reported that fluorescent C-dots (40–800 μg/mL) caused reduction of acidification of synaptic vesicles and increased the ambient level of the neurotransmitters.Citation76
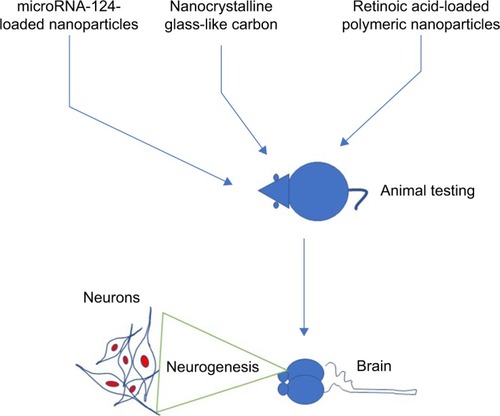
Interestingly, it was reported that treatment of NGF-loaded heparinized cationic solid lipid nanoparticles (HCSLNs) caused differentiation of induced pluripotent stem cells (iPSCs) into neuronal cells.Citation77 In addition, presence of neuron-specific staining in differentiated neuronal cells confirmed that NGF-loaded HCSLNs caused neuronal cell differentiation.Citation77 Recently, it was reported that traceable microRNA-124-loaded nanoparticles, efficiently delivered into neural stem or progenitor cells, promoted neuronal differentiation and maturation.Citation78 Similarly, it was reported that nanocrystalline glass-like carbon (NGLC) can induce neuronal differentiation. It was reported that NGLC caused differentiation of the dopaminergic neurons derived from the substantia nigra of the transgenic mouse embryo’s brain.Citation79 Nanoparticles caused not only the neuronal differentiation but also the formation of new cells. For example, treatment of nanoparticles caused an increased formation of daughter neuronal cells.Citation80 In another report, it was demonstrated that polyvinylidene fluoride and poly vinylidenefluoride-co-trifluoroethylene or BaTiO3 (barium titanate) stimulated and promoted differentiation of SH-SY5Y neuroblastoma cells.Citation81
Nanotopography is also an important factor in neuronal differentiation. For example, nanostructured zirconia surfaces produced by supersonic cluster beam deposition of zirconia nanoparticles promoted neuronal differentiation and maturation of the hippocampus neurons.Citation82 Neurogenic niches constitute a powerful endogenous source of formation of new neurons to repair brain cells. Furthermore, it was reported that retinoic acid nanoparticles (RA-NPs) caused neurogenesis in the neural stem cells when the stem cells were exposed to blue light.Citation83 Application of nanoparticle extracellular matrix along with conductive fiber film promoted neurite adhesion, neural alignment, and elongation of neuritis.Citation84 The NGF-conjugated mesoporous silica nanoparticle was reported to promote neuron proliferation and neurite growth in pheochromocytoma (PC12) cell line.Citation85 In the same study, it was reported that use of NGF-conjugated mesoporous silica nanoparticle significantly promoted differentiation of neuron-like PC12 cells and growth of neurites compared to NGF alone.Citation85 This report suggests that use of nanoparticles along with NGFs improves neuronal cell differentiation many fold. Nanopatterned SU-8 surface using nanosphere lithography was reported to enhance neuronal cell growth.Citation86 Moreover, nanotopography also promoted neuronal differentiation of human iPSCs.Citation87
The treatment of nanoparticles not only induces neuronal differentiation but also improves functional or behavioral recovery in animal models (). For example, Zhang et al reported that treatment of small interfering RNA along with retinoic acid resulted in attenuation of neuronal loss and restoration of memory deficiencies in mice. Moreover, an intracerebroventricular injection of microRNA-124-loaded nanoparticles into a mouse model of Parkinson’s disease caused an increased formation of new neurons in the olfactory bulb.Citation88 In the same study, it was found that microRNA-124-loaded nanoparticles enhanced migration of new neurons into the lesioned striatum of mice and caused improvement of motor function.Citation88 In another study, it was reported that an administration of triiodothyronine in a rat model of ischemic stroke was reported to cause a 34% decrease in tissue infarction and a 59% decrease in brain edema.Citation89
In another report, it was demonstrated that RA-NPs enhanced vascular regulation of neural stem cell and promoted neuronal cell survival and neuronal cell differentiation after ischemia effect.Citation90 In addition, it was found that treatment of RA-NP protected endothelial cells from ischemic death and stimulated the release of prosurvival, proliferation-stimulating factors for neural stem cells.Citation90 It would be interesting to investigate the effect of triiodothyronine or microRNA-124-loaded nanoparticles in other animal models to check whether it can also enhance functional and behavioral recovery. In addition to use of nanoparticles for the neuronal differentiation, nanoparticles have also been used to deliver drugs in the neuronal cells. For example, it was reported that the minicircle DNA and nanoparticles were used to deliver a neurotherapeutic gene into neural stem cells.Citation80 In the same study, it was demonstrated that minicircles DNA along with magnetofection technology caused the overexpression of brain-derived neurotrophic factor gene in neural stem cells.Citation80
We have summarized other nanoparticles based on their stimulatory actions in tabular form. For example, in , we have listed the nanoparticles with stimulatory effects on neurons tested under both in vitro culture and in vivo conditions. The stimulatory effects of nanoparticles caused an increased neuronal cell differentiation and promoted nerve regeneration, hippocampal neuron activity, cell viability, neuronal growth and cerebral neuronal induction, and gene expression in nigral dopaminergic neurons. They also promoted neuronal growth, axonal guidance, Schwann cells’ guidance, neural tissue reconstruction, neuronal–glial interaction, neurogenesis, and neuroprotection. These nanoparticles with different shapes, sizes, and chemical compositions improved nerve regeneration, neuronal recovery, neuronal signaling, neuroprotection, and neurogenesis in various animal models. These nanoparticles were also able to improve functional and behavioral recovery of the motor functions in the animal models of Parkinson’s disease and spinal cord injury.
Table 1 List of various nanoparticles with stimulatory effects on neurons
Inhibitory effect of nanoparticles on neuronal cells
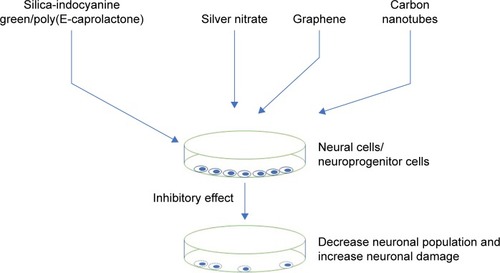
Despite having therapeutic potentials, nanoparticles pose safety concerns. There are few nanoparticles, which are also reported to have inhibitory effects on the neuronal cells. These nanoparticles caused opposite and damaging action on the neuronal differentiation. The inhibitory effect on the neuronal differentiation is diagrammatically depicted in . It was reported that cerium oxide nanoparticles displayed antioxidant properties in both in vitro and in vivo conditions and caused an inhibitory effect on the neural stem cells by inhibiting the neuronal cell differentiation.Citation91 In addition, detailed computational analyses showed that cerium oxide altered pathways and networks relevant to neuronal development and inhibited neuronal differentiation.Citation91 It was found that cerium oxide caused a decrease in neuron-specific β3-tubulin expression, a marker of neuronal differentiation, and glial fibrillary acidic protein, a neuroglial marker.Citation91 In contrast to this report, cerium oxide nanoparticles promoted neurogenesis and abrogated hypoxia-induced memory impairment through AMP-activated protein kinase–protein kinase C–cAMP-response element binding protein (CREB)-binding protein signaling cascade in the rat.Citation92 In another study, nanoparticle exposure did not impair cell viability and neuroinflammation in primary hippocampal cultures, but significantly decreased the neuronal differentiation markers in human SH-SY5Y cells.Citation93 We do not know the reason of the contradicting responses of cerium oxide on neuronal cells, and the possibility of using different concentrations or different sizes of cerium oxide could be one of the reasons. Nevertheless, detailed studies must be undertaken with different sizes of cerium oxide to understand cerium oxide’s role.
Polyamidoamine (PAMAM) dendrimer has many biologic applications that include delivering gene or drug molecules to the cells. Despite having potential therapeutic and diagnostic application, PAMAM also caused some cytotoxic effects. It was reported that PAMAM dendrimer exposure caused an adverse effect on neuronal cell differentiation and adverse effect associated with oxidative stress and DNA damage.Citation94 In addition, PAMAM dendrimer was reported to inhibit neutrosphere growth. In the same study, it was reported that PAMAM reduced number of microtubule-associated protein 2-positive cells after 10 days of differentiation.Citation94 In another report, AgNPs induced inflammatory response in neuronal cells.Citation9 It was reported that AgNPs entered the nuclei of mouse neuronal cells and induced progression of neurodegenerative disorder.Citation9 It was reported that silver nitrate treatment increased cellular superoxide dismutase activity and decreased mitochondrial membrane potential, leading to neuronal death.Citation11 In addition, even a low concentration of AgNPs interrupted early neuronal processes and facilitated neuron apoptosis by increased cellular oxidative stress and mitochondrial disruption.Citation11 In another study, it was reported that silica-indocyanine green/poly (ε-caprolactone) nanoparticles caused no neuronal differentiation because of mitochondrial damage.Citation95 We have summarized other nanoparticles that are having inhibitory and cytotoxic effects on neurons, in tabular forms. For example, inhibitory and cytotoxic effects on neurons studied in in vitro models are shown in , whereas inhibitory and cytotoxic effects studied in animal models are shown in .
Table 2 List of various nanoparticles with neurotoxic effects on neurons tested in in vitro conditions
Table 3 List of nanoparticles with inhibitory effects on neurons, which are tested in animal models
Risks and challenges of nanoparticles on neuronal cells
Despite having so many beneficial properties, the nanoparticles also cause some health concerns because of their small size and chemical compositions. Researchers were interested to find out whether nanoparticles do exert some negative effects on the neuron biology. Recently, it has been reported that the use of low concentration of AgNPs caused neuronal damageCitation96 and also treatment of silica nanoparticles impaired the mitochondrial function during neuronal differentiation.Citation96 In another study, it was reported that PAMAM dendrimers with various surface functional groups caused cytotoxic effects on neuronal differentiation in human neural progenitor cells.Citation94 These nanoparticles upon testing under in vitro conditions promoted neuronal damage and induced neurodegeneration, neuronal cytotoxicity, and neurotoxicity. Like in vitro models, nanoparticles have also been tested in animal models, which induced neuronal damage, neuronal degeneration, neuronal damage, neuronal toxicity, cell death, and impaired BBB. We have listed other nanoparticles that are also reported to cause toxic effects on neuronal cells, in and .
Summary
Nanoparticles have many potential applications, which include the promotion and activation of neuronal cell differentiation as reported in both in vitro and in vivo models. Nanoparticles can also reverse the neurologic impairments in the animal models of neurologic disorders such as brain ischemia and Parkinson’s and Alzheimer’s diseases. Research has shown that many nanoparticles promoted neuronal differentiation and enhanced neuronal survival and neuronal growth and maturation. But there are few nanoparticles that do not promote neuronal differentiation and cause neuronal damage or neurotoxicity. To achieve better response on the neuronal cells, researchers have used different sizes and shapes of nanoparticles. Sometimes one nanoparticle is conjugated with another nanoparticle or biomolecules to enhance the effects. Nanoparticles not only induce neuronal differentiation but also induce functional or behavioral recovery in animal models. The size of nanoparticles is also an important factor for their actions on the neurons. The researchers must know the size of nanoparticles before testing them for anticipated response. Most of the current data are based on morphologic, anatomical, and behavioral parameters, and still we do not know molecular mechanisms behind nanoparticle action on neurons. It would be interesting to study the molecular mechanism of the nanoparticle action on neurons.
Future direction
The nanoparticles hold a great promise for both diagnostic and therapeutic applications for various neurodegenerative diseases. They are also viable candidates to deliver neuroprotective molecules in the body for both diagnostic and therapeutic applications. The success of nanoparticles in neural areas depends on the consistent data generation, which depicts less variability in both in vitro and in vivo models. The cytotoxic effects of nanoparticles also need to be properly studied with proper dosages and correct treatment modalities to minimize the risk. Nanoparticles with stimulatory or inhibitory actions can be first studied through in vitro models, then through in vivo models. The results of both in vitro and in vivo studies must be compared and analyzed before calling nanoparticle stimulators or inhibitors. This strategy would help the researchers to identify and select potential nanoparticles for therapeutic and diagnostic purposes. Finally, nanoparticles with higher efficacy and ability to repair the damaged neurons with the least side effects in both in vitro and in vivo models hold great promise for the patients suffering from various neurodegenerative diseases.
Availability of data and material
The data analyzed are available from the corresponding author upon a request.
Acknowledgments
The authors are thankful to the entire management of the Institute for Research and Medical Consultations (IMRC), Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia, for their support and encouragement.
Disclosure
The authors report no conflicts of interest in this work.
References
- KuangYHChenXSuJRNA interference targeting the CD147 induces apoptosis of multi-drug resistant cancer cells related to XIAP depletionCancer Lett200927618919519097686
- ChowdhuryEHRosliRKarimMESystemic delivery of nanoformulations of anti-cancer drugs with therapeutic potency in animal models of cancerCurr Cancer Ther Rev201612117
- ChowdhuryEHNanotherapeutics: From Laboratory to ClinicBoca Raton, FLCRC Press2016
- MurphyCJJanaNRControlling the aspect ratio of inorganic nanorods and nanowiresAdv Mater20021480
- SunYXiaYShape-controlled synthesis of gold and silver nanoparticlesScience20022982176
- BalasubramanianBKraemerKLRedingNASkomskiRDucharmeSSellmyerDJSynthesis of monodisperse TiO(2)-paraffin core shell nanoparticles for improved dielectric propertiesACS Nano2010441893190020359188
- CaiJMiaoYYuBMaPLiLFanHLarge-scale, facile transfer of oleic acid-stabilized iron oxide nanoparticles to the aqueous phase for biological applicationsLangmuir2017331662166928146360
- SchrittwieserSPelazBParakWJHomogeneous biosensing based on magnetic particle labelsSensors201616828
- LinFDoongRCatalytic nanoreactors of Au@Fe3O4 yolk-shell nanostructures with various Au sizes for efficient nitroarene reductionJ Phys Chem C201712178447853
- HanLZhangYLuXWangKWangZZhangHPolydopamine nanoparticles modulating stimuli-responsive PNIPAM hydrogels with cell/tissue adhesivenessACS Appl Mater Interfaces20168290882910027709887
- GuoXZhangGChenLKhanAAGuBLiBNewborn neurons are damaged in vitro by a low concentration of silver nanoparticles through the inflammatory oxidative stress pathwayDNA Cell Biol201736121062107029058455
- ZhouXYangAHuangZYinGPuXJinJEnhancement of neurite adhesion, alignment and elongation on conductive polypyrrole-poly(lactide acid) fibers with cell-derived extracellular matrixColloids Surf B Biointerfaces201714921722527768911
- ZhaoYZJinRRYangWUsing gelatin nanoparticle mediated intranasal delivery of neuropeptide substance P to enhance neuro-recovery in hemiparkinsonian ratsPLoS One2016112e014884826894626
- KuangYZhangKCaoYHydrophobic IR-780 dye encapsulated in cRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapyACS Appl Mater Interfaces20179122171222628306236
- LinHCHuangCLHuangYJHsiaoILYangCWChuangCYTranscriptomic gene-network analysis of exposure to silver nanoparticle reveals potentially neurodegenerative progression in mouse brain neural cellsToxicol In Vitro20163428929927131904
- WangSZhaoXWangSQianJHeSBiologically inspired poly-dopamine capped gold nanorods for drug delivery and light-mediated cancer therapyACS Appl Mater Interfaces20168243682438427564325
- ChenJWangQZhouJPorphyra polysaccharide-derived carbon dots for non-viral co-delivery of different gene combinations and neuronal differentiation of ectodermal mesenchymal stem cellsNanoscale2017930108201083128726952
- SahaAMohantaSCDekaKDebPDeviPSSurface-engineered multifunctional Eu:Gd2O3 nanoplates for targeted and pH-responsive drug delivery and imaging applicationsACS Appl Mater Interfaces201794126414128098453
- KempJAShimMSHeoCYKwonYJ“Combo” nanomedicine: co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapyAdv Drug Deliv Rev20169831826546465
- HeKMaYYangBLiangCChenXCaiCThe efficacy assessments of alkylating drugs induced by nano-Fe3O4/CA for curing breast and hepatic cancerSpectrochim Acta A20171738286
- ShahabadiNAkbariAJamshidbeigiMFalsafiMFunctionalization of Fe3O4@SiO2 magnetic nanoparticles with nicotinamide and in vitro DNA interactionJ Mol Liq2016224227233
- XingRLiuGZhuJHouYChenXFunctional magnetic nanoparticles for non-viral gene delivery and MR imagingPharm Res2014311377138924065595
- SaeiAABarzegariAMajdMHAsgariDOmidiYFe3O4 nanoparticles engineered for plasmid DNA delivery to Escherichia coliJ Nanopart Res201416111
- AftabSShahANadhmanANanomedicine: an effective tool in cancer therapyInt J Pharm20185401–213214929427746
- AfrimzonEDeutschAShafranYIntracellular esterase activity in living cells may distinguish between metastatic and tumor-free lymph nodesClin Exp Metastasis20082521322418197360
- ZhaoJCastranovaVToxicology of nanomaterials used in nanomedicineJ Toxicol Environ Health B Crit Rev201114859363222008094
- GottliebEArmourSMHarrisMHThompsonCBMitochondrial membrane potential regulates matrix configuration and cytochrome C release during apoptosisCell Death Differ200310670971712761579
- WoodASchneiderJShilatifardACross-talking histones: implications for the regulation of gene expression and DNA repairBiochem Cell Biol200583446046716094449
- SmalleyKSHerlynMTowards the targeted therapy of melanomaMini Rev Med Chem20066438739316613575
- UnfriedKAlbrechtCKlotzLOvon MikeczAGrether-BeckSSchinsRPFCellular responses to nanoparticles: target structures and mechanismsNanotoxicology2007115271
- HanleyCLayneJPunnooseAPreferential killing of cancer cells and activated human T cells using ZnO nanoparticlesNanotech20081929295103
- TurcotteSChanDASutphinPDHayMPDennyWAGiacciaAJA molecule targeting VHL-deficient renal cell carcinoma that induces autophagyCancer Cell20081419010218598947
- WangHWickRLXingBToxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegansEnviron Pollut200915741171117719081167
- RasmussenJWMartinezELoukaPWingettDGZinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applicationsExpert Opin Drug Del20107910631077
- MathewRWhiteEAutophagy in tumorigenesis and energy metabolism: friend by day, foe by nightCurr Opin Genet Dev201121111311921255998
- WangXWangWLiLPerryGLeeHGZhuXOxidative stress and mitochondrial dysfunction in Alzheimer’s diseaseBiochim Biophys Acta2014184281240124724189435
- YaffeePOsipovATanCTuliRHendifarAReview of systemic therapies for locally advanced and metastatic rectal cancerJ Gastrointest Oncol2015618520025830038
- PatiRDasIMehtaRKSahuRSonawaneAZinc-oxide nanoparticles exhibit genotoxic, clastogenic, cytotoxic and actin depolymerization effects by inducing oxidative stress responses in macrophages and adult miceToxicol Sci2016150245447226794139
- BhangSHHanJJangHKpH-triggered release of manganese from MnAu nanoparticles that enables cellular neuronal differentiation without cellular toxicityBiomaterials201555334325934450
- WangKHeXLinthicumWCarbon nanotubes induced fibro-genesis on nanostructured substratesEnviron Sci Nano20174368969928944063
- DanteSPetrelliAPetriniEMSelective targeting of neurons with inorganic nanoparticles: revealing the crucial role of nanoparticle surface chargeACS Nano20171176630664028595006
- GuerzoniLPNicolasVAngelovaAIn vitro modulation of TrkB receptor signaling upon sequential delivery of curcumin-DHA loaded carriers towards promoting neuronal survivalPharm Res201734249250527995523
- SunBTaingALiuHNerve growth factor-conjugated mesoporous silica nanoparticles promote neuron-like PC12 cell proliferation and neurite growthJ Nanosci Nanotechnol20161632390239327455646
- FrancaEJaoPFFangSPScale of carbon nanomaterials affects neural outgrowth and adhesionIEEE Trans Nanobioscience2016151111826829799
- DingSBaoYLinYNeuroprotective effect of functionalized multi-walled carbon nanotubes on spinal cord injury in ratsInt J Clin Exp Pathol2015812157691577726884846
- SinghNChenJKoziolKKChitin and carbon nanotube composites as biocompatible scaffolds for neuron growthNanoscale20168158288829927031428
- SinghAKimWKimYMultifunctional photonics nanoparticles for crossing the blood-brain barrier and effecting optically trackable brain theranosticsAdv Funct Mater201626397057706629081729
- GholamineBKarimiISalimiAMazdaraniPBeckerLANeurobehavioral toxicity of carbon nanotubes in miceToxicol Ind Health201733434035027230352
- WeiMLiSYangZZhengWLeWGold nanoparticles enhance the differentiation of embryonic stem cells into dopaminergic neurons via mTOR/p70S6K pathwayNanomedicine (Lond)201712111305131728520507
- HsiaoILChangCCWuCYIndirect effects of TiO2 nanoparticle on neuron-glial cell interactionsChem Biol Interact2016254344427216632
- LinRLiYMacDonaldTImproving sensitivity and specificity of capturing and detecting targeted cancer cells with anti-biofouling polymer coated magnetic iron oxide nanoparticlesColloids Surf B Biointerfaces201715026127028029547
- SeemayerNHHadnagyWTomingasTEvaluation of health risks by airborne particulates from in vitro cyto- and genotoxicity testing on human and rodent tissue culture cells: a longitudinal study from 1975 until nowJ Aerosol Sci199021Suppl 1S501S504
- SeatonATranLAitkenRDonaldsonKNanoparticles, human health hazard and regulationJ R Soc Interface20107S119S12919726441
- WangSGuanSXuJNeural stem cell proliferation and differentiation in the conductive PEDOT-HA/Cs/Gel scaffold for neural tissue engineeringBiomater Sci20175102024203428894864
- WangKHuangQQiuFSuiMNon-viral delivery systems for the application in p53 cancer gene therapyCurr Med Chem201522354118413626423086
- SeverMTurkyilmazMSevincCRegenerative effects of peptide nanofibers in an experimental model of Parkinson’s diseaseActa Biomater201646799027619838
- BrookingJDavisSSIllumLTransport of nanoparticles across the rat nasal mucosaJ Drug Targeting20019267279
- SharmaHSHussainSSchlagerJAliSFSharmaAInfluence of nanoparticles on blood-brain barrier permeability and brain edema formation in ratsActa Neurochir Suppl201010635936419812977
- TangJXiongLWangSDistribution, translocation and accumulation of silver nanoparticles in ratsJ Nanosci Nanotechnol200994924493219928170
- JakobssonAOttossonMZalisMCO’CarrollDJohanssonUEJohanssonFThree-dimensional functional human neuronal networks in uncompressed low-density electrospunfiber scaffoldsNanomedicine20171341563157328064005
- MarinoATonda-TuroCDe PasqualeDmGelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regenerationBiochim Biophys Acta20171861238639527864151
- XiaHSunXLiuDZhouYZhongDOriented growth of rat Schwann cells on aligned electrospun poly(methyl methacrylate) nanofibersJ Neurol Sci2016369889527653871
- BernsEJÁlvarezZGoldbergerJEA tenascin-C mimetic peptide amphiphile nanofiber gel promotes neurite outgrowth and cell migration of neurosphere-derived cellsActa Biomater201637505827063496
- FengZVChenWSKeratithamkulKDegradation of the electrospun silica nanofiber in a biological medium for primary hippocampal neuron–effect of surface modificationInt J Nanomedicine20161172974127013873
- HackelbergSTuckSJHeLNanofibrous scaffolds for the guidance of stem cell-derived neurons for auditory nerve regenerationPLoS One2017127e018042728672008
- HaddadTNoelSLiberelleBEl AyoubiRAjjiADe CrescenzoGFabrication and surface modification of poly lactic acid (PLA) scaffolds with epidermal growth factor for neural tissue engineeringBiomatter201661e123127627740881
- HuangDLinCWenXGuSZhaoPA potential nanofiber membrane device for filling surgical residual cavity to prevent glioma recurrence and improve local neural tissue reconstructionPLoS One2016118e016143527548322
- StrużyńskaLSkalskaJMechanisms underlying neurotoxicity of silver nanoparticlesAdv Exp Med Biol2018104822725029453542
- MiliBDasKKumarAPreparation of NGF encapsulated chitosan nanoparticles and its evaluation on neuronal differentiation potentiality of canine mesenchymal stem cellsJ Mater Sci Mater Med2017291429204722
- ChenLWatsonCMorschMImproving the delivery of SOD1 antisense oligonucleotides to motor neurons using calcium phosphate-lipid nanoparticlesFront Neurosci20171147628912673
- MarkoutsaEXuPRedox potential-sensitive N-acetyl cysteine-prodrug nanoparticles inhibit the activation of microglia and improve neuronal survivalMol Pharm20171451591160028335600
- SatishAKorrapatiPSTailored release of triiodothyronine and retinoic acid from a spatio-temporally fabricated nanofiber composite instigating neuronal differentiationNanoscale2017938145651458028932862
- WongALiuQGriffinSNichollsARegalbutoJRSynthesis of ultrasmall, homogeneously alloyed, bimetallic nanoparticles on silica supportsScience201735863691427143029170281
- KolarcikCLCattKRostEEvaluation of poly(3,4-ethylenedioxythiophene)/carbon nanotube neural electrode coatings for stimulation in the dorsal root ganglionJ Neural Eng201512101600825485675
- ChenDYangDDoughertyCIn vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal-organic frameworks nanomaterialsACS Nano2017114315432728345871
- BorisovaTNazarovaADekaliukMNeuromodulatory properties of fluorescent carbon dots: effect on exocytotic release, uptake and ambient level of glutamate and GABA in brain nerve terminalsInt J Biochem Cell Biol20155920321525486182
- KuoYCChenCWNeuroregeneration of induced pluripotent stem cells in polyacrylamide-chitosan inverted colloidal crystal scaffolds with poly(lactide-co-glycolide) nanoparticles and transactivator of transcription von Hippel-Lindau peptideTissue Eng Part A2017237–826327428107800
- SaraivaCFerreiraLBernardinoLTraceable microRNA-124 loaded nanoparticles as a new promising therapeutic tool for Parkinson’s diseaseNeurogenesis (Austin)201631e125685528405588
- Rodriguez-LosadaNRomeroPEstivill-TorrúsGGuzmán de VilloriaRAguirreJACell survival and differentiation with nanocrystalline glass-like carbon using substantia nigra dopaminergic cells derived from transgenic mouse embryosPLoS One2017123e017397828334019
- FernandesARChariDMPart II: functional delivery of a neurotherapeutic gene to neural stem cells using minicircle DNA and nanoparticles: translational advantages for regenerative neurologyJ Control Release201623830031027369863
- GenchiGGCeseracciuLMarinoAP(VDF-TrFE)/BaTiO3 nanoparticle composite films mediate piezoelectric stimulation and promote differentiation of SH-SY5Y neuroblastoma cellsAdv Healthc Mater20165141808182027283784
- SchulteCRipamontiMMaffioliEScale invariant disordered nanotopography promotes hippocampal neuron development and maturation with involvement of mechanotransductive pathwaysFront Cell Neurosci20161026727917111
- SantosTFerreiraRQuartinEBlue light potentiates neurogenesis induced by retinoic acid-loaded responsive nanoparticlesActa Biomater20175929330228673742
- ZhaoYJiangYLvWDual targeted nanocarrier for brain ischemic stroke treatmentJ Control Release2016233647127142584
- SunYLiWWuXFunctional self-assembling peptide nanofiber hydrogels designed for nerve degenerationACS Appl Mater Interfaces2016832348235926720334
- KimEYooSJKimENano-patterned SU-8 surface using nanosphere-lithography for enhanced neuronal cell growthNanotechnology2016271717530326984937
- SongLWangKLiYYangYNanotopography promoted neuronal differentiation of human induced pluripotent stem cellsColloids Surf B Biointerfaces2016148495827591570
- SaraivaSMCastro-LópezVPañedaCAlonsoMJSynthetic nanocarriers for the delivery of polynucleotides to the eyeEur J Pharm Sci201710351828263915
- MdzinarishviliASutariyaVTalasilaPKGeldenhuysWJSadanaPEngineering triiodothyronine (T3) nanoparticle for use in ischemic brain strokeDrug Deliv Transl Res20133430931723864999
- FerreiraRFonsecaMCSantosTRetinoic acid-loaded polymeric nanoparticles enhance vascular regulation of neural stem cell survival and differentiation after ischaemiaNanoscale20168158126813727025400
- GligaAREdoffKCaputoFCerium oxide nanoparticles inhibit differentiation of neural stem cellsSci Rep201771928428839176
- AryaAGangwarASinghSKCerium oxide nanoparticles promote neurogenesis and abrogate hypoxia-induced memory impairment through AMPK-PKC-CBP signaling cascadeInt J Nanomedicine2016111159117327069362
- DucrayADStojiljkovicAMöllerAUptake of silica nanoparticles in the brain and effects on neuronal differentiation using different in vitro modelsNanomedicine20171331195120427871963
- ZhangRLiYHuBLuZZhangJZhangXTraceable nanoparticle delivery of small interfering RNA and retinoic acid with temporally release ability to control neural stem cell differentiation for Alzheimer’s disease therapyAdv Mater201628306345635227168033
- DucrayADFelserAZielinskiJEffects of silica nanoparticle exposure on mitochondrial function during neuronal differentiationJ Nanobiotechnology20171514928676089
- GuoJFilpponenIJohanssonLSComplexes of magnetic nanoparticles with cellulose nanocrystals as regenerable, highly efficient, and selective platform for protein separationBiomacromolecules20171889890528199100
- PampaloniNPScainiDPerissinottoFBosiSPratoMBalleriniLSculpting neurotransmission during synaptic development by 2D nanostructured interfacesNanomedicine Epub2017525
- HuFZhangXLiuHNeuronally differentiated adipose-derived stem cells and aligned PHBV nanofiber nerve scaffolds promote sciatic nerve regenerationBiochem Biophys Res Commun2017489217117828549587
- OrlandoACazzanigaETringaliMMesoporous silica nanoparticles trigger mitophagy in endothelial cells and perturb neuronal network activity in a size- and time-dependent mannerInt J Nanomedicine2017123547355928507435
- TomaMBeluAMayerDOffenhäusserAFlexible gold nanocone array surfaces as a tool for regulating neuronal behaviorSmall20171324111
- ChungCYLinMHLeeINLeeTHLeeMHYangJTBrain-derived neurotrophic factor loaded PS80 PBCA nanocarrier for in vitro neural differentiation of mouse induced pluripotent stem cellsInt J Mol Sci2017183E66328335495
- MarcianesPNegroSGarcía-GarcíaLMontejoCBarciaEFernández-CarballidoASurface-modified gatifloxacin nanoparticles with potential for treating central nervous system tuberculosisInt J Nanomedicine2017121959196828331318
- Espadas-AlvarezAJBannonMJOrozco-BarriosCERegulation of human GDNF gene expression in nigral dopaminergic neurons using a new doxycycline-regulated NTS-polyplex nanoparticle systemNanomedicine20171341363137528219741
- LeeSJZhuWHeyburnLNowickiMHarrisBZhangLGDevelopment of novel 3-D printed scaffolds with core-shell nanoparticles for nerve regenerationIEEE Trans Biomed Eng201764240841828113194
- KuoYCRajeshRNerve growth factor-loaded heparinized cationic solid lipid nanoparticles for regulating membrane charge of induced pluripotent stem cells during differentiationMater Sci Eng C Mater Biol Appl20177768068928532079
- NiuSZhangLKZhangLInhibition by multifunctional magnetic nanoparticles loaded with alpha-synuclein RNAi plasmid in a Parkinson’s disease modelTheranostics20177234435628042339
- Aydemir SezerUOzturkKAruBYanıkkayaDemirelGSezerSBozkurtMRZero valent zinc nanoparticles promote neuroglial cell proliferation: a biodegradable and conductive filler candidate for nerve regenerationJ Mater Sci Mater Med20172811928012153
- DefteralıÇVerdejoRMajeedSIn vitro evaluation of biocompatibility of uncoated thermally reduced graphene and carbon nanotube-loaded PVDF membranes with adult neural stem cell-derived neurons and gliaFront Bioeng Biotechnol201649427999773
- KandalamSSindjiLDelcroixGJPharmacologically active microcarriers delivering BDNF within a hydrogel: novel strategy for human bone marrow-derived stem cells neural/neuronal differentiation guidance and therapeutic secretome enhancementActa Biomater20174916718027865962
- SuaratoGLeeSILiWMicellar nanocomplexes for biomagnetic delivery of intracellular proteins to dictate axon formation during neuronal developmentBiomaterials201711217619127768972
- ZennaroCRastaldiMPBakeineGJNanoporous surface is essential for glomerular podocyte differentiation in three-dimensional cultureInt J Nanomedicine2016114957497327757030
- PapadimitriouSARobinMPCericDO’ReillyRKMarinoSResminiMFluorescent polymeric nanovehicles for neural stem cell modulationNanoscale2016839173401734927722391
- XiaoSZhouDLuanPGraphene quantum dots conjugated neuroprotective peptide improve learning and memory capabilityBiomaterials20161069811027552320
- HuangKDelportGOrcin-ChaixLDrummondCLauretJSPenicaudASingle layer nano graphene platelets derived from graphite nanofibresNanoscale20168168810881827065439
- GällentoftLPetterssonLMDanielsenNSchouenborgJPrinzCNLinsmeierCEImpact of degradable nanowires on long-term brain tissue responsesJ Nanobiotechnology20161416427507159
- HsiaoILHsiehYKChuangCYWangCFHuangYJEffects of silver nanoparticles on the interactions of neuron- and glia-like cells: toxicity, uptake mechanisms, and lysosomal trackingEnviron Toxicol20173261742175328181394
- ShiXXiaoYXiaoHHarrisGWangTCheJTopographic guidance based on microgrooved electroactive composite films for neural interfaceColloids Surf B Biointerfaces201614576877627295493
- VedagiriAThangarajanSMitigating effect of chrysin loaded solid lipid nanoparticles against Amyloid β25–35 induced oxidative stress in rat hippocampal region: an efficient formulation approach for Alzheimer’s diseaseNeuropeptides20165811112527021394
- TavakolSMousaviSMMTavakolBHoveiziEAiJSorkhabadiSMRMechano-transduction signals derived from self-assembling peptide nanofibers containing long motif of laminin influence neurogenesis in in-vitro and in-vivoMol Neurobiol20175442483249626984600
- HanafyASFaridRMHelmyMWElGamalSSPharmacological, toxicological and neuronal localization assessment of galantamine/chitosan complex nanoparticles in rats: future potential contribution in Alzheimer’s disease managementDrug Deliv20162383111312226942549
- HesariZSoleimaniMAtyabiFA hybrid microfluidic system for regulation of neural differentiation in induced pluripotent stem cellsJ Biomed Mater Res A201610461534154326914600
- YadavSGandhamSKPanicucciRAmijiMMIntranasal brain delivery of cationic nanoemulsion-encapsulated TNFα siRNA in prevention of experimental neuroinflammationNanomedicine2016124987100226767514
- LiPXuTWuSLeiLHeDChronic exposure to graphene-based nanomaterials induces behavioral deficits and neural damage in Caenorhabditis elegansJ Appl Toxicol201737101140115028418071
- SulejczakDTaraszewskaAChrapustaSJDziewulskaDNakielskiPRafałowskaJNanofiber mat spinal cord dressing-released glutamate impairs blood-spinal cord barrierFolia Neuropathol201654439240428139821
- MareiHEElnegiryAAZaghloulANanotubes impregnated human olfactory bulb neural stem cells promote neuronal differentiation in Trimethyltin-induced neurodegeneration rat modelJ Cell Physiol2017232123586359728121007
- MashockMJZanonTKappellADPetrellaLNAndersenECHristovaKRCopper oxide nanoparticles impact several toxicological endpoints and cause neurodegeneration in Caenorhabditis elegansPLoS One20161112e016761327911941
- CocciniTCaloniFRamírez CandoLJDe SimoneUCytotoxicity and proliferative capacity impairment induced on human brain cell cultures after short- and long-term exposure to magnetite nanoparticlesJ Appl Toxicol201737336137327480414
- WuTHeKAngSImpairments of spatial learning and memory following intrahippocampal injection in rats of 3-mercaptopropionic acid-modified CdTe quantum dots and molecular mechanismsInt J Nanomedicine2016112737275527358562
- LatronicoTDepaloNValenteGCytotoxicity study on luminescent nanocrystals containing phospholipid micelles in primary cultures of rat astrocytesPLoS One2016114e015345127097043
- JeonYMLeeMYAirborne nanoparticles induce autophagic cell death of human neuronal cellsJ Appl Toxicol201636101332134227080386