Abstract
In recent years, the supercritical fluid (SCF) technology has attracted enormous interest from researchers over the traditional pharmaceutical manufacturing strategies due to the environmentally benign nature and economically promising character of SCFs. Among all the SCF-assisted processes for particle formation, the solution-enhanced dispersion by supercritical fluids (SEDS) process is perhaps one of the most efficient methods to fabricate the biomaterials and pharmaceutical compounds at an arbitrary gauge, ranging from micro- to nanoscale. The resultant miniature-sized particles from the SEDS process offer enhanced features concerning their physical attributes such as bioavailability enhancement due to their high surface area. First, we provide a brief description of SCFs and their behavior as an anti-solvent in SCF-assisted processing. Then, we aim to give a brief overview of the SEDS process as well as its modified prototypes, highlighting the pros and cons of the particular modification. We then emphasize the effects of various processing constraints such as temperature, pressure, SCF as well as organic solvents (if used) and their flow rates, and the concentration of drug/polymer, among others, on particle formation with respect to the particle size distribution, precipitation yield, and morphologic attributes. Next, we aim to systematically discuss the application of the SEDS technique in producing therapeutic nano-sized formulations by operating the drugs alone or in combination with the biodegradable polymers for the application focusing oral, pulmonary, and transdermal as well as implantable delivery with a set of examples. We finally summarize with perspectives.
Introduction
Recently, the supercritical fluid (SCF) technology has garnered enormous attention from researchers in numerous fields such as pharmaceutical and food as well as nutraceutical industries intended for various applications.Citation1–Citation3 This high-pressure technology has been widely adopted in the past few decades for obtaining products due to the environmentally benign nature and economically promising character of SCFs.Citation4 Despite their success in particle formation during the processing of pharmaceutical actives, various traditional methods (milling, freeze drying, and spraying) still suffer from several limitations such as formulation instability, broad particle size distribution, and low drug loading efficiency, among others.Citation5 In some cases, the processing of particle formation is extended to obtain uniform size distribution by subsequent milling and sieving, which often result in the damage of sensitive biomolecules due to high shear forces.Citation6,Citation7 In addition, most of these processes usually rely on the utilization of large amounts of organic solvents, which result in product damage, toxicity, inflammability, and biocompatibility issues, among others.Citation8 To this end, SCFs take advantage of the benign solvents, that is, CO2 and water, to circumvent the problems associated with the conventional approaches for precipitation of drugs either alone or in combination with the biodegradable polymers.Citation9 Therefore, these SCFs act as an effective substitute for organic solvents in fabricating the pharmaceutical products.Citation4,Citation6
More often, SCFs as benign solvents offer considerable interest in pharmaceutical manufacturing processes because of their solvating power in separating the components and momentous changes in their physicochemical properties beyond the critical point.Citation10 Moreover, other benefits of SCFs include solubilizing ability and ease of recycling, among others. The physical properties of SCFs (namely, density, viscosity, solvency, and diffusivity) that exist amid both liquid and gas can be easily altered by adjusting the critical conditions (pressure and temperature) during the processing of solutes.Citation8,Citation9,Citation11,Citation12 In this framework, other SCFs such as water and solvents such as CO2/ethanol mixture, acetone, nitrous oxide, propane, diethyl ether, trifluoromethane, and chlorodifluoromethane are operated at their corresponding super-critical conditions.Citation10,Citation12–Citation18 The distinctive properties of these SCFs, including the critical parameters and other attributes such as solubility, have been already reported elsewhere.Citation19 Among all the SCFs available, supercritical CO2 (SC-CO2) has attracted enormous interest from researchers due to its wide adaptability, safety, cost-effectiveness, and requiring mild conditions for operation (temperature 304 K/31.1°C and pressure 7.38 MPa/73.8 bar) under ambient circumstances.Citation10 Moreover, it should be noted that SC-CO2 is recognized as safe by the United States Food and Drug Administration in pharmaceutical manufacturingCitation20 as it is nonreactive, nontoxic, non-polluting, innocuous, and non-flammable.Citation9,Citation10 It also offers several advantages which are highly favorable for particle fabrication, such as high volatility, low cohesive energy density, and low polarizability per unit volume, among others.Citation12 Moreover, the unique physical properties of SC-CO2, such as density, diffusivity solvency, and viscosity, can be manipulated beyond its critical point by adjusting the temperature and pressure.Citation10
Increasing demand for particle fabrication of various active pharmaceutical ingredients (APIs) and investigations on their crystalline morphologiesCitation21 combined with the aim of overcoming the limitations of currently available traditional methods (ie, particle destruction as well as impairment of bioactivity by strong shear forces, varied particle size distribution, and others),Citation5 have garnered enormous attention of researchers toward the SCF technology. This technology is perhaps one of the rapidly evolving processes with many variants since its first report in 1879.Citation22 Different variants of the SCF technology for particle fabrication are categorized based on the behavior of SCF, such as “solvent” (rapid expansion of supercritical solutions),Citation23 “solute” (particle formation from gas-saturated solutions),Citation24 “anti-solvent” (supercritical anti-solvent [SAS],Citation25 and its variantsCitation15,Citation26–Citation31), “reagent”,Citation32 and others such as supercritical-assisted atomization processCitation10,Citation33,Citation34 and depressurization of an expanded liquid organic solution.Citation15,Citation35 Despite the variance in the behavior, SCF acts as a re-precipitation aid for rapid, uniform, as well as smooth nucleation of solutes (drug and/or polymer) in all the above-mentioned processes intended for particle fabrication.Citation10 Moreover, the performance efficacy of these processes utterly depends on the selection of an appropriate solvent and fine-tuning of the critical parameters.Citation22
SAS process
The SAS precipitation process has attracted widespread attention due to its distinctive advantages and ability to circumvent the processing limitations of conventional particle fabrication methods and other SCF processes such as rapid expansion of supercritical solutions.Citation10,Citation36 As SC-CO2 is a relatively poor solvent for most of the pharmaceutical compounds and high-molecular-weight polymers under mild conditions (temperature <100°C, pressure <350 bar) and the utilization of co-solvents became impractical in their solubility improvement, the anti-solvent or non-solvent properties of it have been explored.Citation12 This process is highly suitable to fabricate the solutes that are practically insoluble in SCFs. The name SAS was coined based on the behavior of SCF, where it acts as an anti-solvent with the solute (API/polymer).Citation9 Bleich et al applied the SCF as an anti-solvent for the first time and encapsulated hyoscine butyl bromide into the poly(L-lactic acid) (PLLA) microparticles via co-precipitation using a mixture of solvents (methanol and dichloromethane [DCM]).Citation37 In the past two decades, tremendous progress has been evidenced by the advancements of SAS for fabricating the polymer-based micro- or nano-sized composites.
The particle fabrication via SAS process involves the following sequential stages. Initially, the mixing of dispersed multiphase systems leads to the nucleation of solute, after attaining the supersaturation state. The surrounding molecules of the solute in the medium then start accumulating over the nucleus, and the crystal growth ultimately ends with agglomeration. As the nucleation phenomena are very rapid in this process, the mixing phase is exceptionally crucial, which defines the particle characteristics with respect to morphologic attributes and particle size distribution of the end product.Citation1 The mechanism lying behind particle formation is that the blend of solvent, SCF, and solute expands to the supersaturation state and results in rapid nucleation, indicating the high mass transfer between the SCF and the solvent carrying the solute/API due to the high diffusivity and low viscosity of SCF.Citation9,Citation22 Furthermore, the high diffusivity of SCF sequentially controls the phenomena of particle precipitation by initially increasing the volume of the solvent, which decreases its density and thereby declines the solvating power and eventually leads to the precipitation of the solute.Citation9 Herein, the differences in densities of the solvent and SCF act as a potent driving force in the nucleation of solute. Subsequently, high supersaturation and smaller particle size can be achieved by rapid mixing of SCF–solution mixture.Citation9 Moreover, the yield of this process majorly depends on the order of addition of SCF, solvent, and other substrates if used.Citation10 In addition, various experimental attributes such as the critical conditions (ie, pressure and temperature), the chemical composition of solutes (polymer and/or APIs), and the type of organic solvent are required to be systematically optimized, which have been already discussed elsewhere.Citation9,Citation10
Among all the SCF-based processes for particle fabrication, the SAS process has become the most efficient and highly advantageous approach in producing micro- and nano-sized constructs with desired morphologies.Citation10 This process often results in the formation of small-sized particles that yield a high specific surface area, which improves the release or solubility of APIs by improved mass transfer rates between the particle and the surrounding medium.Citation38 In addition, this process has gained enormous importance due to high drug loading efficiency and rapid precipitation of solutes. More often, the process of particle fabrication is carried out at the ambient circumstances, which are highly suitable to process thermosensitive biomolecules such as genes and proteins. Moreover, this process is highly adaptable in performing continuous operations and due to the ease of scale-up for fine particle production. Other processes that work similar to the SAS technique include precipitation with compressed anti-solventCitation26 and gaseous anti-solvent.Citation27 These methods comparatively have less operational problems than traditional SAS process; moreover, it is easy to practice these particle fabrication strategies at the industrial scale.Citation10,Citation27 Despite the significant advantages, the SAS process still possesses some disadvantages such as larger-sized droplets at the tip of the nozzle and extended washing period, leading to particle aggregation.Citation9,Citation25,Citation39,Citation40 However, this can be minimized by enhancing the mixing of solution using a vibrating ultrasonic processor to break/atomize the solution jet at high turbulence into tiny droplets in the sub-micron range.Citation9 In addition, further advancements have been made to improve the micronization of SCF-insoluble solutes.Citation10 Various modifications have been made in the SAS process to improve its functional attributes. The modified SAS processes include aerosol solvent extraction system,Citation15 supercritical anti-solvent with enhanced mass transfer,Citation31 supercritical fluid-assisted extraction of emulsions,Citation41 and solution-enhanced dispersion by supercritical fluids (SEDS).Citation28–Citation30 Among all these SAS processes available, SEDS is the most advanced anti-solvent process for particle precipitation. In this review, we provide a comprehensive overview of this process based on the literature published in the past two decades related to drug delivery through various routes of administration. In addition, this review highlights the effects of critical parameters on the particle size, providing a vision for the future of SCF technology.
SEDS process
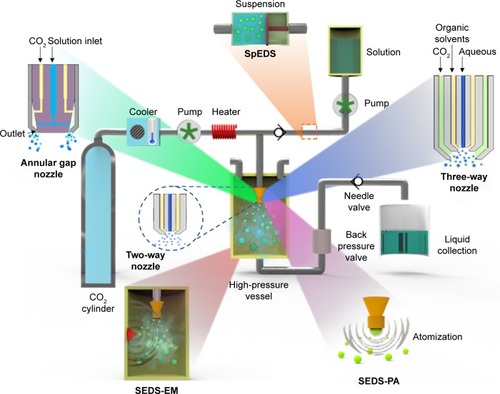
The SEDS process was developed by York and Hanna of Bradford University in the year 1996 to improve the performance efficiency of the traditional SAS process.Citation42 On the other hand, this advanced process also minimizes the operating limitations of the aerosol solvent extraction system process and other SAS processes.Citation10 The SEDS process is usually operated at a lesser drying time with increased mass transfer rates that are significantly different from the conventional SAS process in achieving miniature-sized droplets.Citation14,Citation42,Citation43 The chief aim of the SEDS process is to produce uniform-sized fine particles in a single-phase equilibrium, while removing the organic solvent to deliver them in a dried form. Typically, the components (API and/or excipients) are initially dissolved in a suitable organic solvent by vigorous mixing, and then they are rapidly sprayed along with SCF through a specially designed coaxial nozzle into a high-pressure vessel. The mixture yields small-sized droplets due to jet breakup at the tip of the nozzle, subsequently resulting in the fine particles. Herewith, the nozzle as well as its specifications (predominantly the inner diameters) play a crucial role in the eventual particle size distribution. In addition, various processing conditions such as the flow rates of both SC-CO2 and the drug solution and critical parameters should be optimized to facilitate better control over the particle size and morphology ().Citation38 During the co-precipitation of drug and polymer composite particles in this process, the components are required to be insoluble in SCF and they should possess mutual compatibility as well as excellent thermodynamic properties. Otherwise, it leads to a severe consequence of phase separation resulting in individual particles of drugs and polymers rather than drug–polymer composites.
Figure 1 Conceptual representation of the SEDS process and its various modifications.
Abbreviations: SEDS, solution-enhanced dispersion by supercritical fluids; SEDS-EM, solution-enhanced dispersion by supercritical fluids with enhanced mass transfer; SEDS-PA, solution-enhanced dispersion by supercritical fluids – prefilming atomization; SpEDS, suspension-enhanced dispersion by supercritical fluids.
As mentioned earlier, the particle formation in an anti-solvent process utterly depends on the mass transfer ratio between SCF and the droplet-containing solute, subsequently the rate of solvent transfer into the SCF phase.Citation38,Citation44 Thus, the mass transfer at a high rate allows spontaneous nucleation and results in small-sized particles with no chance of agglomeration.Citation28,Citation38,Citation44,Citation45 To demonstrate these facts, two possible mechanisms have been proposed, such as the drop dispersion followed by the mass transfer between SCF and droplet and the micro-mixing of the solvent with SCF.Citation1,Citation46 However, the miscibility between SCF and the solvent predominantly determines the type of mechanism involved during the particle formation.Citation1
Among all the SAS-based particle fabrication techniques available, the SEDS process holds numerous advantages such as production of ultrafine particles with a narrow and uniform particle size distribution enhancing the dissolution rates of the APIs, high yields of polymer-based micro- and nano-sized composites, minimal agglomeration of particles, acceptable limits of residual solvents when operated at a reduced drying time, and ease of polymer coating over various particulate forms of APIs resulting in core–shell composites with sustained drug release ability, among others.Citation47–Citation50 Moreover, this process is highly suitable for operating the water-soluble compounds by spraying the aqueous solutions along with the organic solvent separately through a coaxial three-compartment nozzle.Citation10,Citation51–Citation55 However, there still exist some problems such as small processing capacity and easy blockage of the nozzle. In addition, the minor disadvantage of this process is that the SCF is completely miscible with the solution feed above the critical pressure (exists as a single phase),Citation1 which may result in a broad particle size distribution during scale-up.Citation46
Modified SEDS processes
One of the critical steps of particle fabrication by SEDS is the formation of smaller-sized droplets at the tip of the nozzle.Citation56 Herewith, the nozzle and its specifications play a critical role during SEDS processing, which can be altered to adjust the jet breakup for fine particle formation. In this vein, various designs of atomizer have been developed, such as coaxial nozzles, internal twin-fluid mixing nozzles, four-pinhole nozzle, and an annular gap nozzle.Citation55,Citation57 In addition, tremendous progress has been evidenced by the advancements of SEDS in enhancing the efficiency of particle formation and overcoming the processing damage issues. Other variants () of the SEDS process include solution-enhanced dispersion by supercritical fluids – prefilming atomization, solution-enhanced dispersion by supercritical fluids with enhanced mass transfer, and suspension-enhanced dispersion by supercritical fluids (SpEDS).
Two-channel nozzle
In the SEDS process, the two-way coaxial nozzle is commonly used to spray the SCF and the solution feed containing the active substrate separately into the high-pressure vessel under controlled critical conditions ().Citation55 During particle formation, the turbulent flow of SCF allows the solution containing the substrates to split rapidly into tiny droplets of partly mixed and highly supersaturated solution that are sprayed into the high-pressure vessel as a jet. The nucleation of solute then starts at the tip of the two-way nozzle chamber, and subsequently, the particle growth ends the process in the precipitation vessel.Citation52,Citation55
Three-channel nozzle
A coaxial three-channel system can be applied for the micronization/nanonization of poorly water-soluble drugs or drug–polymer composites. This system facilitates the flow of three solvents (SC-CO2, aqueous drug solution, and the polymer dissolved in an organic solvent) distinctly into the precipitation vessel to overcome the compatibility issues ().Citation58 In general, the mixture of drug and polymer dissolved in the same solvent (such as acetone, DCM, or a mixture of solvents) is introduced through a two-way channel-assisted spraying method, which leads to the risk of organic solvents damaging the biomolecules. To overcome this limitation, a multi-channel nozzle system is used to spray the sensitive biomolecules (mostly peptides, proteins, and genes) separately during the SEDS processing. However, it should be noted that the geometric dimensions of the nozzle play a crucial role in influencing the morphology of the micro-/nanoparticles in the three-channel nozzle system.Citation58 In one case, Zhang et al fabricated microparticles by using the three-channel nozzle system with different inner diameters in the range of 50–2,000 μm and their ends on the same plane.Citation58 The inner diameters of the different channels in the nozzle had played a crucial role in the size of the initially formed droplets and mass diffusion between the solution containing the APIs and SC-CO2.Citation59 However, in some instances, previous reports have indicated that the initial droplet size had no significant effect on the eventual sizes of the particles.Citation60
Annular gap nozzle
The other interesting type of nozzle in SEDS processing is the annular gap nozzle. This nozzle system has been explicitly designed to enhance the surface area of exposure of the solution with SC-CO2 (). Herein, SC-CO2 and the drug/polymer solution that are introduced separately through different channels are mixed in the annular gap of the nozzle, and they are then sprayed rapidly into the high-pressure vessel to augment the mass transfer rates between the SCF and the solution feed containing the active substrate. Moreover, this approach is highly advantageous over others because the distance within the annular gap of the nozzle can be adjusted based on the particle size requirement.Citation57
Solution-enhanced dispersion by supercritical fluids – prefilming atomization
This process is different from the conventional SEDS process as it utilizes a specialized prefilming twin-fluid atomizer that increases the mass transfer rate between the SCF and the solution feed (). The principle lying behind particle formation is that the fluid intended for atomization is driven along the coaxial annular passage within the nozzle as thin swirl liquid film sheets,Citation61 which separate after interacting with the dense gas at the tip and result in fine droplets. Further, the mixing of both the liquids, that is, the SCF and the solution feed, is intensified for enhancement of mass transfer rates, which eventually result in fine particles.Citation61–Citation63 Previous reports have also indicated that the existence of atomizing air in the nozzle chamber might result in a faster reduction in the breakup length, and this became even stronger when swirling was imparted to the atomizing air.Citation61,Citation64
Solution-enhanced dispersion by supercritical fluids with enhanced mass transfer
The solution-enhanced dispersion by supercritical fluids with enhanced mass transfer process is an enhanced mass transfer precipitation technique that was developed by combining the conventional SEDS process with the auxiliary ultrasonication setup (). The operation of this process is similar to that of the conventional SEDS process, but the mixture of SCF and the solution feed is sprayed onto the vibrating surface at an ultrasonic frequency, which augments the mass transfer between the solution and the SCF, thus resulting in a significant reduction of the particle size approximately by several folds.Citation65 In particular, the size of the droplets in the ultrasonic field is reduced with an increase in the ultrasound energy input, which subsequently improves the mass transfer rate and mixing between the droplets and SC-CO2.Citation65 Hence, this phenomenon demonstrates that the attached ultrasound transducer can efficiently control the particle size.
Suspension-enhanced dispersion by supercritical fluids
SpEDS is one of the most advanced processes that have been developed for broadening the application of SEDS and overcoming its processing damage issues.Citation29 The device setup of this process is almost similar to SEDS, but SpEDS has an auxiliary injector equipped with a piston to efficiently pump the loaded suspension of the drug or the drug–polymer mixture ().Citation29,Citation30 The use of injector setup in this process is highly advantageous as it avoids obstruction of the one-way valve and damage of the high-pressure pump during pumping of the particle suspension into the high-pressure vessel.Citation29,Citation66,Citation67 This process predominantly results in core–shell structured drug–polymer composites at an arbitrary size ranging from microscale to nanoscale with high drug encapsulation efficiency. Moreover, these core–shell nanocomposites offer a significant advantage of sustained drug release from the core (discussed under the “Applications” section).
Effects of various processing parameters on particle size
The SEDS process is often preferred to precipitate particles at an arbitrary size ranging from micro- to nanometer scale with uniform size distribution and smooth surfaces. These fine-sized particles will eventually augment the bioavailability of most of the APIs due to their high surface area. More often, this process is used to operate poorly water-soluble drugs/biodegradable polymers that are aimed for the controlled release of drugs. To achieve this goal, the processing parameters of SEDS must be optimized with good care for fine particle formation, such as suitable operating conditions, appropriate selection of suitable polymers based on their solubility, and selection of the proper solvent. Here-with, we emphasize the effect of various operating parameters of the SEDS process, such as critical conditions (pressure and temperature), solute (drug or polymer) and solution concentration, a type of organic solvent as well as SCF, flow rates of SCF and solutions, and others such as nozzle diameter, atomization frequency, and humidity, on the size of the particle and its morphologic attributes.
Critical conditions
The critical parameters that play a crucial role during operation of the SCF technology are pressure and temperature. These parameters are the predominant deciding factors that indicate the state of existence of any supercritical solvent. Herewith, the solvents above the critical conditions exist as a single phase, where their physical properties are intermediate between liquid and gas and can be easily manipulated by adjusting the critical conditions. This adjustment is beneficial where they could be altered during the processing of pharmaceutical additives for attaining better yields of the product. However, it should be noted that the variations in these conditions often show significant influence on not only the disparity in physical properties of the SCFs but also the morphologic and structural attributes of the formulation.
Pressure
In general, the SCF-assisted processing at increased pressure, that is, above the critical point, often results in a significant reduction of the particle size to the extent of nano-size range due to volumetric expansion of the solvent and its solubility improvement in the SCF. Contrariwise, it results in an irregular morphology below the critical point of SCF. In addition, the increase in pressure augments the diffusion of SCF into the solvent, ensuing supersaturation that subsequently results in small-sized particles.Citation68–Citation70 However, the changes in the pressure had no significant influence on the biological activity of the sensitive molecules such as enzymes.Citation71
Temperature
Temperature is another critical parameter that plays a very crucial role in the particle fabrication processes. The SEDS process is generally operated at around 40°C, where no significant effects are observed on the particle. However, the solute merely experiences reduction in its density with a rise in temperature, which subsequently reduces its solubility in the organic solvent. These consequences result in slower supersaturation and longer growth time, yielding the large-sized particles.Citation72 Herewith, the balance of the growth time and nucleation speed plays a vital role in deciding the eventual particle size of the product.
Similar to pressure, changes in temperature also result in variations in the morphologic and structural attributes of the particles. In some instances, an increase in temperature results in severe aggregation of the particles with irregular surfaces due to collisions among the microparticles and vice versa.Citation68–Citation70 To this end, the drug loading efficiency in the polymeric carriers is reduced during these conditions due to phase separation, which was articulated in a thermodynamics point of view.
Solute
The foremost consideration of any substrate (drug/polymer) during the processing is its solubility in SCF. As SC-CO2 is relatively a poor solvent, the anti-solvent processes are explored to operate the poorly SCF-soluble solutes by using organic solvents such as acetone, DCM, dimethyl sulfoxide (DMSO), and others to solubilize the materials, where SCF acts as an anti-solvent with respect to the substrate. Moreover, it should be noted that the structure and nature of drugs/polymers and their physicochemical properties are essential factors for producing particles with desired drug encapsulation efficiency as well as particle size distribution with the SEDS process. For example, lipophilic drugs or SCF-soluble drugs are profoundly challenging for their precipitation or encapsulation in polymers. On the other hand, compared to the crystalline polymer or semi-crystalline polymer, amorphous polymers (eg, poly(lactic-co-glycolic acid) [PLGA]) are hard to crystallize and precipitate in the high-pressure vessel. These consequences often result in reduced drug loading efficiency and broader particle size distribution.
Multiple polymers
Preceding reports indicated that it is easy to precipitate and produce the small-sized microparticles from polymers in their crystalline form or semi-crystalline form (eg, PLLA). These forms of polymers are recrystallized well in the SEDS process through successive steps: initially nucleation, that is, the formation of the polymeric nucleus, followed by the growth phase, resulting in the desired composite particles in the amorphous form.Citation10 However, it is difficult to process amorphous forms of polymers such as PLGA, as they eventually result in undesirable aggregates.Citation10 To overcome this limitation, the combination of biodegradable polymers (PLLA/PLGA) is preferred in some cases to produce microparticles within the acceptable mean particle size range.Citation73 Moreover, the crystallinity of the polymeric microparticles is reduced after SEDS processing, such that the resultant microparticles in their amorphous state possess high degradation rate in vivo.
Solution concentration
In addition to the nature of the substrate, the concentration of a drug or a polymer in the solvent also plays a crucial role in determining the particle size and its distribution during SEDS processing.Citation63 In an SCF-assisted process, the particle size of a substrate is usually increased with an increase in its concentration, which is correlated to the terms of enhanced nucleation and growth processes. In this framework, the injected dilute solutions often result in delayed saturation and subsequently the slower precipitation during the expansion of the droplet.Citation63 These consequences eventually result in small-sized particles. Thus, nucleation is the dominant plausible mechanism in these circumstances. Contrariwise, increase in concentration results in enhancement of surface tension as well as the viscosity of the solution, which eventually result in the formation of large primary droplets.Citation9,Citation74 Herewith, the mechanism lying behind the particle growth correlates with the nucleation of solute due to rapid supersaturation, yielding the large-sized particles.Citation56,Citation63 Previous reports have indicated that the solutions at a lower concentration were highly beneficial for the formation of particles with uniform size distribution. However, the decrease in solution concentration, in turn, influences the loading efficiency of the drugs in the polymers.Citation68
In an attempt to explore the effect of organic non-solvent concomitantly with the solution concentration on the particle size and shape in SEDS, Chen et al demonstrated that the addition of DCM into the ethanolic solutions resulted in an initial concentration with a high degree of saturation yielding the small-sized particles with a spherical shape.Citation9,Citation74 In this study, the authors described that the effect of solvent ratio was found to be prevailing on the particle sizes compared to that of the solution concentration.Citation74 Moreover, they clarified that the solution concentration showed significant influence on the width of the particle. Together, they concluded that an increase in the degree of saturation with a decrease in the solution concentration had resulted in small-sized spherical particles. The strategy of utilizing non-solvent was highly cost-effective, as it results in the fabrication of particles at the lower consumption of CO2.Citation74
Solvent
In an anti-solvent process, various co-solvents are required to increase the solubility of a solute. The most preferred solvents include acetone, DMSO, methanol, chloroform, ethanol, isopropanol, and DCM, among others. However, the selection of an appropriate solvent plays a crucial role while formulating the drug delivery systems. The selection process primarily depends on the solubility of the solute (polymer/drug) in the solvent and its compatibility with the SCF. In a few instances, a combination of solvents such as acetone/DMSOCitation59 and DCM/DMSOCitation47,Citation59 is also used at a determined ratio, such that one of them is highly volatile to induce significant volume of expansion and easy removal and the other solvent enhances the solubility of a solute.Citation68 Moreover, it should be noted that the solvent selection is also based on the ratiometric solubility of polymer with the drug, because the drug should precipitate earlier for its encapsulation in the polymer. Furthermore, the entrapped drug may also result in diverse polymorphic forms in different solvents, which eventually guide the release of the drugs.Citation75 In this context, some solvents may result in a reduced yield of the end product due to the following reasons: 1) the high viscosity of the solvent that leads to inadequate diffusion between the solvent and the anti-solvent and 2) the high solubility of a solute in the solvent leading to difficulty in its precipitation at a low concentration.Citation47 To overcome this limitation, high saturation is achieved by using a non-solvent at a low concentration of the solute. Thus, the solute can rapidly precipitate and lead to micro-/nano-sized particles with uniform size distribution.Citation76 In addition, the multiphase emulsions can be preferred in some instances instead of organic solvents to overcome the insolubility and inactivation of sensitive molecules such as genes or peptides.
Solution flow rate
In addition to the solute concentration, the particle size of a substrate depends on the solution flow rate during SCF processing. In general, an increase in the solution flow rate results in an increase of the size of the particles due to weak impingement of SC-CO2 on the liquid film.Citation56,Citation72 However, it is also related to other parameters such as surface tension and viscosity of the liquid. In one case, Chen et al reported that the solution flow rate affects the shape of the particles.Citation74 With an increase in flow rate above the normal level, the system resulted in particles with piriform shape, that is, more extended than a sphere. These consequences of the particle with different shapes concerning the flow rate can be explained well in relation to the hydrodynamic aspects of the liquid, that is, the variation in kinetic energy per unit mass of the liquid, which is appropriate to precipitate small-sized particles with narrow size distribution at a low flow rate and vice versa.Citation68 In addition to the particle size, the flow rate also affects the drug loading efficiency in polymer-based delivery systems. In a way, an increase in the solution flow rate results in enhancement of their loading efficacy. In one case, the solution flow rate had shown combinatorial effects on the particle size.Citation63 The particle diameter was increased in the beginning and then decreased with an increase in flow rate, demonstrating the influence of the rapid atomization of formed liquid sheets. The effective mechanisms that played a vital role during atomization were clearly explained by He et al.Citation63 They explained that two different but complementary mechanisms are involved: 1) prompt atomization and 2) the wave mechanism during atomization.Citation63,Citation70 The mechanism of prompt atomization is generally correlated to the speed of liquid sheet at the edge of the atomizer tip, which is maintained low at a lower flow rate, resulting in an intense equivalent momentum transfer between the solution and SC-CO2 and eventually disintegrates the sheet into fine droplets.Citation63,Citation70 The energy per unit mass of liquid in these conditions that gained from the dense gas to fragment the sheet into drops decreases with increase in the solution flow rate at a constant Vc (ie, flow rate of CO2 at the standard state [temperature 273 K, pressure 101, 325 Pa] in L/min), which increases the droplet size and, subsequently, the particle size of the end product.Citation63,Citation70
Supercritical fluid
SCF in these anti-solvent processes acts not only as an anti-solvent, but also as a spray enhancer and a reprecipitation aid. In general, SCFs have no significant influence on the synthesized formulation in the anti-solvent processes. However, the mean diameter of the particles generated from a semi-crystalline form of polymers depends on the density of the SCF.Citation77 During the SEDS processing of substrates, the pumped SCF is completely miscible with the solution feed above the critical pressure and the mixture exists as a single phase. The resultant particles produced by the SEDS process possess attractive physicochemical properties such as reduced tensile strength facilitating these readily dispersible fine particles for aerosol formulations,Citation78 wafer-like morphology with a higher surface area to improve their bioavailability in vivo,Citation79 and producing dosage forms with reduced dose variability.Citation80
The SCF technology is often operated using one of the available supercritical solvents. Interestingly, Ghaderi et al have improved the SEDS process by using a combination of SCFs (N2 and CO2) to overcome the severe flocculation caused during the production of polymeric microparticles.Citation81 The obtained polymer dispersions using a combination of gases were in smaller sizes compared to those of products obtained when CO2 alone was utilized, due to enhancement of the plasticizing effect of SCF. These particles with successful drug entrapment resulted in the controlled release of drugs.Citation81,Citation82
SCF flow rate
Similar to the solution flow rate, the flow rate of SCF has a minor influence on the particle size of the resultant products, which can be adjusted by optimizing the conditions.Citation63 In general, an increase in the flow rate of SCF results in reduction of particle size due to the effects concerning the consolidating forces of surface tension and viscosity of the liquids that sturdily oppose the disrupting action of externally applied aerodynamic forces in a twin-fluid atomization process. Henceforth, the impingement of dense gas on the liquid film is reinforced and results in the formation of fine droplets.Citation63
Miscellaneous factors
Nozzle and atomization
The SEDS process involves the spraying of various constituents through a specially designed coaxial nozzle that is convenient for the parallel flow of both the liquids, that is, SCF as well as the solution containing the active substrate, separately. Despite the advantages, the utilization of coaxial nozzle still faces a limitation of generating a high-shear force during the discharge of the constituents from the pump, which damages the labile biomolecules such as genes and peptides. This could be addressed by utilizing the three-channel nozzle system. In addition, it should be noted that the diameter of the nozzle plays a critical role in the eventual size of the particles.
Furthermore, atomization of liquids in the nozzle is one of the critical steps that plays a crucial role during particle formation in the SEDS process.Citation56 These specially designed devices (ie, atomizers) efficiently atomize a solution of active substances, intensify its mixing with the SCF in the nozzle, and enhance the mass transfer rates between them for fine particle formation.Citation56,Citation57,Citation63 The solution that is intended to be atomized is initially driven along a coaxial annular passage resulting in a thin swirl film at a thickness of 10 μm. The SC-CO2 stream for atomization then impinges the formed thin swirl film at the tip of the atomizer and generates shear forces which disintegrate the film into ultrafine droplets. Further intensification of mixing may result in the formation of uniform-sized particles.Citation56 However, a high quality of atomization could yield ultrafine primary droplets, ensuing enhancement of the two-way mass transfer rates and subsequently resulting in small-sized particles at a significant nucleation rate.Citation56,Citation62
Humidity
Humidity is another crucial factor that significantly influences the quality of the end product during SCF-assisted processing. In general, the particle adhesion scenario and wetting phenomena are the resultant effects of humidity, and they vary with the processing conditions as well. A study has compared the materials obtained from SEDS processing with the products obtained from the conventional micronization method.Citation83 This investigation demonstrated that the product obtained from the SEDS process was more sensitive to humidity. The effects of humidity on particle morphology were captured by atomic force microscopy, indicating that the changes were due to surface chemistry differences involving various factors such as electrostatic, capillary, and van der Waals forces and the surface free energy of the particle, which played critical roles in particle adhesion.Citation83
Problems
Despite their efficacy and advancements in producing various polymeric constructs, the SEDS process still faces a minor problem of poor recovery as single nano-units during the synthesis of nanoparticle formulations.Citation84 More often, the nanoparticles in their dry form tend to disperse in the precipitation chamber and are very difficult to collect. To overcome this limitation, Torino et al proposed an innovative strategy that worked on the principle of processing the soft aggregates of the collected nanoparticles by ultrafiltration, ultracentrifugation, or ultrasound-based techniques for their separation as single nano-units.Citation84
Applications
Drug delivery
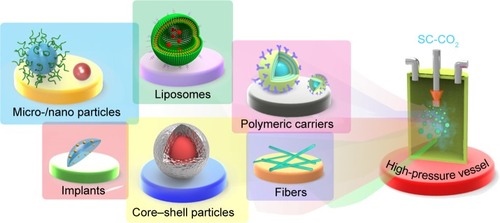
Drug delivery usually relies on various formulations and approaches for conveying APIs (drugs/proteins/genes) to accomplish the desired therapeutic effects.Citation10 The foremost aim of any technology that produces these formulations/dosage forms is the efficient delivery of drugs to the right target as far as the health care field is concerned. The SCF technology is perhaps one of them that has gained enormous attention in the past few decades due to its numerous advantages and ability to overcome the limitations associated with the traditional methods, such as physical instability of the resultant product, multi-step procedures, and usually relying on large amounts of organic solvents, among others.Citation10,Citation12,Citation38,Citation45,Citation85–Citation88 The unique adaptive properties of SCFs, such as solvating power, large compressibility, and others, provide many better ways of particle fabrication for drug delivery.Citation10,Citation88,Citation89 Another significant advantage of this technology is that it allows single-step fabrication of particles with uniform size distribution, which is difficult to achieve by traditional methods. Moreover, the SCF-assisted processes have been used to synthesize various formulations with respect to different categories as well as routes of administration, which have been discussed elsewhere.Citation10 This technology has been used to process the APIs alone or in combination with various biodegradable polymeric carriers for the enhancement of bioavailability.Citation10 In this framework, the SEDS process, the most advanced prototype of the SCF technology, has been widely used in the pharmaceutical manufacturing process for producing the dosage forms intended for oral, pulmonary, and transdermal routes of administration. Herewith, we give a detailed view of processing APIs via the SEDS process with a set of examples.
Micro-/nanonization of pure drug
Drug delivery has been associated with some critical challenges such as solubility and diffusion, which need to be addressed for the effective conveyance of therapeutic cargo. More often, the new chemical entities also suffer from poor solubility and stability issues. In addition, the dissolution of the APIs after delivery has remained a significant concern in formulating dosage forms, especially for the drugs with a narrow therapeutic index. To overcome this limitation, numerous studies have indicated that reduction in the size of the particles is one of the ways for enhancing the therapeutic efficacy of such drugs. This has been well achieved by micronization or nanonization of drugs, which results in enhancement of the available surface area of exposure to the solvent and, subsequently, the dissolution of poorly water-soluble drugs, and eventually their therapeutic efficiency. Moreover, these nano-sized products have been used for various biomedical applications such as bioimaging, tissue engineering, in vitro diagnostics and implants, among others ().Citation3,Citation10,Citation88,Citation90
Table 1 Examples showing the nanonization/micronization of pure drugs using the SEDS process
Various techniques such as grinding, milling, crushing, electrospinning, and spray drying, among others, are also available to produce micro-sized as well as nano-sized particles of various pharmaceutical compounds.Citation10,Citation74 However, the applicability of these conventional processes is limited due to the need for large amounts of organic solvents. In some instances, these processes yield broad particle size distribution in the end product, thermal denaturing owing to high processing temperatures, and excessive surface changes or roughness of products, thereby manipulating the bioavailability of the drugs.Citation10 Moreover, the process may lead to product contamination and damage of the sensitive biomolecules in some cases because of the strong shearing forces and heat generated by them.Citation59,Citation90,Citation91
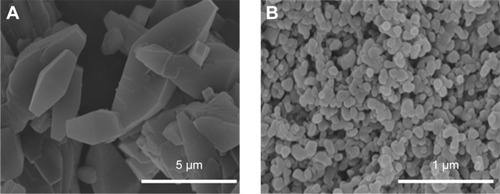
Among all the SCF-assisted processes, the SEDS technique is one of the efficient approaches for the nanonization of drugs as the scope of fine particle formation is significantly high due to excessive mass transfer rates, intensified mixing, and the formation of tiny droplets leading to ultrafine particles with narrow size distribution.Citation59,Citation91 This process often results in fine-sized particulates of drugs at a high efficiency as it utilizes an anti-solvent that acts as a spray enhancer by the mechanical effect.Citation65,Citation92,Citation93 The extemporaneous contact of high-speed liquid jet streams with the SCF generates a uniformly dispersed mixture and eventually leads to prompt precipitation of the nano-sized particles. The customized operation of the SEDS process is very fast, such that it results in a change of physical state of the solute from a regular crystalline state into an amorphous form, where it has no chance and scope to consolidate the solute in its systematic crystalline patterns during recrystallization.Citation59,Citation90,Citation91 In the thermodynamic point of view, the solutes in their crystalline equivalents happen to be in the lowest energy state due to their regularly arranged basic structural units.Citation90 Nevertheless, the transformation in their physical state would result in superfluous free energy and entropy, and these consequences would undoubtedly facilitate an upsurge in the dissolution rate of drugs and eventually their bioavailability ().Citation59,Citation90
Figure 2 FE-SEM photographs of pure (A) and the SEDS process-assisted nanoparticulate forms (B) of methotrexate powder.
Note: Reprinted from J Supercrit Fluids. Vol 67. Chen AZ, Li L, Wang SB, et al. Nanonization of methotrexate by solution-enhanced dispersion by supercritical CO2. Pages 7–13. Copyright 2012, with permission from Elsevier.Citation59
Abbreviations: FE-SEM, field emission-scanning electron microscopy; SEDS, solution-enhanced dispersion by supercritical fluids.
In general, the SEDS process is not only used for operating poorly water-soluble drugs, but also widely applied for the precipitation of protein- and peptide-based drugs that are intended to be absorbed from noninvasive routes of administration such as pulmonary and transdermal delivery.Citation96,Citation97 Despite the success and reliability in formulating the therapeutic proteins by the SEDS process, the long-term stability may be compromised due to the undesirable chemical degradation mechanisms and conformational alterations that can affect the primary, secondary, and/or tertiary structures of a protein.Citation96 However, extensive studies are required to overcome these limitations and explore the processing of sensitive biomacromolecules alone.
Polymeric carriers
Controlled drug release
In recent times, various biodegradable as well as biocompatible polymers have attracted significant attention from researchers in preparing the controlled delivery systems due to the ease of drug impregnation, ability to convey high amounts of therapeutic cargo, sustained release of drugs from the matrix by preserving the levels in the therapeutic window, reduction in side effects of drugs, enhancement of bioavailability, and biodegradability, among others.Citation10,Citation104–Citation106 In addition, the incorporation of drugs in a suitable carrier facilitates the improvement of the fate of the drug by changing its delivery pattern (). However, the ability to achieve control over various physical parameters such as morphology, size, stimuli-responsive and desired release characteristics is highly challenging during the formulation of polymeric carriers. These parameters utterly depend on the selection of the polymers by considering their thermosensitivity as well as polarity. Moreover, the critical aspect that should be considered during the processing of polymeric carriers is their solubility in SCFs. However, in the SEDS process, it could be surpassed by dissolving them in the desired organic solvents, which could be eventually removed through extraction after particle formation. These processes that utilize SCFs as anti-solvents for the production of many pharmaceutical products through co-precipitation or encapsulation strategy involve the momentous solvating power for the efficient separation of the desired mixture of polymers and drugs as micro- or nano-sized particles. In this context, various biodegradable micro-/nano-sized particles that have been prepared by SEDS and its modified processes are used as potential delivery systems to accomplish the therapeutic duties (; ).Citation107 More often, active therapeutic moieties such as drugs, proteins, steroids, carotenoids, and organic pigments have been incorporated using various biodegradable polymeric carriers such as PLLA, PLGA, polyethylene glycol, chitosan, alginate, and lactose, among others, for their controlled delivery.Citation10,Citation52,Citation91,Citation92,Citation108–Citation110 This technology has a scope to adjust the size of the polymeric particles, which makes it highly feasible to attach the targeting ligands for the targeting efficacy, and their smaller size facilitates high dissolution rates and increases the bioavailability of the drugs. These consequences of high dissolution rates and bioavailability enhancement of the drugs subsequently facilitate the enhancement of their therapeutic efficiency at low dosage.Citation107,Citation111 The controlled release of drugs can generally be achieved through different mechanisms, which utterly depend on the method of choice for preparation as well as the type of the polymer selected. More often, the drugs co-precipitated/encapsulated by SEDS and its associated processes are released by slow diffusion from the polymeric matrix. In this framework, the interactions between the polymer and the drug often constitute electrostatic or weak hydrogen bonding interactions that are weakened when exposed to the solvent and facilitate drug release.
Table 2 Examples of drug–polymer conjugates obtained by the SEDS processing
Core–shell particles
In the conventional SEDS process, the composite micro-/nanoparticles formed during the co-precipitation of drug and polymer sometimes result in low drug loading as well as reduced encapsulation efficiency due to washes during depressurization and washing period. In addition, the drug molecules that are dispersed in the polymeric matrix are adsorbed on the surface of the polymer through weakly bound interactions, which often result in rapid release of the entire drug cargo.Citation66 To overcome these limitations, Chen et al fabricated the innovative core–shell formulations using the advanced SEDS process, that is, SpEDS, which resulted in improved drug loading efficiency and showed sustained drug release effect.Citation29 In this process, the drug nanoparticles were initially produced and were then coated with the polymer to produce the core–shell composites. In addition, the authors demonstrated that this method of processing was highly advantageous over other SCF-assisted processes as the drug molecules in the core slowly diffused, after which the outer shell became porous or got degraded by the surrounding fluid. In some instances, rapid degradation phenomenon, that is, the burst-out process, was also achieved with pH/thermosensitive polymers.Citation10
Pulmonary delivery
Biomacromolecules such as proteins, genes, and vaccines are the most promising therapeutic agents that should be delivered in right amounts to the desired sites without losing their biological activity, which results in higher efficacy and lower dosage requirement for administration.Citation126,Citation127 Pulmonary delivery of drugs is a considerable alternative for delivering such therapeutic, sensitive biomolecules as the lung is an appropriate site for the absorption of various therapeutic agents due to its high surface area, high vascularization, receipt of entire cardiac output, thin blood–alveolar barrier, lower enzyme activities, and circumventing the hepatic first-pass metabolism.Citation10,Citation122,Citation128,Citation129 More often, the pulmonary administration of various active moieties for localized as well as systemic therapy is accomplished through various delivery containers such as dry powder inhalers, pressurized metered-dose inhalers, and nebulizers, loaded with powdered formulations. The size of particles plays a crucial role in fabricating the powders for inhalation delivery, and the acceptable size range of these powders should be <7 μm for their efficient deposition in the lungs.Citation42,Citation45,Citation87,Citation129 Among all these SCF-based processes, the SEDS process is one of the most effective methods in synthesizing pharmaceutical formulations intended for inhalation delivery. However, it should be noted that critical care should be taken for avoiding any residual amount of organic solvents, because these remnants may cause undesired severe immune responses in the lungs. In addition, the other notable point of concern that should be considered during the preparation of these delivery systems is strict optimization of the formulation for preserving the biological activity of therapeutic biomolecules. In one case, Tservistas et al prepared dry powder formulation of plasmid DNA using mannitol as the bulk excipient with the modified SEDS process.Citation122 The prominent modification in the process was the utilization of three-channel coaxial nozzle, which resulted in the production of plasmid DNA-loaded particles. Initially, the processed nucleic acid by this high-pressure technology resulted in degradation of the plasmid DNA. However, further investigations revealed that the pH of the medium played a crucial role in the retrieval of intact DNA, and eventually, the supercoiled proportion of DNA was recovered (~80%).Citation122 Many other studies have also reported that the synthesis of inhalation delivery systems of drugs and other therapeutic biomolecules such as genes and proteins was feasible via SEDS and its modified processes.Citation78,Citation80,Citation122,Citation130
Transdermal and implantable delivery
Drug delivery through transdermal route has attracted increasing interest from researchers due to its potential advantages such as circumventing the hepatic first-pass metabolism, sustained drug release, and localized treatment, among others.Citation120,Citation131–Citation133 However, efficient permeation of drugs through the skin is highly limited by a barrier of protection called the stratum corneum. To overcome this limitation, several permeation enhancement techniques, including microneedle,Citation131 electroporation,Citation132 iontophoresis,Citation134 and ultrasound pretreatment,Citation133 among others,Citation135 have been proposed for drug delivery and cosmetic application.Citation135,Citation136 Nevertheless, the applicability of these methods is limited due to their respective limitations, which have been discussed elsewhere.Citation120 To this end, SCF-assisted preparations have been subjected to transdermal drug delivery with enhanced permeation efficiency as they possess several advantages such as high drug loading efficiency in the matrix through diffusion, plasticizing effect, high solubility, low amounts of residual solvent, and narrow as well as uniform particle size distribution resulting in improvement of solubility and permeation of drugs.Citation137 In one case, Chen et al fabricated the silk fibroin (SF) magnetic nanoparticles using the SpEDS process for the delivery of methotrexate.Citation10,Citation127 The transdermal drug delivery was efficiently achieved through a synergistic approach utilizing the combination of the SEDS process-assisted SF nanoparticles with stationary/alternating magnetic fields. Remarkably, the synergistic combination of magnetic fields acting on the magnetic nanoparticles had created a massage-like effect on the skin for the penetration of carriers.Citation127
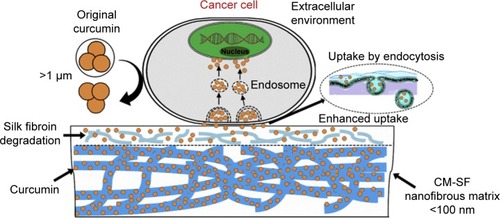
Implantable drug delivery systems are incredible medical devices that are preloaded with drugs or other biomolecules and inserted into the body through surgical procedures for long-term therapeutics. More often, these systems are used for the treatment of postoperative complications such as infections and immune responses.Citation10,Citation138 In addition, these systemic delivery systems are recognized as a potential solution to address the issues associated with various other systemic routes of administration, such as frequent administration, adverse effects due to long-term treatment, and patient compliance.Citation10,Citation118,Citation138 Various methods have been considered for producing the implantable systems, such as solvent casting process and hot-melt extrusion method, among others.Citation10 However, these methods still face certain limitations such as high processing temperatures and remnants of organic solvents that provoke undesired immune responses when implanted. To this end, the SCF technology has attracted considerable interest from researchers for synthesizing these implants, by overcoming the above-mentioned limitations of conventional fabrication methods. This technology is highly beneficial in processing the APIs, the applicability of which is limited due to their poor pharmacokinetic behaviors, such as low solubility and, subsequently, low bioavailability.Citation118 Interestingly, Xie et al developed an implantable polymeric nanofibrous drug delivery platform to enhance the bioavailability of natural polyphenolic compound curcumin (CM) for effective cancer therapeutics.Citation138 This active moiety is known for its effect on a wide range of cancers. In this study, the nanofibrous implantable matrices of CM-incorporated SF carriers by the SEDS process had resulted in controllable release of CM for topical cancer treatment ().Citation117,Citation138
Figure 4 Schematic representation showing the mechanism of enhanced cellular uptake efficiency of implantable nanofibrous drug delivery platform by using the SEDS processing method.
Note: Reprinted from Biomaterials. Vol 103. Xie M, Fan D, Chen Y, et al. An implantable and controlled drug-release silk fibroin nanofibrous matrix to advance the treatment of solid tumour cancers. Pages 33–43. Copyright 2016, with permission from Elsevier.Citation138
Abbreviations: CM-SF, curcumin-silk fibroin; SEDS, solution-enhanced dispersion by supercritical fluids.
Food and nutraceutical industries
Due to its environmentally benign nature, the SCF technology has been widely used in various applications in health care field other than drug delivery.Citation6,Citation10 One among them is the processing of diverse bioactive substances, that is, proteins and carotenoids, for nutraceutical applications, and also their encapsulation in biodegradable polymers to yield particles for the efficient delivery of nutrients.Citation101 In addition, the biodegradable polymers coated over the biomolecules offer them significant protection from the harsh environments in the physiological fluids. Carotenoids, often referred to as nutraceutical compounds, are the most common group of pigments that are widely used in food and nutraceutical industries.Citation101 More often, the presence of carotenoids can inhibit the oxidation of food constituents due to the singlet oxygen quenching activity.Citation109 In addition, these substituents are used to improve the elegance of foods. However, the color intensity of the pigments utterly depends on their physicochemical properties such as particle size and its distribution and morphology.Citation101,Citation109 More often, these water-insoluble commercial carotenoids are present in their crystalline forms that are often processed by using oils and organic solvents, which may lead to the contamination of food and nutrients. To this end, this ecofriendly process can overcome the limitation of food contamination. The SEDS process is the most efficient method for the processing of carotenoids, as this strategy is operated at near-ambient conditions and in an inert environment, where there is no scope of thermal degradation of sensitive carotenoids.Citation65,Citation139 Unlike other conventional methods, the SCF-based processes do not rely on the organic solvents. The micronization or nanonization of these nutraceutical moieties by SEDS results in improvement of their solubility due to the enhanced surface area of exposure in the solvents.Citation6,Citation10 However, it is evident that the applicability of synthetic pigments is restricted due to the health hazards in the food, cosmetic, and pharmaceutical industries. In a case, Hong et al micronized the natural pigment, astaxanthin, by using the solution-enhanced dispersion by supercritical fluids – prefilming atomization method.Citation139 The authors demonstrated that this natural pigment was preferred owing to its different biological functions such as ultraviolet light protection, antioxidant, and anti-inflammatory effects, among others.Citation139 Moreover, the authors claimed that various experimental variables such as initial concentration of the solute, solution flow rate, and critical conditions had shown significant influence in the determination of morphology and particle size of astaxanthin particles.Citation70
The processing of these nutraceutical moieties by traditional fabrication methods often results in undesired effects due to the presence of double bonds in the molecular chains of carotenoids.Citation109 In this framework, the exposure of these sensitive molecules to heat, light, and acids may result in the isomerization of trans-carotenoids (the stable form), which get converted to their cis-form, which are comparatively inferior to the trans-form (ie, diminished color, reduced pro-vitamin activity, and eventually, their efficacy).Citation109,Citation140,Citation141 The encapsulation and co-precipitation of these proteins/carotenoids in biodegradable polymers by the SEDS process is the most efficient method to preserve the effects as well as enhance their efficiency.Citation109,Citation119 In addition, further advancements have been made in formulating the nano-sized inclusion complexes with β-cyclodextrin using the SEDS process.Citation140 At optimized conditions, the lycopene/β-CD nanoparticles of 40 nm were obtained at high temperature and pressure with a low solution flow rate, resulting in enhancement of both the solubility and stability of lycopene.
Conclusion
In summary, the review has given the insights of the SEDS process in generating micro-/nano-sized APIs or their polymeric conjugates that can be administered through oral, pulmonary, and transdermal routes. Furthermore, the specialized formulations such as core–shell formulations and implantable nanofibrous delivery systems have been systematically reviewed. The delivery systems produced by this process are highly advantageous over products that are obtained by other processing techniques of the SCF technology resulting in products with better performances. In addition, we emphasized the effects of various parameters on the particle morphology during SEDS processing.
Despite its success and significant advantages, the SEDS method still faces a significant impediment regarding its processing for scale-up due to the lack of fundamental studies that precisely describe the phase behavior and performance of the multicomponent mixtures. In addition, the certainty of particle characteristics, which include the solubility enhancement of microparticles produced by this process, remains as an obstacle. Therefore, we envision that integrating the conventional process with the SCF process may result in further advancements of the carriers. In addition, it may surpass the complexity of the processing and enable better understanding of the behavioral characteristics of the product. However, with continued innovations, we believe that a promising formulation of biodegradable micro-/nano-sized particles from these SCF-assisted processes will soon become commercially available.
Acknowledgments
The authors acknowledge the financial support from National Natural Science Foundation of China (U1605225, 31570974, and 31470927), Public Science and Technology Research Funds Projects of Ocean (201505029), Promotion Program for Young and Middle-aged Teacher in Science and Technology Research of Huaqiao University (ZQN-PY107), and the Start-Up Fund For Young Researchers (Project No 16BS803).
Disclosure
The authors report no conflicts of interest in this work.
References
- BałdygaJKubickiDShekunovBYSmithKBMixing effects on particle formation in supercritical fluidsChem Eng Res Des201088911311141
- KompellaUBKoushikKPreparation of drug delivery systems using supercritical fluid technologyCrit Rev Ther Drug Carrier Syst200118217319911325031
- ChenBQKankalaRKChenAZInvestigation of silk fibroin nanoparticle-decorated poly(l-lactic acid) composite scaffolds for osteoblast growth and differentiationInt J Nanomedicine2017121877189028331312
- HauthalWHAdvances with supercritical fluids [review]Chemosphere200143112313511233819
- ChenAZZhaoZWangSBLiYZhaoCLiuYGA continuous RESS process to prepare PLA–PEG–PLA microparticlesJ Supercrit Fluids2011599297
- GintyPJWhitakerMJShakesheffKMHowdleSMDrug delivery goes supercriticalMater Today2005884248
- GintyPJHowardDRoseFRAJMammalian cell survival and processing in supercritical CO2Proc Natl Acad Sci2006103197426743116651535
- PasqualiIBettiniRAre pharmaceutics really going supercritical?Int J Pharm2008364217618718597957
- KalaniMYunusRApplication of supercritical antisolvent method in drug encapsulation: a reviewInt J Nanomedicine201161429144221796245
- KankalaRKZhangYSWangSBLeeCHChenAZSupercritical fluid technology: an emphasis on drug delivery and related biomedical applicationsAdv Healthc Mater20176161700433
- WuKLiJPrecipitation of a biodegradable polymer using compressed carbon dioxide as antisolventJ Supercrit Fluids2008462211216
- DaviesORLewisALWhitakerMJTaiHShakesheffKMHowdleSMApplications of supercritical CO2 in the fabrication of polymer systems for drug delivery and tissue engineeringAdv Drug Del Rev2008603373387
- ReverchonEAdamiRNanomaterials and supercritical fluidsJ Supercrit Fluids2006371122
- ByrappaKOharaSAdschiriTNanoparticles synthesis using super-critical fluid technology – towards biomedical applicationsAdv Drug Del Rev2008603299327
- HakutaYHayashiHAraiKFine particle formation using supercritical fluidsCurr Opin Solid State Mater Sci200374–5341351
- MezianiMJRollinsHWAllardLFSunYPProtein-protected nanoparticles from rapid expansion of supercritical solution into aqueous solutionJ Phys Chem B2002106431117811182
- WarwickBDehghaniFFosterNRBiffinJRRegtopHLMicronization of copper indomethacin using gas antisolvent processesInd Eng Chem Res200241819932004
- KröberHTeipelUMaterials processing with supercritical antisolvent precipitation: process parameters and morphology of tartaric acidJ Supercrit Fluids2002223229235
- PerryRHPerry’s Chemical Engineers’ Handbook7th edNew York, NYMcGraw-Hill1997
- DjerafiRMasmoudiYCramponCMeniaiABadensESupercritical anti-solvent precipitation of ethyl celluloseJ Supercrit Fluids20151059298
- TomaskoDLLiHLiuDA review of CO2 applications in the processing of polymersInd Eng Chem Res2003422564316456
- VemavarapuCMollanMJLodayaMNeedhamTEDesign and process aspects of laboratory scale SCF particle formation systemsInt J Pharm20052921–211615725549
- MatsonDWFultonJLPetersenRCSmithRDRapid expansion of supercritical fluid solutions: solute formation of powders, thin films, and fibersInd Eng Chem Res1987261122982306
- FraileMMartínŸDeodatoDProduction of new hybrid systems for drug delivery by PGSS (Particles from Gas Saturated Solutions) processJ Supercrit Fluids201381226235
- BertuccoAPalladoPBenedettiLFormation of biocompatible polymer microspheres for controlled drug delivery by a supercritical antisolvent techniqueProcess Technol Proc199612217222
- FalkRRandolphTWMeyerJDKellyRMManningMCControlled release of ionic compounds from poly (l-lactide) microspheres produced by precipitation with a compressed antisolventJ Control Release19974417785
- YeoSDLimGBDebendettiPGBernsteinHFormation of microparticulate protein powder using a supercritical fluid antisolventBiotechnol Bioeng199341334134618609558
- MargulisKNeofytouEABeyguiREZareRNCelecoxib nanoparticles for therapeutic angiogenesisACS Nano2015999416942626244654
- ChenAZWangGYWangSBLiLLiuYGZhaoCFormation of methotrexate-PLLA-PEG-PLLA composite microspheres by microencapsulation through a process of suspension-enhanced dispersion by supercritical CO2Int J Nanomedicine201273013302222787397
- ChenAZLinXFWangSBBiological evaluation of Fe3O4–poly(L-lactide)–poly(ethylene glycol)–poly(L-lactide) magnetic microspheres prepared in supercritical CO2Toxicol Lett20122121758222609093
- ChattopadhyayPGuptaRBSupercritical CO2 based production of magnetically responsive micro- and nanoparticles for drug targetingInd Eng Chem Res2002412460496058
- BeckmanEJSupercritical and near-critical CO2 in green chemical synthesis and processingJ Supercrit Fluids2004282–3121191
- ReverchonESupercritical-assisted atomization to produce micro-and/or nanoparticles of controlled size and distributionInd Eng Chem Res2002411024052411
- ShenYBDuZWangQGuanYXYaoSJPreparation of chitosan microparticles with diverse molecular weights using supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixerPowder Technol2014254416424
- García-GonzálezCAConcheiroAAlvarez-LorenzoCProcessing of materials for regenerative medicine using supercritical fluid technologyBioconju Chem201526711591171
- YangDLuoWWangJA novel controlled release formulation of the Pin1 inhibitor ATRA to improve liver cancer therapy by simultaneously blocking multiple cancer pathwaysJ Control Release201826940542229170140
- BleichJKleinebuddePMüllerBWInfluence of gas density and pressure on microparticles produced with the ASES processInt J Pharm199410617784
- MishimaKBiodegradable particle formation for drug and gene delivery using supercritical fluid and dense gasAdv Drug Del Rev2008603411432
- AdamiRReverchonEJärvenpääEHuopalahtiRSupercritical antisolvent micronization of nalmefene HCl on laboratory and pilot scalePowder Technol20081821105112
- CampardelliRAdamiRDella PortaGReverchonENanoparticle precipitation by supercritical assisted injection in a liquid antisolventChem Eng J2012192246251
- Della PortaGFalcoNReverchonENSAID drugs release from injectable microspheres produced by supercritical fluid emulsion extractionJ Pharm Sci20109931484149919780130
- YorkPHannaMParticle engineering by supercritical fluid technologies for powder inhalation drug deliveryPaper presented at: Proceedings of the Conference on Respiratory Drug Delivery1996Phoenix, Arizona
- JungJPerrutMParticle design using supercritical fluids: literature and patent surveyJ Supercrit Fluids2001203179219
- MishimaKMatsuyamaKTanabeDYamauchiSYoungTJJohnstonKPMicroencapsulation of proteins by rapid expansion of supercritical solution with a nonsolventAIChE J2000464857865
- ShoyeleSACawthorneSParticle engineering techniques for inhaled biopharmaceuticalsAdv Drug Deliv Rev2006589–101009102917005293
- BałdygaJCzarnockiRShekunovBYSmithKBParticle formation in supercritical fluids-Scale-up problemChem Eng Res Des2010883331341
- YanTChengYWangZHuangDMiaoHZhangYPreparation and characterization of baicalein powder micronized by the SEDS processJ Supercrit Fluids2015104177182
- ChenAZKangYQPuXMYinGFLiYHuJYDevelopment of Fe3O4-poly(l-lactide) magnetic microparticles in supercritical CO2J Colloid Interface Sci2009330231732219036387
- NeromeHMachmudahSWahyudionoNanoparticle formation of lycopene/β-cyclodextrin inclusion complex using supercritical antisolvent precipitationJ Supercrit Fluids20138397103
- LiSZhaoYPreparation of zein nanoparticles by using solution-enhanced dispersion with supercritical CO2 and elucidation with computational fluid dynamicsInt J Nanomedicine2017123485349428496324
- PalakodatySYorkPPritchardJSupercritical fluid processing of materials from aqueous solutions: the application of SEDS to lactose as a model substancePharm Res19981512183518439892466
- JuppoAMBoissierCKhooCEvaluation of solid dispersion particles prepared with SEDSInt J Pharm2003250238540112527165
- HootonJCGermanCSAllenSCharacterization of particle-interactions by atomic force microscopy: effect of contact areaPharm Res200320350851412669976
- KangYWuJYinGPreparation, characterization and in vitro cytotoxicity of indomethacin-loaded PLLA/PLGA microparticles using supercritical CO2 techniqueEur J Pharm Biopharm2008701859718495445
- ChenAZWangGYWangSBFengJGLiuYGKangYQPreparation of Poly-(Methyl vinyl ether-co-maleic Anhydride) nanoparticles by solution-enhanced dispersion by supercritical CO2Materials (Basel)201251018411852
- SuoQLHeWZHuangYCMicronization of the natural pigment-bixin by the SEDS process through prefilming atomizationPowder Technol20051542–3110115
- XiaoKWangWHuDQuYHaoZWangLCefquinome controlled size submicron particles precipitation by SEDS process using annular gap nozzleInt J Chem Eng2017201718
- ZhangYZLiaoXMYinGFPreparation of water soluble drugs-loaded microparticles using modified solution enhanced dispersion by supercritical CO2Powder Technol2012221343350
- ChenAZLiLWangSBNanonization of methotrexate by solution-enhanced dispersion by supercritical CO2J Supercrit Fluids201267713
- RantakyläMJänttiMAaltonenOHurmeMThe effect of initial drop size on particle size in the supercritical antisolvent precipitation (SAS) techniqueJ Supercrit Fluids2002243251263
- HeWZSuoQLHongHLSupercritical antisolvent micronization of natural carotene by the SEDS process through prefilming atomizationInd Eng Chem Res200645621082115
- HeWJiangZSuoQLiGMechanism of dispersing an active component into a polymeric carrier by the SEDS-PA processJ Mater Sci2009452467
- HeWZSuoQLJiangZHShanAHongHLPrecipitation of ephedrine by SEDS process using a specially designed prefilming atomizerJ Supercrit Fluids2004311101110
- HeWZJiangZHSuoQLAnalysis of energy efficiency of air in atomizing pseudoplastic liquid using a specially designed prefilming airblast atomizerInd Eng Chem Res2003421331443149
- JinHYHemingwayMXiaFLiSNZhaoYPProduction of β-carotene nanoparticles by the solution enhanced dispersion with enhanced mass transfer by ultrasound in supercritical CO2 (SEDS-EM)Ind Eng Chem Res201150231347513484
- ChenAZLiYChauFTMicroencapsulation of puerarin nanoparticles by poly(l-lactide) in a supercritical CO2 processActa Biomater2009582913291919463980
- ChenAZPuXMKangYQLiaoLYaoYDYinGFStudy of poly(l-lactide) microparticles based on supercritical CO2J Mater Sci Mater Med200718122339234517569002
- HuDLiuLChenWLiSZhaoYA novel preparation method for 5-aminosalicylic acid loaded Eudragit S100 nanoparticlesInt J Mol Sci20121356454646822754377
- MachadoFRSJrReisDFBoschettoDLEncapsulation of astaxanthin from Haematococcus pluvialis in PHBV by means of SEDS technique using supercritical CO2Ind Crops Prod2014541721
- HongHLSuoQLLiFWWeiXHZhangJBPrecipitation and characterization of chelerythrine microparticles by the supercritical antisolvent processChem Eng Technol200831710511055
- MoshashaeeSBisratMForbesRTNyqvistHYorkPSupercritical fluid processing of proteins. I: lysozyme precipitation from organic solutionEur J Pharm Sci200011323924511042230
- HongHLSuoQLLangZMHanLMLiCPMicronization of the officinal component emodin via the SEDS process through prefilming atomizationCryst Res Technol2008435502507
- KangYYinGOuyangPPreparation of PLLA/PLGA microparticles using solution enhanced dispersion by supercritical fluids (SEDS)J Colloid Interface Sci20083221879418402971
- ChenAZLiYChauFTApplication of organic nonsolvent in the process of solution-enhanced dispersion by supercritical CO2 to prepare puerarin fine particlesJ Supercrit Fluids2009493394402
- VelagaSPBergerRCarlforsJSupercritical fluids crystallization of budesonide and flunisolidePharm Res200219101564157112425477
- ChenAZKangYQWangSBTangNSuXQPreparation and anti-tumor effect evaluation of composite microparticles co-loaded with siRNA and paclitaxel by a supercritical processJ Mater Chem B201533164396447
- GhaderiRArturssonPCarlforsJPreparation of biodegradable microparticles using solution-enhanced dispersion by supercritical fluids (SEDS)Pharm Res199916567668110350010
- ShekunovBYFeeleyJCChowAHLTongHHYYorkPAerosolisation behaviour of micronised and supercritically-processed powdersJ Aerosol Sci2003345553568
- RichardsonCHde MatasMHoskerHMukherjeeRWongIChrystynHDetermination of the relative bioavailability of salbutamol to the lungs following inhalation from dry powder inhaler formulations containing drug substance manufactured by supercritical fluids and micronizationPharm Res200724112008201717510755
- SchiavoneHPalakodatySClarkAYorkPTzannisSTEvaluation of SCF-engineered particle-based lactose blends in passive dry powder inhalersInt J Pharm20042811–2556615288343
- GhaderiRArturssonPCarlforsJA new method for preparing biodegradable microparticles and entrapment of hydrocortisone in dl-PLG microparticles using supercritical fluidsEur J Pharm Sci20001011910699378
- ToropainenTHeikkiläTLeppänenJCrystal Structure Changes of γ-cyclodextrin after the SEDS process in supercritical carbon dioxide affect the dissolution rate of complexed budesonidePharm Res20072461058106617385023
- HootonJCGermanCSAllenSAn atomic force microscopy study of the effect of nanoscale contact geometry and surface chemistry on the adhesion of pharmaceutical particlesPharm Res200421695396115212159
- TorinoEDe MarcoIReverchonEOrganic nanoparticles recovery in supercritical antisolvent precipitationJ Supercrit Fluids2010551300306
- MoribeKTozukaYYamamotoKSupercritical carbon dioxide processing of active pharmaceutical ingredients for polymorphic control and for complex formationAdv Drug Deliv Rev200860332833818006109
- OkamotoHDanjoKApplication of supercritical fluid to preparation of powders of high-molecular weight drugs for inhalationAdv Drug Del Rev2008603433446
- PasqualiIBettiniRGiordanoFSupercritical fluid technologies: an innovative approach for manipulating the solid-state of pharmaceuticalsAdv Drug Del Rev2008603399410
- MaTZhangYSChenACarbon dioxide-assisted bioassembly of cell-loaded scaffolds from polymeric porous microspheresJ Supercrit Fluids201712014351
- ZhangAZhangQBaiHLiLLiJPolymeric nanoporous materials fabricated with supercritical CO2 and CO2-expanded liquidsChem Soc Rev201443206938695325032751
- ChenBQKankalaRKWangSBChenAZContinuous nanonization of lonidamine by modified-rapid expansion of supercritical solution processJ Supercrit Fluids20181331486493
- ChenAZPuXMKangYQLiaoLYaoYDYinGFPreparation of 5-Fluorouracil-Poly(L-lactide) microparticles using solution-enhanced dispersion by supercritical CO2Macromol Rapid Commun2006271512541259
- FranceschiEDe CesaroAMFerreiraSRSVladimir OliveiraJPrecipitation of β-carotene microparticles from SEDS technique using supercritical CO2J Food Eng2009954656663
- ChenAZPuXMYinGFStudy of lysozyme-polymer composite microparticles in supercritical CO2J Appl Polym Sci2012125431753183
- XieMFanDZhaoZNano-curcumin prepared via supercritical: Improved anti-bacterial, anti-oxidant and anti-cancer efficacyInt J Pharm2015496273274026570985
- ZhaoZXieMLiYFormation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2Int J Nanomedicine2015103171318125995627
- VelagaSPCarlforsJSupercritical fluids processing of recombinant human growth hormoneDrug Dev Ind Pharm200531213514915773281
- NestaDPElliottJSWarrJPSupercritical fluid precipitation of recombinant human immunoglobulin from aqueous solutionsBiotechnol Bioeng200067445746410620761
- KodamaTHondaMTakemuraREffect of the Z-isomer content on nanoparticle production of lycopene using solution-enhanced dispersion by supercritical fluids (SEDS)J Supercrit Fluids20181331291296
- MiaoHChenZXuWWangWSongYWangZPreparation and characterization of naringenin microparticles via a supercritical anti-Solvent processJ Supercrit Fluids20181311925
- RehmanMShekunovBYYorkPColthorpePSolubility and precipitation of nicotinic acid in supercritical carbon dioxideJ Pharm Sci200190101570158211745715
- QuanCJohanCCharlottaTCarotenoids particle formation by super-critical fluid technologiesChin J Chem Eng2009172344349
- AguiarGPSBoschettoDLChavesLMPCTrans-resveratrol micronization by SEDS techniqueInd Crops Prod201689350355
- RichardsonCHde MatasMHoskerHMukherjeeRWongIChrystynHDetermination of the relative bioavailability of salbutamol to the lungs following inhalation from dry powder inhaler formulations containing drug substance manufactured by supercritical fluids and micronizationPharm Res200724112008201717510755
- ElizondoESalaSImbuluzquetaEHigh loading of gentamicin in bioadhesive PVM/MA nanostructured microparticles using compressed carbon-dioxidePharm Res201128230932121125416
- GuneyOAkgermanASynthesis of controlled-release products in supercritical mediumAIChE J2002484856866
- UhrichKECannizzaroSMLangerRSShakesheffKMPolymeric systems for controlled drug releaseChem Rev199999113181319811749514
- BaldelliABoraeyMANobesDSVehringRAnalysis of the particle formation process of structured microparticlesMol Pharm20151282562257325685865
- PriamoWLde CezaroAMBenettiSCOliveiraJVFerreiraSRSIn vitro release profiles of β-carotene encapsulated in PHBV by means of supercritical carbon dioxide micronization techniqueJ Supercrit Fluids2011562137143
- PriamoWLde CezaroAMFerreiraSRSOliveiraJVPrecipitation and encapsulation of β-carotene in PHBV using carbon dioxide as anti-solventJ Supercrit Fluids2010541103109
- FranceschiEDe CesaroAMFeitenMPrecipitation of β-carotene and PHBV and co-precipitation from SEDS technique using supercritical CO2J Supercrit Fluids2008472259269
- TienYCSuCSLienLHChenYPRecrystallization of erlotinib hydrochloride and fulvestrant using supercritical antisolvent processJ Supercrit Fluids2010551292299
- ChenAZLiYChenDHuJYDevelopment of core-shell microcapsules by a novel supercritical CO2 processJ Mater Sci Mater Med200920375175818987946
- ZhangCLiGWangYCuiFZhangJHuangQPreparation and characterization of 5-fluorouracil-loaded PLLA-PEG/PEG nanoparticles by a novel supercritical CO2 techniqueInt J Pharm20124361–227228122721846
- PatelJPatilPPreparation and characterization of amoxicillin mucoadhesive microparticles using solution-enhanced dispersion by supercritical CO2J Microencapsul201229439840822292967
- ToropainenTVelagaSHeikkiläTPreparation of budesonide/γ-cyclodextrin complexes in supercritical fluids with a novel SEDS methodJ Pharm Sci200695102235224516883551
- JunSWKimMSJoGHCefuroxime axetil solid dispersions prepared using solution enhanced dispersion by supercritical fluidsJ Pharm Pharmacol200557121529153716354397
- XieMBLiYZhaoZSolubility enhancement of curcumin via supercritical CO2 based silk fibroin carrierJ Supercrit Fluids201510319
- XieMLiYZhaoZDevelopment of silk fibroin-derived nano-fibrous drug delivery system in supercritical CO2Mater Lett2016167175178
- HuDLinCLiuLLiSZhaoYPreparation, characterization, and in vitro release investigation of lutein/zein nanoparticles via solution enhanced dispersion by supercritical fluidsJ Food Eng20121093545552
- ChenAZChenLQWangSBWangYQZhaJZStudy of magnetic silk fibroin nanoparticles for massage-like transdermal drug deliveryInt J Nanomedicine2015104639465126229467
- ChenFYinGLiaoXPreparation, characterization and in vitro release properties of morphine-loaded PLLA-PEG-PLLA microparticles via solution enhanced dispersion by supercritical fluidsJ Mater Sci Mater Med20132471693170523625317
- TservistasMLevyMSLo-YimMYThe formation of plasmid DNA loaded pharmaceutical powders using supercritical fluid technologyBiotechnol Bioeng2001721121811084588
- HuangXZhangYYinGTumor-targeted paclitaxel-loaded folate conjugated poly(ethylene glycol)-poly(L-lactide) microparticles produced by supercritical fluid technologyJ Mater Sci Mater Med20152629525649516
- JacobsonGBGonzalez-GonzalezESpitlerRBiodegradable nanoparticles with sustained release of functional siRNA in skinJ Pharm Sci201099104261426620737633
- YangGZhaoYZhangYDangBLiuYFengNEnhanced oral bioavailability of silymarin using liposomes containing a bile salt: preparation by supercritical fluid technology and evaluation in vitro and in vivoInt J Nanomedicine2015106633664426543366
- OkamotoHDanjoKApplication of supercritical fluid to preparation of powders of high-molecular weight drugs for inhalationAdv Drug Deliv Rev200860343344617996326
- ChenAZTangNWangSBKangYQSongHFInsulin-loaded poly-L-lactide porous microspheres prepared in supercritical CO2 for pulmonary drug deliveryJ Supercrit Fluids2015101117123
- PattonJSTrincheroPPlatzRMSpecial issue proceedings of the sixth international symposium on recent advances in drug delivery systems bioavailability of pulmonary delivered peptides and proteins: α-interferon, calcitonins and parathyroid hormonesJ Control Release19942817985
- LipworthBJPharmacokinetics of inhaled drugsBr J Clin Pharmacol19964266977058971424
- RehmanMShekunovBYYorkPOptimisation of powders for pulmonary delivery using supercritical fluid technologyEur J Pharm Sci200422111715113578
- SivamaniRKLiepmannDMaibachHIMicroneedles and trans-dermal applicationsExpert Opin Drug Deliv200741192517184159
- DenetARVanbeverRPreatVSkin electroporation for transdermal and topical deliveryAdv Drug Deliv Rev200456565967415019751
- ParkEJWernerJSmithNBUltrasound mediated transdermal insulin delivery in pigs using a lightweight transducerPharm Res20072471396140117443398
- MayesSFerroneMFentanyl HCl patient-controlled iontophoretic transdermal system for the management of acute postoperative painAnn Pharmacother200640122178218617164395
- PrausnitzMRLangerRTransdermal drug deliveryNat Biotechnol200826111261126818997767
- García-GonzálezCASampaio da SousaARArgemíAProduction of hybrid lipid-based particles loaded with inorganic nanoparticles and active compounds for prolonged topical releaseInt J Pharm20093821–229630419720123
- DuarteARCManoJFReisRLPreparation of chitosan scaffolds loaded with dexamethasone for tissue engineering applications using supercritical fluid technologyEur Polym J2009451141148
- XieMFanDChenYAn implantable and controlled drug-release silk fibroin nanofibrous matrix to advance the treatment of solid tumour cancersBiomaterials2016103334327376557
- HongHLSuoQLHanLMLiCPStudy on precipitation of astaxan-thin in supercritical fluidPowder Technol20091913294298
- NeromeHMachmudahSWahyudionoNanoparticle formation of lycopene/β-cyclodextrin inclusion complex using supercritical anti-solvent precipitationJ Supercrit Fluids201383Suppl C97103
- JainAThakurDGhoshalGKatareOPShivhareUSMicroencapsulation by complex coacervation using whey protein isolates and gum acacia: an approach to preserve the functionality and controlled release of β-caroteneFood Bioprocess Technol20158816351644