Abstract
Gold (Au) nanoshells with solid silica cores have great potential for cancer photothermal therapy. However, this nanostructure cannot carry enough drugs. Here, we report a Au nanoshell with a hollow silica core for drug loading and cancer therapy. The silica shells were synthesized using nanoliposome templates, and then Au nanoshells were grown on the outer surface of the silica shells. Transmission-electron and scanning-electron microscopy showed that the Au nanoshells were successfully fabricated, and that the liposome/SiO2/Au core-shell nanocomposites were spherical with a narrow size distribution. Images of several broken spheres, and the fact that hollow templates (liposomes) were used, suggest that the fabricated Au nanoshells were hollow. After doxorubicin (DOX) was incorporated into liposome/SiO2/Au, the DOX-loaded Au nanoshells killed cancer cells with high therapeutic efficacy when irradiated with near-infrared light, suggesting that the Au nanoshells delivered both DOX chemotherapy and photothermal therapy with a synergistic effect.
Introduction
Gold (Au) nanoparticles have been widely used in biomedical research areas for biomolecular, cell, and tissue labeling for many decades. In recent years, Au nanomaterials have attracted greater attention from researchersCitation1 because it was demonstrated that Au nanomaterial structures have great potential for cancer photothermal therapy. These special nanostructures, including nanoshells,Citation2–Citation8 nanocages,Citation9,Citation10 nanocubes,Citation11 and nanorods,Citation12,Citation13 have a strong surface plasmon resonance in the near-infrared (NIR) region. This benefits in situ cancer therapy because these Au nanostructures can be excited by NIR radiation, which can penetrate tissue more deeply than irradiation with shorter wavelengths. Au nanoshells are composed of a dielectric nanocore covered by an ultrathin Au shell. The resonant wavelength of the Au nanoshell can be easily turned to the NIR band through adjustments of the shell size and thickness. The shell structures are usually stable because the shells are firmly fixed on water-insoluble solid nanocores.
All Au nanoshells have been synthesized using a template-mediated method. These templates include silica nanospheres,Citation2 polystyrene (PS) nanospheres,Citation4 and poly(lactic-co-glycolic acid) (PLGA) nanospheres.Citation5 The complexity of core-shell formation is mainly dependent on the core (template) formation method. Au shells are synthesized in a similar manner (Au chloride hydrate is reduced by a reducing agent). The formation method of the silica nanocores usually consists of one simple step: the hydrolyzation of tetraethyl orthosilicate (TEOS) in alkaline aqueous solution. This is more convenient and simpler than that of the PLGA or PS nanocores, which are prepared through emulsified-solvent evaporation or emulsion polymerization methods consisting of several steps. However, previously reported Au nanoshells containing silica cores could not be loaded with enough drug for practical use because the inorganic silica nanoparticles are a solid structure.Citation2
In this work, hollow silica cores were synthesized and used as templates to prepare drug-loaded Au nanoshells for cancer therapy. Lipid vesicles (liposomes) have been used as templates to prepare hollow materials.Citation14–Citation16 Therefore, we used nanoscale liposomes as templates for the synthesis of silica shells through the hydrolyzation of TEOS. The obtained silica shells were then coated with Au shells through the reaction of Au chloride tetrahydrate and hydroxylamine. This novel method for the preparation of Au nanoshells with silica inner shells is quite simple. The liposome templates were formed by the self-assembly of a lipid in water and the growth of the silica and Au shells was a heterogeneous nucleation process. Because the liposome cores are hollow, doxorubicin (DOX) can be incorporated into the nanocomposites. It has been demonstrated that NIR lasers can be used to excite the metallic nanoparticles, and induce drug release from the nanocomposites, which supplies a dose of both drugs and metallic nanoparticles.Citation17 The DOX-loaded Au nanoshells killed cancer cells after being irradiated by NIR light and showed a synergistic effect of chemotherapy and photothermal therapy. The novel drug-loaded Au nanoshell structure prepared and applied in this work will be of interest to both material scientists and biologists.
Materials and methods
Materials
The Au chloride tetrahydrate (HAuCl4), hydroxylamine (NH2OH, 50 wt.% solution in water) were purchased from Sigma-Aldrich (St Louis, MO). The soybean lecithin, cholesterol, TEOS, aminopropyltrithoxy silane (KH-550), trichloromethane, ethanol, NaOH, dimethyl sulfoxide (DMSO), and distilled water were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). DOX was obtained from Shanghai Humanity Hospital. The propidine Iodide (PI) and Hoechst33342 were purchased from Sigma-Aldrich. The human liver cancer cells (SMMC-7721) were ordered from the Chinese Academy of Sciences (Shanghai, China). The RPMI-1640 culture medium and fetal calf serum (FCS) were obtained from Gibco (Carlsbad, CA). 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-diphenytetrazoliumromide (MTT) was purchased from Shanghai Haoran Biological Technology Co., Ltd. (Shanghai, China).
Silica-coated liposome nanocomposites synthesis
The soybean lecithin (120 mg) and cholesterol (8 mg) were dissolved in chloroform (1 mL) in a round-bottomed flask and dried via rotary evaporation by blow-drying with flowing nitrogen gas to make a thin film. The film was then further blow-dried for about 30 minutes with nitrogen gas to remove the last traces of chloroform. Distilled water (8 mL) was added to the flask to hydrate the dried lipid film, the contents were gently shaken, and then sonicated for about 30 minutes in nitrogen. The liposome dispersion was extruded through polycarbonate membrane filters (Sinopharm Chemical Regent Co., Ltd), which had pore diameters of 200 nm. The liposome suspension was diluted with distilled water (liposomes: water = 1:3 (v/v)), and the pH was adjusted to 10.8 with NaOH. TEOS (100 μL; containing 33% (v/v) ethanol) was then added into the diluted liposome suspension (4 mL) and gently stirred at room temperature. Twenty-four hours later, the stirring was stopped and the silica-coated liposome (liposome/SiO2) suspension was then stored at 4°C in a refrigerator.
Gold nanoshell synthesis
The distilled-water-diluted KH550 (20 μL, 6 mol/L) was mixed with the liposome/SiO2 suspension (200 μL) for about 2 hours. HAuCl4 aqueous solution (50 μL; 4 mM; pH 6.5, adjusted by NaOH) and NH2OH aqueous solution (25 μL; 2 mmol/L) were added to the mixture under gentle stirring. After a 10-minute incubation, a higher-concentration HAuCl4 solution (50 μL; 40 mM; pH 6.5) was added. Then the NH2OH solution (400 μL; 20 mmol/L) was added to the mixture drop by drop. After the addition of NH2OH was finished, the reaction solution was continuously stirred for 10 minutes. The suspension was precipitated via centrifugation. The precipitate was washed with water and then freeze-dried for 72 hours.
Fabrication of DOX-loaded liposome/SiO2/Au
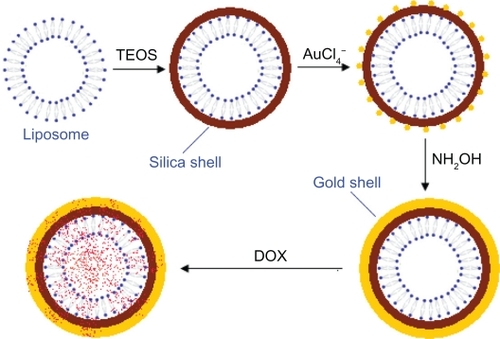
The liposome/SiO2/Au precipitate (5 mg) was added to DOX aqueous solution (200 μL; 1.78 mg/mL) and incubated at room temperature. Twelve hours later, the suspension was precipitated via centrifugation and washed once with water to remove the free DOX. The washed precipitate was then resuspended in a serum-free RPMI 1640 medium before cell experimentation. A schematic of the whole DOX-loaded liposome/SiO2/Au formation process described above is shown in .
Characterization of the liposome/SiO2/Au
The liposome/SiO2 and liposome/SiO2/Au aqueous suspensions were air-dried onto carbon-coated grips, and then examined at 80 kV using a transmission electron microscopy (TEM; JSM-6360LV; JEOL, Tokyo, Japan). The morphology of liposome/SiO2/Au was also observed using a scanning electron microscopy (SEM; JSM-6360LV; JEOL) equipped with energy-dispersive X-ray spectrometry (EDS; JEM-1200EX/S; JEOL). Before SEM observation, the sample was freeze-dried at −70°C for 48 hours. The size distributions were measured via SEM analysis of a 200-particle sample. Ultraviolet–visible (UV–Vis) absorption spectra were taken on a diode array spectrophotometer (UV-2102PC; Unico, Beijing, China) with a deuterium-lamp source. X-ray diffraction (XRD) patterns were recorded on a D8 DISCOVER with a GADDS diffractometer (Bruker AXS, Karlsruhe, Germany) using Cu Kα (λ = 0.154 18 nm) radiation.
In vitro release experiments
A 200-μL DOX-loaded liposome/SiO2/Au suspension with concentration of about 15 mg/mL was loaded into a small plastic centrifugal tube at room temperature, and irradiated using a NIR laser (wavelength: 808 nm; power: 100 mW; spot area: 5 × 8 mm) for 15 minutes in a dark room. The irradiated suspension was then centrifuged immediately. The supernatant solution was measured through UV–Vis absorbance taken on a diode array spectrophotometer (UV-2102PC; Unico, Shanghai, China) with a deuterium lamp source. As a control, a DOX-loaded liposome/SiO2/Au suspension containing the same volume and same concentration as the above suspension was placed at room temperature for 15 minutes in the dark room, and the suspension was also centrifuged and the supernatant solution obtained was then measured through UV–Vis absorbance. All experiments were conducted in triplicate.
Cell experiments
Human liver cancer cells (SMMC-7721) were cultured in a RPMI-1640 medium, which was 10% FCS and 1% antibiotic-antimycotic, at 37°C in an incubation chamber containing 5% CO2. The serum-free RPMI 1640 medium-dispersed DOX-loaded liposome/SiO2/Au (containing 250 μg/mL of liposome/SiO2/Au and 22 μg/mL of DOX), liposome/SiO2/Au (250 μg/mL), and DOX (22 μg/mL) were added into six wells of cells and incubated with the cells. Each type of material was incubated with two wells of cells. After a 1-hour incubation, three wells of cells incubated with DOX-loaded liposome/SiO2/Au, liposome/SiO2/Au, and DOX were irradiated using the 808-nm laser for 15 minutes. As a control, the other three wells of cells were not irradiated by the laser. To evaluate the cell viability, fluorescent dyes (PI [10 μg/mL] and Hoechst33342 [3 μg/mL]) were then added to the above six wells, which were incubated for 20 minutes. The cells were imaged with an upright fluorescent microscope (Leica DME, Wetzlar, Germany). The in vitro cell viability was evaluated using a MTT assay. The experimental details were as follows: SMMC-7721 cells were seeded onto 96-well plates and incubated in 200 μL of the medium at 37°C in 5% CO2 for 24 hours before treatment. There were three treatment groups: cells being incubated with DOX (22 μg/mL in RPMI 1640, 100 μL), liposome/SiO2/Au (250 μg/mL in RPMI 1640, 100 μL), and DOX-liposome/SiO2/Au (272 μg/mL in RPMI 1640, 100 μL), which were irradiated with NIR light for 15 minutes. Another three identical treatment groups were incubated but without NIR laser irradiation. The cells incubated with RPMI 1640 (100 μL for each well) served as the control group. After a 75-minute incubation, MTT (5 mg/mL in phosphate-buffered saline, 20 μL) was added into each well of the plate. Four hours later, the suspensions were replaced with 200 μL of DMSO, and the cell viability of each group was determined by measuring their absorbance at 570 nm using a Flexstation III enzyme-labeled instrument (Molecular Devices, Sunnyvale, CA).
Results and discussion
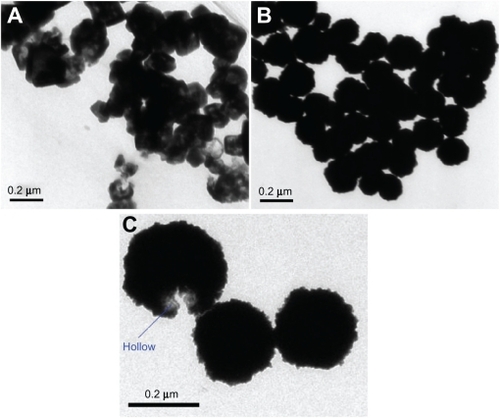
The liposomes/SiO2 before and after Au coating were generally spherical. However, their detailed morphologies are quite different. In the TEM image of the silica-coated liposome (liposome/SiO2), the contrast between the dark edge and pale center suggests that it is hollow (). Liposomes are hollow lipid vesicles, but their membranes are hard to observe via TEM unless the liposomes are negatively stained using a special reagent (such as phosphotungstic acid). Therefore, the dark shells suggest that SiO2 grew on the outer surface of the liposomes. Hydrolyzation of TEOS on the lipid membrane is a heterogeneous nucleation process. The lipid membranes can lower the activation energy of nucleation. Therefore, the nucleation rate of SiO2 deposition on the liposomes may be improved. This may be the main reason why the lipid membrane can be used as template for silica-shell formation. Because a silica shell that is synthesized in alkaline solution is negatively charged, we modified the silica shells with KH550, a silane-coupling agent with an amido group (−NH2). The silica shells with positively charged −NH3+ surface groups in aqueous solution can capture negatively charged AuCl4−. The HAuCl4 was, therefore, reduced by the reducing agent (NH2OH) on the silica shells. After NH2OH was added into the mixture of liposome/SiO2 and HAuCl4, the hollow structures of the liposome/SiO2 were difficult to observe (), which indicated that the hollow silica spheres were successfully coated with Au shells. Au materials are usually black when viewed via TEM. Au nanoparticles have been widely used for cell and tissue labeling because their black appearance can be easily distinguished from the background. The hollow morphology of the nanospheres was masked by the Au nanoparticles and could not be observed directly. Only from broken liposome/SiO2/Au nanospheres (only a small number of broken nanospheres were found in all the collected TEM images) was the hollow nature observed ().
Figure 2 Transmission-electron microscopy images of the A) liposome/SiO2 and B, C) liposome/SiO2/Au nanospheres.
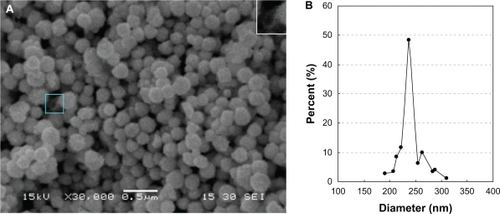
SEM images of the liposome/SiO2/Au nanospheres indicate that the liposome/SiO2 nanospheres were successfully coated with Au shells (). Broken nanospheres served as evidence that the liposome/SiO2/Au nanospheres were hollow (, see the blue frame or the inset at the top right corner of the image). The average diameter, measured from the SEM image, was 238.47 ± 39.08 nm, which indicates that the liposome/SiO2/Au nanospheres had a narrow size distribution ().
Figure 3 A) Scanning-electron microscopy image and B) size distribution of the liposome/SiO2/Au nanoparticles.
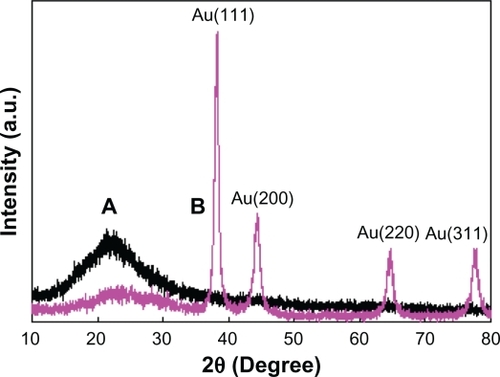
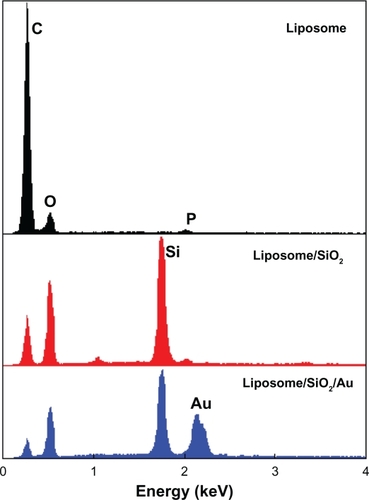
EDS () and XRD () were used to further confirm the formation of the silica and Au shells. After TEOS had been added to the liposome suspension, the EDS spectrum of the product was measured. It showed that the signal intensity corresponding to O was significantly enhanced and a new strong signal (Si) appeared. These results and the fact that the hydrolysate of TEOS is SiO2 imply that the hollow nanoparticles shown in were silica shells. After HAuCl4 and NH2OH were introduced into the liposome/SiO2 suspension, a strong Au signal appeared in the EDS spectrum of the product. This result combined with the TEM images shown in (or ) imply that the liposome/SiO2 nanoparticles were coated with Au shells. Compared with the XRD pattern of the liposome/SiO2 nanospheres, XRD pattern of the liposome/SiO2/Au nanospheres had four additional diffraction peaks at 2θ = 38.2°, 44.4°, 65.5°, and 77.9°. These four peaks perfectly match the face-centered cubic structure of Au, which also indicates that Au nanoshells were deposited on the liposome/SiO2 nanospheres.
Figure 4 Energy dispersive X-ray spectroscopy spectra of the liposome, liposome/SiO2 and liposome/SiO2/Au.
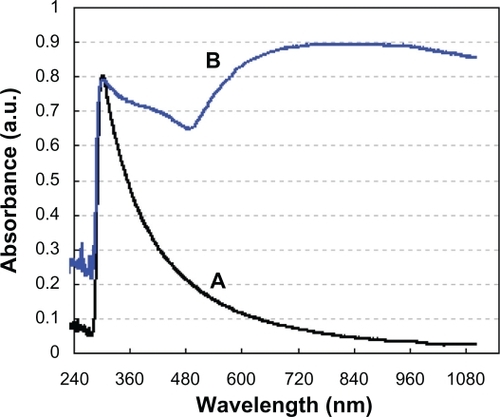
The liposome/SiO2 had only one sharp UV-absorption peak at a wavelength of 302 nm. After being coated with Au shells, the obtained liposome/SiO2/Au not only contained the sharp peak at about 302 nm, but also a broad absorption band between 500 and 1100 nm, which had a maximum at 810 nm (). Dispersed small Au nanoparticles have an absorption peak at 520 nm.Citation10 The broad absorption peak between 500 and 1100 nm implies that most of the small Au nanoparticles on the liposome/SiO2 surfaces were syncretized with each other and formed continuous shells. The fact that the liposome/SiO2/Au nanospheres show strong NIR absorption (>650 nm) is interesting, because NIR light energy can be converted into thermal energy by the Au nanoshells, which provides great potential for photothermal cancer therapy.
Figure 6 Ultraviolet–visible absorption spectra of the A) liposome/SiO2 and B) liposome/SiO2/Au nanoparticles.
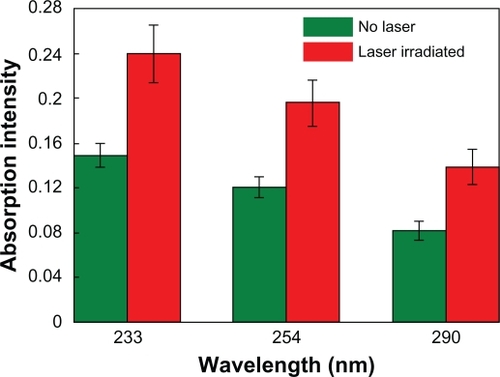
The hollow liposome/SiO2/Au nanoparticles can be used as drug carriers. Here, DOX was selected to be incorporated into these nanocomposites because this water-soluble drug is popularly used for cancer chemotherapy. In this work, 1 mg of liposome/SiO2/Au dry powder was loaded with about 88μg of DOX. DOX has three sharp UV absorption peaks at about 233, 254, and 290 nm, respectively. After the DOX-loaded liposome/SiO2/Au nanoparticles being irradiated by the 808-nm laser and the suspensions being centrifuged, the absorption peak intensities of the supernatant solutions were obviously higher than those of the supernatant solutions obtained from the DOX-loaded liposome/SiO2/Au nanoparticles without laser irradiation (). This indicates that the DOX is rapidly released from the DOX-loaded liposome/SiO2/Au nanoparticles when they are irradiated by NIR light.
Figure 7 Absorption intensities of the supernatant solutions obtained from the DOX-loaded liposome/SiO2/Au nanoparticles with and without 808-nm laser irradiation. The higher the absorption intensity, the more the DOX released.
Abbreviation: DOX, doxorubicin.
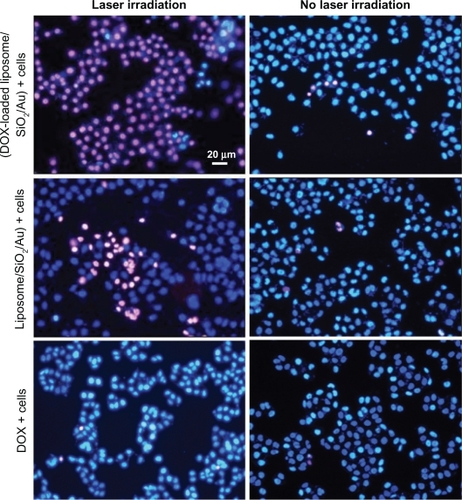
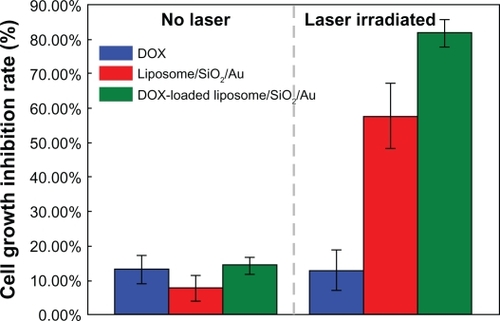
From the fluorescence images shown in , it can be seen that when the liposome/SiO2/Au and DOX-loaded liposome/SiO2/Au nanoparticles were incubated with human liver cancer cells (SMMC-7721), the viability of only a small amount of cells was reduced. This indicates that the liposome/SiO2/Au before and after being loaded with DOX that had not been irradiated had a low toxicity. After being irradiated by the 808-nm laser, however, most of the cancer cells were killed by the DOX-loaded liposome/SiO2/Au. This suggests that the toxicity of the DOX-loaded liposome/SiO2/Au was stronger than that of the liposome/SiO2/Au without DOX. The viability of the cancer cells incubated with DOX with or without NIR light irradiation was not noticeably different. This may have been because of the lower DOX concentration in the culture medium. In addition, the cell viability described above was more accurately evaluated using a MTT assay. From , it can be seen that when the cells were incubated with DOX, liposome/SiO2/Au and DOX-loaded liposome/SiO2/Au without laser irradiation, the cell growth inhibition rates were 13.08% ± 3.97%, 7.63% ± 3.80%, and 14.27% ± 2.47%, respectively. For the case that cells incubated with DOX and irradiated by laser irradiation, the cell growth inhibition rate was 12.91% ± 5.85%, which was similar to the case without laser irradiation. However, for the case of the laser irradiated cells incubated with liposome/SiO2/Au and DOX-loaded liposome/SiO2/Au, the cell growth inhibition rates were 57.76% ± 9.36% and 81.63% ± 4.03%, respectively. These levels were significantly higher than the cases without laser irradiation. Au nanoshells can rapidly convert NIR light energy into local thermal energy and DOX is a common chemotherapy drug that remains a popular choice for cancer therapy. The cell toxicity of the samples with DOX-loaded Au nanoshells irradiated by NIR light was significantly stronger than that of the samples with Au nanoshells or DOX alone when irradiated by the same NIR light. This indicates that the DOX-loaded Au nanoshells delivered both chemotherapy and photothermal therapy with a synergistic effect on the cell growth inhibition rate. Similar results have been reported previously.Citation5 Because the Au nanoshells synthesized in this work were hollow structures, they may be also used as other chemotherapy drug or photosensitizer carriers in the future. If a photosensitizer was loaded, the nanocomposites may exhibit a similar synergistic effect through the delivery of both photodynamic therapy and photothermal therapy, which should be investigated in future work.
Conclusion
In summary, hollow Au nanoshells, composed of lipid vesicle cores (liposomes), silica shell interlayers and a Au shell outer surface, were successfully synthesized, and DOX was subsequently loaded into these nanocomposites. The nanocomposites obtained were spherical with a narrow size distribution (average diameter: 238.47 ± 39.08 nm). When a NIR laser with a wavelength of 808 nm was used to irradiate human liver cancer cells (SMMC-7721) incubated with the DOX-loaded Au nanoshells, Au nanoshells, and DOX, the DOX-loaded Au nanoshells were found to kill the cancer cells more efficiently than the Au nanoshells without DOX or DOX alone. The preparation method of the hollow Au nanoshells described above was simple. In future work, other drugs such as photosensitizers or other chemotherapy agents can be encapsulated into these nanospheres.
Acknowledgements
We thank Yiming Shou for his assistance with the hollow silica nanosphere preparation. This work was supported in part by the Key Technologies R & D Program in the 11th Five-year Plan of China (2009ZX09103-701) and the National Natural Science Foundation of China (81071833).
Disclosure
The authors report no conflicts of interest in this work.
References
- CaiWGaoTHongHSunJApplication of gold nanoparticles in cancer nanotechnologyNanotechnology, Science and Applications200811732
- HirschLRStaffordRJBanksonJANanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidanceProc Natl Acad Sci U S A200310023135491355414597719
- SternJMStanfieldJKabbaniWHsiehJDCadedduJASelective prostate cancer thermal ablation with laser activated gold nanoshellsJ Urol2008179274875318082199
- LiuHYChenDTangFQPhotothermal therapy of Lewis lung carcinoma in mice using gold nanoshells on carboxylated polystyrene spheresNanotechnology2008194551015107
- ParkHYangJLeeJHaamSChoiIHYooKHMultifunctional nanoparticles for combined doxorubicin and photothermal treatmentsACS Nano20093102919292619772302
- WuGMikhailovskyAKhantHAFuCChiuWZasadzinskiJARemotely triggered liposomal release by near-infrared light absorption via hollow gold nanoshellsJ Am Chem Soc2008130268175817718543914
- LoweryARGobinAMDayESHalasNJWestJLImmunonanoshells for targeted photothermal ablation of tumor cellsInt J Nanomedicine20061214915417722530
- GobinAMLeeMHHalasNJJamesWDDrezekRAWestJLNear-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapyNano Lett2007771929193417550297
- ChenJWangDXiJImmuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cellsNano Lett2007751318132217430005
- SkrabalakSEAuLLuXLiXXiaYGold nanocages for cancer detection and treatmentNanomedicine20072565766817976028
- WuXMingTWangXWangPWangJChenJHigh-photoluminescence-yield gold nanocubes: for cell imaging and photothermal therapyACS Nano20104111312020014823
- HauckTSJenningsTLYatsenkoTKumaradasJCChanWCWEnhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermiaAdv Mater2008202038323838
- HuangXEl-SayedIHQianWEl-SayedMACancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorodsJ Am Chem Soc200612862115212016464114
- BéguSAubert PouësselALernerDATourné-PéteilhCDevoisselleJMLiposil, a promising composite material for drug storage and releaseJ Control Release200711811617250924
- ChuMLiuGSynthesis of liposomes-templated CdSe hollow and solid nanospheresMater Lett20066011114
- TanGXuPHeJLawsonLMcPhersonGLJohnVTHighly aspherical silica nanoshells by templating tubular liposomesSoft Matter200953006300920352059
- SkirtachAGJavierAMKreftOLaser-induced release of encapsulated materials inside living cellsAngew Chem Int Ed20064546124617