?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of this work was to investigate the use of nanocrystalline cellulose (NCC) as a drug delivery excipient. NCC crystallites, prepared by an acid hydrolysis method, were shown to have nanoscopic dimensions and exhibit a high degree of crystallinity. These crystallites bound significant quantities of the water soluble, ionizable drugs tetratcycline and doxorubicin, which were released rapidly over a 1-day period. Cetyl trimethylammonium bromide (CTAB) was bound to the surface of NCC and increased the zeta potential in a concentration-dependent manner from −55 to 0 mV. NCC crystallites with CTAB-modified surfaces bound significant quantities of the hydrophobic anticancer drugs docetaxel, paclitaxel, and etoposide. These drugs were released in a controlled manner over a 2-day period. The NCC-CTAB complexes were found to bind to KU-7 cells, and evidence of cellular uptake was observed.
Introduction
Cellulose has a long history of use in the pharmaceutical industry. The material has excellent compaction properties when blended with other pharmaceutical excipients so that drug-loaded tablets form dense matrices suitable for the oral administration of drugs. The form of cellulose used in tablets is termed microcrystalline cellulose (MCC) which is a purified, depolymerized alpha cellulose derived from plant sources.Citation1 Despite an extended history of use in tableting, there is still considerable continuing research into the use of MCC and other types of cellulose in advanced pelleting systems whereby the rate of tablet disintegration and drug release may be controlled by microparticle inclusion, excipient layering or tablet coating.Citation2–Citation11
Derivatized cellulose has also been used extensively in pharmaceutical preparations so that ethyl cellulose, methyl cellulose, carboxymethyl cellulose, and numerous other forms are used in oral, topical, and injectable formulations. For example, carboxymethyl cellulose is the primary component of Seprafilm™, which is applied to surgical sites to prevent post surgical adhesions. Recently, the use of MCC in self-emulsifying drug-delivery systems and semisolid injectable formulations has also been described.Citation2,Citation12 The use of these forms of cellulose in such formulations points to the inertness and excellent biocompatibility of cellulose in humans. Additionally, hydroxypropyl methyl cellulose has recently been used as a hydrogel matrix for chondrocyte implantation into animal joints for cartilage repair.Citation13
However, these uses of cellulose in formulations do not involve direct molecular level control of drug release via binding interactions with the drug. Although the surface of MCC has a slight negative charge due to hydroxyl residues, this charge is confined to a relatively small surface area on a large mass of insoluble MCC and would not likely adsorb or bind significant amounts of drug. The principle of using charged particles to bind acidic or basic drugs is well established as evidenced by the use of ion-exchange resins for the past 50 years to bind and release drugs.Citation14 Drug release from resins is usually rapid once the resin–drug complex reaches the target site, since counter ions present in body fluids displace the drug from the binding site. For extended release, resins have been coated with agents such as ethyl or carboxymethyl cellulose to delay drug elution. Complex polysaccharides such as chitosan have been used extensively in controlled release drug formulations. Such methods rely on a charge interaction between the positive charge on amine groups of each sugar residue in chitosan with negatively charged drugs such as antisense oligonucleotides.Citation15,Citation16 The high positive and negative charges on chitosan and oligonucleotides respectively, allow for a strong binding interaction between the carrier and the drug so that phosphate counterions tend to release a weakly bound fraction rapidly and a tightly bound fraction very slowly.Citation16
Nanocrystalline cellulose (NCC) is extracted from lignocellulosics by acidic extraction methods resulting in the formation of nanodimensional cellulose rods, termed crystallites, with a very high surface to volume ratio.Citation17 Due to its large surface area, high aspect ratio, and tremendous stiffness and strength, NCC is actively being investigated as a nano-composite material. For a thorough review of the preparation, properties, modification, and application of NCC, readers are directed to the excellent review by Habibi et al.Citation18
NCC offers several potential advantages as a drug delivery excipient. The very large surface area and negative charge of NCC suggests that large amounts of drugs might be bound to the surface of this material with the potential for high payloads and optimal control of dosing. Other nanocrystalline materials, such as nanocrystalline clays have been shown to bind and subsequently release drugs in a controlled manner via ion exchange mechanisms and are being investigated for use in pharmaceutical formulations.Citation19 The established biocompatibility of cellulose supports the use of NCC for a similar purpose. The abundant surface hydroxyl groups on NCC provide a site for the surface modification of the m aterial with a range of chemical groups by a variety of methods. Surface modification may be used to modulate the loading and release of drugs that would not normally bind to NCC, such as nonionized or hydrophobic drugs. For example, Lonnberg et al suggested that poly(caprolactone) chains might be conjugated onto nanocrystalline cellulose for such a purpose.Citation20 Additionally, since NCC is a low-cost, readily abundant material from a renewable and sustainable resource, its use provides a substantial environmental advantage compared with other nanomaterials.
The objectives of this work were to investigate the binding and release of a range of ionized drugs from NCC and to surface modify NCC with the surfactant, cetyl trimethylammonium bromide (CTAB) for the binding and release of hydrophobic drugs. To our knowledge, this is the first report of the use of NCC as a drug delivery excipient.
Materials and methods
Materials
Nanocrystalline cellulose was prepared according to the method outlined below by FPInnovations (Vancouver, BC, Canada). A 4 mg/mL suspension in deionized water was used for this investigation. Docetaxel was obtained from Natural Pharma Inc, (Vancouver, BC, Canada). Doxorubicin hydrochloride, paclitaxel, and etoposide were supplied by Polymed Therapeutics, Inc (Houston, TX, USA). Cetyl trimethylammonium bromide and tetracycline hydrochloride were supplied by Sigma Aldrich (Oakville, ON, Canada). Methoxy poly(ethylene glycol)-block-poly (D,L lactide) (MePEG-b-PDLLA) diblock copolymer was synthesized and characterized as previously described.Citation21
NCC preparation
NCC was prepared by a previously reported method.Citation22 Briefly, a fully bleached, commercial softwood was milled to pass through a 0.5-mm screen in a Wiley mill to ensure particle size uniformity and increased surface area. The milled pulp (40.0 g) was hydrolyzed in 64% w/w sulfuric acid (8.75 mL of a sulfuric acid solution/g pulp) at 45°C and stirred at high speed for 25 minutes. The cellulose suspension was then diluted with cold, deionized distilled water (dH2O) and allowed to settle overnight. The clear top layer was decanted and the remaining white cloudy layer was washed with deionized water and centrifuged three times to remove practically all soluble cellulose materials. The resulting thick, white suspension was dialyzed against slow running dH2O for 1–4 days using dialysis tubing with a 12,000–14,000 molecular weight cutoff (Spectra/Por®, Spectrum Laboratories, Inc, Rancho Dominguez, CA, USA). The suspension from the dialysis tubes was dispersed by subjecting it to ultrasound treatment in a Fisher Sonic Dismembrator (Fisher Scientific, Ottawa, ON, Canada) for 10 minutes at 60% power.
X-ray powder diffraction
Diffraction data were collected on films of lyophilized and air dried NCC using a previously described method.Citation23 Briefly, a Bruker D8 Advance powder x-ray diffractometer (Bruker Corporation, Milton, ON, Canada) equipped with a CuXα x-ray tube, a diffracted beam graphite monochromator, and Nal scintillation detector was used with the generator set to 40 kV and 40 mA. Data was collected from 5°–90° 2θ using a step size of 0.02° and a counting time of 1 sec/step. A divergence of 1.00 mm and anti-scatter slits were used with a 0.2 mm receiving slit, and the sample was rotated during data collection. The films were mounted on zero-background Si plates. The peaks were assigned according to the monoclinic unit cell for cellulose I. The crystallinity was obtained from the x-ray intensity, as described by Hamad and Hu.Citation23
Imaging
Air-dried films or a drop of the NCC suspension in dH2O were mounted on aluminum stubs using adhesive carbon tabs, and the stubs were sputter-coated with AuPd. Morphological characteristics were observed using an FEI Quanta 400F field-emission scanning electron microscope (SEM) (FEI Company, Hillsboro, OR, USA), operated in high vacuum mode with a 5 kV accelerating voltage.
NCC drug binding
Doxorubicin hydrochloride (DOX) or tetracycline hydrochloride (TET) were dissolved in either 10 mM phosphate buffered saline (PBS) at pH 7.4, or dH2O with increasing drug concentrations ([drugadded]). Drug solutions (1.5 mL) were added to 0.5 mL of a 4 mg/mL NCC suspension (2 mg of NCC) in a 2 mL microcentrifuge tube and incubated at 37°C with tumbling at 8 rpm for 30 minutes. Suspensions containing PBS or NaCl produced flocculated NCC/drug suspensions, which were centrifuged at 18000 × g for 10 minutes to pellet the NCC and bound drug. The concentration of unbound drug in the supernatant ([drugunbound]) was assayed using a Varian 50 Bio UV Vis spectrophotometer (Varian, Inc., Mississauga, ON, Canada) using wavelengths of 482 nm and 364 nm for DOX and TET, respectively. The concentration of drug bound to the NCC ([drugbound]) was calculated using the following formula:
NCC does not flocculate in dH2O; therefore, the NCC/drug complexes prepared in dH2O could not be separated by microcentrifugation. In this case, the NCC/drug suspensions were transferred to dialysis bags with a molecular weight cutoff of 10,000 Da (Spectra/Por®, Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA) and dialyzed against dH2O overnight in the dark at 4°C. The concentration of unbound drug in the dialysate was determined by UV Vis spectroscopy, allowing for the calculation of the amount of drug bound to the NCC according to EquationEquation 1(1) .
To solubilize the hydrophobic drugs paclitaxel (PTX), docetaxel (DTX), and etoposide (ETOP), the surface of the NCC was first modified with CTAB. This was achieved by incubating increasing amounts of CTAB with 2.5 mg of NCC so the final CTAB concentration varied from 0 mM to 12.9 mM. An aliquot of 100 mM NaCl was added, resulting in a final NaCl concentration of 10 mM, which facilitated flocculation and subsequent separation of the NCC/CTAB nanocomplexes by centrifugation as described above. The NCC/CTAB was incubated with stock solutions of the drugs with increasing concentrations. Since these drugs are characterized by low aqueous solubility, they were solubilized in a minimal amount of either DMSO or MePEG-b-PDLLA diblock copolymer in 10 mM NaCl as previously described.Citation21 The drug/NCC/CTAB suspensions were incubated at 25°C with tumbling at 8 rpm for 30 minutes then centrifuged at 18000 × g for 10 minutes to pellet the NCC/CTAB and bound drug. The amount of unbound drug in the supernatant was determined by high performance liquid chromatography (HPLC) using a Waters HPLC system with Millennium software and UV Vis detection (Waters Ltd, Mississauga, ON, Canada). Separation was achieved using a Novapak C18 column with 20 μL injections and a mobile phase flow rate of 1 mL/min. The DTX and PTX mobile phase consisted of 58% acetonitrile, 37% dH2O, and 5% methanol, with detection at 232 nm. The mobile phase for ETOP was 27% acetonitrile, 1% acetic acid, and 72% dH2O, and detection was at 286 nm. Calibration curves were prepared for all drugs and were linear in the desired concentration range. The amount of drug bound to the NCC/CTAB was determined using EquationEquation 1(1) .
Drug release studies
DOX was bound to NCC for release studies by incubating a solution of 325 μg/mL of DOX in dH2O with a suspension containing 2.5 mg of NCC. To flocculate the NCC and allow for separation of the NCC/DOX nanocomplex, NaCl was added to a final concentration of 10 mM. The suspension was centrifuged at 18000 × g for 10 minutes to pellet the NCC/DOX, and the drug binding was determined by UV Vis spectroscopy as described above. The final mass of DOX bound to the NCC for the release studies was 212 ±3.5 μg. The same procedure was used to prepare TET-bound NCC nanocomplexes for the release studies, with the exception that the initial TET solution used was 1000 μg/mL, which resulted in the binding of 187 ± 2.0 μg of TET. NCC/drug nanocomplexes with DTX, PTX, and ETOP were prepared as described for the drug-binding studies. The concentration of DTX and PTX that was incubated with the NCC suspension was 200 μg/mL, and the concentration of ETOP was 100 μg/mL. The final mass of drug bound to the NCC was 184 ± 4.8 μg, 149 ± 4.8 μg, and 63 ± 0.1 μg for DTX, PTX, and ETOP, respectively. The drug loaded NCC samples were resuspended in 1 mL of PBS followed by incubation at 37°C with tumbling at 8 rpm. At predetermined times, the suspensions were centrifuged at 18000 × g for 10 minutes, and the supernatant was removed for drug quantification by UV Vis for DOX and TET or HPLC for DTX, PTX, and ETOP, as previously described. At each sampling time point, fresh PBS was added to the tubes, and the NCC/drug nanocomplexes were resuspended.
Zeta potential measurements
The effect of CTAB concentration on zeta potential of NCC was determined by incubating 2.5 mg samples of NCC with increasing concentrations of CTAB in dH2O to yield final CTAB concentrations of 0, 0.755, 1.13, 1.51, 1.89, 2.27, and 3.02 mM. The zeta potentials of the samples were measured using a Malvern 3000 HS-Zetasizer (Malvern Instruments Ltd, Malvern, UK).
Cell binding and uptake studies
KU-7 bladder cancer cells were a kind gift from Dr Martin Gleave at the Prostate Centre, Vancouver General Hospital and were grown in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, 5% glutamine, and 5% penicillin/streptomycin. Cells were plated at 10,000 cells per well on a 96-well cell culture plate and allowed to equilibrate overnight. NCC for binding studies was prepared by coating with CTAB followed by fluorescein binding. Briefly, 2 mg of NCC were suspended in a 3 mM CTAB in 5 mM sodium chloride solution for 30 minutes at 37°C. The suspension was centrifuged at 18,000 × g for 30 seconds and the supernatant discarded. The pellet was resuspended in 1 mL of a 5 mM sodium chloride solution containing 100 μg/mL fluorescein and incubated for 30 minutes at 37°C. The suspension was centrifuged at 18,000 × g for 30 seconds, and the supernatant was discarded followed by resuspension in PBS and serial dilution to give concentrations ranging from 10 to 0.06 mg/mL. To each cell culture plate well, 200 μL of each NCC suspension was added, and the cells were allowed to incubate for 24 hours. The media was removed and the cells washed three times with PBS prior to lysis with 2% Triton X-100. The fluorescence intensity of the NCC/CTAB/fluorescein bound to the cells was determined using a fluorescence spectroscopy plate reader with excitation and emission wavelengths of 480 and 520 nm, respectively. These values were converted to mass of NCC/CTAB/fluorescein bound to cells using a previously constructed standard curve of fluorescence intensity as a function of mass of NCC/CTAB/fluorescein. A BCA total protein assay kit (Pierce Chemicals, Rockford, IL, USA) was used to ensure that each well contained the same number of cells.
To visualize cell uptake of NCC, KU-7 cells were cultured as described for the binding studies. Glass cover slips (1 cm diameter) were sterilized and placed in the base of 6 well tissue culture plates followed by the addition of 50,000 cells to each well and incubation overnight. Cells were incubated with a 1.25 mg/mL NCC/CTAB/fluorescein suspension for various times at 37°C under culture conditions. The cover slips were removed, washed three times in PBS and then treated with 4′,6-diamidino-2-phenylindole (DAPI) in Progold stain for 30 minutes, washed again and then treated with 3.7% paraformaldehyde in dH2O for 30 minutes. The cover slips were placed cell side down on microscope slides and sealed with clear nail varnish. Fluorescein- and DAPI-stained nuclei were visualized using an Olympus FV-1000 confocal laser scanning microscope (Olympus Canada, Inc., Markham, ON, Canada).
Results
X-ray powder diffraction and imaging of NCC
The X-ray powder diffraction (XRPD) pattern of NCC was characterized by two major peaks centered around 15° and 23° 2θ (). The degree of crystallinity of the sample was calculated to be greater than 90%. The SEM images of NCC as a dried film show a layered pattern of NCC crystallites oriented in a common direction ().
Figure 1 X-ray powder diffraction pattern of lyophilized nanocrystalline cellulose. Intense peaks at 15° and 23° 2θ, due to constructive interference of diffracted x-rays, in addition to the lack of an amorphous halo, indicate the highly crystalline nature of the material.
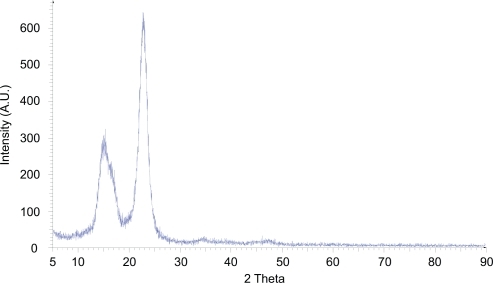
Figure 2 Scanning electron micrograph of a dried film of nanocrystalline cellulose (NCC) (A) and NCC crystallites in deionized distilled water (B).
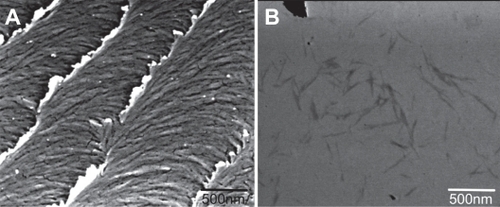
The scanning transmission electron microscopy (STEM) micrograph of the NCC suspension shows individual NCC fibers with approximate dimensions of 10 nm wide by 500 nm in length ().
Drug binding to NCC
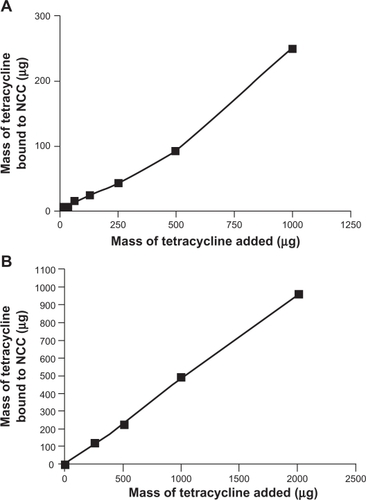
The amount of DOX bound to NCC increased significantly as the mass of drug added to the NCC suspension increased. When the dispersion medium was PBS, a maximum of 122 μg of DOX was bound to NCC representing a 65% binding efficiency (). When dH2O was used as the dispersion medium, a maximum of 1667 μg of DOX was bound to NCC with a binding efficiency of 83% (). It was found that the binding efficiency of TET to NCC was considerably less than that of DOX, regardless of the dispersion medium used (). Using PBS as a dispersion medium, a maximum of 251 μg of TET was bound with a 25% binding efficiency (). When the dispersion medium was dH2O, 959 μg of TET was bound to NCC with a 48% binding efficiency ().
Figure 3 The binding of doxorubicin to 2 mg NCC in 10 mM, pH 7.4 phosphate buffered saline at 25°C (A) and deionized distilled water at 25°C (B).
Abbreviation: NCC, nanocrystalline cellulose.
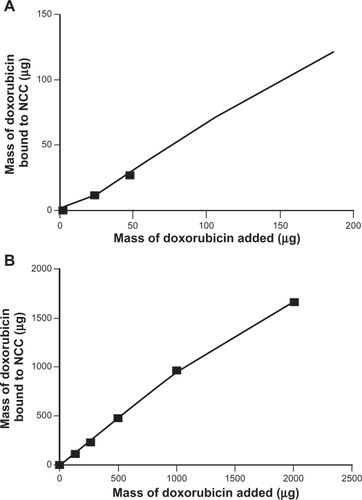
Figure 4 The binding of tetracycline to 2 mg NCC in 10 mM, pH 7.4 phosphate buffered saline at 25°C (A) or deionized distilled water at 25°C (B).
Abbreviation: NCC, nanocrystalline cellulose.
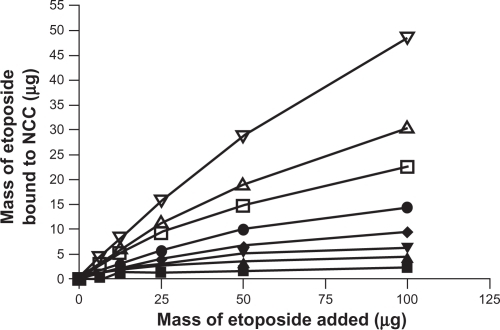
The effect of increasing concentration of CTAB coating on NCC on the binding of the hydrophobic drugs DTX, PTX, and ETOP was investigated (–). In all cases it was found that increased amounts of CTAB resulted in increased drug binding. At the highest CTAB concentration (12.9 mM), the binding efficiency of DTX and PTX to the NCC/CTAB nanocomplexes was approximately 90% up to 100 μg of drug added (). Above this drug concentration, the drug-binding efficiency decreased with saturation of binding occurring at approximately 200 μg (). Much less ETOP was capable of binding to the NCC/CTAB nanocomplexes with a 48% binding efficiency and a maximum of 48 μg bound when 100 μg of drug was added to the NCC/CTAB ().
Figure 5 A) The binding of docetaxel to 2.5 mg of NCC/CTAB nanocomplexes in 10 mM NaCl at 25°C with CTAB concentrations of 0 mM (▪), 0.755 mM (▴), 1.51 mM (▾), 2.27 mM (♦), 4.53 mM (•), 6.79 mM (□), and 12.9 mM (Δ). B) Maximal binding of docetaxel at a CTAB concentration of 12.9 mM.
Abbreviations: CTAB, cetyl trimethylammonium bromide; NCC, nanocrystalline cellulose.
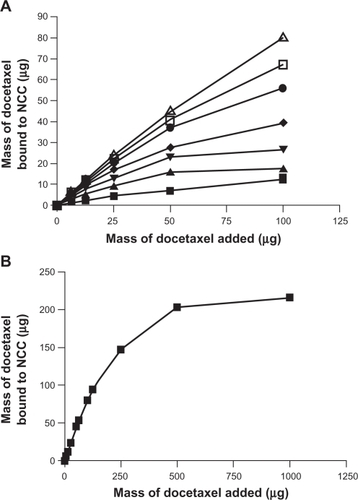
Figure 6 A) The binding of paclitaxel to 2.5 mg of NCC/CTAB nanocomplexes in 10 mM NaCl at 25°C with CTAB concentrations of 0 mM (▪), 0.755 mM (▴), 1.51 mM (▾), 2.27 mM (♦), 4.53 mM (•), 6.79 mM (□), and 12.9 mM (Δ). B) Maximal binding of paclitaxel at a CTAB concentration of 12.9 mM.
Abbreviations: CTAB, cetyl trimethylammonium bromide; NCC, nanocrystalline cellulose.
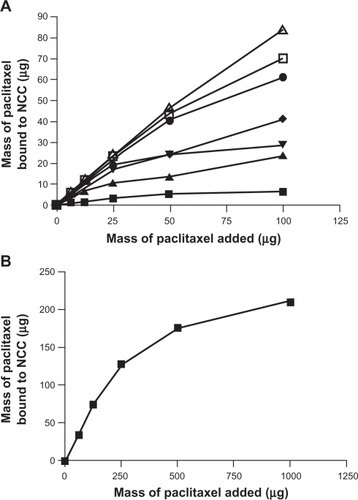
Figure 7 The binding of etoposide to 2.5 mg of NCC/CTAB nanocomplexes in 10 mM NaCl at 25°C with CTAB concentrations of 0 mM (▪), 0.375 mM (▴), 0.755 mM (▾), 1.51 mM (♦), 2.27 mM (•), 4.53 mM (□), and 6.79 mM (Δ), and 12.9 (∇).
Abbreviations: CTAB, cetyl trimethylammonium bromide; NCC, nanocrystalline cellulose.
Release of drugs from NCC
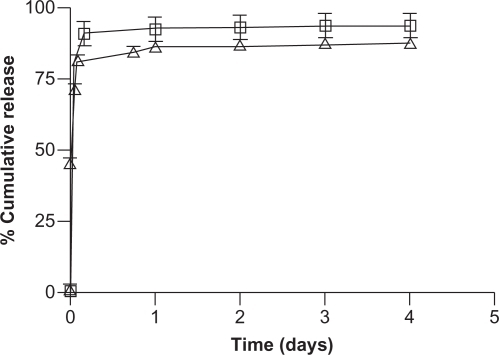
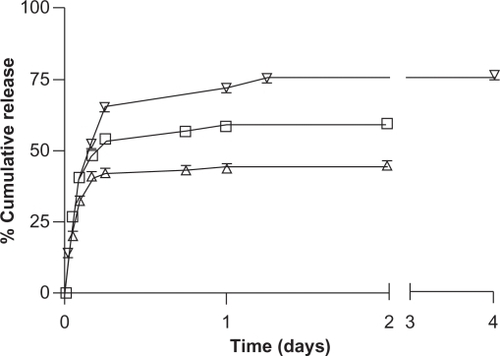
Both DOX and TET released rapidly from NCC, resulting in approximately 90% of bound TET and 85% of bound DOX released in 4 hours (). By day 1, the drug release had plateaued, with 93% and 87% of TET and DOX released, respectively. The release profiles of the hydrophobic drugs DTX, PTX, and ETOP bound to NCC/CTAB are shown in . Approximately 26% of DTX was released within the first hour, followed by a slower but sustained release. In total, 59% of the total bound DTX was released in 2 days. A similar release profile was observed for PTX, which was characterized by a rapid release of 20% of bound drug in the first hour followed by slower release resulting in 44% drug release over 2 days. The release of ETOP was similar to DTX and PTX, with the exception that a total of 75% of the drug was released over 4 days.
NCC/CTAB characterization
In dH2O, NCC remained as a stable colloidal dispersion and did not flocculate or sediment under high-speed centrifugation. However, when 5 mM of NaCl was added, flocculation and subsequent sedimentation by high-speed centrifugation could be achieved. In water, CTAB had the same effect as NaCl so that at approximately 2 mM CTAB, the NCC could be sedimented under centrifugation. At lower concentrations of CTAB, a small amount of NaCl (10 mM) was added to tubes to enable sedimentation.
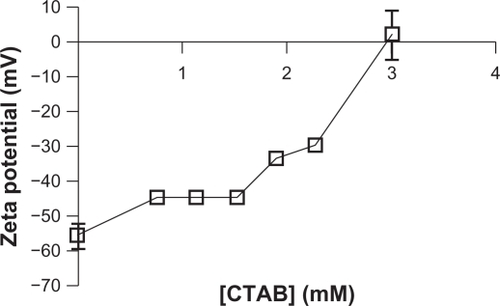
NCC had a strongly negative charge in water as evidenced by a zeta potential of approximately −55 mV. Upon incubation with CTAB, the zeta potential increased in a concentration-dependent manner. At a concentration of 3 mM CTAB, there was complete neutralization of the negative zeta potential ().
Cell binding and uptake studies
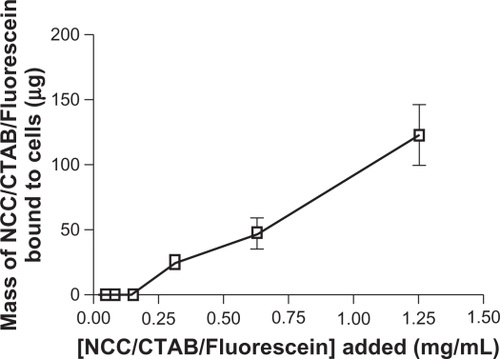
More than 95% of the fluorescein bound to NCC/CTAB remained bound upon resuspension or dilution in aqueous media. When NCC/CTAB/fluorescein was incubated with KU-7 cells, the fluorescence signal was not quantifiable below 0.3 mg/mL; however, above this concentration, the fluorescence increased in a concentration-dependent manner (). At NCC/CTAB/fluorescein concentrations greater than 1.25 mg/mL, there was no linearity of the fluorescein quantitation, and it was not possible to accurately measure fluorescein uptake or binding to the cells.
Figure 11 Mass of NCC/CTAB/fluorescein bound to KU-7 cells as a function of concentration of NCC/CTAB/fluorescein added to cells.
Abbreviations: CTAB, cetyl trimethylammonium bromide; NCC, nanocrystalline cellulose.
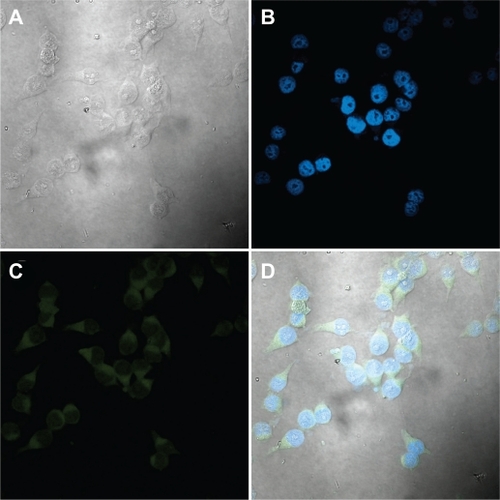
The nuclei of the KU-7 cells displayed pronounced staining with DAPI as shown in . There is clear evidence of cellular uptake of fluorescein as demonstrated by strong fluorescence emission from the cytoplasm of the cells (). The uptake of fluorescein reached a maximum by 2 hours with little increase in cytoplasmic fluorescence emission after longer incubations. Cell uptake was observed using NCC/CTAB/fluorescein nanocomplex concentrations of 0.25, 0.50, and 1.00 mg/mL. There was no evidence of cell lysis with these complexes for up to 24 hours.
Figure 12 Confocal micrographs of KU-7 cells incubated for 2 hours with NCC/CTAB/fluorescein nanocomplexes with a NCC/CTAB/fluorescein concentration of 0.25 mg/mL. A) White light image of KU-7 cells. B) Staining of the nuclei with DAPI. C) Fluorescein in the cytoplasm. D) An overlay of images B and C.
Abbreviations: CTAB, cetyl trimethylammonium bromide; DAPI, 4′,6-diamidino-2-phenylindole; NCC, nanocrystalline cellulose.
Discussion
Due its large surface area, stiffness, strength, and phase behavior, NCC is actively being investigated as a material for use in the packaging, aerospace, automotive, and cosmetic industries. However, to our knowledge, this is the first report of the use of NCC as an excipient for drug binding and subsequent controlled release. The high surface charge of NCC and the established biocompatibility of cellulosic materials suggest significant opportunities for exploration of the potential pharmaceutical applications of NCC. Other workers have described the use of chitosan, a positively charged insoluble polysaccharide, as a binding agent for the controlled release of negatively charged anti-sense oligonucleotide (ASO) drugs.Citation15,Citation16 In such studies, the chitosan/ASO complexes were used either alone or dispersed in a polymeric vehicle for the treatment of cancer.
Using an acid hydrolysis method, cellulose crystallites, or what we termed NCC crystallites, were isolated and investigated for use as a drug delivery excipient. The XRPD pattern, which is characterized by two high intensity peaks, indicates the high degree of crystallinity of the material (). Additionally, the SEM images of these fibers clearly demonstrate the nanoscopic dimensions of this material when in a dH2O suspension () and, as reviewed by Hamad,Citation17 the ability of the material to yield a chiral nematic phase when dried as a film ().
These studies have demonstrated that hydrophilic drugs may bind directly to the surface of NCC at relatively high weight ratios ( and ) (eg, almost 500 μg of TET may be bound to just 2 mg of NCC, offering a 20% w/w drug loading ()). The two hydrophilic drugs studied (TET and DOX) probably bound by an ionic interaction with the negatively charged surface of NCC since DOX is a cationic species slightly positively charged and TET is zwitterionic. Interestingly, considerably less DOX bound to NCC when dispersed in PBS as compared with dH2O. This may be attributed to interference with DOX binding due to the presence of large amounts of counterions in PBS. Both these agents released rapidly from NCC in vitro (), likely due to PBS counterions displacing the drugs via ion exchange. Such rapid release profiles are also seen for acidic or basic drugs bound to ion-exchange resins.Citation14 Nevertheless, these rapid-release profiles observed for NCC may be suitable for potential applications as wound-dressing materials or for implantation into surgical resection voids such as tumor removal sites or periodontal cavities.
This work also demonstrates that NCC can be surface modified to deliver hydrophobic antiproliferative drugs. By coating the negatively charged NCC with a cationic surfactant (CTAB) it was possible to create a hydrophobic domain on the surface of the NCC. A clear interaction between CTAB and NCC was observed by flocculation phenomena at higher CTAB concentrations. Furthermore, the zeta potential (surface charge) on the NCC became increasingly less negative as the concentration of CTAB increased, providing evidence of a binding interaction between the CTAB and the NCC (). The strong association between NCC and CTAB was further supported by washing experiments whereby flocculation phenomena only began to decay when the NCC/CTAB nanocomplexes were washed more than 15 times with PBS (data not shown). Other groups have observed a similar interaction between CTAB and negatively charged gold nanoparticles whereby the surfactant binds to the surface of the particle and may cause concentration dependent particle aggregation.Citation24 Furthermore, Alkilany et alCitation25 described the partitioning of hydrophobic napthol molecules into surfactant-coated gold nanorods. Interestingly, although gold nanoparticles are used for laser-induced thermal ablation of tumors, these particles have also been modified with hydrophobic polymer chains for the purpose of delivering hydrophobic drugs in a manner similar to CTAB use on NCC described in this study.Citation26
The hydrophobic drugs DTX, PTX, and ETOP partitioned strongly into the CTAB domains on NCC using either free drug solutions at low concentrations or micellar solubilized drugs at higher concentrations ( and ). These drugs released more slowly from NCC () than the hydrophilic drugs DOX and TET. However, the release profiles were all characterized by a burst phase of release of between 40% and 75% of the bound drug over the first 2 days followed by an extremely slow rate of release. These profiles suggest a weakly bound fraction of drug releasing quickly and a strongly bound fraction that released very slowly.
These NCC/CTAB nanocomplexes were shown to associate strongly with KU-7 cancer cells (). Because fluorescein was strongly bound within the CTAB coating on the NCC, it was possible to quantitate the cell-bound NCC by measuring the fluorescein emission from the cells. This assay does not differentiate between cell surface association and cellular uptake of NCC but clearly shows that NCC may be used to carry agents (in this case a hydrophobic probe, fluorescein) to cells. This concept is supported by confocal microscopy studies showing a strong fluorescence signal from the cytoplasm of the cancer cells (). In these studies, the nuclear and cytoplasmic regions were differentially stained with DAPI () and fluorescein (), respectively. No fluorescein signal was observed in the location of the nucleus, suggesting the cytoplasm as the location of fluorescein, since surface-bound fluorescein would be observed over the full exposure of the cells. These data indicate cellular uptake of fluorescein but do not differentiate the uptake of free fluorescein from the NCC/CTAB/fluorescein nanocomplexes, as it is possible that fluorescein may partition into the hydrophobic cell membrane following cell binding of the nanocomplexes. Since cellular uptake of fluorescein was almost complete by 2 hours and anticancer drugs such as ETOP, PTX, and DTX release occurred over days (), it may be assumed that NCC/CTAB/drug nanocomplexes offer a viable and novel method of delivering drugs to cells and may actually deliver these anticancer drugs as controlled release systems (NCC/CTAB/drug nanocomplexes) within cells. These confocal studies also indicate the biocompatibility of the NCC/CTAB/nanocomplexes, since cells were intact following incubation with the nanocomplexes for 24 hours. In cytotoxicity studies measuring the release of lactate dehydrogenase (a marker of cytolysis), NCC and NCC-CTAB were found to have no lytic effect at a concentration of 1 mg/mL (data not shown). However, upon dilution in PBS, lower concentrations of NCC/CTAB (not NCC) were observed to cause some background lysis, indicating that some unbound CTAB might interact directly with the cancer cell membranes. The potential cytotoxic effect of CTAB bound to NCC is under further investigation.
Conclusion
In these studies, we investigated the potential of NCC as a drug delivery excipient. It was demonstrated that NCC was capable of binding significant quantities of the ionizable water soluble antibiotics tetracycline and doxorubicin. These hydrophilic drugs were rapidly released with complete release in 1 day. It was shown that the surface of NCC could be modified by binding the cationic surfactant CTAB, resulting in a concentration dependent increase in the zeta potential of the NCC crystallites. CTAB-coated NCC was shown to bind significant quantities of the nonionized hydrophobic anticancer agents docetaxel, paclitaxel, and etoposide and release these drugs in a controlled manner over several days. The NCC/CTAB nanocomplexes bound to KU-7 bladder cancer cells and demonstrated efficient delivery of a hydrophobic fluorescent probe fluoroscein to the cytoplasm of these cells. Overall, these studies have established the potential of NCC as a drug delivery excipient for use alone or in conjunction with other formulations.
Acknowledgments/disclosure
The authors gratefully acknowledge financial support from FPInnovations. JKJ, KL, BZW, LY, and HMB are employees of the University of British Columbia and report no conflicts of interest in this work. WYH is an employee of FPInnovations and an Adjunct Professor with the University of British Columbia.
References
- O’ConnorRESchwartzJBDrug release mechanism from a microcrystalline cellulose pellet systemPharm Res19931033563618464807
- BaumannMDKangCEStanwickJCAn injectable drug delivery platform for sustained combination therapyJ Control Release2009138320521319442692
- BleyOSiepmannJBodmeierRProtection of moisture-sensitive drugs with aqueous polymer coatings: importance of coating and curing conditionsInt J Pharm20093781–2596519477253
- El-MaradnyHAModulation of a pulsatile release drug delivery system using different swellable/rupturable materialsDrug Deliv200714853954618027184
- Gomez-CarracedoASoutoCMartinez-PachecoRConcheiroAGomez-AmozaJLMicrostructural and drug release properties of oven-dried and of slowly or fast frozen freeze-dried MCC-Carbopol pelletsEur J Pharm Sci2007671236245
- JavadzadehYShariatiHMovahhed-DaneshENokhodchiAEffect of some commercial grades of microcrystalline cellulose on flowability, compressibility, and dissolution profile of piroxicam liquisolid compactsDrug Dev Ind Pharm200935224325118785038
- KhanGMZhuJBStudies on drug release kinetics from ibuprofen-carbomer hydrophilic matrix tablets: influence of co-excipients on release rate of the drugJ Control Release19995721972039971903
- KnightPEPodczeckFNewtonJMThe rheological properties of modified microcrystalline cellulose containing high levels of model drugsJ Pharm Sci20099862160216918825774
- KranzHJurgensKPinierMSiepmannJDrug release from MCC- and carrageenan-based pellets: experiment and theoryEur J Pharm Biopharm200973230230919465119
- PodczeckFKnightPENewtonJMThe evaluation of modified microcrystalline cellulose for the preparation of pellets with high drug loading by extrusion/spheronizationInt J Pharm20083501–214515417905548
- WatanabeYMukaiBKawamuraKPreparation and evaluation of press-coated aminophylline tablet using crystalline cellulose and polyethylene glycol in the outer shell for timed-release dosage formsYakuga Zasshi20021222157162
- AbdallaAKleinSMaderKA new self-emulsifying drug delivery system (SEDDS) for poorly soluble drugs: characterization, dissolution, in vitro digestion and incorporation into solid pelletsEur J Pharm Sci200835545746418940249
- VinatierCGauthierOFatimiAAn injectable cellulose-based hydrogel for the transfer of autologous nasal chondrocytes in articular cartilage defectsBiotechnol Bioeng200910241259126718949749
- GuoXChangRKHussainMAIon-exchange resins as drug delivery carriersJ Pharm Sci200998113886390219226637
- SpringateCMJacksonJKGleaveMEBurtHMEfficacy of an intratumoral controlled release formulation of clusterin antisense oligonucleotide complexed with chitosan containing paclitaxel or docetaxel in prostate cancer xenograft modelsCancer Chemother Pharmacol200556323924715864591
- SpringateCMJacksonJKGleaveMEBurtHMClusterin antisense complexed with chitosan for controlled intratumoral deliveryInt J Pharm20083501–2536417890029
- HamadWOn the development and applications of cellulosic nanofibrillar and nanocrystalline materialsCan J Chem Eng200684513519
- HabibiYLuciaLARojasOJCellulose nanocrystals: chemistry, self-assembly, and applicationsChem Rev201011063479350020201500
- ShaikhSBirdiAQutubuddinSLakatoshEBaskaranHControlled release in transdermal pressure sensitive adhesives using organosilicate nanocompositesAnn Biomed Eng200735122130213717786555
- LönnbergHFogelströmaLSamirMASASurface grafting of microfibrillated cellulose with poly(ɛ-caprolactone) – Synthesis and characterizationEur Polym J200844929912997
- ZhangXJacksonJKBurtHMDevelopment of amphiphilic diblock copolymers as micellar carriers of taxolInt J Pharm19961321–2195206
- PanJHamadWStrausSKParameters affecting the chiral nematic phase of nanocrystalline cellulose filmsMacromolecules20104338513858
- HamadWHuTQStructure-property-yield interrelationships in nanocrystalline cellulose extractionCan J Chem Eng2010883392402
- YangYMatsubaraSNogamiMShiJHuangWOne-dimensional self-assembly of gold nanoparticles for tunable surface plasmon resonance propertiesNanotechnology200617112821
- AlkilanyAFreyRFerryJMurphyCGold nanorods as nanoadmicelles: 1-naphthol partitioning into a nanorod-bound surfactant bilayerLangmuir20082418102351023918700748
- KimJ-HLeeTRDiscrete thermally responsive hydrogel-coated gold nanoparticles for use as drug-delivery vehiclesDrug Develop Res2006676169