?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Leishmaniasis is a protozoan disease, affecting 12 million people in different regions of the world with a wide spectrum of diseases. Although several chemotherapeutic agents have been used for treating the disease, long-term therapy, limited efficacy and the development of drug-resistant parasites remain the major limitations.
Methods
To develop a new nanovaccine for leishmaniasis, recombinant Leishmania superoxide dismutase (SODB1) was loaded onto chitosan nanoparticles by the ionotropic gelation method. Size and loading efficiency of the nanoparticles were evaluated and optimized, and an immunization study was undertaken on BALB/c mice. The mice received phosphate buffer saline (PBS), superoxide dismutase B1 (SODB1) in PBS and nanoparticles via subcutaneous injection. Soluble Leishmania Antigens (SLA) and complete Freund’s adjuvant (CFA) were also injected subcutaneously three times every three weeks (some groups received only a single dose). Three weeks after the last injection, blood samples were collected and assessed with ELISA to detect IgG2a and IgG1.
Results
Immunological analysis showed that in single and triple doses of SODB1 nanoparticles, IgG2a and IgG2a/IgG1 were significantly higher than the other groups (P<0.05).
Conclusion
The results revealed that formulations of SODB1 in biodegradable and stable chitosan nanoparticles can increase the immunogenicity toward cell-mediated immunity (TH1 cells producing IgG2a in mice) that is effective in Leishmania eradication and could be presented as a single dose nanovaccine for leishmaniasis.
Introduction
Leishmaniasis is a vector-transmitted protozoan disease distributed throughout the world’s tropical and subtropical regions. Leishmaniasis is caused by 20 different species which are pathogenic to humans, and belong to the genus Leishmania, an intracellular parasite transmitted by the bite of the phlebotomine sandfly. Leishmaniasis currently threatens 350 million people in 88 countries around the world. Two million new cases are considered to occur annually, with an estimated 12 million people presently infected.Citation1–Citation3 There is also a growing number of cases of human immunodeficiency virus (HIV)/leishmaniasis coinfection. Leishmaniasis is an important opportunistic infection in HIV patients, which is potentially fatal, even when treated appropriately. HIV infection can also increase the risk of development of visceral leishmaniasis by 10–100-fold in endemic areas.Citation4 Leishmaniasis is a parasitic disease with a wide range of clinical presentations, including cutaneous, mucocutaneous, and visceral. The most dangerous form of leishmaniasis is kala-azar which, if untreated, has a fatality rate as high as 100% within two years in developing countries.Citation5 Although several therapeutic agents, including paromomycin, antimony-containing compounds, amphotericin B, fluconazole, and pentamidine, are used as drug therapy in leishmaniasis, the need for prolonged regimens, drug-induced adverse effects, limited efficacy, development of drug-resistant parasite species, and scarring the skin remain important limitations of leishmaniasis drug therapy programs.Citation2 On the other hand, immunotherapy could be able to prevent the infection, and an effective vaccine for leishmaniasis would be an effective means of eradication of this infectious protozoan disease.
Although many efforts have been made to formulate a Leishmania vaccine during the last decade, effective immunotherapy against leishmaniasis has not yet been achieved.Citation3,Citation6 First-generation vaccines against leishmaniasis consisted of dead parasites. A vaccine comprising a single dose of whole-cell autoclave-killed Leishmania major was mixed with Bacillus Calmette–Guérin (BCG) vaccine and compared with BCG vaccine alone against leishmaniasis in Bam, Iran.Citation7 However, this vaccine was shown to have low efficacy (54%). Second-generation vaccines used the antigen subunits of the parasite which were naive fractions purified from parasites or synthetic antigens made by DNA recombinant technology. Third-generation vaccines include genes coding for a protective antigen and cloned into a vector containing a eukaryotic promoter. Recombinant second-generation vaccines and third-generation DNA vaccines achieved mean parasite load reductions of 68% and 59%, respectively, in laboratory animal models, but their success in field trials has not yet been reported.Citation8 The first recombinant antigen used to vaccinate against leishmaniasis was leishmaniolysin (gp63), a membrane protease present in the promastigotes of all species, but its immunogenic properties in clinical trials were shown to be limited.Citation9
In the present study, recombinant Leishmania superoxide dismutase B1 (SODB1), an antigen cloned in Iran, was tested as another potential antigen for immunotherapy.Citation10 L. major SODB1 is a 195-amino acid protein with a molecular weight of 21287 Da and an isoelectric pH of 6.31. Superoxide dismutases are a group of metalloenzymes that eliminate superoxide radicals by dismutation into hydrogen peroxide and molecular oxygen. Typically, eukaryotes, including mammals, have Cu/ZnSOD in the cytosol and MnSOD in the mitochondrial matrix, whereas FeSODs have been found in prokaryotes and protozoa, and in the chloroplasts of plants and algae. Two closely related FeSODs (ie, SODB1 and SODB2), have been identified in Leishmania chagasi, localized within the glycosomes in high levels, and have been found to play an important role in the survival and growth of Leishmania within human macrophages.Citation11,Citation12
Unfortunately most protein and peptide vaccines show only low immunological activity when administered alone. Incorporation of antigens with adjuvants can improve the immunological response. Previous studies confirm that use of adjuvants increases the efficacy of purified antigens by up to 82% in vaccines.Citation8 However, the most effective adjuvants, eg, Freund’s adjuvant, generally cause severe inflammation, which may preclude their use in humans because of unacceptable side effects.Citation13
Particulate delivery of antigen is effective for increasing the immunogenicity of vaccine subunits used in combination with an adjuvant. Moreover, phagocytosis of the particles by macrophages are important for induction of TH1 and TH2 responses, probably by affecting initial antigen uptake, processing, and presentation.Citation14
In the present study, chitosan was used as an adjuvant nanoparticulate delivery system for the SODB1 vaccine subunit. Chitosan, α(1–4)2-amino 2-deoxy β-D glucan, is a deacetylated form of chitin, a polysaccharide present in abundance in the shells of crustaceans.Citation15 The cationic nature of chitosan has been conveniently exploited for the development of particulate drug delivery systems. In addition to its ability to complex with negatively charged polymers, an interesting property of chitosan is its ability to form a gel on contact with specific polyanions. Ionotropic gelation of chitosan with tripolyphosphate for drug encapsulation was first reported by Bodmeier et al,Citation16 although their approach aimed at designing chitosan-tripolyphosphate beads rather than nanoparticles.
Another benefit of nanoparticulate delivery of SODB1 is sustained release of the antigen and ongoing stimulation of the immune system. Therefore, the possibility of developing a single-dose vaccine could be examined. Also, the main problem with antigens, ie, lack of stability and loss of potency during handling and transportation, suggesting potentially severe problems with future scale up of recombinant vaccine production, could be overcome by the increased stability of a nanoparticle formulation. Therefore, scale up of this formulation and subunit vaccines with GMP compliance could be possible.
Materials and methods
Materials
Chitosan (high, medium, and low molecular weight), and complete Freund’s adjuvant were purchased from Sigma-Aldrich (St Louis, MO). Tripolyphosphate, bovine serum albumin, Coomassie Brilliant Blue G250, and glacial acetic acid were purchased from Merck Chemicals (Darmstadt, Germany). Recombinant SODB1 was kindly donated by the Autoimmune Diseases Research Center of Shiraz University of Medical Sciences. Rabbit antimouse IgG1 horseradish peroxidase conjugate and goat antimouse IgG2a horseradish peroxidase conjugate were purchased from Invitrogen (Carlsbad, CA). Tetramethylbenzidine was purchased from Zymed Laboratories (South San Francisco, CA). Ultrapure water was used during the all experiments (Direct Q3; Millipore Iberica, Madrid, Spain). All other chemicals were of pharmaceutical grade.
Female BALB/c mice (4–6 weeks of age) were purchased from the Razi Research Institute for Vaccine and Serum in Iran. Each group of mice was kept in a separate cage, with access to water and food under stress-free conditions, in the animal house of the Shiraz University of Medical Sciences.
Preparation of chitosan nanoparticles
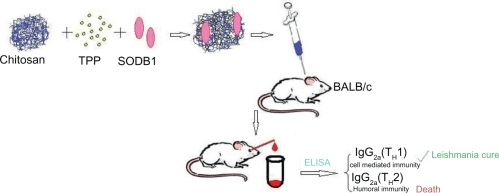
Chitosan nanoparticles were prepared according to the ionotropic gelation process.Citation17,Citation18 Low, medium, and high molecular weight chitosan was dissolved in 0.25% (v/v) acetic acid solution (by mixing at 1500 rpm for two hours) to make chitosan concentrations of 0.05, 0.10, 0.20, 0.30, 0.50, and 0.60% (w/v). Different concentrations of tripolyphosphate solutions in purified water (from 0.02%–2.5%) were added dropwise to the chitosan solution under magnetic stirring at room temperature. Formation of the nanoparticles was the result of interaction between the negatively charged groups of tripolyphosphate and the positively charged amino groups of the chitosan. The nanoparticle suspensions were gently stirred for 60 minutes at room temperature before being subjected to further analysis or application.Citation19,Citation20 Selected concentrations of chitosan and tripolyphosphate which produced appropriate nanoparticles were used to fabricate SODB1-loaded nanoparticles by the same method, except that SODB1 solution was incorporated into tripolyphosphate solution prior to being added to the chitosan solution. In each SODB1 loading experiment, 1 mL of solution containing 2 mg SODB1 was added to tripolyphosphate solution. The zeta potential of the nanoparticles was assessed (Zetasizer; Malvern Instruments, Malvern, UK) in optimized SODB1-loaded chitosan nanoparticles. The preparation procedure and administration of the vaccine in the animal model are presented schematically in .
Characterization of nanoparticles
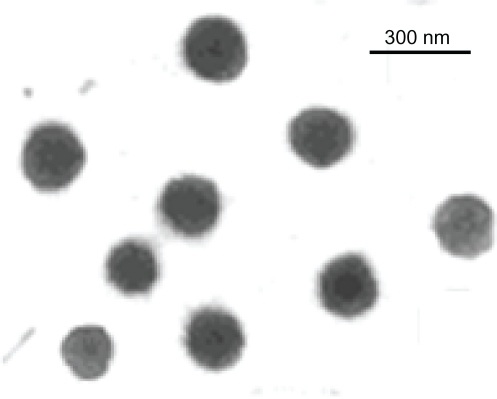
A laser defraction particle size analyzer (SALD 2101; Shimadzu, Tokyo, Japan) was used to measure the mean diameter and size distribution of the nanoparticles. Morphological examination of the nanoparticles was performed by transmission electron microscopy (CM10; Philips, Eindhoven, The Netherlands). The samples were stained with 2% (w/v) phosphotungstic acid and placed on copper grids with Formvar films, to be viewed by transmission electron microscopy.
Evaluation of protein-loading in nanoparticles
The nanoparticles were separated from the solution by ultracentrifugation at 50,000 g and at 10°C for 30 minutes. Supernatant from the centrifugation was carefully separated, and the protein content in the supernatant was analyzed with ultraviolet spectrophotometry at 595 nm using the indirect Bradford protein assay. A calibration curve was plotted with five different concentrations of bovine serum albumin as the standard protein for constructing the standard curve. Triplicate samples were analyzed at each time interval, and the loading efficiency in chitosan-loaded nanoparticles was calculated from the calibration curve. The loading efficiency of the SODB1 nanoparticles was calculated according to the following formula:
Immunization study
Sixty female BALB/c mice were divided into 12 groups, and 5 μg/50 μL of SODB1 was injected subcutaneously via the scruff of the neck in three doses at three-weekly intervals based on guidelines provided in other papers describing similar research.Citation21,Citation22 All of the injections were prepared under aseptic conditions under laminar air flow, and put into insulin syringes (31 gauge, 12.7 mm in length). The different groups of animals receiving the vaccine are summarized in .
Table 1 Nature and composition of the different materials injected into BALB/c mice to assess type and severity of immune response
Three weeks after the final injection, blood samples were collected using the retro-orbital collection method.Citation23 A heparinized microhematocrit tube was inserted carefully into the eye cavity, and a 0.2 mL blood sample was taken from each mouse. Serum was separated by centrifuging at 5000 rpm for 5 minutes and stored at −70°C for further analysis.
Soluble Leishmania antigens were prepared from stationary-phase promastigotes of L. major (IR75), harvested after five passages, washed three times in ice-cold phosphate-buffered saline (PBS; pH 7.2), and finally suspended in PBS buffer at a concentration of 1 × 107 cfu/mL. The suspension was sonicated five times, each step for 30 seconds and, between each step, the samples were kept for 30 seconds on ice and the lysate centrifuged at 12,000 g for 15 minutes at 4°C. After centrifuging, the supernatant was collected and the protein concentrations were estimated by the Bradford method. The extracted proteins were kept at −70°C and used as a positive control as needed.
Enzyme-linked immunosorbent assay
In this study, 96-well flat-bottomed Maxisorb plates (NUNC, Denmark) were coated at 4°C overnight with 50 μL/well of SODB1 at 2 μg/mL in PBS. The plates were washed with 300 μL of PBS containing 0.05% Tween 20 (PBS/T20) three times. They were then blocked for 1.5 hours at 37°C with 200 μL/well of 1% bovine serum albumin in PBS. Plates were washed with PBS/T20 twice. After that, serum samples were diluted to 1:500 with PBS (according to the initial setup) and applied to plates. The plates were incubated at 37°C for two hours and washed with 300 μL of PBS/T20 four times. Horseradish peroxidases-conjugated antibodies were then added for one hour at 37°C using a 1:10000 dilution for rabbit antimouse IgG1 and a 1:2000 dilution for goat antimouse IgG2a. The plates were washed with 300 μL of PBS/T20 six times, 100 μL of tetramethylbenzidine substrate was added, and the plate was kept in the dark until the blue color of the PBS wells (negative control) began to appear. The reaction was stopped with 50 μL of 1 N sulfuric acid, and the plates were read at 450 nm in a microplate reader (Model Synergy; Bio-Rad Laboratories, Hercules, CA).
Statistical analysis
Statistical analysis was performed using the Student’s t-test. Differences were considered significant at P < 0.05.
Results and discussion
Physicochemical characterization and peptide association
Chitosan nanoparticles were developed using a simple and straightforward technique involving the addition of tripolyphosphate solution to chitosan solution at room temperature. The nanoparticles were formed by an ionotropic gelation method. This gelation happens immediately upon mixing of the two phases through intermolecular and intramolecular linkage between negatively charged tripolyphosphates (pH 8) and positively charged chitosan amino groups (pH 4).Citation24
Because the isoelectric pH of SODB1 is 6.31,Citation10 SODB1 is better associated with the nanoparticles when dissolved in alkaline sodium tripolyphosphate solution. This is attributed to the ionic interaction between the negatively charged protein in the sodium tripolyphosphate solution and chitosan upon mixing. Other forces, including hydrogen bonding and hydrophobic forces, could be involved in the association process. The conditions for formation of high yield nanoparticles of a particular nanometric size may vary significantly depending on the purity, acid salt, and molecular weight of the chitosan used. Consequently, the formulation parameters should be optimized for each individual chitosan type.Citation25 We examined high-, medium-, and low-molecular-weight chitosan for nanoparticle preparation and found that low molecular weight chitosan was able to make appropriately sized nanoparticles, so this was subsequently used to prepare nanoparticles within an appropriate size range. After several experiments based on similar studies,Citation17,Citation18 and preparation of more than 31 formulations containing different ratios of chitosan and tripolyphosphate, formulation with an adequate size range of approximately 250–300nm was considered for further studies. Therefore, formulation 15 in was chosen for our loading study. It has been claimed that antigen-containing nanoparticles greater than 225 nm in diameter tend to induce TH1 cytokines, whereas nanoparticles less than 155 nm are more likely to induce TH2-cytokines.Citation14 Therefore, the size of the nanoparticles should be adjusted to 250–300 nm, so that they can be effectively phagocytosed by macrophages after injection, and migrate to their target (ie, the lysosome of the macrophage) to stimulate the immune system against the parasite. The association efficiency was then measured according to the formula described in the Methods section. To ensure that chitosan showed no interference with the results of the protein assay using the Bradford method, protein-unloaded nanoparticles (containing chitosan and tripolyphosphate) were used as a blank solution in the spectrophotometric assay. The results are presented in .
Table 2 Concentration and volume ratios of chitosan and tripolyphosphate and resulting nanoparticle sizes and loading efficiency
SODB1-loaded nanoparticles showed a positive zeta potential of about +17 mV. This positive charge is in favor of the vaccine formulation, and the chances of positively charged nanoparticles are higher than negative charged or neutral nanoparticles for phagocytosis.Citation26 Transmission electron microscopy of the nanoparticles identified 250–300 nm nanoparticles, the morphology of which is shown in . In summary, chitosan nanoparticles obtained by ionic cross-linking with tripolyphosphate display some attractive features which render them promising carriers for the delivery of antigens. These features include ease of formation, a very homogeneous and adjustable size from 200 nm up to 1 mm, a positive surface charge that can be conveniently modulated, a good capacity for association with peptides, proteins, vaccines, oligonucleotides, and plasmids, release of the associated molecules at different rates depending on the composition of the particles, and, finally, retention of integrity and activity of the associated compounds following freeze-drying and reconstitution.Citation25
Immunogenicity
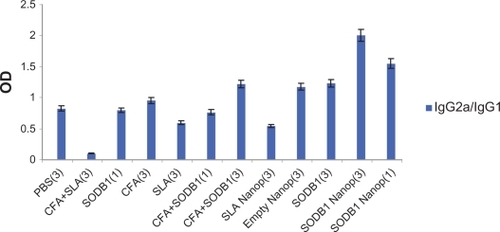
Because leishmaniasis is an intracellular infection, it should be controlled by cell-mediated immunity. T cell responses and cytokine-induced macrophage activation are determinants of cell-mediated immunity.Citation27 Resistance to leishmaniasis is associated with the activation of TH1 CD4+ and CD8+ T cells, leishmanial antigens which can be picked up by antigen-presenting cells (dendritic and Langerhans cells) that transfer to CD4+ and CD8+ T cells inducing production of different cytokines (IL22, IFN-γ, and granulocyte macrophage colony-stimulating factor), thereby activating macrophages to destroy intracellular parasites (cell-mediated immunity) through a nitric oxide-mediated mechanism.Citation2 Conversely, the activation of TH2 cells (humoral immunity) results in increased parasite survival and exacerbation of lesions because of the macrophage-suppressive actions of TH2 cytokines, notably IL-4.Citation28 In mice, IgG2a produced from TH1 cells indicates cell-mediated immunity, and IgG1 produced from TH2 cells indicates humoral immunity.Citation29 Therefore, the balance between TH1 and TH2 cells (TH1/TH2 ratio) that influences the outcome of leishmaniasis can be represented as the IgG2a/IgG1 ratio.
The results of the enzyme-linked immunosorbent assay performed on sera from the 12 groups of mice are presented and compared in and . These show that SODB1 is immunogenic (significantly different from negative controls, P < 0.05), and are in agreement with the theory that virtually any parasite protein may act as an antigen, regardless of its location in the parasite, because T cells are able to recognize peptides derived from cytosolic proteins bound in the major histocompatibility complex Class I groove or peptides derived from the lysosomal compartment bound in the major histocompatibility complex Class II groove on the antigen-presenting cell surface.Citation13 Comparison of the immunogenicity of SODB1 and soluble Leishmania antigens showed that, although SODB1 is less immunogenic than soluble Leishmania antigens, the pathway that SODB1 stimulates in the immune system is towards cellular immunity derived from the IgG2a/IgG1 ratio. SODB1 immunogenicity should be directed towards cellular immunity that is effective against leishmaniasis. The finding that chitosan nanoparticles containing SODB1 induce a stronger response towards cellular immunity and parasite eradication than SODB1 alone in PBS and other control groups is logical. A significant difference (P < 0.05) was observed in the amount of IgG2a in comparison with the other groups, except for the soluble Leishmania antigen-loaded nanoparticle group. The IgG2a/IgG1 ratio was significantly higher (P < 0.05) for the SODB1-loaded nanoparticle group compared with the other groups.
Figure 3 Immunologic responses in animal model on different groups.
Abbreviations: PBS, phosphate-buffered saline; SLA, soluble Leishmania antigen; CFA, complete Freund’s adjuvant; SODB1, superoxide dismutase B1; Nanop, nanoparticles.
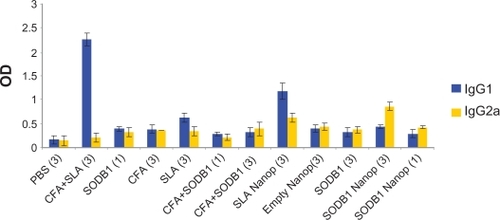
Figure 4 IgG2a/IgG1 ratios in 12 different groups of animals that received the same volumes of different antigenic materials and PBS.
Abbreviations: PBS, phosphate-buffered saline; SLA, soluble Leishmania antigen; CFA, complete Freund’s adjuvant; SODB1, superoxide dismutase B1; Nanop, nanoparticles.
Chitosan not only acts as an adjuvant and activates the immune system, but also increases antigen size, and activates the immune system more strongly by its ability to produce nanoparticles. This indicates that it would be a good substitute for a simple antigenic vaccine (purified or recombinant).
The ability of chitosan nanoparticles to sustain the release of SODB1 is evident when comparing triple doses and single doses of SODB1 nanoparticles. Statistical analysis showed that there was no significant difference between these two dose regimens (P > 0.05), and that both can stimulate the immune system efficiently toward cellular immunity against leishmaniasis. This property induces long-term activation of the immune system, which suggests that a single dose vaccine is feasible. Comparison of a single dose of SODB1 alone with a single dose of SODB1 nanoparticles indicates that the pure peptide cannot stimulate an adequate immune system response. Furthermore, comparison of single dose Freund’s adjuvant + SODB1 with SODB1 nanoparticles showed that the nanoparticles stimulate cellular immunity more effectively (P < 0.05). Moreover, chitosan is a biodegradable, safe, and a relatively low-cost polymer, so that it is a promising candidate for vaccine delivery.
Because SODB1 has a similar structure to many protozoans, it could be used as a potential vaccine for other protozoan infections, including malaria, trypanosomiasis, trichomoniasis, and toxoplasmosis. The structure of protozoan superoxide dismutase is different from that of human superoxide dismutase, so there would be no risk of an autoimmune response.
From the pharmaceutical point of view, mass production of recombinant particulate antigen is safer and more accurate with modern recombination techniques, and better controlled with Good Manufacturing Practice rules than mass production of killed or dangerously modified micro-organisms. For example, active substance assay (for dose adjustment) is possible for recombinant antigens and macromolecules (nanoparticles) by new chemical/biotechnological instrumentation, while for weak or dead microbes, the assay is complicated and imprecise.
Conclusion
Having demonstrated the efficacy of nanoparticulate delivery of a vaccine subunit, we formulated SODB1 in chitosan nanoparticles. Our data show that chitosan is able to increase SODB1 immunogenicity in BALB/c mice, not only by increasing its size and better stimulation of the immune system, but also by the adjuvant properties of chitosan. Both single-dose and triple-dose vaccination with SODB1 chitosan-loaded nanoparticles showed identical results, and were found to be more effective in inducing cell-mediated immunity (TH1 cells that is responsible for Leishmania eradication and could be indicated by IgG2a in mice) than control and other groups which received the soluble form of SODB1 antigen. The present findings also confirm the possibility of a single dose nanovaccine for leishmaniasis and similar protozoan diseases. Moreover, chitosan nanoparticles can improve protein stability in production, transportation, and administration, and a potentially longer shelf life. Also, chitosan is safe, biodegradable, and cost-effective. Finally, the ionotropic gelation method is suitable for protein loading in nanoparticles which exhibited high loading efficiency in this study.
Acknowledgements
The authors acknowledge the financial support of Exir Pharmaceutical Company, the Iran Nanotechnology Initiative Council, Tabriz Research Center for Pharmaceutical Nano-technology, and the Shiraz University of Medical Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
- SoongLModulation of dendritic cell function by leishmania parasitesJ Immunol20081804355436018354154
- El-OnJCurrent status and perspectives of the immunotherapy of leishmaniasisIsr Med Assoc J20091162362720077951
- World Health OrganizationLeishmaniasis. The disease and its epidemiology Available at: www.who.int/leishmaniasis.disease_epidemiology/en/index.html. Accessed on January 25, 2011.
- DaherEFFonsecaPPGerhardESLeitaoTMSilva JuniorGBClinical and epidemiological features of visceral leishmaniasis and HIV co-infection in fifteen patients from BrazilJ Parasitol20099565265519642802
- MarrJNilsenTKomunieckiRMolecular Medical ParasitologySan Diego, CAAcademic Press2003
- OkworIUzonnaJVaccines and vaccination strategies against human cutaneous leishmaniasisHum Vaccin2009529130119221514
- SharifiIFeKriARAflatonianMRRandomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, IranLancet19983511540154310326536
- Palatnik-de-SousaCBVaccines for leishmaniasis in the fore coming 25 yearsVaccine2008261709172418295939
- SpitzerNJardimALippertDOlafsonRWLong-term protection of mice against Leishmania major with a synthetic peptide vaccineVaccine1999171298130010195763
- YeganehFBarkhordariFOmidiMCloning and expression of Leishmania major superoxide dismutase B1: A potential target antigen for serodiagnosis of LeishmaniasisIran J Immunol2009613014019801786
- DufernezFYernauxCGerbodDThe presence of four iron-containing superoxide dismutase isozymes in trypanosomatidae: Characterization, subcellular localization, and phylogenetic origin in Trypanosoma bruceiFree Radic Biol Med20064021022516413404
- GhoshSGoswamiSAdhyaSRole of superoxide dismutase in survival of Leishmania within the macrophageBiochem J.2003369Pt 344745212459037
- HandmanELeishmaniasis: Current status of vaccine developmentClin Microbiol Rev20011422924311292637
- KensilCRMoAXTrunehACurrent vaccine adjuvants: An overview of a diverse classFront Biosci200492972298815353330
- ShahidiFAbuzaytounRChitin, chitosan, and co-products: Chemistry, production, applications, and health effectsAdv Food Nutr Res2005499313515797344
- BodmeierRChenHGPaeratakulOA novel approach to the oral delivery of micro- or nanoparticlesPharm Res198964134172748533
- CalvoPRemunan-LopezCVila-JatoJLAlonsoMJChitosan and chitosan/ethylene oxide-propylene oxide block copolymer nano-particles as novel carriers for proteins and vaccinesPharm Res199714143114369358557
- VilaASanchezAJanesKLow molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in miceEur J Pharm Biopharm20045712313114729088
- GanQWangTChitosan nanoparticle as protein delivery carrier – systematic examination of fabrication conditions for efficient loading and releaseColloids Surf B Biointerfaces200759243417555948
- AktasYAndrieuxKAlonsoMJPreparation and in vitro evaluation of chitosan nanoparticles containing a caspase inhibitorInt J Pharm200529837838315893439
- ZhuBQieYWangJChitosan microspheres enhance the immunogenicity of an Ag85B-based fusion protein containing multiple T-cell epitopes of Mycobacterium tuberculosisEur J Pharm Biopharm20076631832617280823
- ZaharoffDARogersCJHanceKWSchlomJGreinerJWChitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccinationVaccine2007252085209417258843
- HoffJMethods of blood collection in the mouseLab Anim2000294753
- TsaiMLBaiSWChenRHCavitation effects versus stretch effects resulted in different size and polydispersity of ionotropic gelation chitosan-sodium tripolyphosphate nanoparticleCarbohydr Polym2007714481457
- JanesKACalvoPAlonsoMJPolysaccharide colloidal particles as delivery systems for macromoleculesAdv Drug Deliv Rev200147839711251247
- ZahrASDavisCAPishkoMVMacrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol)Langmuir2006228178818516952259
- MurrayHWBermanJDDaviesCRSaraviaNGAdvances in leishmaniasisLancet20053661561157716257344
- AbbasAKLichtmanAHCellular and Molecular Immunology5th edPhiladelphia, PASaunders Co2003
- DayMJImmunoglobulin G subclass distribution in canine leishmaniasis: A review and analysis of pitfalls in interpretationVet Parasitol20071472817467176