Abstract
Background
Amine-modified carbon nanotubes are drug delivery platforms with great potential that have not yet been applied in human clinical trials. Although modified nanotube vectors have the ability to carry multiple effectors, targeting agents, and even wrapped RNA, reports on unmodified, insoluble carbon nanotubes have highlighted inflammation in organs, including the intestine, with disruption of its resident microbiota. Disruption of the microbiota may allow for colonization by pathogenic bacteria, such as Clostridoidies difficile, stimulate immunoinfiltrates into the lamina propria or alter the absorption of therapeutics. Most proposed nanotube drugs are soluble, modified structures that are administered parenterally, and the majority of these soluble macromolecules are renally excreted; however, some are released into the bile, gaining access to the gastrointestinal tract.
Methods
Using environmentally isolated BALB/C mice in oral and intraperitoneal dosing models, high dose (3.80 or 4.25 mg/week), we administered amine-modified, soluble carbon nanotubes for 7 or 8 weeks. The general health and weight of the mice were monitored weekly, and upon killing, the diversity and content of their colonic, cecal, and ileal microbiota were assessed using shotgun 16S DNA sequencing.
Results and conclusion
We show that while oral administration at suprapharmacological doses modestly altered the α- and β-diversity of the mouse microbiome, these changes did not result in observed changes in clinical end points. Intraperitoneally-dosed mice exhibited none of the toxicities assessed.
Introduction
Despite being excellent antioxidants due to their uniform spCitation2 bond structure,Citation1 when inhaled in their unmodified state, fibrillar, insoluble, single-walled carbon nanotubes (SWCNTs) have been associated with inflammation and granuloma formation in the lungs.Citation2,Citation3 They have recently been shown to inflame the intestines of mice and disrupt resident microbiota.Citation4 In contrast, highly soluble, SWCNTs achieved through modification can avoid this toxicity, be biocompatible, and be excreted rapidly.Citation5–Citation9 By combining favorable pharmacokinetic properties with the ability to carry numerous, diverse payloads simultaneously, NTs have been proposed as highly effective platforms for drug delivery.Citation10 Better standardization of length, diameter, degree of imperfection, chirality, and adduct density are continued goals of the field.Citation11,Citation12 Formal toxicity studies are also needed. In this paper, we explore the effects of suprapharmacological intraperitoneal (IP) and oral (PO [per os]) dosing of amine-modified, SWCNTs on bacterial populations of rectal, cecal, and ileal contents in adult mice, in addition to general assessments of health. Closely related multiwalled, unmodified CNTs have also shown inflammation-related toxicity,Citation13 but are larger and more heterogeneous, making them less suitable candidates for drug development. Multiwalled NTs are not included in this investigation.
The mouse digestive tract is the most widely used experimental model for the microbiome, and has led to better understanding of many human pathologies, including obesity,Citation14 inflammatory bowel disease,Citation15 and diabetes.Citation16 However, the mouse digestive tract differs in some significant ways from its human counterpart. Mice have taller villi, larger ceca, decreased rectal goblet cells, and no appendix or haustra, all of which can influence biogeography.Citation17–Citation19 Morphological variation combined with disparate mucosal immunity and diet and frequent coprophagy nurtures a murine microbiota that differs from that of humans in content and diversity.Citation20 Although murine intestines are not perfect models for the human intestine, bacterial communities react similarly to many environmental stressors, allowing for mice as models for microbiota investigations.Citation21
When the microbiota is perturbed by an antibiotic, inflammation, pharmaceutical, or ingested bacteria, the general trends of loss of diversity and the elimination of certain sub groups are often translatable.Citation21,Citation22 For instance, the presence of Clostridium scindens and C. populeti have been shown to be protective factors against C. difficile infections in both mice and humans, while Enterococcus avium encourages the expansion of C. difficile.Citation23 It has generally been observed that diversity is a sign of gut health that limits inflammation and resists domination by pathogenic organisms.Citation24
Most studies on the ability of medications to alter the microbiota have focused on antibiotics, which by their nature can rapidly and drastically alter intestinal populations. However, many compounds make changes to the flora.Citation25,Citation26 The converse is also true: the effects of medications, their uptake, and their metabolism can vary greatly based on the microbiota.Citation27 Here, we explore microbiota changes related to amine-modified SWCNTs, a class of molecules that is under development for a variety of biomedical applications. More specifically, this study will employ lysine-modified SWCNTs (SWCNT-Lys-NH2).Citation7 The effect of these positively charged, primary-amine-coated macromolecules on the microbiota has yet to be examined. This study is more generally an examination of the effect of a large cationic amine load.
While the vast majority of systemically delivered amine-modified NTs are excreted through the kidneys, a small population is released into the bile and thus into the gastrointestinal (GI) tract via a mechanism discovered by this group.Citation28 Distribution, elimination, and toxicity studies have been performed using amine-modified NTs; however, no study detailing the intestinal uptake and bioavailability of PO-administered tubes has been reported in the literature. Though NTs may move from the circulation to the gut, it remains unknown whether the reverse is true.
In this study, PO and systemic routes of NT administration are examined, followed by the collection and analysis of bowel contents from three different bio-geographical regions of the digestive tract. Both routes of administration are important. Most proposed NT-based drugs are administered either intravenously or IP, as bioavailability is unknown and NT-linked adducts may not survive the stomach. Toxicity by systemic administration methods is of greater interest; however, the creation of PO NT-based drugs is a distinct possibility. Furthermore, PO dosing provides far higher amounts of NTs to the GI tract and interacts with the esophagus, stomach, and duodenum, which are avoided in systemic administration. Sequencing three sections of the bowel, each with distinct microbiota populations, allows for more precision in how SWCNT-Lys-NH2 may affect bacterial communities.
Methods
Production of lysine-modified SWCNT solutions
SWCNT-Lys-NH2 were produced using electrocyclic chemistryCitation29 as previously described.Citation7 The NTs had an average length of ~400 nm and an average diameter of 2–3 nm, including adducts (), with an average primary amine density of one amine per 121 carbons (~275 amines per tube) or about one amine for every 1,455 Da. All tubes were solvent exchanged by dialysisCitation30 into a 25% methanol–H2O solution and then frozen before being lyophilized to a brown powder and dissolved into either sterile water or sterile saline for use in this study. pH was then adjusted tô7.4 using Sigma-Aldrich pH-indicator strips (7.2–8.8 range) and HCl or NaOH. Sterile water was reverse osmosis, autoclaved water.Citation31 Solutions were made freshly using lyophilized SWCNT-Lys-NH2 with each set of injections and each cage-water replacement.
Mice
BALB/c mice were purchased from Jackson Laboratories, mated, and females housed individually once confirmed pregnant in pathogen-isolated cages with irradiated food and distilled water. Selected healthy pups born within 72 hours of one another were harmonized to their median birth date. Adolescents were separated into experimental group cages as soon as they were weaned and after 3 days of cohousing with the other broods for randomization and microbiota harmonization. Physical allocation of same-sex isobiotic BALB/c experimental groups occurred before male sexual maturity. Mouse handling and weekly cage changes were performed by investigators wearing sterile gowns, masks, and gloves in a sterile biosafety hood. All animals were maintained in a specific pathogen-free facility at the Memorial Sloan Kettering Cancer Center Animal Resource Center. All experiments were approved by the facility’s Institutional Animal Care and Use Committee and performed according to their approved protocols.
Experimental groups
Experimental groups were designed for 38 healthy pups from 42 littered by 10 mothers (). Once adolescents, nine groups of pups were divided into nine cages in sets of four to five mice based on sex, planned exposure type, and day of life of first experimental exposure. Female mice were divided into groups 1–6, consisting of three PO-dosing groups and three IP dosing groups. Male mice were divided into groups 7–9 and dosed IP only. Delivery of SWCNT-Lys-NH2 by either PO or IP injection methods were started on day 23 or day 30 of life, depending on the group, to examine the effect of stage of development on the introduction of NTs. All mice received irradiated food, and all mice except those in the OS-dosing experimental groups received sterile water, in keeping with microbiome best research practices.Citation31 All mice were weighed in milligrams rounded to the nearest centigram from day 23 of life until the end of the experiment.
Table 1 Experimental groups used
Mice in the PO-dosing experimental groups were housed with sterile water that had been inoculated with SWCNT-Lys-NH2 to a molality of 0.125 g/L. PO solution concentration and dosing were based on a consumption experiment described in Methods section. PO dose was calculated under the assumption that the total volume consumed was divided equally by cagemates, meaning they each drank on average 34 mL tube water per week for a total of 4.25 mg per week.
The mice in IP injection control groups 4 and 7 were injected IP with 200 μL sterile saline once every 7 days from 23 days of life. Mice in IP injection experimental groups 5, 6, 8, and 9 were injected IP with 200 μL saline solution of Lys-modified NTs at a molality of 19 g/L for a total of 3.8 mg per injection from either 23 or 30 days of life. IP dosing was based on an unpublished toxicity dose escalation study and was a third of the morbidity threshold.
Validation of oral-dosing quantity and concentration
In a planning experiment, two sets of four adult female BALB/c mice each were housed in cages where the only source of liquid was a sterile water solution of SWCNT-Lys-NH2 at 0.125 g/L concentration. This was compared with two additional control cages that were given sterile water. All mice were allowed to imbibe ad libitum. The amount of fluid (sterile water or Lys-NT solution) consumed or wasted in the process of drinking was measured every 5 days for 30 days. The difference between cages never exceeded 16%. Stools were assessed qualitatively for gross changes, including liquidity, and no changes were found in any of the four cages. After the allotted 30 days, the mice were weighed. The groups had mean weights within 10% of one another when normalized for starting mean weight.
Sample collection
After 7 or 8 weeks of NT exposure depending on the group (in the 12th week of life), fresh stools were collected in a sterile manner from the three mice with weights closest to the cage mean using a flash-freeze technique that was proven on these mice by pilot sequencing experiments on feces. Subsequently, the mice were killed and the contents of their ceca and ilea extruded in a sterile manner, then flash-frozen. Stool, cecum, and ileum samples were then prepared for sequencing. Liver and kidneys were removed from all female mice, briefly dabbed on gauze, and weighed at time of death.
DNA extraction
Briefly, a frozen aliquot (~100 mg) of each sample was suspended while frozen in a solution containing 500 μL extraction buffer (200 mM Tris, pH 8/200 mM NaCl/20 mM EDTA), 200 μL 20% SDS, 500 μL phenol:chloroform:isoamyl alcohol (25:24:1), and 500 μL 0.1 mm-diameter zirconia/silica beads (BioSpec Products). Microbial cells were lysed by mechanical disruption with a bead beater (BioSpec Products) for 2 minutes, after which two rounds of phenol:chloroform:isoamyl alcohol extraction were performed. DNA was precipitated with ethanol and resuspended in 50 μL TE buffer with 100 μg/mL RNase. Isolated DNA was subjected to additional purification with QiaAmp mini spin columns (Qiagen).
16S rDNA amplification and Illumina sequencing
For each sample, duplicate 50 μL PCRs were performed, each containing 50 ng purified DNA, 0.2 mM dNTPs, 1.5 mM MgCl2, 2.5 U platinum Taq DNA polymerase, 2.5 μL 10× PCR buffer, and 0.5 μM each primer designed to amplify the V4–V5:563F (5′-nnnnnnnn-NNNNNNNNNNNN-AYTGGGYDTAAAGNG-3′) and 926R (5′-nnnnnnnn-NNNNNNNNNNNN-CCGTCAATTYHTTTRAGT-3′). A unique 12-base Golay barcode (Ns) preceded the primers for sample identification,Citation33 and one to eight additional nucleotides were placed in front of the barcode to offset the sequencing of the primers. Cycling conditions were 94°C for 3 minutes, followed by 27 cycles of 94°C for 50 seconds, 51°C for 30 seconds, and 72°C for 1 minute; 72°C for 5 minutes was used for the final elongation step. Replicate PCRs were pooled, and amplicons were purified using the QiaQuick PCR purification kit (Qiagen). PCR products were quantified and pooled at equimolar amounts before Illumina barcodes and adaptors were ligated on using the Illumina TruSeq sample preparation protocol. The completed library was sequenced on an Illumina MiSeq platform following Illumina-recommended procedures with a paired-end 250×250 bp kit.
Sequence analysis
The 16S (V4–V5) paired-end reads were merged and demultiplexed. The Uparse pipelineCitation32 was used toCitation33 perform error filtering, using maximum expected error of 1,Citation32,Citation34 group sequences into operational taxonomic units (OTUs) of 97% distance-based similarity,Citation34 and identify and remove potential chimeric sequences using both de novo and reference-based methods. Prior to clustering, singleton sequences were removed as part of the protocol. In order to gauge the impact of singleton removal on resulting sequences and diversity measures, the analysis was repeated with singletons retained (“Uparse +1” algorithm). Taxonomic assignment to species level was performed for representative sequences from each OTU. This was achieved by using a custom Python script incorporating nucleotide BLAST,Citation35 with NCBI RefSeqCitation36 as the reference training set. We used a minimum E value threshold of 1E–10 for assignments. Sequence designations and identity scores were inspected manually for quality and consistency in terms of taxonomic structure and secondary matches. A phylogenetic tree of OTU-representative sequences was constructed using QIIME. α-Diversity and UniFrac distances (inverse Simpson plots) were calculated using the PhyloSeq R package.Citation37 All statistical analyses were performed using R version 3.2.1. Graphics were made using the GGplot2 package.Citation38 We also made comparisons using linear discriminant analysis effect size (LEfSe): the difference between bacterial groups based on cladogram-based UniFrac distance, operational taxonomic units, quantity, and specific-gene presence.Citation39 One sample from the male IP control group (group 7) did not reach minimum coverage and was excluded from analysis.
Results
Deaths
One mouse from the female sterile saline injection control group (group 4) died rapidly after IP injection on day 58 of life. The mouse was excluded from analysis. This was likely a result of large-vessel trauma during the injection.
Gross toxicity
Gross toxicity was assessed by total mouse weight and comparison of liver and kidney weights using all mice in each female group. When results were separated by sex, there were no significant differences between any of the male or female groups by Student’s t-test (). Whole-organ liver and kidney weights were compared for the female mice. Organ weights between treatment groups of mice also were not statistically significantly different ().
Figure 2 Group mean weights of mice over time (n=4 or five per data point).
Notes: Closed symbols represent PO groups. Open symbols represent IP groups. Dotted lines represent control groups. Pink groups are female, and blue groups are male. 95% CI bars have been removed for clarity. There were no significant differences in any of the final male or female mean group weights compared with other groups within their sex by Student’s t-test. Data analysis performed using GraphPad Prism version 7.02. See for chart displaying 95% CI bars.
Abbreviations: PO, per os (oral); IP, intraperitoneal.
Microbiota diversity
α-Diversity comparisons were segregated by sex and exposure type. Analyses employed inverse Simpson to determine α-diversity and Kruskal–Wallace to determine significance. IP female groups (4–6) and IP male groups (7–9) had no significant differences in diversity between them for any part of the bowel. IP injection group α-diversity with Kruskal–Wallis-derived P-values may be found in .
Female PO groups had significant differences between experimental and relevant control groups. There was a significant reduction in diversity in the cecum of mice receiving PO SWCNT-Lys-NH2 P23 (group 2) when compared with PO sterile water (group 1), but no significant change in colon or ileum diversity (). There was also a significant reduction in diversity in the colons of mice receiving PO SWCNT-Lys-NH2 P30 (group 3) when compared with PO sterile water (group 1). Female PO group α-diversity with Kruskal–Wallis-derived P-values may be found in .
Table 2 α-Diversity of PO groups
Differences in bacterial content
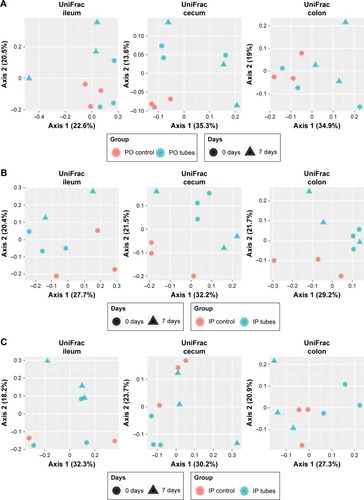
Principal coordinate analysis (PCoA) employing UniFrac distances is a way of clustering bacteria by the degree of their many disparate features, including phylogenetic differences. This is a measure of β-diversity. UniFrac PCoA analysis is provided for each sequenced female sample, organized by section of the bowel, and a second PCoA analysis separated by intervention type (ie, SWCNT-Lys-NH2 delivery or lack thereof) is also provided in .
When the PCoA colored by bowel section was compared to the PCoA separated by intervention type, there was clustering of bacterial contents taken from the ceca and colons of mice as being separate from contents taken from their ilea, but there was no such clustering between intervention type and controls. If differences in colon contents between mice given PO SWCNT-Lys-NH2 and their controls were great enough, the specimens from these groups would cluster together. However, examination of all samples by biogeography failed to cluster the samples. This means that the introduction of SWCNT-Lys-NH2 did not affect the microbiota drastically enough to make it distinct from controls by either IP or PO administration methods. In other words, the differences between experimental groups and controls were not great enough to overshadow the regional differences.
In order to examine smaller effects, when the female PO groups (1–3), female IP groups (4–6), and male IP groups (7–9) were analyzed by region of the bowel (), subtle microbiota differences became evident. Mirroring the α-diversity differences, there was tight clustering of PO control cecal samples compared with experimental groups 2 and 3. There were no other tight sample clustering that separated groups. There was a regional association of IP control cecal samples compared with experimental groups 5 and 6, but identification of this as a notable difference in β-diversity would require a higher power study. This regional association was not true of IP-injected male counterparts, also shown in .
Figure 4 PCoA plots of all samples divided into sets of groups – A (1–3), B (4–6), and C (7–9) – and separated by biogeography.
Abbreviation: PCoA, principal coordinate analysis.
UniFrac PCoA and inverse Simpson analysis measure group differences as a whole and may overlook clinically significant individual differences, such as increases in pathogenic species. For this reason, genus- and species-level bacterial content percentage plots along with LEfSe analyses were compared. LEfSe analyses in part reflect manyfold differences in the abundance of bacterial phylogenetic subgroups, and do not equate to gross content. No LEfSe differences in known pathogenic bacteria were identified between any of the groups.
Discussion
This is a first-reported attempt to understand the effect of amine-modified CNTs on the resident bacteria of the intestines. Three different intestinal regions, differences in sex of experimental mice, and time of first exposure to SWCNT-Lys-NH2 were additional variables in this analysis. SWCNT-Lys-NH2 are highly stable, large, polycationic amine structures unlike any naturally ingested material in terms of size and charge capacity. There was reason to suspect these structures might affect the microbiota. Ammonia cations are excellent antimicrobials, and so are polycationic polymers used as surface materials.Citation40 Cationic antimicrobial peptides, such as neutrophil-generated indolicidin, have the potential to inhibit calmodulin,Citation41 of which bacteria have analogs.Citation42
The effects of prolonged high-dose PO administration of SWCNT-Lys-NH2 on microbiota were nontoxic by our metrics, with the exception of decreased diversity of bacteria in the ceca and colons of some female mice receiving PO tubes compared with controls. IP-dosed females did not display reportable differences in α- or β-diversity by our measures. PCoA UniFrac analysis showed that changes in microbiota of treated mice were dwarfed by the natural difference in microbiota in different biogeographical regions of the intestine. In mice with significantly perturbed microbiota, the level of change in α- and β-diversity was more prominent than regional differences. When small group-content differences were examined, the clustering of PO female control cecal samples reinforced that experimental groups were different from controls, if only in subtle ways. Specific increases and decreases in bacterial content as assessed by LEfSe analysis were not revealing for known pathogenic bacteria in any case.
The features of the SWCNT-Lys-NH2 that caused the changes in diversity were not investigated, but could have been size, charge, electron-scavenging potential, or an interaction with bacterial surface membranes or receptors. Importantly, there were no gross changes in the weight or observed health of the mice in any treated group when compared with untreated controls, including the PO groups. Mice injected with SWCNT-Lys-NH2 IP did not demonstrate significantly altered microbiota compared to controls by inverse Simpson, LEfSe, or PCoA UniFrac analysis. Secondary forms of toxicity related to the microbiome, such as susceptibility to infection, were not assessed. Our results stand as a counterpoint to resent research by Chen et alCitation4 that employed unmodified SWCNTs and showed intestinal inflammation and significant microbiota alterations. Our study used a weekly total NT dose that was more than 10-fold the weekly dose used in Chen et al’s highest dosed mice, and a total dose close to 100-fold. Another recent studyCitation43 examined the microbiota toxicity of PO-dosed, unmodified multiwalled CNTs. This study did not show toxicity to the microbiome by PCoA analysis, but was conducted on a different variety of NTs at weekly NT doses that were a tenth of the doses used in Chen et al, and conducted with half the total dose.
Although this manuscript used small data sets, mice in experimental groups were exposed to suprapharmacological levels of NTs, >1.5 g/kg, over the course of the experiment. These doses are substantially beyond the proposed doses of modified CNTs as drug delivery vehicles, and should increase the sensitivity and specificity of detecting microbiota alterations or gross toxicity in experimental groups. Importantly, mice in these experimental groups showed no effect on their gross weight, organ weights, or symptom-assessed well-being.
The time points for first NT administration were chosen in order to interrogate whether SWCNT-Lys-NH2 affected development. A time point of 23 days was as close to possible to the date of weaning when the mouse was still truly an adolescent. Differences between mice that received first administration at 23 days and those that received first administration at 30 days of life were found to be insignificant in these experiments. The number of male and female groups were determined by the availability of pups, of which many more were female.
Conclusion
Parenterally delivered amine-modified NTs were nontoxic at the dosage and time frames investigated and did not have a major effect on the microbiota. PO-administered NTs may perturb the α- and β-diversity of the microbiota in the lower-GI tract but were also nontoxic at the levels and time frames investigated.
Supplementary materials
Figure S1 Organ-weight toxicity analysis: mean organ weights upon death for each female group of all mice in the group (n=4 or 5).
Notes: Bars represent SD. No mean weight was statistically different by Student’s t-test from any other mean weight within the colored groupings. Data analysis performed using GraphPad Prism version 7.02.
Abbreviation: IP, intraperitoneal.
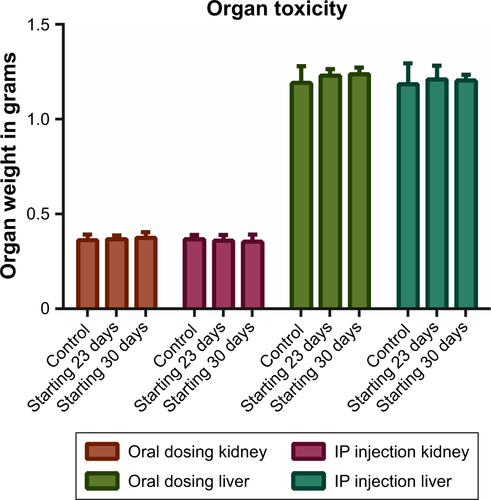
Figure S2 Mouse weights by group with 95% CI bars.
Abbreviations: PO, per os (oral); IP, intraperitoneal.
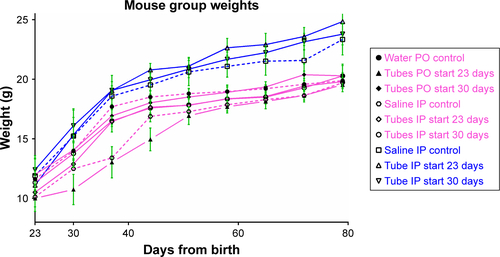
Figure S3 UniFrac PCoA analyses.
Notes: (A) Female samples colored by organ and differentiated by either control groups or day of first exposure. (B) Female samples colored by intervention. Shapes identify day of first exposure.
Abbreviations: PCoA, principal coordinate analysis; PO, per os (oral); IP, intraperitoneal.
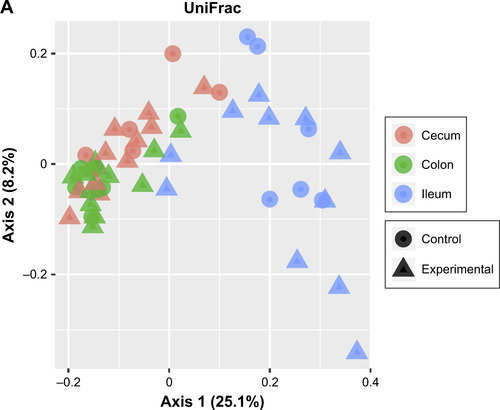
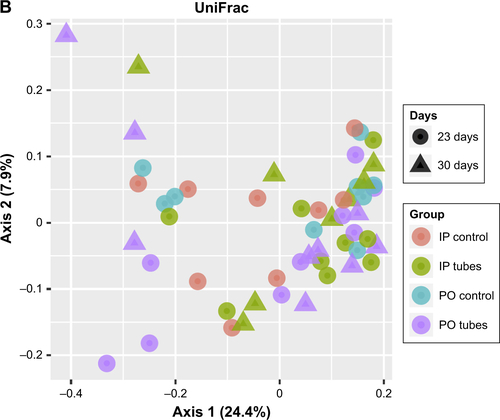
Table S1 α-Diversity of IP injection groups
Disclosure
The authors report no conflicts of interest in this work.
References
- Lucente-SchultzRMMooreVCLeonardADAntioxidant single-walled carbon nanotubesJ Am Chem Soc2009131113934394119243186
- BrownDMKinlochIABangertUAn in vitro study of the potential of carbon nanotubes and nanofibres to induce inflammatory mediators and frustrated phagocytosisCarbon N Y200745917431756
- BhattacharyaKAndónFTel-SayedRFadeelBMechanisms of carbon nanotube-induced toxicity: focus on pulmonary inflammationAdv Drug Deliv Rev201365152087209723751779
- ChenHZhaoRWangBAcute oral administration of single-walled carbon nanotubes increases intestinal permeability and inflammatory responses: association with the changes in gut microbiota in miceAdv Healthc Mater2018713e170131329388390
- MulveyJJVillaCHMcdevittMREscorciaFECaseyEScheinbergDASelf-assembly of carbon nanotubes and antibodies on tumours for targeted amplified deliveryNat Nanotechnol201381076377124077028
- RuggieroAVillaCHBanderEParadoxical glomerular filtration of carbon nanotubesProc Natl Acad Sci U S A201010727123691237420566862
- MulveyJJFeinbergENAlidoriSMcdevittMRHellerDAScheinbergDASynthesis, pharmacokinetics, and biological use of lysine-modified single-walled carbon nanotubesInt J Nanomedicine201494245425525228803
- Ali-BoucettaHNunesASainzRAsbestos-like pathogenicity of long carbon nanotubes alleviated by chemical functionalizationAngew Chemie Int Ed Engl201352822742278
- BattigelliAMénard-MoyonCda RosTPratoMBiancoAEndowing carbon nanotubes with biological and biomedical properties by chemical modificationsAdv Drug Deliv Rev201365151899192023856410
- TibbittMWDahlmanJELangerREmerging frontiers in drug deliveryJ Am Chem Soc2016138370471726741786
- LiuHNishideDTanakaTKatauraHLarge-scale single-chirality separation of single-wall carbon nanotubes by simple gel chromatographyNat Commun2011230921556063
- Sanchez-ValenciaJRDienelTGröningOControlled synthesis of single-chirality carbon nanotubesNature20145127512616425100481
- ZhouLFormanHJGeYLunecJMulti-walled carbon nanotubes: a cytotoxicity study in relation to functionalization, dose and dispersionToxicol In Vitro20174229229828483489
- TurnbaughPJLeyREMahowaldMAMagriniVMardisERGordonJIAn obesity-associated gut microbiome with increased capacity for energy harvestNature200644471221027113117183312
- WlodarskaMKosticADXavierRJAn integrative view of microbiome-host interactions in inflammatory bowel diseasesCell Host Microbe201517557759125974300
- LivanosAEGreinerTUVangayPAntibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in miceNat Microbiol20161111614027782139
- LinnenbrinkMWangJHardouinEAKünzelSMetzlerDBainesJFThe role of biogeography in shaping diversity of the intestinal microbiota in house miceMol Ecol20132271904191623398547
- DonaldsonGPLeeSMMazmanianSKGut biogeography of the bacterial microbiotaNat Rev Microbiol2016141203226499895
- StearnsJCLynchMDSenadheeraDBBacterial biogeography of the human digestive tractSci Rep2011117022355685
- XiaoLFengQLiangSA catalog of the mouse gut metagenomeNat Biotechnol201533101103110826414350
- BecattiniSTaurYPamerEGAntibiotic-induced changes in the intestinal microbiota and diseaseTrends Mol Med201622645847827178527
- LozuponeCAStombaughJIGordonJIJanssonJKKnightRDiversity, stability and resilience of the human gut microbiotaNature2012489741522023022972295
- BuffieCGBucciVSteinRRPrecision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficileNature2015517753320520825337874
- BuffieCGPamerEGMicrobiota-mediated colonization resistance against intestinal pathogensNat Rev Immunol2013131179080124096337
- DevkotaSPrescription drugs obscure microbiome analysesScience20163513516272452453
- MaierLPruteanuMKuhnMExtensive impact of non-antibiotic drugs on human gut bacteriaNature2018555769862362829555994
- ServickKMouse microbes may make scientific studies harder to replicate2016 Available from: http://www.sciencemag.org/news/2016/08/mouse-microbes-may-make-scientific-studies-harder-replicateAccessedJuly 25, 2018
- AlidoriSBowmanRLYarilinDDeconvoluting hepatic processing of carbon nanotubesNat Commun201671234327468684
- TsugeOKanemasaSRecent advances in azomethine ylide chemistryKatritzkyARAdvances in Heterocyclic Chemistry45San DiegoAcademic Press1989231349
- MulveyJFeinbergEMcdevittMScheinbergDDialytic separation of bundled, functionalized carbon nanotubes from carbonaceous impuritiesCrystals201444450465
- DubéP2017 Microbiome in Mouse Models Workshop review – best practices2017 Available from: https://www.taconic.com/taconic-insights/microbiome-and-germ-free/2017-microbiome-workshop-review-part-three.htmlAccessed July 25, 2018
- EdgarRCUPARSE: highly accurate OTU sequences from microbial amplicon readsNat Methods2013101099699823955772
- CaporasoJGLauberCLWaltersWAUltra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platformsISME J2012681621162422402401
- EdgarRCFlyvbjergHError filtering, pair assembly and error correction for next-generation sequencing readsBioinformatics201531213476348226139637
- AltschulSFGishWMillerWMyersEWLipmanDJBasic local alignment search toolJ Mol Biol199021534034102231712
- TatusovaTCiufoSFedorovBO’NeillKTolstoyIRefSeq microbial genomes database: new representation and annotation strategyNucleic Acids Res201442D1D553D55924316578
- McmurdiePJHolmesSPhyloseq: an R package for reproducible interactive analysis and graphics of microbiome census dataPLoS One201384e6121723630581
- WickhamHGgplot2: Elegant Graphics for Data AnalysisHeidelbergSpringer2009
- SegataNIzardJWaldronLMetagenomic biomarker discovery and explanationGenome Biol2011126R6021702898
- Carmona-RibeiroAMCarrascoLDCationic antimicrobial polymers and their assembliesInt J Mol Sci20131459906994623665898
- SitaramNSubbalakshmiCNagarajRIndolicidin, a 13-residue basic antimicrobial peptide rich in tryptophan and proline, interacts with Ca2+-calmodulinBiochem Biophys Res Commun2003309487988413679055
- SwanDGHaleRSDhillonNLeadlayPFA bacterial calcium-binding protein homologous to calmodulinNature1987329613484853627246
- ChristophersenDVJacobsenNRAndersenMHCardiovascular health effects of oral and pulmonary exposure to multi-walled carbon nanotubes in ApoE-deficient miceToxicology2016371294027725195