Abstract
Purpose
The delivery of transgenes into human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes (hiPSC-CMs) represents an important tool in cardiac regeneration with potential for clinical applications. Gene transfection is more difficult, however, for hiPSCs and hiPSC-CMs than for somatic cells. Despite improvements in transfection and transduction, the efficiency, cytotoxicity, safety, and cost of these methods remain unsatisfactory. The objective of this study is to examine gene transfection in hiPSCs and hiPSC-CMs using magnetic nanoparticles (NPs).
Methods
Magnetic NPs are unique transfection reagents that form complexes with nucleic acids by ionic interaction. The particles, loaded with nucleic acids, can be guided by a magnetic field to allow their concentration onto the surface of the cell membrane. Subsequent uptake of the loaded particles by the cells allows for high efficiency transfection of the cells with nucleic acids. We developed a new method using magnetic NPs to transfect hiPSCs and hiPSC-CMs. HiPSCs and hiPSC-CMs were cultured and analyzed using confocal microscopy, flow cytometry, and patch clamp recordings to quantify the transfection efficiency and cellular function.
Results
We compared the transfection efficiency of hiPSCs with that of human embryonic kidney (HEK 293) cells. We observed that the average efficiency in hiPSCs was 43%±2% compared to 62%±4% in HEK 293 cells. Further analysis of the transfected hiPSCs showed that the differentiation of hiPSCs to hiPSC-CMs was not altered by NPs. Finally, robust transfection of hiPSC-CMs with an efficiency of 18%±2% was obtained.
Conclusion
The difficult-to-transfect hiPSCs and hiPSC-CMs were efficiently transfected using magnetic NPs. Our study offers a novel approach for transfection of hiPSCs and hiPSC-CMs without the need for viral vector generation.
Introduction
Human induced pluripotent stem cells (hiPSCs) are human somatic cells that are genetically reprogrammed into an embryonic-like, pluripotent state capable of differentiating into all three germ layers. Since the original description of induced pluripotent stem cells (iPSCs),Citation1 the field has greatly expanded.Citation2–Citation4 The hiPSC technology has the potential to revolutionize regenerative and precision medicine by providing differentiated cells for cell-based therapy, disease modeling, drug testing, and high-throughput drug discovery in a patient-specific manner.
A large number of studies have refined the techniques for efficient directed- differentiation of hiPSCs into cardiomyocytes.Citation5 In addition, multiple studies have provided evidence for the application of hiPSC-derived cardiomyocytes (hiPSC-CMs) in cardiac transplantation in animal models.Citation6 One of the main advantages of utilizing hiPSC-CMs, as opposed to undifferentiated hiPSCs, is the elimination of the risk of teratoma formation. Moreover, hiPSC-CMs serve as an unlimited source of committed human cardiomyocytes.
Genetic modification of hiPSCs represents an essential tool for the study of hiPSCs. Expression of different markers is required in cell-based therapy applications of hiPSCs and hiPSC-CMs. With the advent of genome editing using CRISPR-Cas9 technology,Citation7 it has become increasingly feasible to correct disease-causing mutations in patient-specific hiPSCs, in order to create isogenic lines for disease modeling or potential therapeutics. However, there are significant technical challenges for the transfection of hiPSCs and hiPSC-CMs, since these cells are known to be difficult to transfect.Citation8–Citation10 Although multiple methods of genetic modifications exist (ie, nucleofection, lipofectamine-mediated transfection, and viral-based transduction), their efficiency, cytotoxicity, safety, and cost remain unsatisfactory.Citation8 Increasingly, nanoparticles (NPs) have been used in biomedical research as powerful tools for drug delivery and personalized medicine.Citation11 The objective of this study is to examine the efficiency of gene transfection in hiPSCs and hiPSC-CMs using magnetic NPs. Our study offers a novel approach to introduce desired genes into hiPSCs and hiPSC-CMs without the need for viral vector generation.
Materials and methods
Cell culture
HiPSCs (19-9-7T and 6-9-9, WiCell, Madison, WI, USA) were plated in feeder-free conditions using matrigel-coated culture dishes and chemically defined medium, mTeSR™ 1 (Stemcell Technologies, Inc., Cambridge, MA, USA). Cardiac myocytes were generated using a directed differentiation protocol.Citation12 Briefly, differentiation of confluent (80%–90%) cells was initiated by adding RPMI/B27 medium (Thermo Fisher Scientific, Waltham, MA, USA) lacking insulin and containing the CHIR99021 (Tocris, Minneapolis, MN, USA) for 24 hours, followed by RPMI/B27 media with an inhibitor of Wnt signaling, IWR-1-endo (Tocris). Differentiated hiPSCs were replated on a coverslip prior to transfection and action potential (AP) recordings.
Magnetic-assisted transfection using nanoparticles
The transfection was conducted following the manufacturer’s instructions (Neuromag, OZ Biosciences Inc., San Diego, CA, USA) and published methods.Citation13,Citation14 The NPs are positively charged, with a zeta >+30 mV in water. The size of the NPs ranges from 140 to 200 nm with the majority around 160 nm, and the particle population is rather homogeneous. Briefly, plasmid DNAs (pIRES2-EGFP, Clontech Laboratories, Inc., Mountain View, CA, USA) or a double fusion construct (an integrating vector) with green fluorescence protein (GFP)Citation15 were diluted in cell culture medium, and the NP reagent was added to the culture medium containing DNA. DNA handling followed NIH guidelines. After brief vortexing and 20-minute incubation at room temperature, the medium containing the DNA/nanoparticle complexes was added to the cell culture dish. The dish was then placed on a magnetic plate and incubated in a cell culture incubator for 1, 2, and 4 hours. Cells were harvested or differentiated after 24–48 hours of transfection. For comparison, lipofectamine-2000 and -3000 (Thermo Fisher Scientific) were used.
Flow cytometric analysis
Cells were trypsinized and analyzed for GFP signal using a standard FACScan cytometer (BD Biosciences, San Jose, CA, USA), as we have described.Citation16 Briefly, cells were fixed with 0.4% paraformaldehyde (PFA) before treating with anti-myosin heavy chain antibody (Developmental Studies Hybridoma Bank, Iowa city, IA, USA) in PBS with 5% donkey serum and 20 µg/mL DNAse-free RNAse (Sigma-Aldrich Co., St Louis, MO, USA), overnight at 4°C. Cells were also stained with 40 µg/mL 7-aminoactinomycin D (7AAD, BD Biosciences) to measure the DNA content. Data were collected using a standard FACScan cytometer (BD Biosciences) upgraded to a dual laser system with the addition of a blue laser (15 mW at 488 nm) and a red laser (25 mW at 637 nm Cytek Development, Inc., Fremont, CA, USA). Data were acquired using CellQuest software (BD Biosciences) and analyzed using FlowJo software (Ver9.4 Treestar Inc., San Carlos, CA, USA). Cells stained with isotype-matched IgG antibodies were used as controls to determine the positive cell population.
Immunofluorescence confocal microscopy
Expression of troponin T in hiPSC-CMs was detected by using mouse monoclonal anti-cardiac troponin T antibody (Abcam, Burlingame, CA, USA). Images were taken using Zeiss LSM 700 confocal microscope (Carl Zeiss, Oberkochen, Germany).
Electrophysiologic recordings
Spontaneous action potentials (APs) of hiPSC-CMs were recorded using the perforated-patch recording technique at 35°C, as we have described.Citation17 Briefly, the patch-pipettes were backfilled with amphotericin (200 µg/mL). The pipette solution contained (mM) K-glutamate 120, KCl 25, MgCl2 1, CaCl2 1, HEPES (N-2-hydroxyethylpiperazine-N’-2-ethanesulphonic acid) 10, pH 7.4 with KOH. The external solution contained (in mM): NaCl 138, KCl 4, MgCl2 1, CaCl2 2, NaH2PO4 0.33, glucose 10, HEPES 10, pH 7.4 with NaOH. The recording was performed using an Axopatch 200A amplifier (Molecular Devices, San Jose, CA, USA). The signal was filtered at 1 kHz using a 4-pole Bessel filter and digitized at sampling frequency of 2 kHz. Data analysis was carried out using Clampfit 10 software and graphics software (Origin Lab, Origin 6.0, Northampton, MA, USA).
Statistical analysis
Data are presented as mean ± standard error (SE). Statistical comparisons were analyzed by Student’s t-test or one-way ANOVA followed by Bonferroni tests for post hoc comparison. Statistical significance was considered to be achieved when P<0.05, and n represents the number of independently repeated experiments.
Results
Efficient transfection of hiPSCs using magnetic nanoparticles
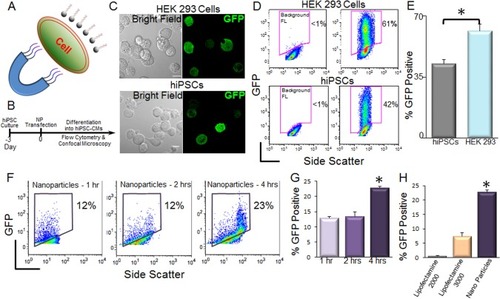
NPs have recently been used as powerful tools for drug and gene delivery.Citation11,Citation18 Magnetic NPs have been successfully used for transfection of difficult-to-transfect primary neurons.Citation13,Citation14 However, the use of magnetic NPs for transfection of hiPSCs remains unknown. We therefore tested the transfection of hiP-SCs using magnetic nanoparticles. shows a diagram depicting magnet-assisted transfection (magnetotransfection) using NPs. A schematic representation of the experimental protocol is shown in . We first tested the double fusion construct, and the confocal microscopic images of transfected cells are shown in . Flow cytometric analyses were used to directly quantify nanoparticle-mediated transfection in HEK 293 cells and hiPSCs (). We further tested the transfection using a non-integrating GFP construct (pIRES2-EGFP) and optimized the time needed for magnetotransfection (). We note a significant increase in percentages of GFP-positive hiPSCs using 4 hours of magnetotransfection. Importantly, there was a significant increase in the efficiency of transfection using magnetotransfection compared to lipofetamine-2000 and -3000 ().
Figure 1 Transfection of hiPSCs using magnetic nanoparticles.
Notes: (A) Diagram depicting magnetic nanoparticle-mediated transfection. (B) Schematic representation of the experimental protocol. (C) Confocal laser scanning microscopic images of double fusion construct-transfected HEK 293 cells (upper) and hiPSCs (lower). The left panels show the corresponding bright-field images of the cells. Scale bar is 10 µm. (D) Flow cytometric analyses of transfection efficiencies. Magnetic nanoparticle-treated cells without GFP plasmids were used as control for background fluorescence (Background FL) shown in the left panel. GFP signals were detected from the GFP expression in the cells. (E) Summary data from D (*P<0.05 by Student’s t-test, n=3–7). (F) Flow cytometric analysis of pIRES2-EGFP-transfected hiPSCs using 1, 2, and 4 hours of magnetotransfection. (G) Summary data from F (*P<0.05 by ANOVA, n=3–7). pIRES2-EGFP vector has a lower transfection efficiency compared to double fusion construct (D and E) after 4 hours of magnetotransfection. (H) Comparison of the transfection efficiency of hiPSCs using pIRES2-EGFP vector and lipofectamine-2000, -3000 and nanoparticle-mediated transfections. Four hours of transfection was used for all the conditions (*P<0.05 by ANOVA, n=3–7).
Transfection with NPs did not alter the differentiation of hiPSCs to hiPSC-CMs
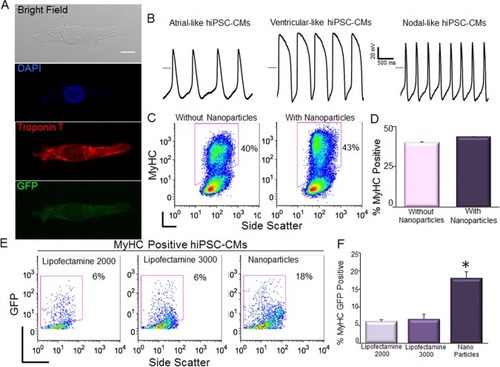
One possible concern using magnetotransfection is whether the procedure may alter the differentiation efficiency of hiPSCs. Here, we directly compared the differentiation efficiency between control (non-transfected) and transfected hiPSCs into cardiomyocytes (CMs). The differentiated CMs exhibited large beating clusters with spontaneous firing APs, consistent with populations of ventricular-like, atrial-like, and nodal-like APs ().Citation19 There were no significant differences in the efficiency of differentiation into CMs between control hiPSCs and GFP-transfected hiPSCs using NPs ().
Figure 2 Transfection of hiPSC-CMs using magnetic nanoparticles.
Notes: (A) Confocal laser scanning microscopic images of GFP-transfected hiPSC-CM. Scale bar is 10 µm. (B) hiPSC-CMs exhibit spontaneous APs with ventricular-like, atrial-like, and nodal-like characteristics. The dotted line represents 0 mV. (C, D) Assessment of the efficiency of differentiation into CMs in control hiPSCs compared to hiPSCs transfected with double fusion construct using nanoparticles by analysis of myosin heavy chain (MyHC) positive cells. Summary data are shown in the right panels. (E, F) Comparison of the transfection efficiency in double positive hiPSC-CMs (MyHC+/GFP+) using pIRES2-EGFP vector and lipofectamine-2000, -3000, and nanoparticle-mediated transfections. Data were collected 4 hours after transfection (*P<0.05 by ANOVA, n=3).
Magnetotransfection was more efficient than lipofectamine in hiPSC-CMs
HiPSCs can be differentiated into multiple cell types. HiPSC-CMs have potential for many applications in cardiac regeneration, as well as serving as models for cardiovascular diseases.Citation6,Citation20 HiPSC-CMs, as differentiated cells, are even more difficult to transfect compared to hiPSCs. It is critical to further evaluate the efficiency of magnetotransfection in hiPSC-CMs. By using flow cytometric analysis of both myo-sin heavy chain (MyHC) and GFP positive cells, we directly demonstrated that magnetotransfection was more efficient than lipofectamine not only in hiPSCs, but also in hiPSC-CMs () using pIRES2-EGFP construct.
Discussion
Since the original description of iPSCs,Citation1 the wide-reaching potentials of the technology have been rapidly realized for both regenerative and precision medicine. The utilization of hiPSCs enables the development of an unlimited source of any human cell types needed for therapeutic and precision medicine. Genetic modification and expression of different reporters are essential for studies to evaluate cell-based therapy applications of hiPSCs and hiPSC-CMs. However, significant technical challenges exist for the transgene delivery into hiPSCs.Citation8–Citation10 Several methods have been developed for transgene delivery, which are mainly catogorized into viral and non-viral methods.Citation8–Citation10 Three types of viral vectors are widely used in the transgene delivery, including adenoviral, lentiviral, and adenoassociated viral vectors. The advantage of viral vectors lies in their high delivery efficacy, but their use also raises safety concerns including the cytotoxicity, cellular immune responses, and transgene integration into host genome. Non-viral methods, including mechanical and electrical methods such as injection or electroporation, and chemical methods such as lipofection, are potentially safer alternatives for transgene delivery into iPSCs, but the transfection efficiency is relatively lower.Citation9,Citation10 For iPSC-CMs and embryonic stem cell-derived cardomyocytes (ESC-CMs), viral transduction and nucleofection are commonly used for transgene delivery.Citation21–Citation24
Magnetic NPs have been used for the transfection of cell lines and primary cells including neurons.Citation13,Citation14,Citation25 Magnetic nanoparticle-mediated gene transfer offers significant advantages over other gene transfer methods, such as high efficiency, low cytotoxicity, low cost, directional and distal controllability, efficient in vivo applications, and lack of immune responses.Citation25–Citation28 Recently, magnetic NPs have been used for gene delivery to neural precursor/stem cells.Citation26,Citation27,Citation29 However, whether magnetic nanoparticles can be efficiently used for transfection of iPSCs and iPSC-CMs has not been addressed and reported. Here, we demonstrate that the difficult-to-transfect hiPSCs and hiPSC-CMs can be efficiently transfected using magnetic NPs.
Our study offers a novel approach to introduce trans-genes into hiPSCs and hiPSC-CMs without the need for viral vector generation. Indeed, our findings transcend the benefits for hiPSCs and hiPSC-CMs. The technique may represent a non-viral method for the generation of hiPSCs and thus avoid the risk of genomic insertions inherent in some integrating viral methods. The potential application will be the genetic modification of hiPSCs and hiPSC-CMs for in vitro guided differentiation and in vivo transplantation for tissue regeneration and repair. The distal control of nanoparticles by a magnetic field will further potentiate the in vivo application and delivery of nucleic acids to specific organs for targeted gene therapy. The possible limitation may come from the cytotoxicity of magnetic NPs, which results from the accumulation of NPs in endosomes and/or vacuoles in cells. However, NPs will be degraded through normal iron metabolism over time, although the mechanism is still not well understood.Citation11,Citation25 For future studies, transfection efficiencies between different types of constructs need to be addressed.
Author contributions
Zhang XD developed the concept and designed the study, performed cell transfection, confocal imaging and patch clamp recordings, made figures, wrote and revised the manuscript; Sirish P helped to design the study and performed the hiPSC and hiPSC-CM culture and flow cytometric analysis, made figures, and revised the manuscript; Yamoah MA helped to design the study, performed the major magnetic transfection experiments, confocal imaging and part of flow cytometric analysis, and wrote and revised the manuscript; Maryam Moshref performed part of the magnetic transfection experiments, conducted immunostaining and confocal imaging; Sharma J performed part of the hiPSC-CM culture; Chen WC helped on patch clamp recordings and cell line culture; Ledford HA helped on the cell line culture and lipofactamine transfection, and revised the manuscript; Lee JH helped on the confocal imaging; Chavez KS helped on the hiPSC-CM culture; Wang W helped on the improvement of magnetic transfection protocol; López JE helped on the improvement of hiPSC and hiPSC-CM culture protocols, and revised the manuscript; Lieu DK helped on the improvement of hiPSC and hiPSC-CM culture protocols. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Dr Joseph Wu (Stanford University) for providing the double fusion construct, and Dr Nipavan Chiamvimonvat for her constructive advice on the study. This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) (grant number R56HL138392 to XDZ), American Heart Association (AHA) (grant number 14BGIA18870087 to XDZ), NIH K12 (grant number UL1 TR000002 to JEL) and Harold Amos Medical Faculty Development Program, RWJ Foundation (to JEL), California Institute of Regenerative Medicine (CIRM) (grant number RB4-05764 to DKL), Postdoctoral Fellowship Awards to PS and JS from CIRM Stem Cell Training Program to UC Davis (grant number TG2-01163), NIH NRSA F31 Predoctoral Award (F31 HL136120 to HAL), Predoctoral (HAL) and Postdoctoral (PS) Fellowship Award from NIH T32 Training Grant in Basic and Translational Cardiovascular Science (grant number T32 HL08350), AHA Postdoctoral Research Award (grant number 16POST26970007 to PS), AHA Career Development Award (grant number 18CDA34110060 to PS), and Harold S. Geneen Charitable Trust Award (PS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
Deborah Lieu reports personal fees from Novoheart Ltd, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- TakahashiKYamanakaSInduction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factorsCell2006126466367616904174
- YuJVodyanikMASmuga-OttoKInduced pluripotent stem cell lines derived from human somatic cellsScience200731858581917192018029452
- TakahashiKTanabeKOhnukiMInduction of pluripotent stem cells from adult human fibroblasts by defined factorsCell2007131586187218035408
- ShiYInoueHWuJCYamanakaSInduced pluripotent stem cell technology: a decade of progressNat Rev Drug Discov201716211513027980341
- LianXHsiaoCWilsonGRobust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signalingProc Natl Acad Sci U S A201210927E1848E185722645348
- LalitPAHeiDJRavalANKampTJInduced pluripotent stem cells for post-myocardial infarction repair: remarkable opportunities and challengesCirc Res201411481328134524723658
- CongLRanFACoxDMultiplex genome engineering using CRISPR/Cas systemsScience2013339612181982323287718
- ChatterjeePCheungYLiewCTransfecting and nucleofecting human induced pluripotent stem cellsJ Vis Exp2011563110
- FontesALakshmipathyUAdvances in genetic modification of pluripotent stem cellsBiotechnol Adv2013317994100123856320
- RaptiKStillitanoFKarakikesIEffectiveness of gene delivery systems for pluripotent and differentiated cellsMol Ther Methods Clin Dev201521406726052535
- ZhangLGuFXChanJMWangAZLangerRSFarokhzadOCNanoparticles in medicine: therapeutic applications and developmentsClin Pharmacol Ther200883576176917957183
- LianXZhangJAzarinSMDirected cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditionsNat Protoc20138116217523257984
- UnderhillSMWheelerDSLiMWattsSDIngramSLAmaraSGAmphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neuronsNeuron201483240441625033183
- WangRPalaviciniJPWangHRanBP9 overexpression accelerates loss of dendritic spines in a mouse model of Alzheimer’s diseaseNeurobiol Dis20146916917924892886
- LeeASWuJCJcWImaging of embryonic stem cell migration in vivoMethods Mol Biol201175010111421618086
- SirishPLiNTimofeyevVMolecular Mechanisms and New Treatment Paradigm for Atrial FibrillationCirc Arrhythm Electrophysiol201695e00372127162031
- ZhangXDTimofeyevVLiNCritical roles of a small conductance Ca2+-activated K+ channel (SK3) in the repolarization process of atrial myocytesCardiovasc Res2014101231732524282291
- ChouLYMingKChanWCStrategies for the intracellular delivery of nanoparticlesChem Soc Rev201140123324520886124
- ZhangJWilsonGFSoerensAGFunctional cardiomyocytes derived from human induced pluripotent stem cellsCirc Res20091044e30e4119213953
- DoyleMJLohrJLChapmanCSKoyano-NakagawaNGarryMGGarryDJHuman Induced Pluripotent Stem Cell-Derived Cardiomyocytes as a Model for Heart Development and Congenital Heart DiseaseStem Cell Rev201511571072726085192
- LieuDKFuJDChiamvimonvatNMechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytesCirc Arrhythm Electrophysiol20136119120123392582
- SaitoYNakamuraKYoshidaMEnhancement of Spontaneous Activity by HCN4 Overexpression in Mouse Embryonic Stem Cell-Derived Cardiomyocytes – A Possible Biological PacemakerPLoS One2015109e013819326384234
- SchweizerPADarcheFFUllrichNDSubtype-specific differentiation of cardiac pacemaker cell clusters from human induced pluripotent stem cellsStem Cell Res Ther20178122929037217
- StillitanoFKarakikesIHajjarRJGene Transfer in Cardiomyocytes Derived from ES and iPS CellsMethods Mol Biol2017152118319327910049
- PlankCZelphatiOMykhaylykOMagnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospectsAdv Drug Deliv Rev20116314–151300133121893135
- PickardMRBarraudPChariDMThe transfection of multipotent neural precursor/stem cell transplant populations with magnetic nanoparticlesBiomaterials20113292274228421193228
- AdamsCFPickardMRChariDMMagnetic nanoparticle mediated transfection of neural stem cell suspension cultures is enhanced by applied oscillating magnetic fieldsNanomedicine20139673774123751375
- CorcheroJLVillaverdeABiomedical applications of distally controlled magnetic nanoparticlesTrends Biotechnol200927846847619564057
- PickardMRAdamsCFChariDMMagnetic Nanoparticle-Mediated Gene Delivery to Two- and Three-Dimensional Neural Stem Cell Cultures: Magnet-Assisted Transfection and Multifection Approaches to Enhance OutcomesCurr Protoc Stem Cell Biol2017402D19.12D.19.16
