Abstract
Obesity is a global epidemic that poses a serious health concern due to it being a risk factor for life-threatening chronic diseases, such as type 2 diabetes, cancer, and cardiovascular diseases. Pharmacotherapy remains the mainstay for the management of obesity; however, its usefulness is limited due to poor drug efficacy, non-specificity and toxic side effects. Therefore, novel approaches that could provide insights into obesity and obesity-associated diseases as well as development of novel anti-obesity treatment modalities or improvement on the existing drugs are necessary. While the ideal treatment of obesity should involve early intervention in susceptible individuals, targeted nanotherapy potentially provides a fresh perspective that might be better than the current conventional therapies. Independent studies have shown improved drug efficacy by using prohibitin (PHB)-targeted therapy in obese rodents and non-human primates, thus providing a proof of concept that targeted nanotherapy can be a feasible treatment for obesity. This review presents a brief global survey of obesity, its impact on human health, its current treatment and their limitations, and the role of angiogenesis and PHB in the development of obesity. Finally, the role and potential use of nanotechnology coupled with targeted drug delivery in the treatment of obesity are discussed.
Introduction
Obesity has reached epidemic proportions worldwideCitation1 and contributes to the increased mortality rates through its association with chronic diseases such as type 2 diabetes (T2D),Citation2 cardiovascular diseases (CVDs),Citation3 cancer,Citation4 and hypertension.Citation5 The current treatment of obesity involves lifestyle modification, pharmacotherapy, and surgery. Pharmacotherapy is the cornerstone of obesity treatment and can be used alone or in combination with diet and surgery.Citation6,Citation7 However, the drugs used for the management of obesity are tainted by the adverse health risks associated with drug treatment due to their off-target side effects, and lack of specificity which reduces the sensitivity and efficacy of the drugs.Citation8–Citation10 Due to adverse effects, many anti-obesity drugs have been removed from the market, leaving only orlistat for long-term treatment of obesity.Citation10–Citation15 Moreover, the pharmaceutical drugs are useful for a limited period (≤2 years) to avoid detrimental health effects. As such, the drugs cannot be used indefinitely, and discontinuation of treatment is often followed by disease relapse.Citation11
Alternative or novel strategies that can treat obesity and sustain the weight loss are required. Such treatments are of utmost importance in health economics as they can reduce the burden of chronic diseases, improve life expectancy, and reduce mortality rates. For this reason, vascular targeted therapy is receiving a considerable amount of attention as a potential anti-obesity therapy. The ability to ferry therapeutic materials directly to pathological cells with no effect on healthy tissues could overcome the drawbacks associated with the conventional pharmacotherapy.Citation16 Strategies that target prohibitin (PHB) as a vascular marker for obesity have been explored using a PHB targeting ligand (adipose-homing peptide, AHP) to develop targeted therapyCitation16 as well as nanotherapyCitation17,Citation18 and are discussed later. Between the two systems, the nanotechnology-based therapy was able to mask the therapy from biodegradation, reduced early drug clearance from the blood system, increased half-life of the nanotherapy, and demonstrated enhanced therapeutic index. These characteristics can be attributed to the unique properties of the nanosystems.Citation17,Citation18 The feasibility of the vascular targeted nanotherapy in obesity is reviewed in this paper.
Obesity: a global epidemic
Obesity is a metabolic and genetic disorder that results from positive energy balance,Citation1,Citation19,Citation20 whereby energy intake exceeds energy expenditure for a prolonged period.Citation21 If not properly managed, obesity leads to the development of chronic diseases such as T2D,Citation2 CVDs,Citation3 various forms of cancer,Citation4 and hypertension.Citation5 These chronic diseases are further associated with adverse health events, increased medical care costs, decreased quality of life, and reduced life expectancy.Citation6,Citation7
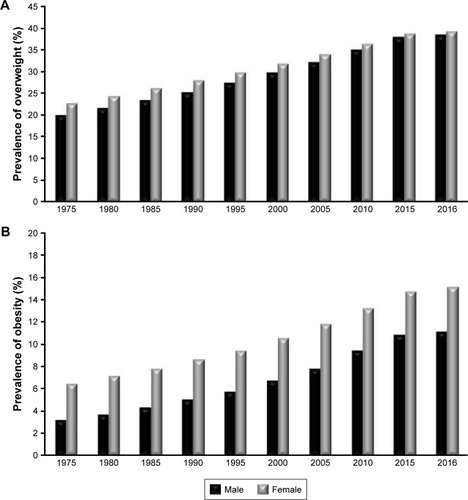
The prevalence of obesity has increased dramatically worldwide and has become a fast growing epidemic in both developed and developing countries.Citation22 The increase in obesity rates can be attributed to gene-by-environment interactions, urbanization, lack of physical activity, and easy access to fast foods.Citation22 The epidemic is beginning to show its ugly face even in children.Citation23 Globally, women are generally more prone to becoming obese than men. This translates to 70% of women and 40% of men classified as either overweight or obese.Citation24,Citation25 The WHO classified 650 million adults (≥18 years, males and females) as obese and 1.9 billion as overweight in 2016 worldwide.Citation24,Citation25 These rates have been steadily growing since 1975 to date as shown in . The overall rates indicate that the USA, China, India, and Brazil have the highest population who are either overweight or obese. Korea and Japan have the least obese and overweight population. Similar trends in the prevalence of overweight and obesity in South African adults have been reported, indicating that South Africa (SA) is not immune to this epidemic.Citation24 Without strategies to combat these rates, WHO predicts that by 2025, 60% of deaths worldwide will be caused by obesity-associated chronic diseases (CVDs, T2D, stroke, and cancers).Citation24,Citation25 In SA, chronic diseases currently account for 43% mortality rate.Citation26 These projections highlight the urgent need and significance to address the obesity epidemic.Citation23,Citation24
Figure 1 The global prevalence of overweight (A) and obesity (B) in both male and female adults.
Notes: The data represent 1975 to 2016 statistics for adults aged ≥18 years. Data adapted from: (A) World Health Organization (WHO). Global Health Observatory data repository. Prevalence of overweight among adults, BMI ≥ 25, age-standardized. Estimates by WHO Region. Available from: http://apps.who.int/gho/data/view.main.REGION2480A?lang=en. Accessed June 4, 2018. © Copyright World Health Organization (WHO), 2017. All Rights Reserved.Citation24 And from: (B) World Health Organization (WHO). Global Health Observatory data repository. Prevalence of obesity among adults, BMI ≥ 30, age-standardized. Estimates by WHO region. Available from: http://apps.who.int/gho/data/view.main.REGION2480A?lang=en. Accessed June 4, 2018. © Copyright World Health Organization (WHO), 2017. All Rights Reserved.Citation26
Pathophysiology of obesity
Physiologically, the white adipose tissues (WATs) play a crucial role in the development of obesity and could serve as the best target for obesity interventions.Citation27 A better understanding of the development and physiological roles of WAT is important, because this will help to unveil possible new therapeutic approaches for obesity, as well as the prevention of progression to obesity-related diseases.
Adipose tissue (AT) as an endocrine organ
There are two types of AT in mammals, namely the WAT and brown adipose tissue (BAT). WAT mainly stores excess energy, whereas the BAT serves as an energy dissipating organ as shown in . The WATs are responsible for obesity and obesity-induced disorders;Citation28 hence, the focus of this study is mainly on the WAT and its effect on the development of obesity and obesity-induced diseases. WAT is a loose connective tissue comprised of various cell types held together by a matrix of collagen fibers. The mature, lipid-filled fat cells or adipocytes make up two thirds of the entire tissue.Citation29 The remaining one third is made up by pre-adipocytes, endothelial cells (ECs), fibroblasts, mesenchymal stem cells, nerve fibers, monocytes, macrophages, pericytes, and immune cells.Citation29 Anatomically, the WATs are located primarily in three major areas: the subcutaneous, intradermal, and intraperitoneal/visceral depots.Citation30 The subcutaneous WAT depot is three- to fourfold larger than the visceral depot, but the two function in a coordinated and compensatory manner in the development of obesity and obesity-related diseases.Citation28
Figure 2 Adipose tissue structure and function and the pathophysiology of obesity.
Notes: During positive energy balance, increase in adipocyte number or size in the WAT leads to the development of obesity and WAT dysfunction. Adipocyte size is reduced during energy restriction or starvation, which leads to normalization of WAT function. ↑ and ↓ arrows indicate the increase and decrease of adipokines during obesity development.
Abbreviations: +ve, positive; AT, adipose tissue; bal, balance; bat, brown adipose tissue; TAGs, triacylglycerols; WAT, white adipose tissue.
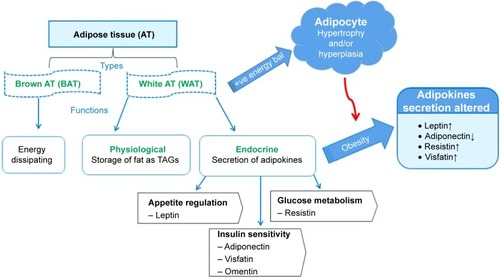
Physiological functions of WATs include the provision of insulation to internal organs, mechanical support to the organs, and also serve as a storage organ. The WATs store excess energy in the form of triacylglycerols (TAGs) and release it during starvation in the form of free fatty acids and glycerol.Citation31 Through the action of insulin, the excess calories are converted into TAGs and stored in the WATs during a chronic positive energy balance. When the energy is in demand, the TAGs are rapidly mobilized via the actions of catecholamines and other lipolytic hormones.Citation29 Because it secretes factors such as cytokines (adipokines), growth factors, acylation stimulating protein, plasminogen activator inhibitor-1, and lipoprotein lipase, recently, the WAT is recognized as an endocrine organ.Citation31 The adipokines and other WAT-secreted factors influence a variety of biological and physiological processes, including metabolism and energy homeostasis. As depicted in , various adipokines play different roles within the body such as food intake (leptin), insulin action (adiponectin), glucose metabolism (adiponectin and resistin), and angiogenesis (chemerin).Citation32–Citation35 In essence, chronic hypertrophy (increase in size of adipocytes) and hyperplasia (increase in number of adipocytes) in the WAT during the development of obesity results in dysregulated secretion of adipokines and malfunctioning of the WAT.Citation32–Citation37 As such, WAT cellular components especially the vascular system can serve as an attractive target for intervention and effective treatment for obesity and its associated disorders.Citation27,Citation33–Citation37,Citation43
Conventional treatment of obesity
Obesity is a chronic disease, and there is currently no treatment that can sustain weight loss for a prolonged period. It can be managed through lifestyle modification, pharmacotherapy, and bariatric surgery.Citation6,Citation7 Lifestyle modification involves a combination of behavioral changes, diet, and physical activity. Lifestyle modification on its own can reduce body weight by 7%, improve blood glucose levels and insulin sensitivity,Citation38 and thus reduce the risk of CVDs and T2D.Citation39,Citation40
Pharmacotherapy is an option for patients with body mass index (BMI) ≥27 kg/m2, with at least one obesity-related chronic disease risk factor.Citation10 The treatment is recommended for patients who had gone through lifestyle modification for at least 6 months with no success. It was initially an option for obese adults; however, it is now also prescribed to obese children and adolescents at a greater health risk.Citation6,Citation41 The anti-obesity drugs are reliable for the period of use, ≤6 months for short term and ≤2 years for long term.Citation10 However, discontinuation of medication usually leads to weight regain within a year.Citation11 Until 2010, only two drugs were approved by U.S. Food and Drug Administration (FDA) for long-term treatment of obesity: orlistat and sibutramine. Due to negative cardiovascular effects that resulted in the death of patients, sibutramine has since been withdrawn from the market.Citation12–Citation15 Sibutramine was successful in reducing body weight in obese subjects since its approval by FDA in 1997. Unfortunately, after its termination only orlistat remains for a long-term management of obesity.Citation10,Citation15
Bariatric surgery is recommended for morbidly obese patients (BMI ≥40 kg/m2) or patients with BMI of 30–40 kg/m2 and at least one life-threatening health risk associated with obesity. It involves modifying the gastrointestinal tract to reduce caloric intake or nutrient absorption by the body, thereby reducing body weight.Citation42,Citation43 Attempts to treat obesity by reducing food intake, with or without anti-obesity drugs are well-known, as are their limitations, difficulties, and general lack of success in maintaining weight loss.Citation10,Citation44 Surgical procedures are usually expensive and sometimes associated with gastrointestinal and neurological complications.Citation45,Citation46 This clearly predicates the need and urgency to find novel or alternative approaches that could be used to treat and/or prevent obesity at an early stage and improve human health and quality of life. Among the most important goals of obesity treatment are: 1) a preferential reduction of abdominal fat/WAT, 2) an amelioration of obesity-related health risks, 3) an improvement in comorbidities and quality of life, and 4) a reduction in mortality rate.Citation6,Citation47 Approaches targeted at the root cause of obesity (ie, the pathological WAT) have been shown to be successful in reducing body weight with minimal adverse effects.Citation16,Citation17
Angiogenesis as a target for vascular targeted therapy
Angiogenesis, also known as neovascularization, refers to the formation of new blood vessels from pre-existing capillaries.Citation48 It is a physiological process that plays an important role in the distribution of oxygen and nutrients to different tissues and organs throughout the bodyCitation49,Citation50 and is crucial for cell growth and development. The blood vessels grow or expand through the interaction between the surrounding cells and the extracellular matrix. This process is activated by the ECs lining the blood vessels. The ECs are actively involved in several regulatory processes within the body and serve as a selective passageway for nutrients, oxygen, and waste removal, to and from the surrounding cells. All physiological angiogenic functions such as the regulation of hemodynamics, vascular remodeling during ovulation, wound healing, and cell growth are executed through the ECs.Citation48,Citation51–Citation55 The occurrence of angiogenesis is highly dependent on activation, proliferation, adhesion, migration, and maturation of the ECs. Therefore, altering any of these functions through angiogenic inhibitors will result in blood vessel malformation and reversal of the effects of the disease.Citation52,Citation53,Citation55
Angiogenesis is also a classical hallmark of cancer,Citation56,Citation57 and its inhibitors have shown great potential as treatment of cancer in preclinical and clinical studies.Citation58 These inhibitors are mostly targeted at arresting the growth of vascular ECs, by so doing they prevent cellular growth and metastasis of the fast growing cancer cells.Citation59 It is known that the ECs through their lining of blood vessels interact with several cells and provide necessary oxygen and nutrients to these cells. Therefore, prevention of their proliferation will starve the diseased cells leading to their death. Since physiological angiogenesis is minimal in adults, vascular targeting could reduce side effects associated with long-term treatment observed with conventional therapeutic methods.Citation60 Moreover, the ECs are always in contact with the blood vessels and the surrounding tissues, and targeting them will increase the accessibility and sensitivity of the systemically administered treatment. Disrupting the functions of ECs will indirectly hamper the survival and growth of the unwanted cells.Citation61 The improved efficacy of vascular targeting in various cancers has been demonstrated through preclinical and clinical studies,Citation58,Citation62,Citation63 by using anti-angiogenic agents individuallyCitation69,Citation70 or in combination with chemotherapy.Citation58
Anti-angiogenic strategies that aim to target biomarkers that are either upregulated or exclusively expressed in pathological vascular systems have been explored for targeted drug discovery and treatment of chronic diseases such as cancer.Citation62,Citation63 Diseased tissues and organs in various chronic diseases are highly dependent on their vascular system for growth, proliferation, and survival. Diabetic retinopathy, rheumatoid arthritis, cancer, obesity, and autoimmune disease are some of the diseases in which angiogenesis is switched on.Citation16 These diseases are characterized by continuous growth of cells and tissues,Citation63–Citation65 as such, treatment strategies that target the vasculature of the diseased cells provide a better approach with sustainable health benefits.
Angiogenesis in obesity
Angiogenesis is an important factor in the expansion of WATs.Citation65–Citation67 AT growth and expansion through either hypertrophy or hyperplasia is highly dependent on the plasticity of the vasculature within this tissue. Hypertrophic and hyperplastic growth of adipocytes are excessive during the development of obesity. This requires an expansion of blood vessels (angiogenesis) to allow nutrients and oxygen to reach the newly formed and expanding adipocytes.Citation66
Like cancer, obesity is associated with vascular disorders,Citation53–Citation55 and since obesity progression is highly dependent on angiogenesis, it could be reversed by using treatment strategies that inhibit this process.Citation50 In obesity, the WATs are highly vascularized in order to support the expanding tissues.Citation66 As a result, the WATs are vulnerable at the level of their blood supply due to excessive blood vessel growth. The angiogenic process is mostly vulnerable at the stage of proliferation of the ECs, which is accompanied by the overexpression of certain cell surface receptors during the development of obesity. The receptors could be useful as targets for a therapeutic intervention.Citation49,Citation66 Thus, inhibition of angiogenesis in the obese WAT vasculature serves as a plausible therapeutic option for treatment of obesity.Citation65,Citation66
Angiogenesis in WATs, like in cancer, is dependent on the balance between proangiogenic and anti-angiogenic factors. Increase in proangiogenic factors in the WATs initiate angiogenesis.Citation67 The WATs secrete a number of proangiogenic factors including vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, hepatocyte growth factor, placental growth factor, fibroblast growth factor-2, angiopoietin (1 and 2), leptin, tumor necrosis factor-α, and matrix metalloproteinases.Citation67 Since adipogenesis is linked to angiogenesis, inhibition of angiogenesis by targeting of proangiogenic factors was shown to regulate WAT mass in animal models of obesity and in vitro.Citation33,Citation67,Citation68
The widely studied angiogenesis inhibitors TNP 470, angiostatin, and endostatinCitation69 have anti-angiogenic effects in obese subjects and cause reduction in body mass. Different angiogenesis inhibitors have different targets and modes of action.Citation68,Citation69 TNP 470 and its analog CDK732, for instance, target methionine aminopeptidase-2 in the ECs.Citation64 Moreover, CDK732 acts by decreasing food intake, the size of adipocytes, fat mass, and body weight in selected rodent models.Citation70 Angiostatin and endostatin reduce WAT mass by inhibiting the proliferation of vascular ECs and causing apoptotic cell death.Citation69 Although, their mechanism is elusive, vascular remodeling in the WAT of treated mice was observed to validate their (angiostatin and endostatin) anti-angiogenic activity. Their actions do not affect or alter food intake or induce any visible signs of side effects.Citation69
Antibodies or ligands that target angiogenic growth factors (such as VEGF-2 receptor) in the WAT vasculature have been shown to inhibit angiogenesis by blocking activities of target receptors when conjugated to apoptotic agents, thus preventing WAT growth in genetic and diet-induced animal models of obesity.Citation67,Citation71 PHB was also explored as an anti-angiogenic target in obesity. Kolonin et alCitation16 discovered that PHB is overexpressed in the WAT vasculature of obese mice and further demonstrated that PHB-targeted pro-apoptotic agent D(KLAKLAK)2 or KLA can be delivered into the WAT vasculature of diet-induced obese mice and reverse obesity. The KLA peptide is only toxic to mammalian cells when it is internalized, and once inside the cells, KLA disrupts the mitochondrial function leading to cell death through apoptosis.Citation16 Thus, the direct delivery of the therapy to diseased tissues could result in selective targeting that has little or no effect on normal or the surrounding cells or tissues.Citation16 For this purpose, angiogenesis proved to be an attractive target for therapeutic intervention for reversal of obesity. Several studies demonstrated the efficiency of anti-angiogenic therapy in animal models of obesity as discussed in the following section.Citation16,Citation66
PHB and its role in obesity
The role and function of PHB in cancer and obesity has been extensively reviewed.Citation72–Citation75 PHB is a 30-kDa multifunctional protein that plays a crucial role in cellular processes such as cell proliferation, endoplasmic reticulum (ER) stress, transcription, apoptosis, and tumor suppression. It is expressed in various cellular compartments including the mitochondria, nucleus, ER, the Golgi complex, endosomes, and plasma membrane.Citation76–Citation78 Most of the cellular functions mentioned above are credited to the mitochondrial and nuclear PHB. The mitochondrial PHB sustains the livelihood of the cell by keeping the mitochondrial function intact, while the nuclear PHB regulates cellular transcriptional activity. PHB protects cells from cellular stress generated during mitochondrial dysfunction such as oxidative stress, electrical stimulation, and hypoxic ischemia. When cells are exposed to any of these stressors, the level of PHB is increased, which in turn promotes survival of the cells. Mitochondrial dysfunction has been associated with the development of various diseases including cancer, diabetes, inflammatory diseases, CVDs, neurodegenerative diseases, and reproductive dysfunction.Citation76,Citation77
Some of the diseases in which PHB is implicated are shown in . PHB was reported to be overexpressed in obesity,Citation16 cancer, and diabetes and could serve as a potential biomarker for therapeutic intervention.Citation74,Citation78,Citation79 Molecules that bind to PHB in various diseases are also highlighted in ; they include AHP,Citation16 flavaglines (FLs),Citation80 and miR-361.Citation77 These ligands were used to deliver therapy or gene silencing molecules to PHB for the treatment of diseases. For instance, knocking down PHB expression in gall bladder cancer (GBC) cells resulted in reduced cancer proliferation, migration, and invasion of surrounding tissues. As such, upregulation of PHB in GBC patients can serve as both prognostic and therapeutic biomarker.Citation79 Lentiviral induced PHB overexpression in diabetic cardiomyopathy, demonstrated protective effects in vitro, and improved the myocardial function in vivo. Thus, PHB could be a potential target for the treatment of diabetic cardiomyopathy and T2D.Citation77 FLs, a class of plant-derived compounds with medicinal properties including anticancer, anti-inflammatory, cardioprotection, and neuroprotection, were demonstrated to bind to PHB with high specificity. FLs (FL3 and FL37) exerts their cytoprotective effects by binding to molecular targets such as PHB and translation initiation factor eIF4A.Citation78,Citation81 The FLs have selective toxicity toward colon cancer cells, bind to PHB in in vitro (Caco-2 BBE cells) and in vivo (chemically induced colitis in mice) models of inflammatory bowel diseases, and inhibit cancer cell proliferation.Citation78 PHB is also upregulated in colorectal cancer (CRC) stages I–III.Citation82 Targeting PHB with AHP containing a pro-apoptotic (KLA) molecule inhibited proliferation of Caco-2 colon cancer cells.Citation83
Table 1 PHB expression and its role in various diseases
PHB is also involved in the development of obesity and WAT remodeling.Citation16 Since this discovery, PHB was used as a vascular marker for obesity. Independent studies confirmed that molecules attached to AHP with amino acid sequence CKGGRAKDC are capable of targeting PHB expressed on the surface of ECs in the WAT of obese mice,Citation16,Citation84 rats,Citation85 and monkeys.Citation86 The role of PHB in the regulation of biological processes leading to the development of diseases implicates PHB as a putative target in drug discovery. Thus, therapeutic strategies that target PHB may unfold the molecular mechanisms and pathogenesis of these diseases.Citation16,Citation77,Citation78,Citation87 Targeted nanotherapy is currently being explored as a potential treatment for obesity in a rodent and non-human primate models of diet-induced obesity, by targeting the vascular system that supports the growth of hypertrophic adipocytes.Citation17,Citation18,Citation78,Citation85
Disease-associated biomarkers play a crucial role in targeted drug delivery, tissue imaging, and diagnostics. Biomarkers are able to discriminate between a physiological and the pathological state of an organism.Citation88,Citation89 These biomarkers are either exclusively expressed or overexpressed by the diseased tissues compared to normal tissues. In biological samples, the concentration of biomarker can be indicative of the presence, absence, or severity of a pathological state.Citation89 Targeting can be achieved by using antibodies, peptides, or aptamers that bind to receptors overexpressed by the diseased cells or tissues with high specificity.Citation90,Citation91 These receptors could serve as targets for diagnosis and therapeutic intervention in various chronic diseases.Citation16,Citation65
Nanotechnology and its application in medical research
Nanotechnology is a multidisciplinary field of science devoted to the development and application of atoms and molecules at a nanometer scale.Citation92,Citation93 Various nanomaterials exist for a wide range of applications in medicine, electronics, water, and so on. Due to their unique and impressive physicochemical properties, they have been widely used in medicine for diagnosis, drug delivery, and molecular imaging of diseases. The most commonly studied nanomaterials in medicine are liposomes, polymers, silver nanoparticles, gold nanoparticles (AuNPs), and quantum dots (QDs).Citation94 These nanomaterials possess unique properties that are important in medical research, for example, their large surface area-to-volume ratio allows for multiplexing applications, due to their high payload capacity.Citation94,Citation95 The NPs (AuNPs and liposomes) can be used as targeted delivery agents when attached to targeting moieties (antibodies, ligand, and aptamers) or as contrast agents (AuNPs and QDs) to monitor cellular events in biological processes. These NPs are capable of transporting materials in areas that would have been otherwise impossible using bulk materials.Citation92,Citation93 Due to their tiny size, the NPs can transverse through cellular barriers and deliver their cargo to the desired target with ease. Nanomaterials ranging from 10 to 20 nm in size are able to pass through blood vessel walls when administered through intravenous,Citation95,Citation96 intramuscular, and subcutaneous routes for biological applications.Citation97 While NPs ≤50 nm can penetrate most cellular barriers with ease.Citation98
Application of nanotechnology in medical sciences (better known as nanomedicine) has been growing in recent years. Nanotechnology has shown significant impact in cancer research, where a number of nanodrugs (eg, Doxil [Janssen, New Jersey, NJ, USA], Myocet [Teva, Castleford, UK], and Abraxane [Celgene, New Jersey, NJ, USA]) have been approved for clinical treatment of cancer.Citation99 The small size of the nanodrug composites allows them to penetrate pathological cells through their leaky vasculature, by taking advantage of the enhanced permeability and retention (EPR) effect on the vasculature of the diseased tissue.Citation95–Citation98,Citation100 In a pathological state, EPR is characterized by pathological and excessive angiogenesis and increased secretion of various permeability mediators that can be used as targets for diagnosis or treatment of a disease. These characteristics do not occur in normal tissues or organs and provide an opportunity for more selective targeting of NPs to diseased tissues and allow efficient delivery of therapeutic molecules to the target passively.Citation101
Unlike the EPR effect, targeted drug delivery is a better strategy because it directs the delivery of the drug specifically to diseased cells. Targeted delivery can be achieved through the exploitation of disease-specific biomarkers, which discriminate between normal and diseased cells. Active targeting of diseased cells will minimize systemic side effects, thus achieving a balance between efficacy and drug toxicity.Citation93,Citation102 Despite the progress made by targeted therapy, there are still concerns due to adverse effects of the therapy.Citation103 Research into NP-based drug delivery systems is mounting because these systems can provide better efficacy and limit cytotoxicity to normal cells or tissues.Citation93
Liposomes and polymeric NPs have been used successfully as drug delivery agents in clinical studies for targeted delivery of anti-cancer drugs.Citation94,Citation103 The drug-targeted NPs can be developed with qualities that can help overcome some of the drawbacks associated with the current therapies, deliver smaller doses of drugs directly to the diseased tissues while monitoring their effect in biological system. The use of such systems as vaccine and chemotherapy carriers for cancer treatment has been extensively studied.Citation104,Citation105 The NPs are effective in the prevention of drug degradation and solubility, thus increasing the dosage reaching the diseased tissue.Citation97 Biodistribution of the nanomaterials is dependent on their physicochemical properties such as size, charge, shape, etc. These properties can be manipulated to help increase their circulation times within the body.Citation95 Nanomedicine, therefore, offers higher drug efficacy with reduced side effects to the normal and surrounding cells or tissues. Moreover, these effects can be monitored in real time. Thus, while nanotechnology has shown power to revolutionize medicine,Citation94,Citation106,Citation107 it can provide an essential breakthrough in the fight against obesity and metabolic diseases through targeted drug delivery.Citation94
Potential application of nanotechnology in obesity
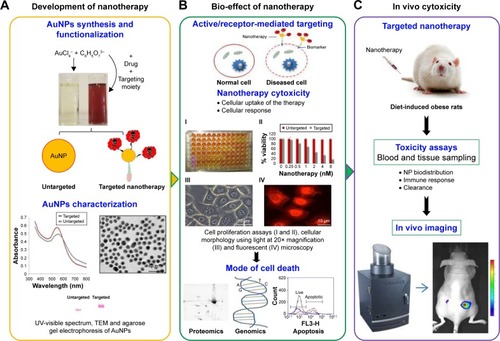
Preclinical studies have covered the basis and paved avenues for vascular targeted nanotherapy as a treatment option for obesity in animal models.Citation18,Citation84,Citation108 summarizes the initial steps toward developing a successful vascular targeted nanotherapy, in order to assess the feasibility of the strategy for the treatment of obesity as demonstrated by independent studies.Citation16–Citation18,Citation84,Citation85 Targeted nanotherapy can be developed by attaching a targeting peptide and a therapeutic peptide onto the NPs, the NPs serve as drug carriers. The biological effect of the NP-conjugates is validated in vitro and in vivo before they can be considered for clinical use. These systems, especially liposomes and polymeric NPs, have been approved by FDA for clinical trials in cancer therapy.Citation99,Citation109–Citation111
Figure 3 Initial steps in the development and assessment of targeted nanotherapy in obesity.
Notes: Preclinical phase in nanotherapy development involves synthesis, functionalization, and characterization of NP conjugates (A). The efficacy and toxicity of the nanotherapy is tested first in vitro using molecular techniques (B) followed by in vivo models of the disease (C).
Abbreviations: NP, nanoparticle; AuNPs, gold nanoparticles; TEM, transmission electron microscopy.
Nanotherapy targeted at ECs in the WAT vasculature for reversal of obesity in preclinical studies has been documented; most of the studies targeted PHB as the WAT vascular marker.Citation17,Citation18,Citation84,Citation85 By using PHB-targeting ligand (AHP) conjugated to a KLA peptide (AHP-KLA), the therapeutic peptide (KLA) was able to selectively induce cell death in the WAT vascular system of obese mice.Citation16 Disrupting the WAT vasculature led to fat resorption and reduced WAT mass with the consequent reduction of the total body weight and obesity reversal.Citation16 Incorporating nanotechnology into the same strategy enhanced the efficacy of the treatment, which was threefold higher than the AHP-KLA bi-conjugate.Citation18
The beneficial effect of PHB-targeted nanotherapy in obesity treatment is summarized in . The researchers demonstrated that when AHP (PHB-targeting ligand) and KLA (pro-apoptotic peptide) are attached to the NPs, the drug’s efficacy is enhanced when compared to AHP-KLA biconjugate. The same effect was reported and reproducible in mice and rats using different nanocarriers. PHB has been used as a vascular marker since its discovery in 2004, at the time AHP was used as a PHB-targeting ligand. Since then a list of interesting PHB ligands were identified, such as oncomirs (miR-361)Citation77 and FLs (FL3 and 37)Citation80,Citation81 which showed more or less the same effects as AHP.
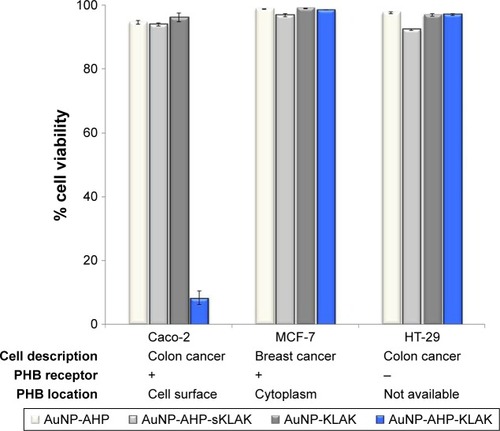
In obesity, the widely used ligand is AHP which demonstrated high specificity for PHB. Specificity of AHP for PHB is unquestionable, and its binding efficiency has been demonstrated in various cells that express PHB extracellular receptor (Caco-2,Citation83 ECs,Citation17 and WAT vasculature in obese mice,Citation16,Citation17 ratsCitation85 and monkeysCitation86). Phage display was used to identify the receptor (PHB) which AHP binds to in vivo,Citation16 the binding was confirmed in vitro and in vivo either alone or when attached to other molecules. And when attached to KLA, it was shown to bind and exert KLA apoptotic effects only on the WAT endothelium of obese subjects.Citation16,Citation18 The specificity and sensitivity of the AHP-nanocomposite was also demonstrated in our previous study. In this study, two PHB-expressing cell lines (MCF-7 and Caco-2) and non-PHB expressing cell line (HT-29) were exposed to various AuNPs for 24 hours. Post treatment, the effects of the AHP-AuNP-KLA were more pronounced on Caco-2 cells (express PHB on cell surface) than the other two control cell lines as shown in .Citation83 Although MCF-7 cells express PHB,Citation112 the cells were spared from the AHP-AuNP-KLA therapeutic effect as the cells express PHB in the cytoplasm.Citation83 These data further demonstrated the specificity of the ligand to PHB, and the potential AHP and other ligands (mentioned in ) might have when used for PHB-targeted therapy.
Figure 4 Specificity of PHB-targeted nanotherapy for extracellular PHB-expressing cells.
Notes: Data adapted from Sibuyi et al.Citation83 The three cell lines were exposed to 8 nM targeted and untargeted AuNPs with and without KLA peptide. Cell viability was assessed 24 hours post treatment.
Abbreviations: AuNP, gold nanoparticles; AHP, adipose-homing peptide; KLA, D(KLAKLAK)2; SKLA, scrambled KLA peptide; AuNP–AHP, AHP targeted AuNP; AuNP–AHP–KLA, AuNP with AHP and a pro-apoptotic peptide; AuNP–AHP–SKLA, AuNP with AHP and a scrambled peptide; AuNP–KLA, AuNP with a pro-apoptotic peptide; PHB, prohibitin; +, present; -, absent.
Interestingly, independent case studies () demonstrated that by combining nanotechnology and vascular targeted therapy, the therapeutic index of the treatment was enhanced. Similar effects were shown when various NP systems were used, ranging from AuNPs,Citation85 liposomes,Citation1,Citation17 and polymeric NPs.Citation108 As such, PHB-targeted nanotherapy could be the key to combat obesity and obesity-associated diseases (cancer, CVDs, and diabetes). PHB proved to be a key factor that links these diseases,Citation76 as such the therapy can be useful in situations where an obese patient suffers from secondary diseases. The PHB-targeted nanotherapy case studies shown in highlight the potential and feasibility of vascular-targeted nanotherapy for the treatment of obesity. The case studies for PHB-targeted nanotherapy in obesity are discussed below:
Table 2 PHB-targeted NP for the treatment of obesity
Case study 1: KLA-encapsulated liposomes for PHB targeted delivery
NP description
Liposomes were synthesized from phosphatidylcholine and cholesterol (Chol). A therapeutic peptide (KLA) was encapsulated within the liposomes, and the targeting peptide (AHP) was attached on the liposomal surface.Citation84
Therapeutic effect
KLA was used as a therapeutic agent. It induced apoptosis once internalized by ECs and inhibit weight gain in diet-induced obese mice.Citation16
Action of targeted liposomes
In vitro, the AHP-targeted nanocarrier was specifically taken up by ECs isolated from inguinal WAT obtained from obese mice when compared to EC cell lines derived from brain and fetal lung.Citation84 In vivo, AHP-targeted liposomes accumulated in the WAT vasculature. Surprisingly, the NPs without a targeting peptide were also detected in the WAT vasculature as well as angiogenic clusters of obese adipocyte, an indication that the NPs are also taken up via EPR effect through passive targeting.Citation18 PHB-mediated uptake of the NPs by ECs resulted in significantly reduced body weight of diet-induced obese mice after 18 days of treatment. The efficacy of the NP conjugate was greatly enhanced with 14% reduction in animal body weight compared with 5% of AHP-KLA (AHP conjugated to KLA) on its own. Body weight reduction in mice treated with NP-conjugate (AHP attached to KLA-encapsulated liposomes) was accompanied by reduced levels of leptin, adipocyte size, macrophage content, adipogenic/angiogenic clusters, reduced ectopic fat deposits in liver and muscle, and increased levels of adiponectin. Subcutaneous and epididymal WAT mass was also reduced. All these changes were not evident in mice treated with AHP-KLA for the same duration.Citation18
Case study 2: AHP-targeted delivery of AuNPs to PHB in obese rats
NP description
AuNPs were synthesized following Turkevich method, polyethylene glycol (PEG) was used to passivate the NPs. AHP was attached to the surface of the NPs for targeted delivery to PHB.Citation85
Therapeutic effect
AHP-AuNPs were designed for targeted delivery in the WAT vasculature of obese rats, and the biodistribution of PHB-targeted AuNP was analyzed 24 hours post treatment.Citation85
Action of NP biconjugate
AHP enhanced the uptake and accumulation of the AuNPs by the WATs of diet-induced obese Wistar rats after 24 hours. Targeted AuNPs showed reduced non-specific uptake by reticuloendothelial system organs compared with non-targeted AuNPs. Non-targeted NPs accumulated mostly in the liver, spleen, lungs, kidneys, and pancreas, whereas AHP-targeted NPs accumulated in the WAT depots (inguinal, perirenal, retroperitoneal, mesenteric, and epidydimal). Similar effects were observed in vitro, where high concentrations of gold were detected on cells treated with AHP-AuNPs.Citation85 The findings indicated that the AHP-AuNP preferentially bound to PHB expressing cells (Caco-2Citation83,Citation85 and vascular ECs from WATs).Citation85
Case study 3: vascular-targeted polymeric NPs for the transformation of WAT to BAT
NP description
Biodegradable polymeric NPs were made from a poly(lactide-coglycolide)-b-PEG copolymer. Therapeutic compounds, rosiglitazone (Rosi), and 16,16-dimethyl prostaglandin E2 (PGE2) analogs were encapsulated within the NPs. Vascular targeting peptides iRGD (amino acid sequence: CRGDK/RGPD/EC) and AHP were attached to the surface. AHP and iRGD recognize PHB and integrin αvβ3/β5 receptors, respectively. The iRGD peptide is further cleaved into the CRGDK fragment which then binds to neuropilin-1; CRGDK increases uptake and internalization of the drug-loaded NPs into the local tissues.Citation108
Therapeutic effect
Rosi (Avandia) is used for the treatment of T2D, either alone or in combination with other drugs.Citation113 It binds and activates PPAR-γ receptors in the WAT, and this leads to the increase in insulin sensitivity. Both Rosi and PGE2 analogs were used to induce angiogenesis and transformation of WAT to BAT, in order to reduce body weight in obese mice. Rosi and PGE2 analogs act on PPAR-γ and prostaglandin receptor, respectively, and increase the expression of uncoupling proteins (UCPs) and transform WAT to BAT.Citation108
Action of polymeric NPs
AHP and iRGD-targeted NPs home to their respective targets and deliver their cargo (Rosi and PGE2) within the WAT vasculature. Treatment of stromal vascular fractions with Rosi and Rosi-NPs stimulated expression of integrin αvβ3/β5 at RNA and protein levels in vitro. In the WAT, Rosi induced transformation of the tissue into BAT and increased angio-genesis in vivo. Although food intake remained the same for all the groups, the targeted NPs inhibited body weight gain in mice by 10% after 25 days of treatment. Phenotypic changes observed in inguinal and epididymal tissues of mice exposed to targeted NPs included the intense reddish-brown color development, shrinkage of adipocytes, increase in number of blood vessels, and transformation of white adipocytes to brown. Compared to untreated and the free-drug-treated mice, treatment with Rosi and PGE2-loaded NPs reduced serum levels of Chol, TAGs, and insulin.Citation108 Therefore, this indicated that obesity can be reduced by coupling vasculature-targeted nanomedicine and AT transformation.
Interestingly, BAT is thermogenically active and capable of increasing energy expenditure bŷ20% while an individual is at rest. Thus, activation of BAT or transformation of WAT to BAT presents another therapeutic target for obesity. Through cold exposure or stimulation of the sympathetic nervous system, white adipocytes can be converted into brown-like (also called “beige” or “brite”) adipocytes which have similar activities as BAT.Citation114 The beige adipocytes can generate heat that in turn increases energy expenditure. This thermogenic activity is attributed to the mitochondrial UCP-1 which is expressed mostly by the brown adipocytes, and to a lesser extent the beige adipocytes.Citation115 Browning of WAT can also be achieved by using thermogenic drugs such as thyroid hormone receptorCitation116 and β-adrenergic receptor agonists.Citation117 Transformation of white adipocytes to beige adipocytes is accompanied by increased metabolic rate, decreased fat mass,Citation116 increased glucose uptake, and improved insulin sensitivity.Citation108,Citation117
In summary, the three case studies demonstrate that vascular targeted strategies in conjunction with nanotechnology have potential for the treatment of obesity and its comorbidities.Citation17,Citation18,Citation77,Citation85,Citation108 This vascular-targeted strategy has more benefits when compared to the targeted therapy alone, some of the benefits are summarized in Box 1. Targeted drug delivery coupled with nanotechnology increased the selectivity and sensitivity of the treatment, with minimal bystander toxicity effects. Candidate drugs for this strategy are not limited to the ones mentioned in this review, but other anti-obesity drugs (current or withdrawn) can also be loaded into the nanocarriers for disease treatment. In fact, multiple molecules can be incorporated into one NP due to their larger surface area. Withdrawn drugs with anti-obesity effects could be revived by this novel, attractive, and plausible strategy for the treatment of obesity.Citation6,Citation17,Citation18,Citation108 Strategies that manipulate angiogenesis, such as anti-angiogenic inhibitors and angiogenesis activators not only reduce body weight, but also inhibit adipogenesis and ectopic fat deposition, improve insulin sensitivity, and normalize the body’s metabolic/physiological functions.Citation18,Citation108 The use of nanocarriers in combination with active targeting emerge as safer and efficient vehicles for drug delivery and offer dual targeting capability. The nanosystems can significantly enhance the drug’s availability to target organs or tissues by increasing the drug’s EPR effect, retention time and accumulation in the diseased organs. Targeted nanotherapy showed improved and exciting clinical benefits in cancer therapy.Citation99,Citation109,Citation110 The three case studies discussed in the review also demonstrate the strategy’s potential for treatment of obesityCitation17,Citation18,Citation98 and could potentially pave the way toward improving the treatment of obesity and its related metabolic diseases.
Conclusion
The current anti-obesity drugs are limited by short circulation time, insolubility, and non-selectivity of the drugs, which in turn reduce the efficacy of the drugs.Citation6,Citation47 More significant, however, are the bystander toxicity effects to healthy tissues that have led to the discontinuation of many anti-obesity drugs.Citation6,Citation10,Citation12–Citation15 This review shows that targeted therapeutic strategies can be used to overcome these drawbacks. Targeted therapy could be based on the use of homing peptides, antibodies, or aptamers that are specific for disease-associated biomarkers. Active targeting can help confine the effects of drugs only on the diseased cells, while sparing the normal cells. This will increase the efficacy of the treatment and reduce toxicity toward non-diseased cells. Vascular targeting showed promising results in the reversal of diet-induced obesity in miceCitation16 and non-human primates.Citation86 Therefore, targeted nanotherapy offers a great opportunity for the treatment of obesity through increased selectivity and sensitivity of the treatment. The concept of targeted drug delivery coupled with nanotechnology is thus an attractive and plausible strategy for the treatment of obesity.Citation17,Citation18,Citation83,Citation85
Acknowledgments
The authors would like to acknowledge South African Department of Science and Technology/Mintek Nanotechnology Innovation Centre, National Research Foundation, and the University of the Western Cape for their financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
- ApovianCMRiffenburgKMPerspectives on the global obesity epidemicCurr Opin Endocrinol Diabetes Obes201724530730928841635
- EckelRHKahnSEFerranniniEObesity and Type 2 Diabetes: What can be unified and what needs to be individualized?J Clin Endocrinol Metab20119661654166321602457
- LavieCJMilaniRVVenturaHOObesity and cardiovascular disease: risk factor, paradox, and impact of weight lossJ Am Coll Cardiol200953211925193219460605
- BrayGAThe underlying basis for obesity: relationship to cancerJ Nutr200213211 Suppl3451S3455S12421869
- NarkiewiczKObesity and hypertension – the issue is more complex than we thoughtNephrology Dialysis Transplantation2006212264267
- BrayGAA concise review on the therapeutics of obesityNutrition2000161095396011054601
- CannonCPKumarATreatment of overweight and obesity: Lifestyle, pharmacologic, and surgical optionsClin Cornerstone200994557119789064
- van GaalLDirinckEPharmacological Approaches in the Treatment and Maintenance of Weight LossDiabetes Care201639Suppl 2S260S26727440841
- AdanRAHMechanisms underlying current and future anti-obesity drugsTrends Neurosci201336213314023312373
- KangJGParkC-YAnti-obesity drugs: a review about their effects and safetyDiabetes Metab J2012361132522363917
- Ioannides-DemosLLPiccennaLMcneilJJPharmacotherapies for obesity: past, current, and future therapiesJ Obes2011201115118
- WilliamsGWithdrawal of sibutramine in EuropeBMJ20103403c82420144986
- AbbotAbbott to voluntarily withdraw Meridia® (Sibutramine) in the U.S.10102010 Available from: http://www.disabled-world.com/medical/recalls/meridia-sibutramine-withdraw.php#ixzz28ih0rGMMAccessed November 30, 2017
- MedsafeWithdrawal of Sibutramine (Reductil) in New Zealand10112010 Available from: http://www.medsafe.govt.nz/hot/media/2010/SibutramineOct2010.aspAccessed November 30, 2017
- Therapeutic Goods AdministrationSibutramine (Reductil) – withdrawal in Australia1082010 Available from: http://www.tga.gov.au/safety/alerts-medicine-sibutramine-101008.htmAccessed November 30, 2017
- KoloninMGSahaPKChanLPasqualiniRArapWReversal of obesity by targeted ablation of adipose tissueNat Med200410662563215133506
- HossenMNKajimotoKAkitaHHyodoMIshitsukaTHarashimaHLigand-based targeted delivery of a peptide modified nanocarrier to endothelial cells in adipose tissueJ Control Release2010147226126820647023
- HossenMNKajimotoKAkitaHHyodoMHarashimaHA comparative study between nanoparticle-targeted therapeutics and bioconjugates as obesity medicationJ Control Release2013171210411223871959
- ArnerPObesity – a genetic disease of adipose tissue?Br J Nutr200083S1S9S1610889786
- O’RahillySFarooqiISGshYChallisBGHuman obesity-lessons from monogenic disordersEndocrinology20031443757376412933645
- TrayhurnPAdipocyte biologyObes Rev20078s14144
- RacetteSBDeusingerSSDeusingerRObesity: Overview of prevalence, etiology, and treatmentPhysical Therapy200383327628812620091
- PulgarónERChildhood obesity: a review of increased risk for physical and psychological comorbiditiesClin Ther2013351A18A3223328273
- World Health Organization (WHO)Global Health Observatory (GHO) data2017 Available from: http://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adults/en/Accessed June 4, 2018
- World Health Organization (WHO)Global Health Observatory data repositoryPrevalence of obesity among adults, BMI ≥ 30 age-standardized. Estimates by WHO region Available from: http://apps.who.int/gho/data/view.main.REGION2480A?lang=enAccessed June 4, 2018
- Department of Health (South Africa)World Obesity Day 20162016 Available from: http://www.health.gov.za/index.php/gf-tb-program/323-world-obesity-day-2016Accessed November 20, 2017
- IwenKAHPerwitzNKrausDFasshauerMKleinJPutting fat cells onto the road map to novel therapeutic strategiesDiscov Med20066758117234130
- GustafsonBHammarstedtAAnderssonCXSmithUTissueIAA Culprit Underlying the Metabolic Syndrome and AtherosclerosisArterioscler Thromb Vasc Biol2007272276228317823366
- HaunerHThe new concept of adipose tissue functionPhysiol Behav200483465365815621071
- HausmanDBDigirolamoMBartnessTJHausmanGJMartinRJThe biology of white adipocyte proliferationObes Rev20012423925412119995
- Vázquez-VelaMEFTorresNTovarARWhite adipose tissue as endocrine organ and its role in obesityArch Med Res200839871572818996284
- BozaogluKCurranJEStockerCJChemerin, a Novel Adipokine in the Regulation of AngiogenesisJ Clin Endocrinol Metab20109552476248520237162
- HajerGRvan HaeftenTWVisserenFLJAdipose tissue dysfunction in obesity, diabetes, and vascular diseasesEur Heart J200829242959297118775919
- KrugAWEhrhart-BornsteinMNewly discovered endocrine functions of white adipose tissue: possible relevance in obesity-related diseasesCell Mol Life Sci200562121359136215924267
- SethiJKVidal-PuigAJAdipose tissue function and plasticity orchestrate nutritional adaptationJ Lipid Res2007481253126217374880
- Guerre-MilloMAdipose tissue and adipokines: for better or worseDiabetes Metab2004301131915029093
- LefterovaMILazarMANew developments in adipogenesisTrends in Endocrinol Metab200920310711419269847
- Diabetes Prevention Program Research GroupReduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or MetforminN Engl J Med Overseas Ed20023466393403
- KleinSSheardNFPi-SunyerXWeight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategiesDiabetes Care2004272067207315277443
- WaddenTAMcguckinBGRothmanRASargentSLLifestyle modification in the management of obesityJ Gastrointest Surg20037445246312763398
- HainerVToplakHMitrakouATreatment modalities of obesityDiabetes Care200831S269S27718227496
- DemariaEJBariatric Surgery for Morbid ObesityN Engl J Med Overseas Ed20073562121762183
- FisherBLSchauerPMedical and surgical options in the treatment of severe obesityAm J Surg20021846BS9S16
- Foster-SchubertKECummingsDEEmerging Therapeutic Strategies for ObesityEndocr Rev200627777979317122357
- AbellTLMinochaAGastrointestinal complications of bariatric surgery: diagnosis and therapyAm J Med Sci2006331421421816617237
- BergerJRThe Neurological Complications of Bariatric SurgeryArch Neurol20046181185118915313834
- St JeorSTHaymanLLDanielsSRPrevention conference VII: obesity, a worldwide epidemic related to heart disease and stroke: Group II: age-dependent risk factors for obesity and comorbiditiesCirculation200411018e471e47515520331
- GuptaKZhangJAngiogenesis: a curse or cure?Postgrad Med J20058195423624215811887
- GriffioenAWMolemaGAngiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammationPharmacol Rev20005223726710835101
- LiekensSde ClercqENeytsJAngiogenesis: regulators and clinical applicationsBiochem Pharmacol200161325327011172729
- GoligorskyMSEndothelial cell dysfunction: can’t live with it, how to live without itAm J Physiol Renal Physiol20052885F871F88015821252
- SengenèsCMiranvilleALolmèdeKCuratCABouloumiéAThe role of endothelial cells in inflamed adipose tissueJ Intern Med2007262441542117875177
- CaballeroAEEndothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart diseaseObes Res200311111278128914627747
- SinghDKWinocourPFarringtonKReview: Endothelial cell dysfunction, medial arterial calcification and osteoprotegerin in diabetesBr J Diabetes Vasc Dis20101027177
- MatherKJSteinbergHOBaronADWeight loss and endothelial function in obesityDiabetes Care20032661927192812766136
- MuellerJGaertnerFCBlechertBJanssenKPEsslerMTargeting of tumor blood vessels: a phage-displayed tumor-homing peptide specifically binds to matrix metalloproteinase-2-processed collagen IV and blocks angiogenesis in vivoMol Cancer Res2009771078108519584266
- WangZDabrosinCYinXBroad targeting of angiogenesis for cancer prevention and therapySemin Cancer Biol201535S224S24325600295
- NingY-MGulleyJLArlenPMPhase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancerJ Clin Oncol201028122070207620308663
- RosenLAntiangiogenic strategies and agents in clinical trialsOncologist2000590001202710804087
- TassiEWellsteinATumor Angiogenesis: Initiation and Targeting – Therapeutic Targeting of an FGF-Binding Protein, an Angiogenic Switch Molecule, and Indicator of Early Stages of Gastrointestinal AdenocarcinomasCancer Res Treat200638418919719771241
- OkajiYTsunoNHSaitoSVaccines targeting tumour angiogenesis – a novel strategy for cancer immunotherapyEur J Surg Oncol200632436337016520018
- GotoHYanoSMatsumoriYOgawaHBlakeyDCSoneSSensitization of tumor-associated endothelial cell apoptosis by the novel vascular-targeting agent ZD6126 in combination with cisplatinClin Cancer Res200410227671767615570000
- LiuYDeisserothATumor vascular targeting therapy with viral vectorsBlood200610783027303316373660
- CarmelietPJainRKAngiogenesis in cancer and other diseasesNature2000407680124925711001068
- DaquinagACZhangYKoloninMGVascular targeting of adipose tissue as an anti-obesity approachTrends Pharmacol Sci201132530030721349592
- CaoYAdipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseasesNat Rev Drug Discov20109210711520118961
- ChristiaensVLijnenHRAngiogenesis and development of adipose tissueMol Cell Endocrinol20103181–22919686803
- BråkenhielmECaoRGaoBAngiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in miceCirc Res200494121579158815155527
- RupnickMAPanigrahyDZhangCYAdipose tissue mass can be regulated through the vasculatureProc Natl Acad Sci U S A20029916107301073512149466
- KimYMAnJJJinY-JAssessment of the anti-obesity effects of the TNP-470 analog, CKD-732J Mol Endocrinol200738445546517446235
- TamJDudaDGPerentesJYQuadriRSFukumuraDJainRKBlockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cellsPLoS One200943e497419333381
- ThuaudFRibeiroNNebigilCGDésaubryLProhibitin ligands in cell death and survival: mode of action and therapeutic potentialChem Biol201320331633123521790
- KoushyarSJiangWGDartDAUnveiling the potential of prohibitin in cancerCancer Lett2015369231632226450374
- MishraSNyombaBLGProhibitin – At the crossroads of obesity-linked diabetes and cancerExp Biol Med20172421111701177
- AndeSRNguyenKHNyombaBLGMishraSProhibitin in Adipose and Immune FunctionsTrends Endocrinol Metab201627853154127312736
- ChowdhuryIThompsonWEThomasKProhibitins role in cellular survival through Ras-Raf-MEK-ERK pathwayJ Cell Physiol20142298998100424347342
- DongWQChaoMLuQHProhibitin overexpression improves myocardial function in diabetic cardiomyopathyOncotarget201671668026623724
- GiannottaMFragassiGTamburroAVanessaCLuiniASalleseMProhibitin: A novel molecular player in KDEL receptor signallingBiomed Res Int2015201573194541326064897
- CaoYLiangHZhangFProhibitin overexpression predicts poor prognosis and promotes cell proliferation and invasion through ERK pathway activation in gallbladder cancerJ Exp Clin Cancer Res201635356827084680
- HanJZhaoQBasmadjianCDésaubryLTheissALFlavaglines ameliorate experimental colitis and protect against intestinal epithelial cell apoptosis and mitochondrial dysfunctionInflamm Bowel Dis2016221556726398710
- BasmadjianCThuaudFRibeiroNDésaubryLFlavaglines: potent anticancer drugs that target prohibitins and the helicase eIF4AFuture Med Chem20135182185219724261894
- ChenDChenFLuXIdentification of prohibitin as a potential biomarker for colorectal carcinoma based on proteomics technologyInt J Oncol201037235536520596663
- SibuyiNRThovhogiNGabuzaKBPeptide-functionalized nanoparticles for the selective induction of apoptosis in target cellsNanomedicine201712141631164528635372
- HossenMNKajimotoKAkitaHHyodoMHarashimaHVascular-targeted nanotherapy for obesity: Unexpected passive targeting mechanism to obese fat for the enhancement of active drug deliveryJ Control Release2012163210111022982237
- ThovhogiNSibuyiNMeyerMOnaniMMadieheATargeted delivery using peptide-functionalised gold nanoparticles to white adipose tissues of obese ratsJ Nanopart Res2015172112
- BarnhartKFChristiansonDRHanleyPWA peptidomimetic targeting white fat causes weight loss and improved insulin resistance in obese monkeysSci Transl Med20113108108ra112-112
- RuoslahtiETargeting tumor vasculature with homing peptides from phage displaySemin Cancer Biol200010643544211170865
- StrimbuKTavelJAWhat are biomarkers?Curr Opin HIV AIDS20105646346620978388
- ByrnesSAWeiglBHSelecting analytical biomarkers for diagnostic applications: a first principles approachExpert Rev Mol Diagn2018181192629200322
- GerberDETargeted therapies: A new generation of cancer treatmentAm Fam Physician20087731131918297955
- HilgenbrinkARLowPSFolate receptor-mediated drug targeting: from therapeutics to diagnosticsJ Pharm Sci200594102135214616136558
- PalDNayakAKNanotechnology for targeted delivery in cancer therapeuticsInt J Pharm Sci Rev Res2010117
- ProvenzaleJMSilvaGAUses of nanoparticles for central nervous system imaging and therapyAJNR Am J Neuroradiol20093071293130119617446
- MoghimiSMHunterACMurrayJCNanomedicine: current status and future prospectsThe FASEB Journal200519331133015746175
- HardmanRA toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factorsEnviron Health Perspect2006114216517216451849
- de JongWHHagensWIKrystekPBurgerMCSipsAJAMGeertsmaREParticle size-dependent organ distribution of gold nanoparticles after intravenous administrationBiomaterials200829121912191918242692
- PraetoriusNMandalTEngineered nanoparticles in cancer therapyRecent Pat Drug Deliv Formul200711375119075873
- ShangLNienhausKNienhausGUEngineered nanoparticles interacting with cells: size mattersJ Nanobiotechnology2014147731551215
- BoboDRobinsonKJIslamJThurechtKJCorrieSRNanoparticle-based medicines: A review of FDA-approved materials and clinical trials to datePharm Res201633102373238727299311
- WangECWangAZNanoparticles and their applications in cell and molecular biologyIntegr Biol201461926
- KingsleyJDDouHMoreheadJRabinowBGendelmanHEDestacheCJNanotechnology: A Focus on Nanoparticles as a Drug Delivery SystemJ Neuroimmune Pharmacol20061334035018040810
- BazakRHouriMEl AchySKamelSRefaatTCancer active targeting by nanoparticles: a comprehensive review of literatureJ Cancer Res Clin Oncol2015141576978425005786
- YezhelyevMVGaoXXingYAl-HajjANieSO’ReganRMEmerging use of nanoparticles in diagnosis and treatment of breast cancerLancet Oncol20067865766716887483
- OjedaRde PazJLBarrientosAGMartín-LomasMPenadésSPreparation of multifunctional glyconanoparticles as a platform for potential carbohydrate-based anticancer vaccinesCarbohydr Res20073423–444845917173881
- TomuleasaCSoritauOOrzaAGold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cellsJ Gastrointestin Liver Dis20122118719622720309
- BriggerIDubernetCCouvreurPNanoparticles in cancer therapy and diagnosisAdv Drug Deliv Rev200254563165112204596
- CaruthersSDWicklineSALanzaGMNanotechnological applications in medicineCurr Opin Biotechnol2007181263017254762
- XueYXuXZhangXQFarokhzadOCLangerRPreventing diet-induced obesity in mice by adipose tissue transformation and angiogenesis using targeted nanoparticlesProc Natl Acad Sci U S A2016113205552555727140638
- AnselmoACMitragotriSNanoparticles in the clinicBioeng Transl Med201611102929313004
- PillaiGNanomedicines for cancer therapy: An update of FDA approved and those under various stages of developmentSOJ Pharm Pharm Sci20141213
- AnselmoACMitragotriSA Review of clinical translation of inorganic nanoparticlesAaps J20151751041105425956384
- PengXMehtaRGDifferential expression of prohibitin is correlated with dual action of Vitamin D as a proliferative and antiproliferative hormone in breast epithelial cellsJ Steroid Biochem Mol Biol20071033–544645017207617
- MalinowskiJMBolestaSRosiglitazone in the treatment of type 2 diabetes mellitus: a critical reviewClin Ther200022101151116811110228
- LeePGreenfieldJRHoKKFulhamMJA critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humansAm J Physiol Endocrinol Metab20102994E601E60620606075
- WuJBoströmPSparksLMBeige adipocytes are a distinct type of thermogenic fat cell in mouse and humanCell2012150236637622796012
- LinJZMartagónAJCiminiSLPharmacological Activation of Thyroid Hormone Receptors Elicits a Functional Conversion of White to Brown FatCell Rep20151381528153726586443
- KieferFWThe significance of beige and brown fat in humansEndocr Connect201765R70R7928465400
