Abstract
Background
Traditional physicochemical approaches for the synthesis of compounds, drugs, and nanostructures developed as potential solutions for antimicrobial resistance or against cancer treatment are, for the most part, facile and straightforward. Nevertheless, these approaches have several limitations, such as the use of toxic chemicals and production of toxic by-products with limited biocompatibility. Therefore, new methods are needed to address these limitations, and green chemistry offers a suitable and novel answer, with the safe and environmentally friendly design, manufacturing, and use of minimally toxic chemicals. Green chemistry approaches are especially useful for the generation of metallic nanoparticles or nanometric structures that can effectively and efficiently address health care concerns.
Objective
Here, tellurium (Te) nanowires were synthesized using a novel green chemistry approach, and their structures and cytocompatibility were evaluated.
Method
An easy and straightforward hydrothermal method was employed, and the Te nanowires were characterized using transmission electron microscopy, scanning electron microscopy, energy-dispersive X-ray spectroscopy, Fourier-transform infrared spectroscopy, X-ray powder diffraction, X-ray photoelectron spectroscopy, and optical microscopy for morphology, size, and chemistry. Cytotoxicity tests were performed with human dermal fibroblasts and human melanoma cells (to assess anticancer properties). The results showed that a treatment with Te nanowires at concentrations between 5 and 100 μg/mL improved the proliferation of healthy cells and decreased cancerous cell growth over a 5-day period. Most importantly, the green chemistry -synthesized Te nanowires outperformed those produced by traditional synthetic chemical methods.
Conclusion
This study suggests that green chemistry approaches for producing Te nanostructures may not only reduce adverse environmental effects resulting from traditional synthetic chemistry methods, but also be more effective in numerous health care applications.
Introduction
In a world of constant duality where the population density increases as dramatically as natural resources disappear, there are some concerns that arise from the increased use of synthetic chemicals in society. Traditional chemistry has undoubtedly enhanced the quality of life, but at a price of threatening the environment and, in some cases, human health. Chemical processes, to a certain extent, are responsible for the contamination of all the known ecosystems all over the world.Citation1–Citation3
This is especially true for nanotechnology or nanomaterials. Nanotechnology produces materials at a small scale that have novel properties much different from those of the bulk materials. In recent years, the increased implementation of nanotechnology has provided solutions for specific problems including antimicrobial resistance,Citation4–Citation6 cancer treatments,Citation7–Citation9 bioimaging,Citation10,Citation11 and drug delivery.Citation12–Citation14 The traditional physicochemical approaches for the synthesis of nanomaterials involve easy and straightforward protocols, using available techniques such as laser ablationCitation15,Citation16 or chemical vapor deposition.Citation17,Citation18 Nevertheless, there is a cost associated with these synthesis methods, such as the production of toxic by-products, the use of harsh chemicals, and lack of biocompatibility of the nanomaterials, if not pure.
Another worldwide concern is cancer, which is defined as the uncontrollable growth of cells and the abnormal behavior of cells.Citation19–Citation21 It is stated to be the second cause of death in the USA, with >1.7 million estimated cases each year.Citation22,Citation23 There are potential known cancer treatments such as chemotherapy,Citation24 radiotherapy,Citation25 and surgery;Citation26 nevertheless, they present a huge variety of side effects including fatigue, anemia, and death.Citation27,Citation28 Therefore, there is a need to find alternative treatments that avoid these limitations. Here, nanotechnology – with the synthesis of different nanostructures – can be considered a solution, as cancer cells do not have the mechanism to eliminate some metallic structures, unlike normal cells that have this ability, leading to selective cancer cell death while maintaining the viability of healthy cells.Citation29,Citation30
Therefore, new methods are needed, and green chemistry offers a suitable and novel answer, with the safe and environmentally friendly design, manufacturing, and use of chemical products that do not produce toxic and harsh by-products.Citation31,Citation32
Green chemistry is considered an alternative approach in that it combines materials coming from synthetic chemistry and nature to overcome issues from traditional synthesis. Green chemistry approaches are especially useful for the generation of metallic nanoparticles (NPs), which have nanometric structures that effectively and efficiently address concerns in both health care and industrial applications.Citation33–Citation35 Therefore, these approaches offer the chance to use living organisms (such as bacteria,Citation36 human cells,Citation37,Citation38 fungi,Citation39 or plantsCitation40,Citation41) as well as dietary and organic natural compounds (such as coffee,Citation42 tea, or honey extractsCitation43) or biological waste materials produced from industrial alimentary plants,Citation44 to naturally synthesize NPs. These nanostructures exhibit antibacterial and/or anticancer properties and can be used as drug delivery carriers and in industrial applications. These approaches are cost-effective and environmentally friendly, using standard reaction parameters in redox-reduction or hydrothermal techniques to generate specifically metallic nanostructures with a high throughput.
Several studies have implemented green nanotechnology for the synthesis of nanostructures to exhibit low cytotoxicity to human cells. For instance, garlic extract has been used to synthesize silver NPs with no cytotoxicity for fibroblasts,Citation45 and Mukherjee et al used gold NPs synthesized using plant leaves to show both biocompatibility and anticancer properties against breast cancer cells.Citation46 With such a promise shown in previous studies, there has been an increased interest in the generation of green NPs that also have anticancer properties. For example, copper oxide NPs made by Rehana et alCitation47 and silver NPs produced by Kelkawi et alCitation48 that were both synthesized using different plant extracts showed attractive anticancer properties.
There are also plenty of studies that compare the effect of the NP synthesis method (ie, green or traditional synthetic chemistry) on the anticancer, antimicrobial, and biocompatibility properties. These studies have shown continuously that NPs made using green approaches are nontoxic or less toxic than those produced by traditional synthetic processes. For example, nickel NPs made from root extracts were nontoxic to mammalian cells compared with the same ones made using conventional chemistry.Citation49
During the past years, as mentioned earlier, different methodologies have been studied to produce NPs. For instance, Liu et al prepared tellurium (Te) nanotubes with a controllable size and morphology by using a microwave approach and monosaccharides as reducing agents.Citation50 Another approach is via hydrothermal reactions, which use high pressure and temperature in addition to water and the organic compound to obtain metallic NPs.Citation51,Citation52 Different materials have been reported to work, such as platinumCitation53 or titanium oxideCitation54 NPs for the catalytic industry, and Te, a rare metalloid, is also considered a good candidate.Citation55,Citation56 Te nanowires (TeNWs) have been synthesized by employing hydrothermal reactions mainly for semiconductor and catalytic applications;Citation57,Citation58 however, their biomedical applications are largely unknown, as Te – even has similarities with oxygen, selenium, or sulfur – is not a trace element, and it has not been considered for biochemical interests. Nevertheless, some compounds of Te including tellurides (Te2−) and tellurites (TeO32−) are known to inhibit different proteins and enzymes and are capable of killing microorganisms such as bacteria and plasmodia. Besides, they can induce apoptosis in certain cancer cells.Citation59 As Sredni mentioned, two Te compounds – ammonium trichloro(dioxoethylene-O,O’)tellurate (AS101) and octa-O-bis-(R,R)-tartarate ditellurane – have been reported as anticancer agents by modulating the enzymatic activity and inhibiting tumor proliferation.Citation60
As a consequence, Te has been considered a good candidate for biological applications. There are only a few reports on their antibacterial properties against Escherichia coli and Staphylococcus aureus showing much promise, using TeNWs nanowires made from the fungus Aspergillus welwitschiaeCitation61 and a combination of Te and Au nanowires over a carbon surface using a chemical approach with hydrazine.Citation62 Low cytotoxicity on mammalian epithelial cells was reported for the latter.
In this research, for the first time, TeNWs were synthesized using both traditional synthetic and green approaches, and their properties were compared. The two ways to synthesize such nanowires were as follows: 1) a clean (green) and cost-effective hydrothermal synthesis employing Te salt and starch and 2) a traditional approach using metallic salt and chemical synthetic reducing agents such as ammonia (NH3) and hydrazine. Both methods were similar in subsequent reaction conditions. After purification, the nanostructures were characterized using transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) spectroscopy, Fourier transform infrared spectroscopy (FT-IR), X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and optical microscopy regarding morphology, size, and composition. Cytocompatibility and anticancer properties of the chemically synthesized TeNWs (CHEM-TeNWs) and green-synthesized TeNWs (GREEN-TeNWs) were compared. Cytotoxicity assays were performed over 5 days with both human dermal fibroblast (HDF) cells and human melanoma cancer cells. The results showed much promise for the further study of GREEN-TeNWs compared with CHEM-TeNWs for numerous medical applications.
Materials and methods
TeNW synthesis and purification
Following a variation of the protocol described by Hong et al,Citation55 in a typical process of integration, sodium tellurite (Na2TeO3; Sigma-Aldrich, St Louis, MO, USA) was mixed with 1 g of polyvinylpyrrolidone (PVP; Sigma-Aldrich) and dissolved in 30 mL of deionized water. Next, 1.5 mL of hydrazine hydrate (Sigma-Aldrich) and 3 mL of an NH3 (Sigma-Aldrich) solution (25% w/w) were added. The solution was stirred at a room temperature, then transferred into a teflon-lined stainless steel reactor and placed into an oven at 180°C for 4 hours. After the reaction, the mixture was allowed to cool down at a room temperature.
For the green synthesis route, the procedure was followed as described by Lu et al.Citation56 with some modifications. Briefly, telluric acid (H2TeO4; Sigma-Aldrich) was mixed with 0.15 g of a starch (Sigma-Aldrich) solution in deionized water. Then, the mixture was transferred into a teflon-lined stainless steel reactor and placed into an oven at 160°C for 15 hours. The mixture was then allowed to cool down at a room temperature.
At a room temperature, the final products from both synthesis methods were purified using the same protocol. The nanowire solutions were centrifuged at 10,000 rpm for 20 minutes, and the pellet was subsequently washed twice with water and centrifuged again at the same rate and time. Finally, the precipitate was resuspended in 35 mL of deionized water. The resulting solution containing the nanowires was transferred into a 20-mL glass vial, which was then placed in a freezer at −80°C for 4 hours and lyophilized overnight. The final powder was weighed and resuspended in a suitable amount of deionized and autoclaved water to reach the final concentration needed for further experiments.
Instruments and characterization
A Heratherm™ General Protocol Oven (Thermo Fisher Scientific, Waltham, MA, USA) was used to produce the hydrothermal reaction in both chemical synthetic and green methodologies. An Eppendorf™ Model 5804-R Centrifuge was used for the centrifugation of the samples. A FreeZone Plus 2.5-L Cascade Console Freeze Dry System was used to purify the samples and to obtain the final TeNW structures.
TeNWs prepared by both chemical synthetic and green approaches were properly characterized using a JEM-1010 transmission electron microscope (JEOL USA Inc., Peabody, MA, USA). For sample preparation, purified nanostructures were air-dried on 300-mesh copper-coated carbon grids (Electron Microscopy Sciences, Hatfield, PA, USA). The samples were then imaged up to an 80,000× magnification with an accelerating voltage of 80.0 kV.
EDX spectroscopy analysis was performed using a dedicated EDX detector coupled with a Hitachi S-4800 scanning electron microscope. TeNW samples were affixed to 300-mesh copper-coated carbon grids and placed into an aluminum pin mount. An accelerating voltage of 10.0 kV was used to obtain an elemental spectrum for the NPs.
FT-IR spectra were recorded using a PerkinElmer Spectrum 400 FT-IR/FT-near infrared in attenuated total reflectance mode. For FT-IR spectroscopy measurements, 5 μg of the dried sample was used.
Powder XRD patterns were obtained using a Rigaku Miniflex 600 (Rigaku Co. Tokyo, Japan) operating at a voltage of 40 kV, a current of 15 mA, and a Cu-Kα radiation of 1.542 Å. All XRD patterns were recorded at a room temperature with a step width of 0.05 (2) and a scan speed of 0.2°/min. The samples were used from the powder obtained after the purification process.
In the XPS, drops of both compounds dispersed in water were deposited on clean copper substrates for sample preparation. After water evaporation, the samples were loaded in a vacuum load lock chamber and then transferred to the XPS ultra-high vacuum chamber with a base pressure of 10−10 mbar. The XPS chamber is equipped with a hemispherical electron energy analyzer (SPECS Phoibos 100 spectrometer, Berlin, Germany) and an Mg-Kα (1,253.6 eV) X-ray source. The angle between the hemispherical analyzer and the plane of the surface was kept at 60°. Wide scan spectra were recorded using an energy step of 0.5 eV and a pass energy of 40 eV, while specific core level spectra (Te 3d, O 1s, and C 1s) were recorded using an energy step of 0.1 eV and a pass energy of 20 eV. The absolute binding energies of the photoelectron spectra were determined by referencing to the Te 3d 5/2 metallic core level at 573 eV.Citation63 Data processing was performed with CasaXPS software (Casa software Ltd., Cheshire, UK). The contributions of the Mg-Kα satellite lines were subtracted.
Optical microscopy analysis was done on a phase contrast mode using an Axio Observer Z1 Inverted Fluorescence Microscope (Carl Zeiss, Oberkochen, Germany). For sample preparation, cells were grown on a 6-well plate with the presence of different GREEN-TeNWs and CHEM-TeNWs for 1, 3, and 5 days. Images were taken at these time points.
In vitro cytotoxicity assays with TeNWs
Cytotoxicity assays were performed with primary HDF cells (human skin fibroblast/human skin cells, strain CCL-110; American Type Culture Collection® [ATCC®], Manassas, VA, USA) and human melanoma cells (Human Malignant Melanoma (A375), strain CRL-1619; ATCC®). The cells were cultured in DMEM (Thermo Fisher Scientific), supplemented with 10% FBS (ATCC® 30–2020™) and 1% penicillin/streptomycin (Thermo Fisher Scientific). MTS assays (CellTiter 96® Aqueous One Solution Cell Proliferation Assay; Promega, Madison, WI, USA) were carried out to assess cytotoxicity. The cells were seeded onto tissue-culture-treated 96-well plates (Thermo Fisher Scientific) at a final concentration of 5,000 cells per well in 100 μL of cell culture medium. An incubation period of 24 hours at 37°C in a humidified incubator with 5% carbon dioxide was employed. The culture medium was then replaced with 100 μL of fresh cell culture medium containing concentrations from 5 to 100 μg/mL of either CHEM-TeNWs or GREEN-TeNWs.
The cells were cultured for three different periods of time to compare the effects of the nanostructures on the cells after days 1, 3, and 5 following exposure.
The cells were washed with PBS, and the original media were replaced with 100 μL of the MTS solution (prepared using a mixing ratio of 1:5 of MTS/medium). After the addition of the solution, the 96-well plate was incubated for 4 hours in the incubator to allow for a color change. Then, the absorbance was measured at 490 nm on an absorbance plate reader (SpectraMAX® M3; Molecular Devices, San Jose, CA) for cell viability after exposure to the TeNWs. Cell viability was calculated by dividing the average absorbance obtained for each sample by that of the control sample and then multiplied by 100. Controls containing cells and media, only media and nanowires in media, were also added in the 96-well plate to identify the healthy growth of cells without nanowires and to determine the absorbance of both the media and the nanowires.
Statistical analysis
All experiments were repeated in triplicate (N=3) to ensure the reliability of the results. Statistical significance was assessed using Student’s t-tests, with P<0.05 being statistically significant. The results are displayed as mean ± SD.
Results and discussion
Synthesis of green biogenic TeNWs
The hydrothermal synthesis of TeNWs was successfully completed using both chemical and green synthesis protocols. shows the comparison between the methods.
Table 1 Synthesis conditions of the protocols used to prepare TeNWs
One of the most remarkable differences between the two synthesis methods is the relative cost and quantity of the reducing agents needed to reduce ionic Te to elemental Te. On one hand, CHEM-TeNWs were generated in an aqueous solution with the presence of two reducing agents (hydrazine and NH3) and PVP as stabilizing and structure-directing agent. Liu et alCitation64 found that PVP has a steric effect on the growth of Te along the [0001] direction. On the other hand, GREEN-TeNWs were generated under the action of starch as unique reducing and stabilizing agent, which makes the synthetic approach cheaper and more environmentally-friendly that the one followed for the production of CHEM-TeNWs.
In the chemical synthesis, using hydrazine and NH3 raises a huge safety concern when performing this process, as both are flammable, extremely toxic, and hazardous. Hydrazine in particular produces explosive vapors/air mixtures at about 38°C. Besides, it is corrosive to metals and skin and a real threat to the environment. Face shields and full-face respirators are needed to work with this chemical. PVP is however a water-soluble polymer that contributes to the formation of the nanostructures, and it is US Food and Drug Administration–approved.
On the other hand, the green synthetic approach needs only starch that is clearly a safe, naturally occurring reducing and stabilizing agent. This polymeric carbohydrate consists of a large number of glucose units that can reduce the Te ions to their elemental form. Therefore, because no additional agent is needed, this method holds advantages over the chemical synthesis method in terms of both cost and safety. Another concern to note is the production of toxic by-products. While waste produced from CHEM-TeNWs synthesis should be considered hazardous, the generation of reaction-derived products from the green synthesis process developed should not be viewed as an environmental threat.
Regarding the time for the reactions, the chemical synthesis of TeNWs took 4 hours at 160°C, while the green process took 15 hours at 180°C. This extra time can be explained by the reducing power of starch, which is lower than when using hydrazine and NH3; however, considering the hazards of the chemicals and by-products of the chemical synthesis approach, the green methodology still has plenty of advantages such as being more economical and easy and following a straightforward hydrothermal synthesis method. Nevertheless, the green synthetic approach employed can be made quicker since the time used for the growth of the nanowires can be reduced to a couple of hours. It was hypothesized that the density of wires would be less than that using 15 hours; however, this is a hypothesis that needs further development.
Moreover, in the control experiment, a colorless solution was obtained in comparison with the dark one of both GREEN-TeNWs and CHEM-TeNWs () after the reaction process.
Figure 1 Solutions of GREEN-TeNWs after the reaction. The colorless solution without starch (left) and dark solution containing starch (right).
Abbreviation: GREEN-TeNWs, green-synthesized tellurium nanowires.

The control experiment was also characterized with TEM, and no nanostructures were found; therefore, no nanowires were obtained without the use of starch. Consequently, the role of starch is crucial to reduce the metal and obtain the nanostructures.
Characterization
Morphological characterization was completed for both of the synthesis approaches with size and coating characteristics reported in .
Table 2 Data from transmission electron microscopy for size and coating
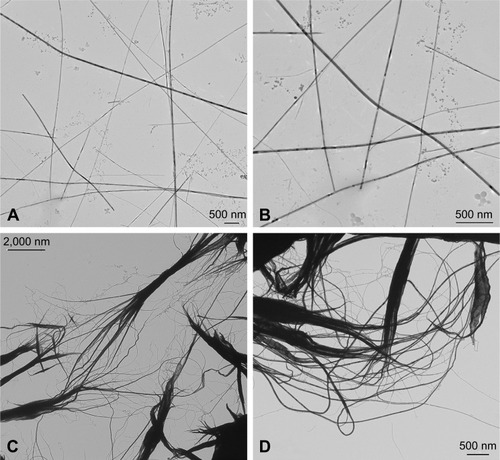
Specifically, CHEM-TeNWs () had an average diameter of 30 nm with a length of several micrometers. The wires showed a low degree of aggregation. On the other hand, GREEN-TeNWs () showed an average diameter of about 20 nm with a similar length. The peculiarity of these structures was due to their morphology. It seemed that nanowires started growing from a particular point, and they extended as far as they could. The main trunk was divided into several smaller sections at the end of the structure as ramifications, which produced branches that grew alongside one another.
Figure 2 Transmission electron microscopy images of TeNWs. CHEM-TeNWs with a low degree of aggregation were found dispersed within the solution (A) and (B). GREEN-TeNWs (C and D) showed a higher degree of aggregation, and the presence of an organic coating was observed.
Abbreviations: CHEM-TeNWs, chemically synthesized TeNWs; GREEN-TeNWs, green-synthesized TeNWs; TeNWs, tellurium nanowires.
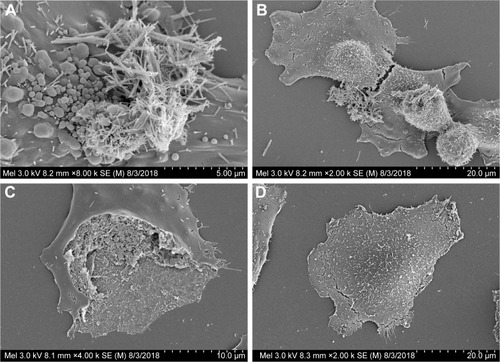
SEM characterization was performed on the GREEN-TeNWs (). The wires started growing from a cluster and extended for several micrometers, with a star-shaped uniform structure that was difficult to observe when the clusters were close to one another.
Figure 3 SEM images of CHEM-TeNWs (A and B) and GREEN-TeNWs (C and D). GREEN-TeNWs grew with a star-shaped structure, while CHEM-TeNWs had different morphologies.
Abbreviations: CHEM-TeNWs, chemically synthesized TeNWs; GREEN-TeNWs, green-synthesized TeNWs; SEM, scanning electron microscopy; TeNWs, tellurium nanowires.
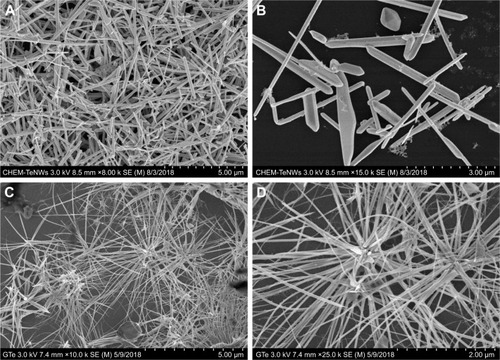
When CHEM-TeNWs were observed, the structures were found to grow on the top of each other generating a cluster that could affect cell viability (). Besides, different morphologies () – crystals, nanowires (NWs) with variable widths and irregular structures – were found in solution which can lead to potential distinct performance regarding their biomedical applications.
It has been hypothesized that the differences between both structures came from the presence of starch. This polymer is known to have ramifications, and it has been elucidated that TeNW grow following the template of starch. Moreover, the uniformity of the GREEN-TeNWs morphology is thought to enhance their action in anticancer and biocompatibility properties in comparison with the chemically synthesized products.
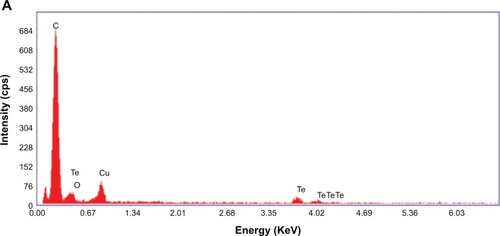
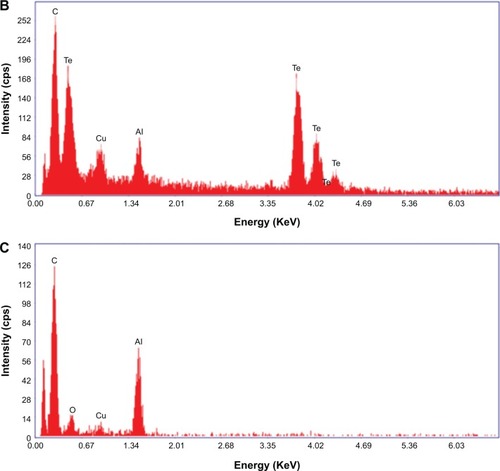
EDX spectroscopy measurements were completed for both the chemically synthesized and green-synthesized nanostruc-tures. For CHEM-TeNWs (), EDX spectroscopy analysis showed that the electron-dense nanowires had specific Te absorption peaks. For GREEN-TeNWs, the peaks for Te were higher due to the higher concentration of Te within the sample (). When the analysis focused on the surrounding areas of the nanostructures, the carbon peak was significantly raised compared with the measurement of the Te metallic core. This, aside from the absence of a Te peak, indicated an organic composition of the coating ().
Figure 4 Energy-dispersive X-ray of TeNWs. CHEM-TeNWs were characterized (A) as well as GREEN-TeNWs in both the metallic core (B) and the surrounding coating (C).
Abbreviations: CHEM-TeNWs, chemically synthesized TeNWs; GREEN-TeNWs, green-synthesized TeNWs; TeNWs, tellurium nanowires.
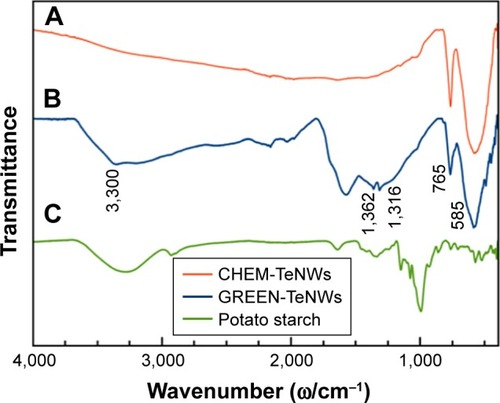
In , we compare the FT-IR spectra of CHEM-TeNWs, GREEN-TeNWs, and potato starch. The CHEM-TeNWs and GREEN-TeNWs samples show absorption bands of Te oxide at around 765 and 585 cm−1, which can be related to the symmetrical equatorial and asymmetrical axial stretching frequencies of the Te–O bond, respectively.Citation65,Citation66 The FT-IR spectrum of GREEN-TeNWs presents a vibrational band at around 3,300 cm−1 related to the O–H stretching mode of starch. The vibrational bands at the region of 1,300–1,400 cm−1 are due to C–O–H bending and CH2 twisting of the starch structure.Citation67
Figure 5 FT-IR spectra of (A) CHEM-TeNWs, (B) GREEN-TeNWs, and (C) potato starch.
Abbreviations: CHEM-TeNWs, chemically synthesized TeNWs; FI-TR spectra, Fourier-transform infrared spectra; GREEN-TeNWs, green-synthesized TeNWs; TeNWs, tellurium nanowires.
shows the XRD patterns for CHEM-TeNWs and GREEN-TeNWs. Practically, all the diffraction peaks in both experimental XRD patterns can be indexed to hexagonal Te structure (h-Te, space group P3121).Citation68 The lattice parameters calculated for both the samples are in good agreement with the reported values for h-Te,Citation68 as shown in . In the case of the XRD pattern of GREEN-TeNWs the presence of foreign phases related to Te-based oxides compounds were also detected ().
Table 3 Hexagonal lattice parameters calculated for GREEN-TeNWs and CHEM-TeNWs
Figure 6 Comparison between the experimental XRD patterns for GREEN-TeNWs and CHEM-TeNWs and the calculated XRD patterns for bulk hexagonal Te (h-Te).Citation68 The diffraction peaks marked with (*) may be related to Te-based oxides.
Abbreviations: CHEM-TeNWs, chemically synthesized TeNWs; GREEN-TeNWs, green-synthesized TeNWs; TeNWs, tellurium nanowires; XRD, X-ray powder diffraction.
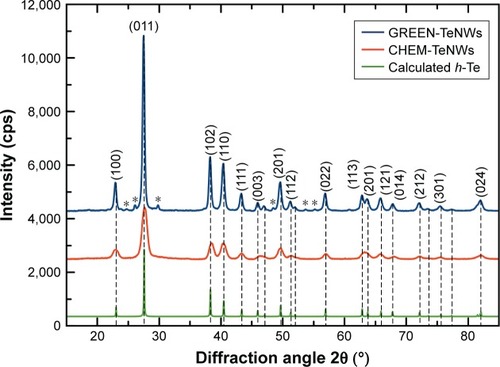
For CHEM-TeNWs, three different phases were identified corresponding to elemental Te, sodium tellurate hydrate, and an organic compound. This last one was present on a relatively little amount compared with the other ones; it has been hypothesized that its presence came from a hydrocarbon-based deposition of organic matter on the top of the sample either after the purification or after the preparation of the sample. On the other hand, GREEN-TeNWs present elemental Te, Te oxide, and organic compound phases. The presence of Te oxide has been related to the use of starch as a reducing agent as oxygen was integrated into the structure. It has been elucidated that the organic compound was associated with the use of starch. Additional information can be find in Supplementary Material (– and –).
XPS was used to characterize the chemical composition and electronic states of both CHEM-TeNWs and GREEN-TeNWs.
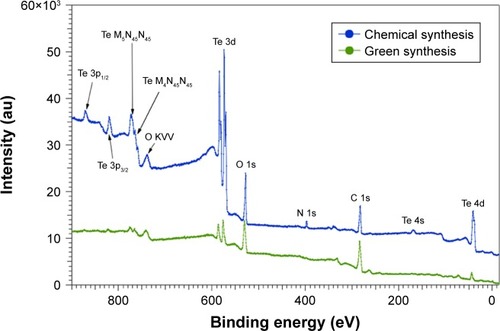
displays the wide scans that have been normalized arbitrarily to the C 1s core level peak for comparison. The main features have been identified in the spectra.
Clear differences can be observed between the two compounds. As the spectra are normalized to the C 1s core level peak, the higher intensity at the Te 3d core level for CHEM-TeNWs indicates that this compound has a higher content of Te than that in GREEN-TeNWs. In addition, the double structure for the Te 3d core level peaks (CHEM-TeNWs) is likely to indicate that this element is partially oxidized. Finally, it clearly appears that GREEN-TeNWs have no nitrogen (absence of N 1s core level peak) as compared to the CHEM-TeNWs; these differences can be explained due to the use of hydrazine and NH3 as reductor agents in the chemical synthetic approach.
The chemical analysis performed at the Te 3d, O 1s, N 1s, and C 1s core level peaks revealed the composition given in .
Table 4 Chemical composition (in percentage atomic concentration) extracted from the Te 3d, O 1s, N 1s, and C 1s core level peaks
Differences between the two compounds are better appreciated when comparing the Te 3d core levels as shown in . The spectra have been normalized to the Te 3d5/2 oxide component (at a binding energy of 576 eV) and shifted vertically for comparison.
Figure 8 XPS spectra recorded at the Te 3d core levels for samples synthesized through the chemical and green routes.
Abbreviation: XPS, X-ray photoelectron spectroscopy.
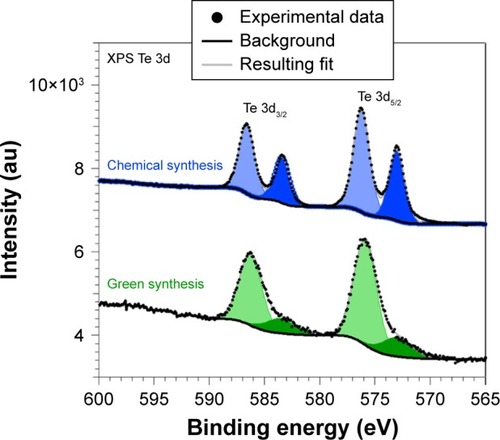
As can be observed, metallic and oxide components could clearly be identified. The metallic components are located at 573 eV (chosen as a reference in order to correct the charging effects) and 583.4 eV for the Te 3d5/2 and Te 3d3/2 peaks, respectively, whereas the oxide components are located at 576 eV and 586.6 eV for the Te 3d5/2 and Te 3d3/2 structures, respectively, which are in agreement with the previous results.Citation63,Citation69 It also clearly appears that GREEN-TeNWs have more of a Te oxide (79%) component than the CHEM-TeNWs (61% oxide) as seen previously on the XRD results.
The two compounds also present differences at the C 1s and O 1s core levels ().
Figure 9 XPS spectra recorded at the C 1s (left) and O 1s (right) core levels for samples synthesized through the chemical and green routes.
Abbreviation: XPS, X-ray photoelectron spectroscopy.
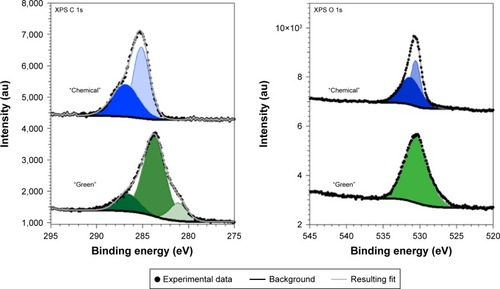
The C 1s core levels spectra (, left) could be fitted with the following minimum number of components at 281.1 eV (13%), 283.7 eV (71%), and 286.6 eV (16%) for GREEN-TeNWs and 285.1 eV (57%) and 286.8 eV (43%) for CHEM-TeNWs. The peaks located at 286.6 eV and 286.8 eV can be ascribed to hydroxyl (C–O/C–OH) groups,Citation70,Citation71 meaning that CHEM-TeNWs have more hydroxyl groups than GREEN-TeNWs. At a lower binding energy, a peak located at 285.1 eV could be identified in CHEM-TeNWs. This peak corresponds to the presence of either C–C or C–H bonds in the spCitation3 tetrahedral configuration,Citation71 and surprisingly, it was not observed in GREEN-TeNWs. Instead, two peaks at lower binding energies of 281.1 eV and 283.7 eV were identified on it. The peak at 283.7 eV could be ascribed to C–C bonds in the sp2 configuration, while the less abundant component with an XPS peak at 281.1 eV could not be clearly identified. It is inferred that this last component corresponds to C atoms weakly bonded. Thus, no evidence of the presence of carbonyl (C=O at 288.1 eV) or carboxyl (O–C=O at 289.0 eV) groups could be observed.Citation71
In the case of the O 1s core level spectra (, right), the spectrum corresponding to GREEN-TeNWs could be fitted with a single component at 530 eV, while CHEM-TeNWs have two components located at 530.6 eV (44%) and 531.3 eV (56%). These relatively weak binding energies suggest that the oxygen atoms are weakly bonded and do not correspond to O–C bonds that would appear at a binding energy of around 533 eV.Citation70 This in turn agrees with the absence of carbonyl and carboxyl groups in the C 1s spectra.
In vitro cytotoxicity of TeNWs
In vitro cytotoxicity assays were performed with HDF cells and melanoma cells (ATCC® CRL-1619). Data from the nanowire treatment were compared with a control that contained only cells and media. A comparison was made between HDF and melanoma cells with the aim of determining potential anticancer activity. The same experiments were performed using chemical and green-synthesized nanowires to determine the effect of the natural coating present on GREEN-TeNWs on the proliferation of the cells.
For HDF experiments, nanowires at a concentration between 5 and 100 μg/mL were tested. For CHEM-TeNWs, the same proliferation trend was observed within the third and fifth day for concentrations up to 15 μg/mL compared with the control (). However, the number of cells was less than that for the control in all the cases. Larger concentrations showed degeneration of the cell proliferation. When GREEN-TeNWs were tested on the cells, a similar proliferation trend was observed for each of the concentrations (). An especially unusual behavior was shown at levels between 15 and 75 μg/mL, with a higher number of cells growing within the third and the fifth day compared with the first 24 hours. When both nanostructures were compared, it can be concluded that the green-synthesized structures enhanced cell proliferation over a broader range of concentrations compared with the chemical synthetic ones.
Figure 10 MTS assays on HDF (A, B) and melanoma (C, D) cells in the presence of CHEM-TeNWs (A, C) and GREEN-TeNWs (B, D) at concentrations ranging from 5 to 100 μg/mL. N=3. Data are presented as mean ± SD; *P<0.01, **P<0.005.
Abbreviations: CHEM-TeNWs, chemically synthesized TeNWs; GREEN-TeNWs, green-synthesized TeNWs; HDF, human dermal fibroblasts; TeNWs, tellurium nanowires.
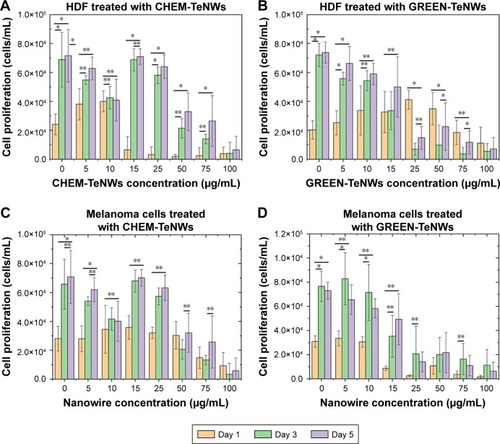
The cytotoxic effect of Te nanostructures is the result of active physicochemical interactions of elemental Te with the functional groups of intracellular proteins and the bases and phosphate groups in DNA.Citation60 While cytotoxicity was apparent for the CHEM-TeNWs (), we hypothesized that the enhanced biocompatibility in GREEN-TeNWs () is most likely due to the presence of a natural, organic coating that encompasses the Te core. The presence of a carbon layer as the natural coating introduces a biodegradable material that can enhance cell proliferation.
When melanoma cells were treated with CHEM-TeNWs, cell proliferation showed a similar trend as that of the control at concentrations up to 25 μg/mL (). GREEN-TeNWs showed a similar behavior (). Nonetheless, levels between 10 and 100 μg/mL showed a delay in the cell proliferation compared with the control over the tested time period. It has been hypothesized that TeNWs have the potential ability to slow down the signaling processes present in cancerous cells due to the effect of Te.Citation72,Citation73 Therefore, we propose that both synthesis methods produce nanostructures with anticancer properties, but specifically this behavior was particularly enhanced in the case of the green synthetic structures.
In this report, the cytocompatibility and anticancer activity of Te nanostructures were observed with a potentially improved performance using GREEN-TeNWs. Thus, not only were the GREEN-TeNWs produced with significantly fewer toxic materials, but their properties toward enhancing healthy cell proliferation and decreasing cancer cell proliferation were also greater.
A comparison was done on the fifth day of the experiments between samples to show the tendency of both cell compatibilities with HDF cells () and cytotoxic effects with melanoma cells ().
Figure 11 Comparison between CHEM-TeNWs and GREEN-TeNWs for HDF (A) and melanoma (B) cells at the fifth day of experiment. Data from MTS assays on HDF and melanoma cells in the presence of TeNWs.
Abbreviations: CHEM-TeNWs, chemically synthesized TeNWs; GREEN-TeNWs, green-synthesized TeNWs; HDF, human dermal fibroblasts; TeNWs, tellurium nanowires.
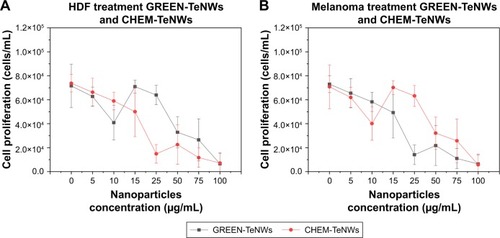
The low cytotoxicity for HDF cells can be appreciated especially at high concentrations of TeNWs, with a noticeable difference between CHEM-TeNWs – with higher cytotoxicity for HDF – and GREEN-TeNWs, which allow for a high cell proliferation. At low concentrations – between 5 and 15 μg/mL – the difference in terms of biocompatibility was almost inappreciable. The biggest difference was observed at a concentration of 25 μg/mL. For experiments with cancerous cells, the opposite behavior occurred. GREEN-TeNWs did not allow for the proliferation of melanoma cells at concentrations ranging between 15 and 100 μg/mL. As with HDF cells, the bigger difference was observed for a concentration of 25 μg/mL. Therefore, it was possible to hypothesize that at a concentration of 25 μg/mL of GREEN-TeNWs, both the maximum biocompatibility for HDF and the maximum cytotoxic effect for melanoma cells were achieved in comparison with the same concentration of CHEM-TeNWs. As a general view, the behavior of GREEN-TeNWs after 5 days in terms of low and high cytotoxicity for HDF and melanoma cells, was enhanced in comparison with CHEM-TeNWs.
IC50 values were calculated for all the experiments in , with the aim of showing the minimum inhibitory concentration for both HDF and melanoma cells. This value was obtained after 5 days of experiments, measuring the potency of the nanowires to inhibit the normal biological functioning of the cells.
Table 5 IC50 values for both CHEM-TeNWs and GREEN-TeNWs in experimental assays with HDF and melanoma cells after 5 days of experiments
The interaction between the cells and the nanostructures was studied using SEM imaging of melanoma and HDF cells. Different concentrations – 100, 50, and 0 μg/mL – of TeNWs were tested in order to elucidate the mechanism of cell death on both cell types. SEM imaging allowed the observation of modifications in the membrane and shape of cells.
For HDF cells, NWs were found to be in contact with the cells; however, no interaction between them was observed at lower concentrations. GREEN-TeNWs surrounded the cells without producing any damage () when compared to the control (). The membrane remained intact without any sign of swelling or blebbing. Thus, it has been hypothesized that the presence of starch encores the TeNWs and prevents their interaction with HDF cells.
Figure 12 Interaction between HDF cells and GREEN-TeNWs. Interaction between 50 μg/mL (A, B), 0 μg/mL (C), and 100 μg/mL (D) of GREEN-TeNWs and HDF cells was analyzed.
Abbreviations: GREEN-TeNWs, green-synthesized TeNWs; HDF, human dermal fibroblasts; TeNWs, tellurium nanowires.
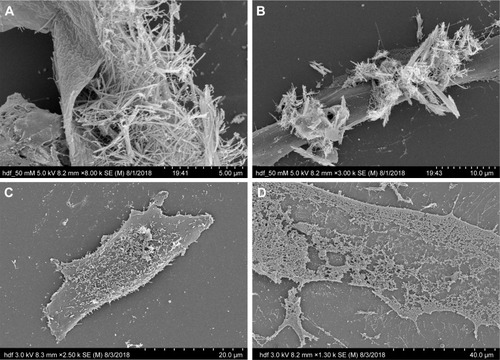
Nevertheless, higher concentrations of 100 μg/mL NPs seem to produce necrosis on cells (). As shown in the literature, swelling of the cell due to a hydration process and discontinuities on the membrane previous to its rupture are dictated as signs of necrosis.Citation74,Citation75 SEM captured the different stages of the rupture of the cytoplasmic membrane due to a swelling process (); hence, the mechanism of death was interpreted as necrosis.
In contrast, blebbing () was observed on melanoma cells in comparison with the control (), indicating a process of apoptosis. These formations on the membrane are associated with a rearrangement in the cytoskeleton, which eventually ended in the rupture in fragments of the cell.Citation76,Citation77 It has been hypothesized that differently than HDF, NWs interact with melanoma cells by inducing chemical signaling, which leads to apoptosis, hence reducing cell proliferation by breaking cells apart (). It has been elucidated that different mechanisms of death are possible due to the use of different kinds of cells.
Figure 13 Interaction between melanoma cells and GREEN-TeNWs. Bubbling (A) and rupture (B and C) of the cell membrane were observed in comparison with the control (D).
Abbreviation: GREEN-TeNWs, green-synthesized tellurium nanowires.
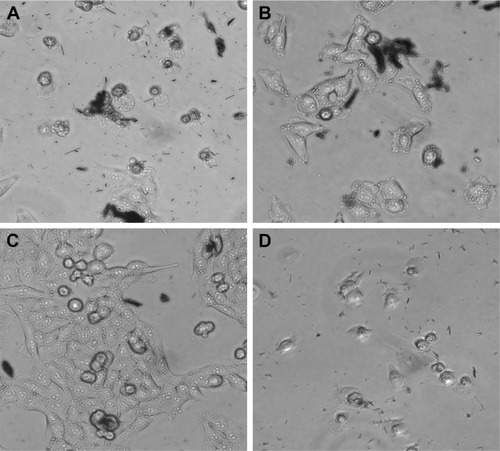
Optical microscopy showed preliminary results of how different cells behaved in the presence of both GREEN-TeNWs and CHEM-TeNWs. After 24 hours of treatment, 50 μg/mL CHEM-TeNWs () showed a remarkable disturbance in the growth of HDF cells after 24 hours of treatment, with poor cell development, while the same concentration of GREEN-TeNWs () allowed the development of the cells to a large extent. When melanoma human cells were tested with the same concentration of CHEM-TeNWs (), the cells showed better development than the ones cultured with GREEN-TeNWs () for the same period.
Figure 14 Optical microscopy characterization of HDF (A, B) and human melanoma (C, D) cells cultured in the presence of both CHEM-TeNWs and GREEN-TeNWs for 24 hours.
Abbreviations: CHEM-TeNWs, chemically synthesized TeNWs; GREEN-TeNWs, green-synthesized TeNWs; HDF, human dermal fibroblasts; TeNWs, tellurium nanowires.
Conclusion
Nanotechnology has taken an advantage of traditional chemical synthesis methods, both physical and chemical, to solve many of the biomedical and technological challenges that society faces today. Nevertheless, there is an environmental impact associated with such synthesis. Concerns such as the production of toxic by-products and harsh reaction conditions should be addressed. Therefore, new and alternative approaches are on the rise. One of the most promising comes from the use of green chemistry.
In this work, we successfully explored and compared two different synthesis approaches (a traditional chemical method and a green chemistry method) to elucidate the differences between the cytocompatibility and anticancer behavior of the respective NPs produced from each process. Experiments with healthy fibroblasts and cancerous melanoma cells were performed over a range of concentrations between 5 and 100 μg/mL and showed both an improvement in the cell proliferation of HDF cells and a decrease in the proliferation of cancerous cells when GREEN-TeNWs were compared with CHEM-TeNWs. An enhancement of healthy fibroblast proliferation was observed for the green synthesis of TeNWs compared with the CHEM-TeNWs, which suggests that these should be explored for a wide range of medical applications. Therefore, we propose that the present green synthetic approach offers important improvements in terms of safety, economy, efficiency, and biocompatibility for numerous biomedical applications, overcoming the main drawbacks of traditional Te nanowire chemical approaches.
Acknowledgments
This work was supported by the Chemical Engineering Department at Northeastern University. Funding from MINECO (MAT2014-59772-C2-1-P and MAT2014-59772-C2-2-P) is acknowledged. We also thank the service from the MiNa Laboratory at Instituto de Micro y Nanotecnología funded by CM (S2013/ICE2822), MINECO (CSIC13-4E-1794), and the EU (FEDER, FSE). JMGM thanks MECD (PRX16/00383) and Fulbright Commission for his stay at Northeastern University.
JLCD acknowledges the financial support provided by the School of Engineering and Sciences of Tecnologico de Monterrey through the Research Group on Optics and Lasers. The authors thank Alfonso Nieto-Argüello for FT-IR spectroscopy and XRD measurements.
Supplementary materials
X-ray diffraction peak list of identified samples:
Green-synthesized tellurium nanowires
Chemically synthesized tellurium nanowires Peak list:
Figure S1 XRD pattern for GREEN-TeNWs.
Abbreviations: GREEN-TeNWs, green-synthesized tellurium nanowires; XRD, X-ray powder diffraction.
Figure S2 XRD pattern for GREEN-TeNWs.
Abbreviations: GREEN-TeNWs, green-synthesized tellurium nanowires; XRD, X-ray powder diffraction.
Figure S3 XRD pattern for GREEN-TeNWs.
Abbreviations: GREEN-TeNWs, green-synthesized tellurium nanowires; XRD, X-ray powder diffraction.
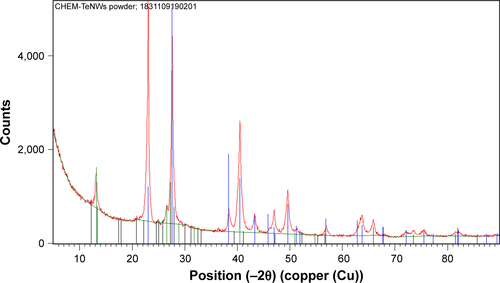
Figure S4 XRD pattern for GREEN-TeNWs.
Abbreviations: GREEN-TeNWs, green-synthesized tellurium nanowires; XRD, X-ray powder diffraction.
Table S1 Peak identification list for XRD pattern for GREEN-TeNWs
Table S2 List of identified patterns on XRD analysis of GREEN-TeNWs
Table S3 Peak identification list for XRD pattern for CHEM-TeNWs
Table S4 List of identified patterns on XRD analysis of CHEM-TeNWs
Disclosure
Dr María Ujué González reports grants from MINECO, MINECO + EU, and the Government of Autonomous Region of Madrid, CM, during the conduct of the study. Dr Yves Huttel reports grants from MINECO during the conduct of the study. Dr José Miguel García-Martín reports grants from MINECO, MECD, and Fulbright Commission, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- NorthEJHaldenRUPlastics and environmental health: the road aheadRev Environ Health20132811810.1515/reveh-2012-003023337043
- BijlsmaNCohenMMEnvironmental chemical assessment in clinical practice: unveiling the elephant in the roomInt J Environ Res Public Health201613218110.3390/ijerph1302018126848668
- LopezJBJacksonDGammieABadrickTReducing the environmental impact of clinical laboratoriesClin Biochem Rev201738131128798502
- WangLChenHShaoLThe antimicrobial activity of nanoparticles: present situation and prospects for the futureInt J Nanomedicine2017121227124910.2147/IJN.S12195628243086
- HemegHNanomaterials for alternative antibacterial therapyInt J Nanomedicine2017128211822510.2147/IJN.S13216329184409
- GravesJLThomasMEwunkemJAntimicrobial nanomaterials: why evolution mattersNanomaterials(Basel)2017710283
- JainSHirstDGO’SullivanJMGold nanoparticles as novel agents for cancer therapyBr J Radiol201285101010111310.1259/bjr/5944883322010024
- CaiWGaoTHongHSunJApplications of gold nanoparticles in cancer nanotechnologyNanotechnol Sci Appl20081173210.2147/NSA.S378824198458
- NuneSKGundaPThallapallyPKLinY-YLaird ForrestMBerklandCJNanoparticles for biomedical imagingExpert Opin Drug Deliv20096111175119410.1517/1742524090322903119743894
- EstelrichJSánchez-MartínMJBusquetsMANanoparticles in magnetic resonance imaging: from simple to dual contrast agentsInt J Nanomedicine2015101727174125834422
- ChoiHSFrangioniJVNanoparticles for biomedical imaging: fundamentals of clinical translationMol Imaging20109629131021084027
- ParkKFacing the truth about nanotechnology in drug deliveryACS Nano2013797442744710.1021/nn404501g24490875
- De JongWHBormPJADrug delivery and nanoparticles: application-sand HazardsInt J Nanomedicine20083213314910.2147/IJN.S59618686775
- WangYFLiuLXueXLiangXJNanoparticle-based drug delivery systems: what can they really do in vivo?F1000Res2017668110.12688/f1000research.10493.228620465
- KöglerMRyabchikovYVUusitaloSBare laser-synthesized Au-based nanoparticles as nondisturbing surface-enhanced raman scattering probes for bacteria identificationJ Biophotonics2018e20170022510.1002/jbio.20170022529388744
- Fernandez-BravoASivakumarPMelikechiNMohamedAAFemtosecond laser ablation synthesis of aryl functional group substituted gold nanoparticlesJ Nanosci Nanotechnol20171742852285610.1166/jnn.2017.1390029668202
- BukhtiarAZouBThe preparation and optical properties of Ni(II) and Mn(II) doped in ZnTe nanobelt/nanorod by using chemical vapor depositionJ Nanosci Nanotechnol20181874700471310.1166/jnn.2018.1528329442648
- IslamMAchourASaeedKBoujtitaMJavedSDjouadiMMetal/Carbon hybrid nanostructures produced from plasma-enhanced chemical vapor deposition over nafion-supported electrochemically deposited cobalt nanoparticlesMaterials201811568710.3390/ma11050687
- SudhakarAHistory of cancer, ancient and modern treatment methodsJ Cancer Sci Ther20091214 PMC20740081
- ArrueboMVilaboaNSáez-GutierrezBAssessment of the evolution of cancer treatment therapiesCancers2011333279333010.3390/cancers303327924212956
- AslanBOzpolatBSoodAKLopez-BeresteinGNanotechnology in cancer therapyJ Drug Target2013211090491310.3109/1061186X.2013.83746924079419
- Center for Disease Control and Prevention (CDC) Available from: https://www.cdc.gov/drugresistance/about.htmlAssessed June 5, 2018
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin201868173010.3322/caac.2144229313949
- RamirezLYHuestisSEYapTYZyzanskiSDrotarDKodishEPotential chemotherapy side effects: what do oncologists tell parents?Pediatr Blood Cancer200952449750210.1002/pbc.v52:419101994
- BaskarRLeeKAYeoRYeohK-WCancerand radiation therapy: current advances and future directionsInt J Med Sci20129319319910.7150/ijms.363522408567
- TohmeSSimmonsRLTsungASurgery for cancer: a trigger for metastasesCancer Res20177771548155210.1158/0008-5472.CAN-16-153628330928
- De AngelisCSide effects related to systemic cancer treatment: are we changing the promethean experience with molecularly targeted therapies?Current Oncol2008154198199
- PearceAHaasMVineyRIncidence and severity of self-reported chemotherapy side effects in routine care: a prospective cohort studyPLoS One20171210e018436010.1371/journal.pone.018436029016607
- ShiJKantoffPWWoosterRFarokhzadOCCancer nanomedicine: progress, challenges and opportunitiesNat Rev Cancer201717203710.1038/nrc.2016.10827834398
- GmeinerWHGhoshSNanotechnology for cancer treatmentNanatechnol Rev201532111122
- KulkarniNMuddapurUBiosynthesis of metal nanoparticles: a reviewJ Nanotechnol201420141810.1155/2014/510246
- AiJBiazarEJafarpourMNanotoxicology and Nanoparticle Safety in Biomedical DesignsInt J Nanomedicine201161117112710.2147/IJN.S2564621698080
- ShahMFawcettDSharmaSTripathySPoinernGGreen synthesis of metallic nanoparticles via biological entitiesMaterials20158117278730810.3390/ma811537728793638
- RiehemannKSchneiderSLugerTGodinBFerrariMFuchsHNanomedicine-challenge and perspectivesAngew Chem Int Ed200948587289710.1002/anie.v48:5
- HussainISinghNBSinghASinghHSinghSCGreen synthesis of nanoparticles and its potential applicationBiotechnol Lett201638454556010.1007/s10529-015-2026-726721237
- CruzMDavidGMWebsterTJSynthesis and characterization of biogenic selenium nanoparticles with antimicrobial properties made by Staphylococcus Aureus, Methicillin-Resistant Staphylococcus Aureus (MRSA), Escherichia Coli, and Pseudomonas AeruginosaJ Biomed Mater Res Part A201810651400141210.1002/jbm.a.36347
- Larios-RodriguezERangel-AyonCCastilloSJZavalaGHerrera-UrbinaRBio-synthesis of gold nanoparticles by human epithelial cells, in vivoNanotechnology2011223535560110.1088/0957-4484/22/35/35560121817787
- El-SaidWAChoH-YYeaC-HChoiJ-WSynthesis of metal nanoparticles inside living human cells based on the intracellular formation processAdv Mater201426691091810.1002/adma.v26.624338869
- MolnárZBódaiVSzakacsGGreen synthesis of gold nanoparticles by thermophilic filamentous fungiSci Rep201881394310.1038/s41598-018-22112-329500365
- MakarovVVLoveAJSinitsynaOV“Green” nanotechnologies: synthesis of metal nanoparticles using plantsActa Naturae201461354424772325
- SinghPKimY-JZhangDYangD-CBiological synthesis of nanoparticles from plants and microorganismsTrends Biotechnol201634758859910.1016/j.tibtech.2016.02.00626944794
- DhandVSoumyaLBharadwajSChakraSBhattDSreedharBGreen synthesis of silver nanoparticles using coffea Arabica seed extract and its antibacterial activityMater Sci Eng C Mater Biol Appl201658364310.1016/j.msec.2015.08.01826478284
- WuLCaiXNelsonKA green synthesis of carbon nanoparticles from honey and their use in real-time photoacoustic imagingNano Res20136531232510.1007/s12274-013-0308-823824757
- SurendraTVRoopanSMAl-DhabiNAArasuMVSarkarGSuthindhiranKVegetable peel waste for the production of ZnO nanoparticles and its toxicological efficiency, antifungal, hemolytic, and antibacterial activitiesNanoscale Res Lett201611154610.1186/s11671-016-1750-927933594
- GurunathanSHanJParkJHKimJ-HA green chemistry approach for synthesizing biocompatible gold nanoparticlesNanoscale Res Lett20149124810.1186/1556-276X-9-24824940177
- MukherjeeSSushmaVPatraSGreen chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapyNanotechnology2012234545510310.1088/0957-4484/23/45/45510323064012
- RehanaDMahendiranDKumarRSRahimanAKEvaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extractsBiomed Pharmacother2017891067107710.1016/j.biopha.2017.02.10128292015
- KelkawiAAbdHAbbasi KajaniABordbarA-KGreen synthesis of silver nanoparticles using mentha pulegium and investigation of their antibacterial, antifungal and anticancer activityIET Nanobiotechnol201711437037610.1049/iet-nbt.2016.010328530184
- SudhasreeSShakila BanuABrindhaPKurianGASynthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicityToxicol Environ Chem201496574375410.1080/02772248.2014.923148
- LiuTZhangGSuXChenXWangDQinJTellurium nanotubes synthesized with microwave-assisted monosaccharide reduction methodJ Nanosci Nanotechnol2007772500250517663271
- TippayawatPPhromviyoNBoueroyPChompoosorAGreen synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activityPeerJ20164e258910.7717/peerj.258927781173
- RamimoghadamDHusseinMZBTaufiq-YapYHHydrothermal synthesis of zinc oxide nanoparticles using rice as soft biotemplateChem Cent J2013713610.1186/1752-153X-7-13623919386
- JiWQiWTangSPengHLiSHydrothermal synthesis of ultrasmall Pt nanoparticles as highly active electrocatalysts for methanol oxidationNanomaterials2015542203221110.3390/nano504220328347116
- HayashiHHakutaYHydrothermal synthesis of metal oxide nanoparticles in supercritical waterMaterials2010373794381710.3390/ma307379428883312
- HongWWangJWangEFacile synthesis of PdAgTe nanowires with superior electrocatalytic activityJ Power Sources201427294094510.1016/j.jpowsour.2014.09.029
- LuQGaoFKomarneniSA green chemical approach to the synthesis of tellurium nanowiresLangmuir200521136002600510.1021/la050594p15952853
- TsaiHWYaghoubiAChanTCElectrochemical synthesis of ultrafast and gram-scale surfactant-free tellurium nanowires by gas-solid transformation and their applications as supercapacitor electrodes for p-doping of graphene transistorsNanoscale20157177535753910.1039/c5nr00876j25832197
- HeZYangYLiuJWYuSHEmerging tellurium nanostructures: controllable synthesis and their applicationsChem Soc Rev201746102732275310.1039/c7cs00013h28425532
- BaLADöringMJamierVJacobCTellurium: an element with great biological potency and potentialOrg Biomol Chem20108194203421610.1039/c0Ob00086h20714663
- SredniBImmunomodulating tellurium compounds as anti-cancer agentsSemin Cancer Biol2012221606910.1016/j.semcancer.2011.12.00322202556
- Abo ElsoudMMAl-HagarOEAAbdelkhalekESSidkeyNMSynthesis and investigations on tellurium myconanoparticlesBiotechnol Rep201818e0024710.1016/j.btre.2018.e00247
- ChouT-MKeY-YTsaoY-HLiY-CLinZ-HFAbrication of Te and Te-Au nanowires-based carbon fiber fabrics for antibacterial applications. Ed. Jason K, LevyInt J Environ Res Public Health201613220210.3390/ijerph1302020226861380
- SilvaRRMejiaHAGRibeiroSJLFacile synthesis of tellurium nanowires and study of their third-order nonlinear optical propertiesJ Braz Chem Soc20172815867
- LiuZShuLYangYShape-controlled synthesis and growth mechanism of one-dimensional nanostructures of trigonal telluriumNew J Chem200327174810.1039/b306782c
- CarotenutoGPalombaMDe NicolaSAmbrosoneGCosciaUStructural and photoconductivity properties of tellurium/PMMA filmsNanoscale Res Lett20151031310.1186/s11671-015-1007-z
- El-MallawanyRATheoretical and experimental IR spectra of binary earth tellurite glassesInfrared Phys19892978178510.1016/0020-0891(89)90125-5
- KizilRIrudayarajJSeetharamanKCharacterization of irradiated starches by using FT-Raman and FTIR spectroscopyJ Agric Food Chem2002503912391810.1021/jf011652p12083858
- AdenisCLangerVLindqvistOReinvestigation of the structure of telluriumActa Crystallogr C19894594194210.1107/S0108270188014453
- VelázquezJMGaikwadAVRoutTKBaierREFurlaniESBanerjeeSNanotexturation-induced extreme wettability of an elemental tellurium coatingJ Mater Chem2012223335333910.1039/C1JM14664E
- GarciaRLosillaNSMartínezJNanopatterning of carbonaceous structures by field-induced carbon dioxide splitting with a force microscopeAppl Phys Lett20109614311010.1063/1.3374885
- NevshupaRMartínezLÁlvarezLInfluence of thermal ageing on surface degradation of ethylene-propylene-diene elastomerJ Appl Polym Sci201111924225110.1002/app.v119:1
- YangDWongWPSmall but mighty: nanoparticles probe cellular signaling pathwaysDev Cell201637539739810.1016/j.devcel.2016.05.02127270039
- MaranoFHussainSRodrigues-LimaFBaeza-SquibanABolandSNanoparticles: molecular targets and cell signallingArch Toxicol201185773374110.1007/s00204-010-0546-420502881
- SunHJiaJJiangCZhaiSGold nanoparticle-induced cell death and potential applications in nanomedicineInt J of Mol Sci2018193E75429518914
- BurattiniSFalcieriEAnalysis of cell death by electron microscopyMethods Mol Biol20131004778910.1007/978-1-62703-383-1_723733571
- TaatjesDJSobelBEBuddRCMorphological and cytochemical determination of cell death by apoptosisHistochem Cell Biol20081291334310.1007/s00418-007-0356-918000678
- SusanEApoptosis: A review of programmed cell deathToxicol Pathol20073549551610.1080/0192623070132033717562483