Abstract
The application of “green” chemistry rules to nanoscience and nanotechnology is very important in the preparation of various nanomaterials. In this work, we successfully developed an eco-friendly chemistry method for preparing silver nanoparticles (Ag-NPs) in natural polymeric media. The colloidal Ag-NPs were synthesized in an aqueous solution using silver nitrate, gelatin, and glucose as a silver precursor, stabilizer, and reducing agent, respectively. The properties of synthesized colloidal Ag-NPs were studied at different reaction times. The ultraviolet-visible (UV-vis) spectra were in excellent agreement with the obtained nanostructure studies performed by transmission electron microscopy (TEM) and their size distributions. The prepared samples were also characterized by X-ray diffraction (XRD) and atomic force microscopy (AFM). The use of eco-friendly reagents, such as gelatin and glucose, provides green and economic attributes to this work.
Introduction
Nanomaterials have received much attention because their structure and properties differ significantly from those of atoms, molecules, and bulk materials.Citation1 The synthesis of metal nanoparticles has been widely discussed in the literature due to their unique physical and chemical properties, which have many potential applications.Citation2–Citation4 The use of inexpensive chemicals and non-toxic solvents – environmentally friendly and renewable/biodegradable – are central to materials synthesis and processing, considering the green nature of these strategies. The reducing agent, reaction medium, and stabilizer are three key factors in the synthesis and stabilization of metallic nanoparticles.Citation5
Natural polymers such as gelatin, chitosan, proteins, and starch are all interesting materials for medical applications because they are biodegradable and bioabsorbable with degradation products that are non-toxic.Citation6 Gelatin is a natural biopolymer extracted from the partial hydrolysis of collagen which has good biocompatibility and biodegradability, and has been widely used in wound dressings, drug carriers, and tissue scaffolds in recent years.Citation7 There are many methods for synthesizing metal nanoparticles, including photo reduction,Citation8 laser ablation,Citation9 chemical reduction in aqueous media with different polymer surfactants,Citation10,Citation11 reduction of chemicals in soft matrices,Citation12–Citation14 reduction of chemicals in solid matrices (eg, mesoporous silicate),Citation15 and chemical vapor deposition.Citation16 Silver nanoparticles (Ag-NPs) are widely used as catalysts,Citation17–Citation19 photo-catalysts,Citation20–Citation22 and in surface-enhanced Raman spectroscopyCitation24–Citation26 as well as chemical analysis.Citation27
The use of gelatin as a capping agent and glucose as a reducing agent to synthesize Ag-NPs in aqueous solutions is attractive because organic solvents are not used and no corresponding pollutants are made; moreover, the resulting nanoparticles in gelatin matrix are biologically compatible. The main objective of this work is to apply the principles of green chemistryCitation28 in the synthesis of Ag-NPs, such as when using an eco-stabilizer and reducing agent. The synthesis method presented could be useful in providing an economic method for the industrial preparation of highly monodispersed, biocompatible, and stable colloidal Ag-NPs. The smallest nanoparticles of about 5 nm having totally monodispersity could be obtained. Other advantages of this approach include the physical conditions of the synthesis, such as the moderate preparation temperature, the lack of need for an additional flow of inert gas, and the use of atmospheric pressure.
Experimental
All reagents in this work were analytical-grade and were used as received without further purification. AgNO3 (99.98%) was used as a silver precursor, and was provided by Merck, Germany. Gelatin (type B) was used as a stabilizer for the preparation of Ag-NPs and was purchased from Sigma-Aldrich, MO, USA. D-Glucose was used as a reducing agent for the reduction of silver ions to Ag atoms and was obtained from BDH Chemical Ltd., Poole, UK. All solutions were freshly prepared using double distilled water and kept in the dark to avoid any photochemical reactions. All glassware used in experimental procedures were cleaned in a fresh solution of HNO3/HCl (3:1, v/v), washed thoroughly with double distilled water, and dried before use.
For synthesis of Ag-NPs, 2.0 g gelatin was added to 190 mL H2O in a flask, and the solution was stirred and heated at 40°C to obtain a clear solution. To obtain the Ag+/gelatin solution, the silver solution (10 mL, 1M) was added to the gelatin solution with continuous stirring. Then, 20 mL of glucose solution (2 M) was added to the Ag+/gelatin solution. The solution obtained was distributed into 5 cuvettes, and the prepared solutions were stirred and maintained for different periods of time: 1 (S1), 3 (S2), 6 (S3), 24 (S4), and 48 hours (S5), respectively. Throughout the reduction process, all solutions were kept at a temperature of 60°C in the dark to avoid any photochemical reactions.
The prepared colloidal Ag-NPs were characterized by using ultraviolet-visible (UV-vis) spectroscopy, X-ray diffraction (XRD), transmission electron microscopy (TEM), and atomic force microscopy (AFM). To ensure the formation of nanosilver, the particles were tested for their optical absorption property using a Lambda 35-Perkin Elmer UV-vis spectrophotometer over the range of 300 to 700 nm. The crystalline structure of Ag-NPs was characterized with an XRD (Philips, X’pert, Cu Kα). The XRD patterns were scanned at a speed of 2°/min. The AFM experiments were carried out on an Ambios-Q scope (SPM) machine. TEM observations were performed with a Hitachi H-7100 electron microscopy, and the particle size distributions were determined using UTHSCSA Image Tool software (Ver. 3.00).
Results and discussion
The color of Ag+/gel solutions over different periods of time gradually changed from colorless to light brown, then to brown, and finally dark brown, indicating the formation of Ag-NPs in the gelatin solution (). Glucose, as an aldehyde, was able to reduce Ag+ ions to Ag0, and through this reaction, glucose can be oxidized to gluconic acid. The gradual formation of Ag-NPs was investigated by UV-vis spectroscopy, which has proven to be a useful spectroscopic method for the detection of prepared NPs over time. In UV-vis spectra, the Ag-NPs can be shown by a surface plasmon resonance (SPR) peak at around 400 nm, but a small shift (blue-shift or red-shift) in the wavelength of the peak could be related to obtaining Ag-NPs in different shapes, sizes, or solvent dependences of prepared Ag-NPs.
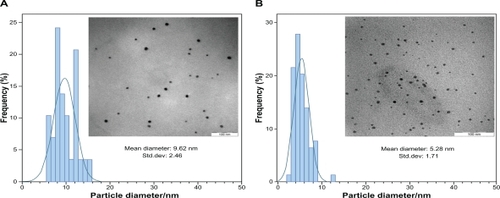
After reaction at 60°C for 1 hour, the Ag-NPs obtained showed a UV-vis absorption peak, a characteristic SPR band for Ag-NPs, centered at 400 nm (). As shown in , the intensity of the SPR peak increased as the reaction time increased, which indicated the continued reduction of the silver ions, and the increase of the absorbance with the reaction time indicates that the concentration of Ag-NPs increases.Citation29 When the reaction time reached 3 hours () the absorbance was increased, and the λmax value was slightly blue-shifted to 438 nm. For reaction times of 6 () and 24 hours (), the absorbance was also increased and blue-shifted to 435 and 431 nm, respectively. This phenomenon indicated that the size of particles was decreased because the absorbance peak due to the SPR of metal nanoparticles shows the blue-shift with decreasing particle size.Citation30 At the end of the reaction (48 hours, ), the absorbance was considerably increased and there was no significant change in λmax value (430 nm), compared with the 24 hour reaction time. At the initial stage of the reaction, the Ag-NPs formed with a broad size distribution, which led to a SPR peak at about 440 nm. After this stage, the Ag-NPs could dissociate due to heating to form smaller particles stabilized by the amine pendant groups on the gelatin, which leads to the formation of gelatin-stabilized stable Ag-NPs.Citation31 The TEM images demonstrate the formation of Ag-NPs at different periods of time. shows typical TEM images and the corresponding particle size distribution of the prepared Ag-NPs at different times. The TEM results indicate that the samples obtained over a longer time period retained a narrower particle size distribution; the average size of all prepared Ag-NPs was <20 nm; and a smaller average size (about 5 nm) was obtained for S5.
Figure 1 The UV-vis absorption spectra of the colloidal Ag-NPs synthesized using glucose at different reaction times.
Abbreviations: Ag-NPs, silver nanoparticles; UV-vis, ultraviolet visible.
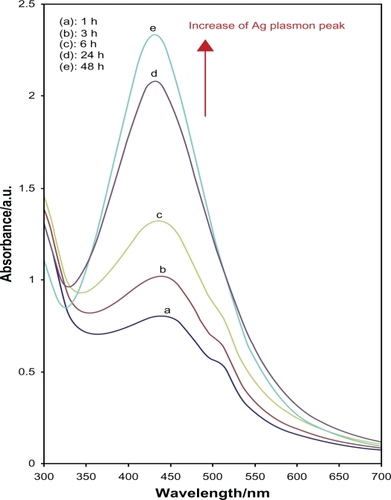
Figure 2 The typical TEM images and corresponding particles size distribution of Ag-NPs at different reaction times; A) S3 and B) S5.
Abbreviations: Ag-NPs, silver nanoparticles; TEM, transmission electron microscopy.
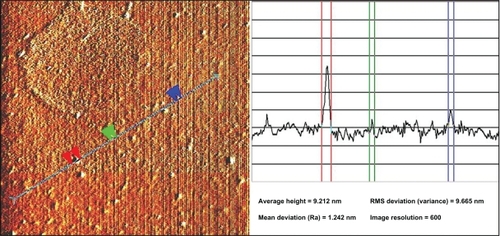
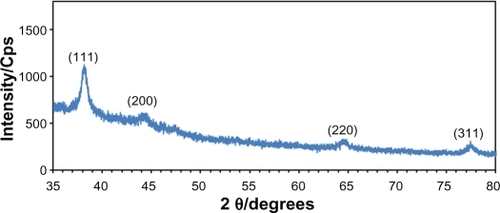
shows the XRD patterns of Ag-NPs formed in S5, which indicates the formation of the silver crystalline structure. The XRD peaks at 2θ degrees of 38.1, 44.3, 64.5, and 77.5 can be attributed to the (111), (200), (220), and (311) crystalline planes of face-centered-cubic (fcc) crystalline structure of metallic silver, respectively (JCPDS file no. 00-004-0783). The AFM result shows the surface morphology of the well-dispersed Ag-NPs formed in gelatin media. As observed in , the value determined by the AFM was close to the TEM determined, and the films of gelatin containing Ag-NPs displayed a densely uniform packed structure. Thus, the Ag-NPs–gelatin films could provide a biocompatible and rough surface for special biological applications, such as cell immobilization.
Figure 3 The XRD pattern of prepared Ag-NPs (S5).
Abbreviation: Ag-NPs, silver nanoparticles; Cps, Counts per second; XRD, X-ray diffraction
Conclusion
We have described a simple and eco-friendly time-dependent method to synthesize colloidal Ag-NPs in corresponding metal solution using green agents, which requires no special physical conditions. The Ag-NPs obtained in this work are uniform and have a narrow particle size distribution, with a small average particle diameter of about 5 nm. This preparation method is general and may be extended to other noble metals, such as Au, Pd, and Pt, and may possibly find various additional medicinal, industrial, and technological applications.
Disclosure
The authors disclose no conflicts of interest.
References
- RoseiFNanostructured surfaces: challenges and frontiers in nanotechnologyJ Phys Condens Matter200416S1373S1436
- PappSPatakfalviRDekanyIMetal nanoparticle formation on layer silicate lamellaeColloid Polym Sci2008286314
- ChimentaoRJMedinaFSueirasJEFierroJLGCesterosYSalagrePEffects of morphology and cesium promotion over silver nanoparticles catalysts in the styrene epoxidationJ Mater Sci20074233073314
- ChenDQiaoXQiuXChenJSynthesis and electrical properties of uniform silver nanoparticles for electronic applicationsJ Mater Sci20094410761081
- LiuJHeFGunnTMZhaoDRobertsCBPrecise seed-mediated growth and size-controlled synthesis of palladium nanoparticles using a green chemistry approachLangmuir2009257116712819309120
- MozafariMRNanomaterials and Nanosystems for Biomedical ApplicationsDordrecth, The NetherlandsSpringer2007
- XuXZhouMAntimicrobial gelatin nanofibers containing silver nanoparticlesFiber Polym20089685690
- DarroudiMAhmadMBShameliKAbdullahAHIbrahimNASynthesis and characterization of UV-irradiated silver/montmorillonite nanocompositesSolid State Sci20091116211624
- DarroudiMAhmadMBZamiriRPreparation and characterization of gelatin mediated silver nanoparticles by laser ablationJ Alloy Compd201150913011304
- AiharaNTorigoeKEsumiKPreparation and characterization of gold and silver nanoparticles in layered laponite suspensionsLangmuir19981449454949
- LinXZTengXYangHDirect synthesis of narrowly dispersed silver nanoparticles using a single-source precursorLangmuir2003191008110085
- ZhuangXChengBKangWXuXElectrospun chitosan/gelatin nanofibers containing silver nanoparticlesCarbohydr Polym201082524527
- DarroudiMAhmadMBAbdullahAHIbrahimNAShameliKEffect of accelerator in green synthesis of silver nanoparticlesInt J Mol Sci2010113898390521152307
- HebeishAAEl-RafieMHAbdel-MohdyFAAbdel-HalimandESEmamHECarboxymethyl cellolose for green synthesis and stabilization of silver nanoparticlesCarbohydr Polym201082933941
- DagOSamarskayaOCoombsNOzinGAThe synthesis of meso-structured silica films and monoliths functionalised by noble metal nanoparticlesJ Mater Chem200313328334
- SzlykEPiszczekPGrodzickiACVD of Ag-I complexes with tertiary phosphines and perfluorinated carboxylates-A new class of silver precursorsChem Vapor Depos20017111116
- ChimentaoRJKirmIMedinaFSensitivity of styrene oxidation reaction to the catalyst structure of silver nanoparticlesAppl Surf Sci2005252793800
- JiangZJLiuCYSunLWCatalytic properties of silver nanoparticles supported on silica spheresJ Phys Chem B20051091730173516851151
- YanJTaoHZengMPVP-capped silver nanoparticles as catalyst for oxidative coupling of thiols to disulfidesChin J Catal200930856858
- SclafaniAMozzanegaMNPichatPEffect of silver deposits on the photocatalytic activity of titanium dioxide samples for the dehydrogenation or oxidation of 2-propanolJ Photoch Photobio A-Chem199159181189
- SclafaniAHerrmannJInfluence of metallic silver and of platinum-silver bimetallic deposits on the photocatalytic activity of titania (anatase and rutile) in organic and aqueous mediaJ Photochem Photobiol A-Chem1998113181188
- TadaHTeranishiKItoSAdditive effect of sacrificial electron donors on Ag/TiO2 Photocatalytic Reduction of Bis(2-dipyridyl)-disulfide to 2-Mercaptopyridine in Aqueous MediaLangmuir19991570847087
- TadaHTeranishiKInubushiYItoSAg nanocluster loading effect on TiO2 photocatalytic reduction of Bis(2-dipyridyl)disulfide to 2-mercaptopyridine by H2OLangmuir20001633043309
- BrightRMMusickMDNatanMJPreparation and characterization of Ag colloid monolayersLangmuir19981456955701
- ShirtcliffeNNickelUSchneiderSReproducible preparation of silver sols with small particle size using borohydride reduction: For use as nuclei for preparation of larger particlesJ Colloid Interf Sci1999211122129
- NickelUCastellAZPopplKSchneiderSA silver colloid produced by reduction with hydrazine as support for highly sensitive surface-enhanced Raman spectroscopyLangmuir20001690879091
- PalTGelatin-A compound for all reasonsJ Chem Educ199471679681
- AnastasPTWarnerJCGreen Chemistry: Theory and PracticeNew YorkOxford University Press1998
- BohrenCFHuffmanDRAbsorption and Scattering of Light by Small ParticlesNew YorkJohn Wiley & Sons Inc1998
- HeathJRSize-dependent surface-plasmon resonances of bare silver particlesPhys Rev B19894099829985
- ZhangJJGuMMZhengTTZhuJJSynthesis of gelatin-stabilized gold nanoparticles and assembly of carboxylic single-walled carbon nanotubes/Au composites for cytosensing and drug uptakeAnal Chem2009816641664820337377