Abstract
Purpose
To develop and demonstrate the effectiveness of a novel dexamethasone (Dex) nanoformulation for treating uveitis.
Materials and methods
We designed and screened a dexamethasone-peptide conjugate (Dex-SA-FFFE), formed via a biodegradable ester bond linkage, that could spontaneously form high drug payload nanoparticles in aqueous solution for treating uveitis.
Results
An in vitro release study indicated that Dex and Dex-SA-FFFE sustainably released from Dex-SA-FFFE nanoparticles over a 48 h study period. Meanwhile, the formed Dex-SA-FFFE nanoparticles hardly caused cytotoxicity in human corneal epithelial cell at drug concentrations up to 1 mM after 24 h of incubation but reduced cell viability after 48 h and 72 h of incubation. An in vitro anti-inflammatory efficacy assay showed that the Dex-SA-FFFE nanoparticles exhibited a comparable anti-inflammatory efficacy to that of Dex in lipopolysaccharide (LPS)-activated RAW264.7 macrophages via significant decreases in the secretion of various pro-inflammatory cytokines (e.g., nitric oxide, tumor necrosis factor-α, interleukin-6). Topical instillation of Dex-SA-FFFE nanoparticles showed good ocular tolerance without causing changes in corneal thickness and intraocular pressure during the entire study period. Furthermore, topical instillation of Dex-SA-FFFE nanoparticles displayed a comparable in vivo therapeutic efficacy to that of dexamethasone sodium phosphate (Dexp) aqueous solutions in an endotoxin-induced uveitis (EIU) rabbit model.
Conclusion
Based on these results, it is reasonable to believe that the proposed Dex-SA-FFFE nanoparticles might have great application for the treatment of anterior uveitis.
Introduction
Uveitis, a term encompassing a variety of intraocular inflammatory disorders, is one of the leading causes of blindness in developed countries.Citation1–Citation3 Even when the underlying cause is unknown, steroids (e.g., dexamethasone [Dex] and triamcinolone acetonide) are still first-line treatment in the clinical therapy of uveitis.Citation4–Citation8 Because they can induce significant systemic side effects, treatments using systemic steroids are extremely limited.Citation4,Citation7,Citation9 Topical steroid treatments in the form of eye drops, suspensions, or ointments are less likely to cause significant side effects than systemic steroid treatment and are more acceptable for the patient; however, they also suffer from low ocular bioavailability owing to the rapid precorneal clearance and poor corneal permeability of the drugs.Citation9–Citation12
In the past several decades, a variety of strategies including the use of adhesive additives, micro/nanotechnologies, and hydrogelation have been explored to extend the precorneal residence and increase the corneal permeability of steroids, thus enhancing ocular bioavailability.Citation13–Citation17 For instance, Kalam reported a Dex sodium phosphate (Dexp)-loaded chitosan/hyaluronic acid nanoparticle synthesized via an ionotropic gelation technique to improve precorneal retention and corneal permeability.Citation18 Nagai et al designed an ophthalmic formulation containing Dex-loaded solid nanoparticles for extending precorneal retention and enhancing ocular bioavailability after topical instillation.Citation19 Similarly, a cationic nanocrystal formulation composed of Dex acetate nanocrystals and polymyxin B was proposed through a self-developed small-scale method for increasing the mucoadhesion of drugs after topical ocular delivery.Citation20 More recently, we proposed a Dex prodrug supramolecular hydrogel formed by a pH hydrolytic strategy for ophthalmic drug delivery. This hydrogel, acting as a “self-delivery” system, combined the advantages of nanoparticles and hydrogels to greatly increase corneal permeability and significantly prolong precorneal retention.Citation21
Although significant advances in pharmaceutical technologies for ophthalmic steroid delivery have been achieved, the development of a novel, superior system with high drug payload for ocular steroid delivery is still urgently required. More recently, drug-peptide conjugates as an effective prodrug strategy represent an important class of therapeutic formulation owing to their high drug payload and lower amount of inert materials.Citation22–Citation27 Since the peptide is derived from amino acids, it is highly biodegradable and biocompatible and should not elicit unexpected side effects after in vivo application. Moreover, the diversity of amino acid combinations permits the facile preparation of various types of peptide-drug conjugates.Citation28–Citation31 For instance, Li et al described a hydrogelator, composed of a D-amino acid and a nonsteroidal anti-inflammatory drug, that had enhanced selectivity as a cyclooxygenase-2 inhibitor.Citation32 Cui et al reported a series of drug-peptide conjugates that could self-assemble into various nanostructures (e.g., nanofiber and nanosphere) to improve the drug’s water solubility, cellular uptake, and potency against cancerous cells.Citation33–Citation35
Encouraged by the results of these studies, herein, we designed and screened a Dex-peptide conjugate (Dex-SA-FFFE), formed via a cleavable ester bond linkage (), that could spontaneously form high drug payload nanoparticles in aqueous solution. Owing to the absence of a drug vehicle, the formed Dex-SA-FFFE nanoparticles significantly alleviate the safety concerns associated with vehicles and give a precise and relatively high drug payload. Furthermore, the absence of any organic solvents and surfactants during the fabrication procedure might be beneficial for practical applications. To assess the potential therapeutic efficacy of Dex-SA-FFFE nanoparticles, in vitro anti-inflammatory assays in lipopolysaccharide (LPS)-activated RAW264.7 macrophages and in vivo anti-inflammatory tests in an endo-toxin-induced uveitis (EIU) rabbit model were performed.
Materials and methods
Materials
Succinated Dex (Dex-SA) was synthesized by using a method described in our previous study.Citation21 The 2-chlorotrityl chloride resin and N-Fmoc-protected amino acids were provided by GL Biochem (Shanghai) Ltd., (Shanghai, China). Porcine liver esterase, cell counting kit-8 (CCK-8), and LPS were purchased from Sigma-Aldrich Co., (St Louise, MO, USA). Dexp and dialysis bags (molecular weight cutoff: 3,500) were provided by Dalian Meilun Biology Technology Co., Ltd., (Liaoning Province, China). All other chemical reagents used are of analytical grade.
Synthesis of Dex-SA-FFFE
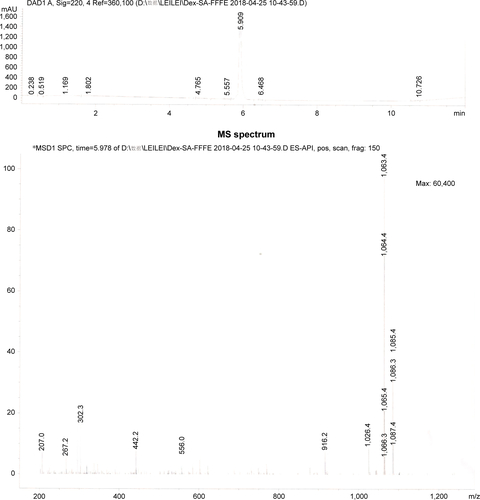
According to a previous study, a Dex-SA-FFFE was synthesized by a classic solid-phase peptide synthesis method using 2-chlorotrityl chloride resin and N-Fmoc-protected amino acids.Citation32 The 2-chlorotrityl chloride resin was first allowed to swell in dry dichloromethane (DCM) for 20 minutes, and then the first amino acid was loaded onto the resin in a dimethyl formamide (DMF) solution of Fmoc-protected amino acids (1.5 equiv) and N,N-diisopropylethylamine ([DIEA]; two equiv) for 2 hours. After washing with DCM five times, the unreactive sites of resin were blocked by the blocking solution (DCM/methanol/DIEA =17:2:1) for 10 minutes. Thereafter, the Fmoc protective group was removed by the addition of 20% piperidine (in DMF), followed by the coupling of Fmoc-protected amino acids (two equiv) to the free amino group on the resin using DIEA (two equiv) and O-benzotriazol-1-yl-tetramethyluronium hexafluorophosphate (HBTU) (one equiv) as coupling agents in DMF for 2 hours. These two steps were repeated to elongate the peptide chain. Finally, Dex-SA was coupled onto the peptide using DIEA (two equiv) and HBTU (one equiv) as the coupling agent in DMF for 2 hours, and the resultant Dex-SA-FFFE was cleaved from the resin with a washing solution (trifluoroacetic acid/triisopropylsilane/H2O=95/2.5/2.5). The obtained crude product was purified by reverse phase high-performance liquid chromatography (HPLC) and characterized by mass spectrometry (MS). MS (m/z): [M+H]+ calculated for C58H67FN4O14: 1,063.46; found: 1,063.4 ().
Formation of Dex-SA-FFFE nanoparticles
Thirty milligrams of Dex-SA-FFFE powder was suspended in 1 mL of phosphate buffered saline (PBS, pH=7.4), followed by the addition of 1 mM Na2CO3 aqueous solution (one molar equivalent to Dex-SA-FFFE). The resulting suspension was heated up to 95°C and cooled down to room temperature to generate Dex-SA-FFFE nanoparticles.
Characterization
The size distribution and zeta potential of Dex-SA-FFFE nanoparticles were analyzed by a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK).
The micromorphology of Dex-SA-FFFE nanoparticles was observed by a transmission electron microscope (TEM, Tecnai F20; FEI, Hillsboro, OR, USA). Ten microliters of Dex-SA-FFFE nanoparticles was pipetted onto the grid and stained with 0.5 wt% phosphotungstic acid before TEM observation.
In vitro release study
In vitro release profiles of the drugs (Dex and Dex-SA-FFFE) from the Dex-SA-FFFE nanoparticles were investigated in both PBS (pH =7.4) and PBS (pH =7.4) containing 20 U/mL porcine liver esterase at 37°C. Briefly, 0.15 mL of Dex-SA-FFFE nanoparticles (23.4 mg/mL Dex-SA-FFFE) was sealed into a dialysis bag (molecular weight cutoff: 3,500) and immersed into 5 mL of either PBS or PBS containing 20 U/mL porcine liver esterase at 37°C for the period of in vitro release study. At predetermined time points, a 1-mL aliquot of the release medium was collected and the amount of the released drugs was quantified by HPLC with a reversed-phase C18 column (ZORBAX Eclipse XDB-C18, 150×4.6 mm i.d., 5 µm; Agilent Technologies, Santa Clara, CA, USA). The residual release medium was completely replaced with 5 mL of either freshly prepared PBS or PBS containing 20 U/mL porcine liver esterase at regular intervals of the study. The mobile phase for HPLC analysis was composed of acetonitrile and 0.1% triethylamine/phosphoric acid (55/45; v/v). Aliquots of 20 µL of tested samples were injected for HPLC analysis. The eluent was detected at 240 nm by a diode array detector.
In vitro cytotoxicity assay
To assess the in vitro cytotoxicity of Dex-SA-FFFE nanoparticles, CCK-8 assay was used to detect the viability of human corneal epithelial cell (HCEC) line after exposure to various drug concentrations. HCEC line was purchased from Chinese Academy of Sciences Cell Bank (Shanghai, China). Briefly, cells were seeded into 96-well plates (5×103 cells/well) with 100 µL of cell culture medium per well and incubated in air containing 5% CO2 at 37°C. Thereafter, 100 µL of cell culture medium containing different concentrations of Dex-SA-FFFE nanoparticles ranging from 0 to 2 mM was added to each well. After 24, 48, and 72 hours of incubation, 100 µL of the CCK-8 solution was added to each well, followed by the incubation for another 2 hours. Cells that were not manipulated were used as control. The absorbance of each well was recorded by a microplate reader (model SpectraMax M5; Molecular Devices LLC, Sunnyvale, CA, USA) at 450 nm. Finally, cell viability was calculated using the following equation: cell viability (%) = absorbance of tested sample/absorbance of control sample ×100. All experiments were performed in triplicate (mean ± SD, six wells for each drug concentration).
In vitro anti-inflammation test
To evaluate the in vitro anti-inflammatory effect of Dex-SA-FFFE nanoparticles, we measured the pro-inflammatory cytokines in the culture medium after exposure to different drug concentrations in LPS-activated RAW264.7 macrophages. RAW264.7 macrophages were provided by Chinese Academy of Sciences Cell Bank. Briefly, RAW264.7 macrophages were seeded into 24-well plates at a density of 1×105 cells/well and incubated overnight in air containing 5% CO2 at 37°C. In addition, the cells were pre-treated with 0.01 mM or 0.1 mM drug (Dex-SA-FFFE nanoparticles or Dex in DMSO) for 2 hours, followed by challenge with 1 µg/mL LPS for 24 hours. Thereafter, the cell culture medium was collected, and the levels of nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) were measured by the Griess assay and an ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA), respectively.
Intraocular biocompatibility
We investigated the intraocular biocompatibility and ocular irritation of Dex-SA-FFFE nanoparticles in healthy Japanese big-eared white rabbits after topical instillation. Japanese big-eared white rabbits (weight: 2.0–2.5 kg) were provided by Wenzhou Medical University Laboratory Animal Center (Wenzhou, China). All animal experiments were conducted with the Guidelines for Care and Use of Research Animals established by Wenzhou Medical University and approved by the Ethical Committee of Wenzhou Medical University. Prior the in vivo applications, the formed Dex-SA-FFFE nanoparticles were sterilized by 0.22 µm filter membrane. The right eyes of the rabbits were topically instilled with 50 µL of Dex-SA-FFFE nanoparticles (30 mg/mL Dex-SA-FFFE) four times daily (6 mg Dex-SA-FFFE per eye daily) for three successive days, while the left eyes were topically instilled with 50 µL of PBS (pH =7.4). Clinical signs including conjunctival hyperemia, corneal edema, and lens opacity were monitored daily by a slit-lamp (SLM-4ER; Chongqing Kanghua Ruiming S&T Co., Ltd., Chongqing, China). The integrity of the corneal epithelium was checked by a fluorescein staining assay. Meanwhile, changes in intraocular pressure and corneal thickness were measured by a handheld tonometer (Tono-Pen AVIA®, Reichert Technologies, Depew, NY, USA) and an iVue (Optovue, Fremont, CA, USA), respectively.
EIU rabbit model and medication
We assessed the in vivo anti-inflammatory efficacy of Dex-SA-FFFE nanoparticles in an EIU rabbit model. Eighteen rabbits were intravitreally injected with 100 ng of LPS to establish the EIU model and then randomly divided into three groups (n=6): 1) eyes topically instilled with 50 µL of Dex-SA-FFFE nanoparticles (30 mg/mL Dex-SA-FFFE) four times daily; 2) eyes topically instilled with 50 µL of Dexp (14.6 mg/mL Dexp; one molar equivalent to Dex-SA-FFFE) solution four times daily; and 3) eyes topically instilled with 50 µL of PBS (pH =7.4) four times daily. After 24 hours, the clinical signs of an inflammatory response in the anterior chamber (e.g., miosis, iris hyperemia, and protein leakage) were observed with a slit lamp (SLM-4ER; Chongqing Kang-hua Ruiming S&T Co., Ltd) by an experienced ophthalmologist. Additionally, 100 µL of aqueous humor was collected with a 30-gauge insulin syringe, followed by the quantification of inflammatory cells, TNF-α, and IL-6 levels in the sample by a cell counting assay and ELISA kits (Shanghai MLBIO Biotechnology Company, China and R&D, USA). Finally, the rabbits from each group were sacrificed, and whole eyeballs were harvested for pathological observation by the hematoxylin and eosin (H&E) staining assay.
Statistical analysis
The data were analyzed by SPSS version 22 (IBM Corporation, Armonk, NY, USA) and expressed as the mean and Standard deviation (SD). The significant differences between control groups and experimental groups were analyzed by one-way ANOVA, and a P-value <0.05 was considered to indicate statistical significance.
Results and discussion
Dex-SA-FFFE nanoparticle formation and characterization
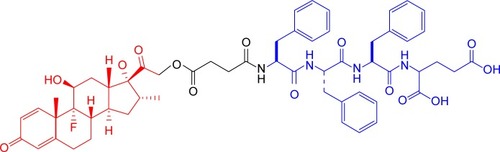
Since prodrugs provide a versatile approach to improve the clinical usefulness of many therapeutic agents by improving water solubility and permeability, sustained release, drug targeting, and the metabolic stability of the parent drug, prodrug design should be considered at the early stages of preclinical development.Citation36–Citation39 Amino acid prodrugs have been illustrated to significantly improve the oral delivery of drugs that have poor solubility and permeability (e.g., valacyclovir and valganciclovir).Citation40,Citation41 More importantly, the diversity of amino acids offers a great opportunity to finely tune the amphiphilicity of drugs, thus altering their self-assembly behavior in aqueous solution to generate various nanostructures (e.g., nanotubes, nanospheres, and nanofibers).Citation35,Citation42 In this article, we designed and screened a Dex-SA-FFFE prodrug via a cleavable ester bond linkage (), which could spontaneously self-assemble into nanoparticles in an aqueous solution with prodrug concentrations up to 100 mg/mL ( and ). Unlike the conventional encapsulation approach, Dex-SA-FFFE nanoparticles formed by the prodrug itself significantly alleviate the safety concerns associated with carriers (e.g., biocompatibility and biodegradability) and provide a precise and relatively high drug payload.
As presented in , the formed Dex-SA-FFFE nanoparticles displayed a uniform spherical shape with a diameter of ~30 nm. Conversely, dynamic light scattering (DLS) analysis indicated that the mean diameter of the formed Dex-SA-FFFE nanoparticles was 150±3.4 nm with polydispersity index of 0.26±0.09 (). The formed Dex-SA-FFFE nanoparticles were negatively charged (−17.8±1.2 mV) owing to the ionized carboxylate groups of the amino acid residues present on the surface of the nanoparticles. The discrepancy between particle sizes measured by TEM and DLS might be ascribed to the high vacuum conditions of TEM as well as to hydrodynamic and electrokinetic effects during the DLS measurements.Citation43 Additionally, the pH value and osmotic pressure of formed Dex-SA-FFFE nanoparticles are 7.3 and 298±2 mOsm·L−1, respectively.
In vitro release study
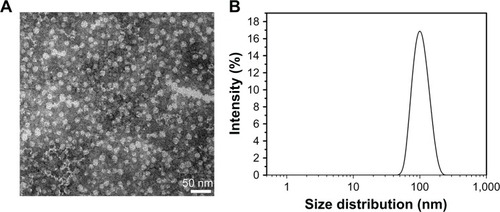
The drug release profiles of Dex and Dex-SA-FFFE from the Dex-SA-FFFE nanoparticles in PBS (pH =7.4) and PBS (pH =7.4) containing 20 U/mL esterase at 37°C are presented in . It is clearly observed that almost 100% of the total drug (Dex or Dex-SA-FFFE) was completely released from the Dex-SA-FFFE nanoparticles in either PBS (pH =7.4) or PBS containing 20 U/mL esterase during the 48-hour study. Despite the absence of esterase, Dex-SA-FFFE underwent obvious hydrolysis in PBS (pH =7.4) to release Dex at each time point (). This result seems to indicate that Dex-SA-FFFE was susceptive to the weak alkali condition, leading to the hydrolysis of Dex-SA-FFFE and the release of its native drug form (Dex). Not surprisingly, with the addition of 20 U/mL of esterase in PBS (pH =7.4), a higher ratio of Dex in the released drug was observed, which indicated that esterase could greatly accelerate the hydrolysis of Dex-SA-FFFE ().
In vitro cytotoxicity
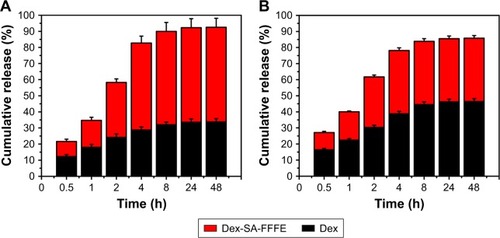
shows the cell viability of Dex-SA-FFFE nanoparticles in HCEC after 24, 48, and 72 hours of incubation. The results indicated that Dex-SA-FFFE nanoparticles were not toxic against HCEC at drug concentrations up to 1 mM after 24 hours of incubation. However, HCEC showed reduced cell viability after 48 and 72 hours of incubation, which might be ascribed to the cleavage and subsequent hydrolysis of Dex-SA-FFFE by esterase to release the native, potentially toxic, Dex.
In vitro anti-inflammation test
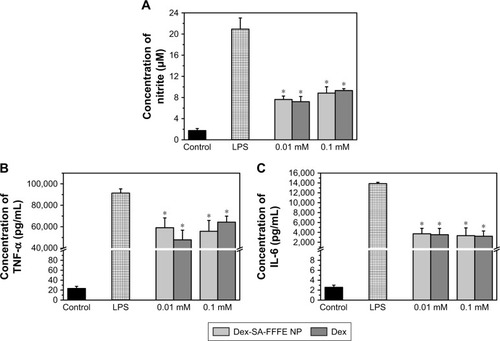
To assess the in vitro anti-inflammatory efficacy of Dex-SA-FFFE nanoparticles with respect to its native drug form (Dex), we monitored the secretion of pro-inflammatory cytokines such as NO, IL-6, and TNF-α in LPS-activated RAW264.7 macrophages. As depicted in , RAW264.7 macrophages challenged with LPS significantly elevated the levels of NO, IL-6, and TNF-α compared to control cells. Treatments of both Dex and Dex-SA-FFFE nanoparticles at dosages of 0.01 and 0.1 mM were able to remarkably inhibit NO, IL-6, and TNF-α production compared to the LPS group (P<0.05). However, there was no significant difference between the Dex group and the Dex-SA-FFFE nanoparticles group at the tested concentrations. These results seem to imply that the Dex-SA-FFFE nanoparticles displayed nearly identical anti-inflammatory efficacy as the native drug (Dex). Early studies have illustrated that the inhibition of IL-6 and TNF-α production has a protective effect in the control of ocular inflammation.Citation5,Citation6,Citation10 Therefore, it is reasonable to believe that the anti-inflammatory activity of the proposed Dex-SA-FFFE nanoparticles is not compromised in terms of its effects on pro-inflammatory cytokines (e.g., TNF-α, and IL-6) and that Dex-SA-FFFE nanoparticles might be a promising candidate for the therapy of ocular inflammatory disorders.
Figure 5 Inhibition of (A) NO, (B) TNF-α, and (C) IL-6 production by Dex-SA-FFFE nanoparticles and Dex in LPS-stimulated RAW264.7 macrophages. *P<0.05, when compared to LPS group.
Abbreviations: Dex-SA-FFFE, dexamethasone-peptide conjugate; IL-6, interleukin-6; LPS, lipopolysaccharide; NO, nitric oxide; TNF-α, tumor necrosis factor-α.
Intraocular biocompatibility
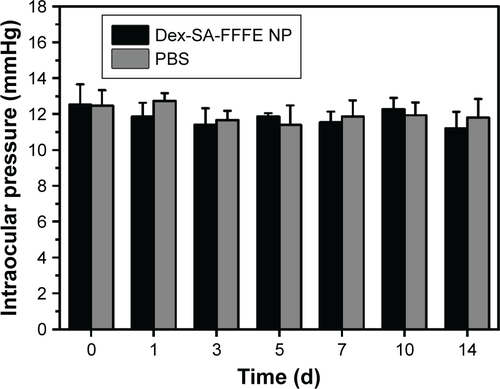
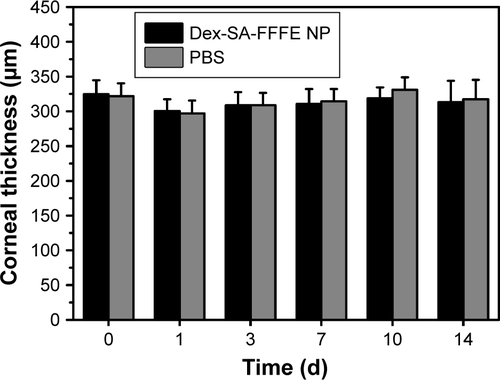
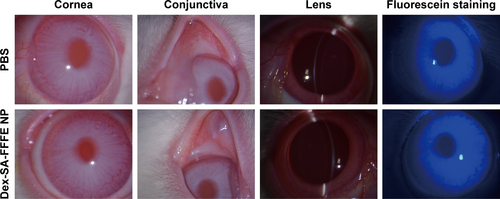
The intraocular biocompatibility of the Dex-SA-FFFE nanoparticles after topical instillation have been investigated in detail. As shown in , the eyes treated by Dex-SA-FFFE nanoparticles did not show any apparent ocular irritation (eg, corneal edema, conjunctival swelling, and lens opacity) during the entire study period. Fluorescein sodium staining indicated that the architecture of the corneal epithelium layer was kept normal and intact, without any obvious defects. Together with the general observation, there are no significant changes in the corneal thickness or intraocular pressure after topical instillation of Dex-SA-FFFE nanoparticles ( and ). Based on these results, we could conclude that the proposed Dex-SA-FFFE nanoparticles are a well-tolerated formulation for ocular therapy.
EIU rabbit model and medication
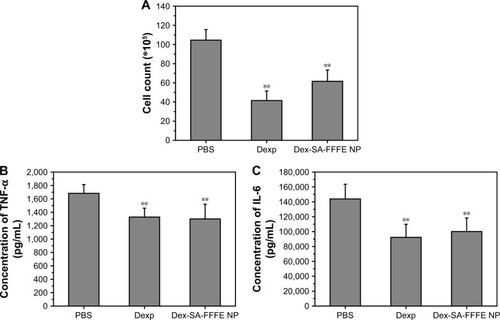
At 24 hours after intravitreal injection of endotoxin in rabbits, the eyes from the PBS group exhibited a severe inflammatory cellular response in the anterior chamber, whereas the eyes from the Dexp and Dex-SA-FFFE nanoparticles groups displayed a slight inflammatory response with minimal inflammatory exudate in the anterior chamber (). This result clearly indicated that treatments using both Dexp and Dex-SA-FFFE nanoparticles could significantly reduce the severity of clinical signs. Early studies have demonstrated that the main cause for the initiation of inflammatory symptoms in uveitis is the breakdown of the blood–aqueous humor barrier, and inflammation may subsequently occur owing to the migration of white blood cells such as leukocytes.Citation44–Citation46 We, therefore, quantified inflammatory cells in the aqueous humor at 24 hours of intravitreal injection of endotoxin (). The inflammatory cell count in the aqueous humor of eyes from the PBS group was (104.5±11) ×105 cells. The eyes treated by Dexp and Dex-SA-FFFE nanoparticles showed significantly lower inflammatory cell counts (P<0.05), but there was no significant difference between the Dexp group and the Dex-SA-FFFE nanoparticles group.
Figure 6 (A) Clinical signs of anterior uveitis in rabbits treated with PBS, Dexp, and Dex-SA-FFFE nanoparticles. White arrowheads indicate inflammatory exudates in the anterior chamber. (B) H&E sections of rabbit eyes treated with PBS, Dexp, and Dex-SA-FFFE nanoparticles at 24 hours after challenge with LPS. White arrowheads indicate inflammatory cells infiltration in the anterior chamber.
Abbreviations: Dex-SA-FFFE, dexamethasone-peptide conjugate; Dexp, Dex sodium phosphate; H&E, hematoxylin and eosin; LPS, lipopolysaccharide.
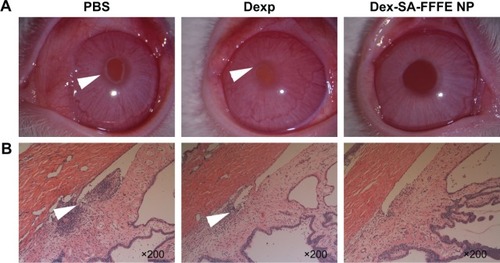
Figure 7 (A) Leucocytes counts, (B) TNF-α, and (C) IL-6 levels in the aqueous humor of EIU rabbits after topical treatment by PBS, Dexp, and Dex-SA-FFFE nanoparticles. **P<0.05, when compared to PBS group.
Abbreviations: Dex-SA-FFFE, dexamethasone-peptide conjugate; Dexp, Dex sodium phosphate; EIU, endotoxin-induced uveitis; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
In line with clinical observations, histopathologic observations () indicated that there were severe inflammatory changes with extensive inflammatory cell infiltration of the anterior chamber in the eyes treated by PBS. Conversely, the eyes treated by Dexp and Dex-SA-FFFE nanoparticles showed very minimal inflammatory changes with an absence of inflammatory cell infiltration in the anterior chamber. Notably, the Dex-SA-FFFE nanoparticles exhibited nearly identical in vivo anti-inflammatory efficacy as Dexp, indicating that the conjugation of Dex to peptide did not compromise the biological activity of Dex.
Although the exact pathogenic mechanisms underlying uveitis are poorly understood, several cytokines, including TNF-α, IL-1, IL-2, and IL-6 have been regarded as very important mediators in the pathogenesis of uveitis.Citation2,Citation5–Citation8,Citation10 We, thereafter, measured the levels of the cytokines TNF-α and IL-6 in the aqueous humor at 24 hours after intravitreal injection of endotoxin (). Both Dexp and Dex-SA-FFFE nanoparticles significantly downregulated TNF-α and IL-6 levels in the aqueous humor compared to the PBS treatment (P<0.05).
Conclusion
In this paper, we proposed the formation of a Dex-SA-FFFE, via an ester bond linkage, that could self-assemble into nanoparticles, acting as a “self-drug” delivery system for treating anterior uveitis. Both Dex and Dex-SA-FFFE were sustainably released from Dex-SA-FFFE nanoparticles in PBS or PBS containing 20 U/mL esterase in a 48-hour study, and the addition of esterase in PBS significantly influenced the ratio of Dex/Dex-SA-FFFE in the released drug, as indicated by the in vitro release study. In vitro cytotoxicity assays indicated that the formed Dex-SA-FFFE nanoparticles hardly caused cytotoxicity in HCEC cells at drug concentrations below 1 mM within 24 hours. Meanwhile, Dex-SA-FFFE nanoparticles displayed a comparable in vitro anti-inflammatory efficacy to that of Dex and significantly inhibited NO, IL-6, and TNF-α production in LPS-activated RAW264.7 macrophages. On the other hand, the formed Dex-SA-FFFE nanoparticles showed good ocular tolerance, as they appeared to exert no deleterious influence on corneal thickness or intraocular pressure in in vivo ocular biocompatibility tests. More importantly, Dex-SA-FFFE nanoparticles provided nearly identical in vivo anti-inflammatory efficacy as an aqueous solution of Dexp and downregulated the levels of cytokines (TNF-α and IL-6) in aqueous humor, indicating that the conjugation of Dex to peptide did not compromise the biological activity of Dex in vivo. In conclusion, the proposed Dex-SA-FFFE nanoparticles might have great application for the treatment of anterior uveitis.
Acknowledgments
This research was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant No LR18H300002) and the National Natural Science Foundation of China (Grant No 31671022).
Supplementary materials
Figure S2 Appearance of Dex-SA-FFFE suspension before (left) and after (right) heating-cooling circle.
Figure S3 LC-MS spectra of Dex-SA-FFFE nanoparticles formed after heating-cooling circle.
Abbreviation: Dex-SA-FFFE, dexamethasone-peptide conjugate.
Figure S4 Slit-lamp image and fluorescein staining of cornea at 3days post topical instillation of phosphate buffered saline (PBS) and Dex-SA-FFFE nanoparticles.
Abbreviation: Dex-SA-FFFE, dexamethasone-peptide conjugate.
Disclosure
The authors report no conflicts of interest in this work.
References
- ReddyAKEngelhardSBShahCTSimAJThorneJEMedical malpractice in uveitis: a review of clinical entities and outcomesOcul Immunol Inflamm201826224224827715388
- SamudreSSLattanzioFAWilliamsPBSheppardJDComparison of topical steroids for acute anterior uveitisJ Ocul Pharmacol Ther200420653354715684812
- MiserocchiEFogliatoGModoratiGBandelloFReview on the worldwide epidemiology of uveitisEur J Ophthalmol201323570571723661536
- Rodríguez VillanuevaJRodríguez VillanuevaLGuzmán NavarroMVillanuevaJRVillanuevaLRNavarroMGPharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trendsInt J Pharm20175161–234235127889587
- GhasemiHRoles of IL-6 in Ocular Inflammation: A ReviewOcul Immunol Inflamm2018261375028146368
- ImpellizzeriDAhmadABruschettaGThe anti-inflammatory effects of palmitoylethanolamide (PEA) on endotoxin-induced uveitis in ratsEur J Pharmacol2015761283525934566
- LinPSuhlerEBRosenbaumJTThe future of uveitis treatmentOphthalmology2014121136537624169255
- ZhengCLeiCChenZTopical administration of diminazene aceturate decreases inflammation in endotoxin-induced uveitisMol Vis20152140325883526
- CaoJNaeemMNohJ-KLeeEHYooJWDexamethasone phosphate-loaded folate-conjugated polymeric nanoparticles for selective delivery to activated macrophages and suppression of inflammatory responsesMacromol Res2015235485492
- Barbosa SalibaJVieiraLFernandes-CunhaGMAnti-Inflammatory Effect of Dexamethasone Controlled Released From Anterior Suprachoroidal Polyurethane Implants on Endotoxin-Induced Uveitis in RatsInvest Ophthalmol Vis Sci20165741671167927054520
- RenfroLSnowJSOcular effects of topical and systemic steroidsDermatol Clin19921035055121617809
- McgheeCNDeanSDanesh-MeyerHLocally administered ocular corticosteroids: benefits and risksDrug Saf2002251335511820911
- GaudanaRJwalaJBodduSHMitraAKRecent perspectives in ocular drug deliveryPharm Res20092651197121618758924
- LiuSJonesLGuFXNanomaterials for ocular drug deliveryMacromol Biosci201212560862022508445
- YuanXMarcanoDCShinCSOcular drug delivery nanowafer with enhanced therapeutic efficacyACS Nano2015921749175825585134
- SoutoEBDoktorovovaSGonzalez-MiraEEgeaMAGarciaMLFeasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugsCurr Eye Res201035753755220597640
- SwaminathanSVaviaPRTrottaFCavalliRNanosponges encapsulating dexamethasone for ocular delivery: formulation design, physico-chemical characterization, safety and corneal permeability assessmentJ Biomed Nanotechnol201396998100723858964
- KalamMADevelopment of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasoneInt J Biol Macromol20168912713627126165
- NagaiNNakazawaYItoYKanaiKOkamotoNShimomuraYA Nanoparticle-Based Ophthalmic Formulation of Dexamethasone Enhances Corneal Permeability of the Drug and Prolongs Its Corneal Residence TimeBiol Pharm Bull20174071055106228674248
- RomeroGBKeckCMMüllerRHBou-ChacraNADevelopment of cationic nanocrystals for ocular deliveryEur J Pharm Biopharm201610721522227388629
- ZhangZYuJZhouYSupramolecular nanofibers of dexamethasone derivatives to form hydrogel for topical ocular drug deliveryColloids Surf B Biointerfaces201816443644329438842
- AnLShah GilaniMRLiangGGilaniHSRehanMPeptide-based nanostructures for cancer diagnosis and therapyCurr Med Chem201421212453246624524760
- DuXZhouJShiJXuBSupramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterialsChem Rev201511524131651330726646318
- MeiBMiaoQTangALiangGEnzyme-instructed self-assembly of taxol promotes axonal branchingNanoscale2015738156051560826359218
- SundarSChenYTongYWDelivery of therapeutics and molecules using self-assembled peptidesCurr Med Chem201421222469247924358972
- VemulaPKWiradharmaNAnkrumJAMirandaORJohnGKarpJMProdrugs as self-assembled hydrogels: a new paradigm for biomaterialsCurr Opin Biotechnol20132461174118223465753
- YangCWangZOuCChenMWangLYangZA supramolecular hydrogelator of curcuminChem Commun2014506694139415
- GyntherMLaineKRopponenJLarge neutral amino acid transporter enables brain drug delivery via prodrugsJ Med Chem200851493293618217702
- PochopinNLCharmanWNStellaVJAmino acid derivatives of dapsone as water-soluble prodrugsInt J Pharm19951212157167
- SanterVdel Río SanchoSLaptevaMKaliaYNTargeted intracorneal delivery-Biodistribution of triamcinolone acetonide following topical iontophoresis of cationic amino acid ester prodrugsInt J Pharm20175251435328414134
- AdelliGRBhagavPTaskarPDevelopment of a Δ9-Tetrahydrocannabinol Amino Acid-Dicarboxylate Prodrug With Improved Ocular BioavailabilityInvest Ophthalmol Vis Sci20175842167217928399267
- LiJKuangYGaoYduXShiJXuBD-amino acids boost the selectivity and confer supramolecular hydrogels of a nonsteroidal anti-inflammatory drug (NSAID)J Am Chem Soc2013135254254523136972
- LinRCuiHSupramolecular nanostructures as drug carriersCurr Opin Chem Eng201577583
- MaWCheethamAGCuiHBuilding Nanostructures with DrugsNano Today2016111133027066106
- WangYCheethamAGAngacianGSuHXieLCuiHPeptide-prug conjugates as effective prodrug strategies for targeted deliveryAdv Drug Deliver Rev2011110112126
- RautioJKumpulainenHHeimbachTProdrugs: design and clinical applicationsNat Rev Drug Discov20087325527018219308
- RautioJKärkkäinenJSloanKBProdrugs – Recent approvals and a glimpse of the pipelineEur J Pharm Sci201710914616128782609
- HamadaYRecent progress in prodrug design strategies based on generally applicable modificationsBioorg Med Chem Lett20172781627163228285913
- AbetVFilaceFRecioJAlvarez-BuillaJBurgosCProdrug approach: An overview of recent casesEur J Med Chem201712781082727823878
- SugawaraMHuangWFeiYJLeibachFHGanapathyVGanapathyMETransport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2J Pharm Sci200089678178910824137
- DiasCNashedYAtluriHMitraAOcular penetration of acyclovir and its peptide prodrugs valacyclovir and val-valacyclovir following systemic administration in rabbits: An evaluation using ocular microdialysis and LC-MSCurr Eye Res200225424325212658558
- VigBSHuttunenKMLaineKRautioJAmino acids as promoieties in prodrug design and developmentAdv Drug Deliv Rev201365101370138523099277
- DobrovolskaiaMAPatriAKZhengJInteraction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profilesNanomedicine20095210611719071065
- BararJJavadzadehAROmidiYOcular novel drug delivery: impacts of membranes and barriersExpert Opin Drug Deliv20085556758118491982
- RatayMLBellottiEGottardiRLittleSRModern Therapeutic Approaches for Noninfectious Ocular Diseases Involving InflammationAdv Healthc Mater20176231700733
- Sánchez-LópezEEspinaMDoktorovovaSSoutoEBGarcíaMLLipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye – Part I – Barriers and determining factors in ocular deliveryEur J Pharm Biopharm2017110707527789358