Abstract
Background
Lipid nanoemulsions that bind to low-density lipoprotein receptors can concentrate chemotherapeutic agents in tissues with low-density lipoprotein receptor overexpression and decrease the toxicity of the treatment. The aim of this study was to develop a new formulation using a lipophilic derivative of methotrexate, ie, didodecyl methotrexate (ddMTX), associated with a lipid nanoemulsion (ddMTX-LDE).
Methods
ddMTX was synthesized by an esterification reaction between methotrexate and dodecyl bromide. The lipid nanoemulsion was prepared by four hours of ultrasonication of a mixture of phosphatidylcholine, triolein, and cholesteryloleate. Association of ddMTX with the lipid nanoemulsion was performed by additional cosonication of ddMTX with the previously prepared lipid nanoemulsion. Formulation stability was evaluated, and cell uptake, cytotoxicity, and acute animal toxicity studies were performed.
Results
The yield of ddMTX incorporation was 98% and the particle size of LDE-ddMTX was 60 nm. After 48 hours of incubation with plasma, approximately 28% ddMTX was released from the lipid nanoemulsion. The formulation remained stable for at least 45 days at 4°C. Cytotoxicity of LDE-ddMTX against K562 and HL60 neoplastic cells was higher than for methotrexate (50% inhibitory concentration [IC50] 1.6 versus 18.2 mM and 0.2 versus 26 mM, respectively), and cellular uptake of LDE-ddMTX was 90-fold higher than that of methotrexate in K562 cells and 75-fold in HL60 cells. Toxicity of LDE-ddMTX, administered at escalating doses, was higher than for methotrexate (LD50 115 mg/kg versus 470 mg/kg; maximum tolerated dose 47 mg/kg versus 94 mg/kg) in mice. However, the hematological toxicity of LDE-ddMTX was lower than for methotrexate.
Conclusion
LDE-ddMTX was stable, and uptake of the formulation by neoplastic cells was remarkably greater than of methotrexate, which resulted in markedly greater cytotoxicity. LDE-ddMTX is thus a promising formulation to be tested in future animal models of cancer or rheumatic disease, wherein methotrexate is widely used.
Introduction
Methotrexate (2,4-diamino,N10-methylpteroyl glutamic acid) is an antiproliferative and immunosuppressive agent widely used against a broad spectrum of diseases. Methotrexate is a potent inhibitor of dihydrofolate reductase, and can also inhibit thymidylate synthase and 5-aminoimidazole carboxamide ribotide transformylase. These enzymes are involved in the synthesis of thymidylate, purines, methionine, and serine.Citation1 Methotrexate was introduced in the 1940s, and is still used extensively in clinical practice. Methotrexate has relatively low toxicity compared with other chemotherapeutic agents,Citation2 but is not devoid of side effects, including nephrotoxicity, hepatotoxicity, myelotoxicity, interstitial pneumonitis, and chronic interstitial obstructive pulmonary disease.Citation3
The effectiveness of methotrexate is often hampered by development of drug resistance mechanisms, such as inhibition of active transport involving the cell folate receptor that mostly internalizes methotrexate, and is hydrophilic and poorly diffused through cell membranes. Aiming to bypass drug resistance and yet retain cytotoxic activity, several strategies have been attempted. One of these strategies consists of chemical modification of methotrexate to improve lipophilicity. In this regard, the γ-carbonyl position is preferentially targeted to attach lipophilic groups to the methotrexate molecule, and in fact modification at the γ and, to a lesser extent, the α-carbonyl position did not decrease the drug cytotoxicity.Citation4 Drug delivery systems have also been used to improve cytotoxicity and reduce toxicity, such as loading of methotrexate into liposomes,Citation4,Citation5 dendrimers,Citation6 micelles,Citation7 chitosan polymers,Citation8 and tuftsin-like peptide carriers,Citation9 as well as attachment to polymeric chains. Other formulations have been described, such as chylomicron-like emulsions, for oral use.Citation10 The use of a solid lipid nanoparticle system as a methotrexate carrier was reportedly shown to improve drug pharmacokinetics and to achieve regression of Ehrlich ascites carcinoma in mice.Citation11,Citation12
The demonstration by Ho et alCitation13 that low-density lipoprotein receptors are overexpressed in neoplastic cells, paved the way for the use of lipoproteinsCitation14–Citation17 and subsequently of lipoprotein-like nanoemulsionsCitation18,Citation19 as vehicles to concentrate chemotherapeutic agents in neoplastic tissues. Taking this approach, the low-density lipoprotein receptor-mediated endocytic pathway is used to internalize drug-loaded lipid particles.Citation14–Citation17 We showed that cholesterol-rich nanoemulsions, with a lipid structure mimicking low-density lipoprotein, has the ability to bind to low-density lipoprotein receptors. A lipid nanoemulsion is made without protein but, in contact with plasma, acquires apolipoprotein E, which is recognized by low-density lipoprotein receptors.Citation19,Citation20 In preclinical studies using chemotherapeutic agents, such as carmustineCitation21 and lipophilic derivatives of paclitaxelCitation22 and etoposide,Citation23,Citation24 the toxicity of these agents was markedly reduced by association with a lipid nanoemulsion, while the pharmacological action was maintained or even increased. In clinical studies enrolling patients with breast and ovary cancer, the amount of lipid nanoemulsion accumulated in tumor tissue was markedly greater than in normal tissue.Citation25–Citation28 In clinical trials enrolling patients with advanced solid tumors and hematological neoplasias,Citation21,Citation29 we showed that the toxicity of high-dose preparations of carmustine, paclitaxel, and etoposide associated with a lipid nanoemulsion was minimal.
This study aimed to investigate whether the association of methotrexate and a lipid nanoemulsion could increase neoplastic cell uptake and cytotoxicity, and decrease the toxicity of the drug. To improve the association yield and stability of the formulation, two dodecyl groups were attached to the methotrexate molecule.
Methods
Materials
Methotrexate (Miantrex®) was purchased from Pfizer (Bentley, Australia); triolein, cholesteryloleate, cholesterol, phosphatidylcholine, dimethyl sulfoxide, and cesium carbonate from Sigma (St Louis, MO); methanol and acetonitrile from Merck (Darmstadt, Germany); and methotrexate sodium salt from Deg Importacao De Produtos Quimicos Ltd (São Paulo, Brazil).
Nuclear magnetic resonance (NMR) of 1H and 13C used to characterize the ddMTX structure was performed using a Bruker DPX-300 instrument (Brucker, Karlsruhe, Germany). Purity was analyzed by high-performance liquid chromatography in a Shimadzu SPD-10 AV column (Shimadzu, Columbia, ML). The structure of the ddMTX was also confirmed by mass spectrometry using a Q-TOF Ultima (Micromass, Manchester, UK) and elemental analysis using a Perkin Elmer CHN 2400 elemental analyzer (Perkin Elmer, Sciex, Shelton, CT). Transmission electron microscopy imaging of LDE-MTX was performed using a LEO 906E transmission electron microscope (Zeiss, Oberkochen, Germany) at 100 kV.
Isogenic female Balb/c mice (±20 g, aged 30–45 days) were supplied by the Butantan Institute (São Paulo, Brazil). All animals used in this study were housed in a temperature-controlled room, with a 12-hour light/dark cycle, and food and water available ad libitum. The ethics committee of University of Sao Paulo Medical School Hospital approved all the animal experiments.
Synthesis of ddMTX
Briefly, ddMTX was synthesized following the method described by Rosowsky et al.Citation30 Methotrexate sodium salt (2.0 g, 4.1 mmol) was diluted in 120 mL of dimethylsulfoxide, and over it were added cesium carbonate (1.43 g, 4.40 mmol) and dodecyl bromide (2.5 g, 10 mmol). The mixture was stirred for 24 hours at room temperature, and 100 mL of water was added to quench the reaction. The product was extracted with chloroform (5 × 50 mL). The organic phase was washed with a saturated sodium chloride solution (3 × 100 mL), dried with magnesium sulfate, filtered, and concentrated. The product was purified by liquid chromatography using silica gel (230–400 mesh) with methanol/chloroform, from 2.5% to 100% of methanol. Pf 122°C–124°C. Yield was 98%.
1H δ (ppm): 0,86 (6H, t, J = 6.0 Hz), 1.23 (36H, s); 1.50–1.68 (4H, m), 2.08–2.20 (1H, m), 2.21–2.32 (2H, m), 2.42–2.50 (1H, m), 3.12 (3H, s), 4.00 (2H, t, J = 6.0 Hz), 4.13 (2H, t, J = 6.0 Hz), 4.67 (2H, s), 4.75–4.83 (1H, m), 5.98 (2H, bs), 6.69 (2H, d, J = 6.0 Hz); 7,02 (1H, bs), 7.69 (1H, d, J = 6.0 Hz), 8.56 (1H, s).13C δ (ppm): 13.99, 22.54, 25.75, 27.31, 28.42, 29.15, 29.24, 29.53, 30.55, 31.80, 38.90, 52.16, 55.68, 64.85, 65.66, 111.21, 121.37, 121.87, 128.79, 146.65, 146.71, 149.10, 151.31, 154.86, 162.51, 162.89, 166.96, 172.63, 173.21. Mass: 338.31, 381.27, 675.63, 791.48 (100), 792.49, 793.52. IV ν (cm−1): 3478.8, 2924.2, 2855.9, 1737.5, 1630.7, 1512.6, 1445.9, 1204.3, 1096.0, 816.9. Elemental analysis: Calc: 66.80 (C), 8.92 (H), 14.16 (N). Expt: 66.50 (C), 8.68 (H), 14.10 (N).
High-pressure liquid chromatography measurements
A high-pressure liquid chromatographic column equipped with an ultraviolet detector at 300 nm was used to analyze and quantify methotrexate and ddMTX. Chromatographic separation was achieved with a ShimPack C18 (2) 5 μm (15 cm × 6 mm) analytical column (Phenomenex, Torrance, CA) protected by a Luna C18 (2) guard cartridge. The mobile phase was methanol at a flow rate of 1.0 mL/minute for ddMTX and buffer of sodium acetate:acetonitrile (90:10) for methotrexate. For quantification of both compounds, a calibration curve was constructed at concentration ranging from 78.12 ng to 50,000 ng.
Distribution coefficient
The distribution coefficient is the log distribution coefficient at a particular pH. Log D at pH 7.4 is often quoted to give an indication of the lipophilicity of a drug at the pH of blood plasma. In aqueous/organic systems, the organic phase concentration is, by convention, the numerator, and the aqueous phase concentration is the denominator. 1.0 mL of n-octanol and 1.0 mL of sodium phosphate buffer, pH 7.4 were added to 1 mg of ddMTX. The mixture was gently shaken for 24 hours at 37°C. The amount of drug in each phase was quantified by high-pressure liquid chromatography.
Preparation of lipid nanoemulsion and LDE-ddMTX
In brief, the lipid nanoemulsion was prepared from a lipid mixture composed of 40 mg cholesteryloleate, 20 mg egg phosphatidylcholine, 1 mg triolein, and 0.5 mg cholesterol. Emulsification of lipids by prolonged ultrasonic irradiation in aqueous media and the procedure of two-step ultracentrifugation of the crude emulsion with density adjustment by addition of KBr to obtain a lipid nanoemulsion were carried out as described previously.Citation20 The lipid nanoemulsion was dialyzed against Tris solution (pH 8.05) and passed through a 0.22 μm filter for the experiments.
ddMTX 6 mg was incorporated into the lipid nano-emulsion (1 mL, 30 mg of total lipids) by solubilization of ddMTX in ethanol (10% v:v) and adding it into the emulsion. The solution was then sonicated for one hour at 70°C using a Branson Sonifier 450 (Danbury, CT), equipped with a 1 cm flat titanium probe. LDE-ddMTX was centrifuged at 3500 rpm for 15 minutes to separate the LDE-ddMTX from the free form of the drug that precipitated to the bottom of the tube. LDE-ddMTX was then passed through a 0.22 μm pore polycarbonate filter and kept at 4°C until it was used. The yield of each batch was assayed before use. For quantification of ddMTX-loaded LDE, 10 μL LDE-ddMTX were dissolved in 1.0 mL of methanol to break the formulation. The ddMTX mass contained in the lipid nano-emulsion was measured in 20 μL of the previous solution using a calibration curve. The amount of drug associated to the lipid nanoemulsion was always measured by high-performance liquid chromatography before the experiments.
LDE-ddMTX particle size
The average size of the LDE-ddMTX particles was measured using a zeta potential analyzer (Brookhaven Instruments Corporation, Holtsville, NY) at 25°C. All the samples were kept at 4°C and diluted and filtrated immediately prior to diameter measurement. In order to evaluate the stability of the formulation, the mean diameters of the lipid nanoemulsion and LDE-ddMTX particles were measured over 45 days.
Transmission electron microscopy of LDE-ddMTX
Five microliters of LDE-ddMTX at approximately 0.2 mg/mL were applied on parlodium-carbon-coated copper grids of 300 or 400 meshes. After 1–2 minutes, the excess liquid is blotted with tissue paper, leaving a small amount of residual fluid. Negative staining was done with a 5 μL drop of 2% ammonium molybdate pH 7.2 for 5–10 seconds and then blotted dry. Grids were examined under a LEO 906E transmission electron microscope (Zeiss, Oberkochen, Germany) at 100 kV acceleration voltage. Images were acquired by a charge- coupled device camera (MegaView III) through the universal transmission electron microscope imaging platform program (Olympus Soft Imaging Solutions GMBh, Oberkochen, Germany), and saved in TIF extension.
In vitro release of ddMTX from lipid nanoemulsion
LDE-ddMTX was dialyzed against human plasma to evaluate drug release from the lipid nanoemulsion. The dialysis bags (Seamless cellulose tubing, Sigma) were soaked in deionized water before placing 1.0 mL of LDE-ddMTX into the bag. The bags were placed into a tube containing 20 mL of human plasma under magnetic stirring for 48 hours at 37°C. Samples of 5 μL were collected at 0.5, 1, 2, 3, 4, 5, 6, 20, 24, 48 and 72 hours and analyzed using high-performance liquid chromatography for content mass of ddMTX in the nanoemulsion.
Cytotoxic activity assays
Human erythroleukemia cells (K562) and human promyelocytic leukemia cells (HL60) were cultured in RPMI 1640 medium supplemented with streptomycin 50 μg/mL, penicillin 50 IU/mL, and 10% (v/v) fetal calf serum (all from Invitrogen, Carlsbad, CA). Cells were maintained at 37°C, 5% CO2, and 100% relative humidity, and subcultivated 2–3 times a week, with 0.4% Trypan blue staining to assess cell growth and viability.
The starting inoculums of 5 × 105 K562 and HL60 cells/mL were seeded in the exponential phase of growth into 96-well culture plates (Nunc TM, Roskilde, Denmark) with LDEdd-MTX (0.05–0.5 mM), methotrexate (2.5–37.5 mM), and lipid nanoemulsion, at the same volume as the lipid nanoemulsion with the associated drug, were added to the cells incubated. After 48 hours of incubation, the medium was removed and the number of living tumor cells was determined by the colorimetric MTT assay.Citation31 Briefly, MTT solution was added to each well (1.2 mg/mL) and incubated for 4 hours. MTT was reduced by mitochondrial dehydrogenase in the viable cells to a purple formazan product, which was dissolved in 100 μL dimethyl sulfoxide for measurement of absorbance at 570–655 nm in an enzyme-linked immunosorbent assay plate reader.
Cell viability of each well was expressed as the survival index. The IC50 was determined as the drug concentration required to achieve 50% cell growth inhibition.
LDE-ddMTX uptake by neoplastic cells
HL60 and K562 cell suspensions were seeded in 35 mm Petri dishes at 105 × cells/mL and incubated at 37°C, 5% CO2, and 100% relative humidity. After 24 hours of cell growth, the medium was replaced by fresh medium supplemented with 10% lipoprotein-deficient serum, and the cells were incubated for 24 hours in the same conditions. The medium was replaced by fresh medium supplemented with 10% fetal bovine serum, and increasing amounts of methotrexate and LDE-ddMTX were added at final drug concentrations ranging from 0.15 to 2.0 mM. After 4 hours of incubation at 37°C, the cells were washed three times with cold phosphate-buffered saline, centrifuged at 1500 rpm for 3 minutes, and then lysed in 1.0 mL of phosphate-buffered saline using a Branson Sonifier 450 sonicator for 10 seconds. Protein precipitation was performed with the addition of 500 μL 50:50 isopropyl alcohol to ethyl acetate and acetonitrile for ddMTX and methotrexate, respectively. The samples were mixed for one minute and centrifuged at 13000 rpm for 10 minutes, and the supernatant was dried under nitrogen flow. Methanol and buffer of sodium acetate:acetonitrile (90:10) were used for ddMTX and methotrexate, respectively, as the mobile phase after extracting the drugs using Stracta cartridges (Phenomenex). The organic phase was concentrated and reconstituted in 0.2 mL of methanol and quantified by high-performance liquid chromatography. The Lowry protein quantification method was used to correct drug/mg of protein concentrations.Citation32
Maximum tolerated dose and 50% lethal dose of LDE-ddMTX
Groups of 10 mice were intravenously injected via the retro-orbital sinus, following a dose-escalation schedule based on three dose levels of methotrexate and LDE-ddMTX (47, 94, and 180 mg/kg). The studies were performed in two schedules, ie, a single-dose and a three-day multiple-dose schedule. Lipid nanoemulsion and saline control were administered. Animals were observed until day 7 and body weight measurements were performed every 2 days. The maximum tolerated dose (defined as the dose that resulted in approximately 15% loss in body weight and does not cause lethality). The lethal dose of 50% (LD50) was defined as the administered dose which caused death of 50% of the animals, calculated by interpolation of results (death percentage in the group/dose).
Hematological toxicity
Blood samples were collected 24 hours after injection of a single 180 mg/kg dose of LDE-ddMTX and methotrexate by retro-orbital sinus puncture, using heparinized Helmington microcapillary tubes. Hematology parameters included erythrocyte, leukocyte, leukocyte differential count, and platelets were performed using a hemocytometer (Optik Labor, Friedrichshafen, Germany). Briefly, blood was 1/1000 diluted in saline for erythrocyte count, 1/100 in Becker reagent for platelets, and 10 μL was added to the hemocytometer. For leukocytes, blood was 1/100 diluted in Turk reagent, and an aliquot of nondiluted blood was used on a glass slide for leukocyte differential count. Slides were stained using a Panoptic kit (Laborclin, Parana, Brazil). Erythrocytes and leukocytes were determined by counting the cells in the large squares, and platelets were counted in the central squares of the hemocytometer.
Statistical analysis
Cell uptake and IC50 data were expressed as the mean ± standard error of the mean, and were analyzed by the nonparametric t-test. Kaplan–Meier analysis was used to assess survival in the different treatment groups. Maximum tolerated dose, LD50, and hematological profile values were also expressed as the mean ± standard error of the mean, and were analyzed by analysis of variance followed by Bonferroni’s test. In all analyses, differences of 0.05 or less were considered to be statistically significant.
Results
Synthesis of LDE-ddMTX
The methotrexate derivative, ddMTX, was obtained by esterification of the α- and γ-carbonyl groups of the glutamic acid residue in the presence of cesium carbonate and dodecyl bromide (). The ddMTX structure was confirmed by 1H and 13C NMR, in which characteristic peaks corresponding to the dodecyl group were present. Esterification achieved a 98% yield and a high purity rate, as confirmed by high-performance liquid chromatography analysis. Mass spectrometry showed a signal 791.48 m/z corresponding to the molecular weight of the derivative. Those results were corroborated by the elemental analysis.
The esterification reaction markedly enhanced the methotrexate distribution coefficient, which was –1.4 before and 3.9 after the reaction. These data confirmed successful and optimized association with the lipid nanoemulsion, which attained a 98% association rate.
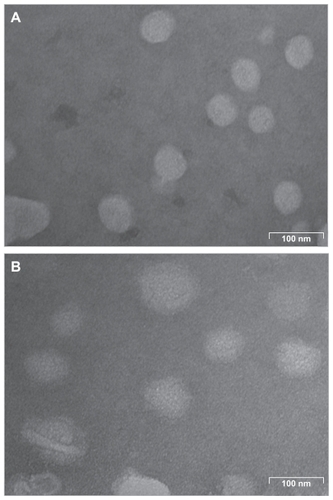
As analyzed by laser light scattering, the average particle size of the lipid nanoemulsion without ddMTX was approximately 40 nm and that of the lipid nanoemulsion with ddMTX was approximately 60 nm. Transmission electron microscopy showed particles of quasispherical shape, with diameters in the same range as those measured by laser light scattering ().
Stability of LDE-ddMTX
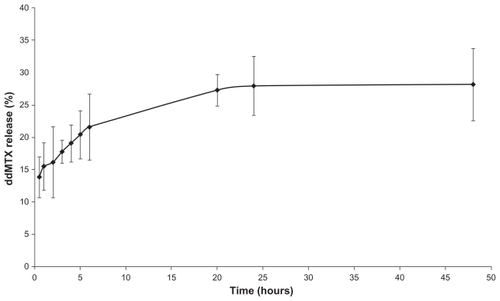
During the process of association of the drug with the lipid nanoemulsion, which comprised an ultrasonication step, there was no structural modification of the drug, a finding that was subsequently confirmed after 45 days of storage at 4°C. On the other hand, the average size of LDE-ddMTX increased from 60 nm to 70 nm. The rate of dissociation of ddMTX from the lipid nanoemulsion was 10% ± 5%, as observed at 4°C storage for 45 days. The ddMTX that dissociated from the lipid nanoemulsion was macroscopically observed by the appearance of a yellow powder of ddMTX at the bottom of the flask. The stability of the LDE-ddMTX association was analyzed over 48 hours of dialysis against human plasma. Free ddMTX was released from the dialysis bag at a slow rate of 28%, and most of the dissociation occurred in the first 24 hours of the dialysis period ().
In vitro studies
The cytotoxicity of LDE-ddMTX was markedly higher than that of methotrexate. For K562 cells, the cytotoxicity of the new formulation was approximately 10-fold higher and for HL60 was 100-fold higher than for methotrexate (, see ). Incubation of HL60 and K562 with the lipid nanoemulsion alone did not inhibit cell growth. It is worth-while to point out that the cytotoxicity of free unassociated ddMTX was not tested because of its high lipophilicity that does not favor appropriate conditions for incubation with cells. For this purpose, it would be necessary to use organic solvents that would be toxic to the cells.
Table 1 IC50 values of methotrexate, ranging from 2.25 to 22.5 mM and of LDE-dMTX, ranging from 0.1 to 2 mM in K562 and HL60 cell lines. Cells were incubated for 48 hours at 37°C
Figure 4 Cytotoxicity of (A) LDE (o), LDE-ddMTX (●), and methotrexate (■) in (B) 562 (I) and HL60 (II) cell lines. The cells were incubated with the formulations (final concentration range 0.1–2.0 mM) for 4 hours at 37°C, and survival was quantified by enzyme-linked immunosorbent assay. Results are presented the mean ± standard error of the mean of three experiments.
Abbreviations: ddMTX, didodecyl methotrexate; LDE, lipid nanoemulsion.
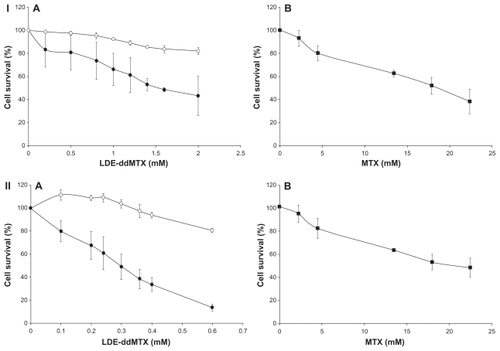
The IC50 for K562 cells incubated with LDE-ddMTX was 1.6 mM, whereas the IC50 for cells incubated with methotrexate was 18.2 mM (P < 0.0001). For the HL60 cells, the IC50 was 0.2 mM for incubation with LDE-ddMTX and 26 mM for incubation with methotrexate (P = 0.0002).
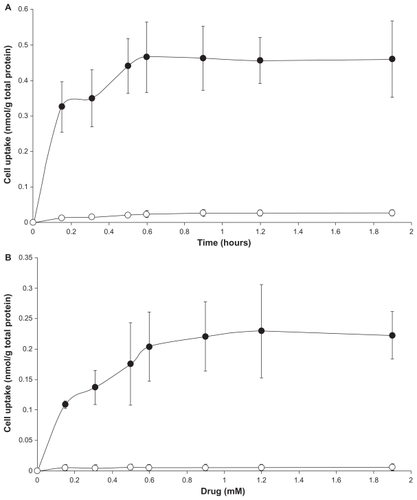
shows the uptake of LDE-ddMTX and methotrexate by K562 and HL60 cells 4 hours after incubation with increasing amounts of both preparations. The uptake amount refers to the nonmetabolized methotrexate and ddMTX found within the cells. It is notable that the uptake of LDE-ddMTX by K562 cells was 90-fold higher than that of methotrexate (P < 0.0001) while the uptake by HL60 was 75-fold higher (P < 0.0001).
Figure 5 Uptake of LDE-ddMTX (●) and methotrexate (○) 4 hours by (A) K562 and (B) HL60 cell lines. Cells were incubated with the formulations (final concentration range 0.15–2.0 mM) for 4 hours at 37°C, lysed, and the drugs were then quantified by high-performance liquid chromatography. Results are presented the mean ± standard error of the mean of three experiments.
Abbreviations: ddMTX, didodecyl methotrexate; LDE, lipid nanoemulsion.
In vivo toxicity
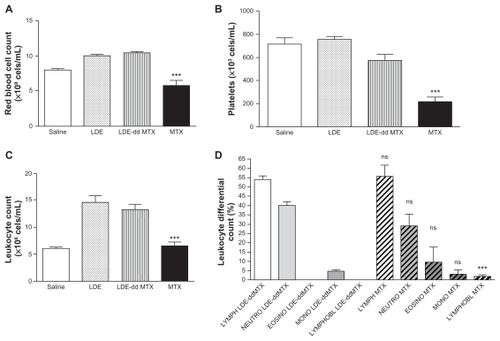
The toxicity of both LDE-ddMTX and methotrexate to mice was tested in two escalating dose schedules, namely single-dose and multiple-dose schedules. Using the single-dose schedule, the tolerability of methotrexate was greater than that of LDE-ddMTX, as evaluated by smaller weight loss and greater survival, despite the hematological toxicity of LDE-ddMTX being lesser than that elicited by methotrexate (). In the multiple-dose scheme, with both LDE-ddMTX and methotrexate treatments, all the animals died by the end of 7 days of observation. As shown in , both the MTD and the LD50 were higher for methotrexate than for LDE-ddMTX.
Table 2 Maximum tolerated dose and 50% lethal dose values of methotrexate and LDE-ddMTX intravenously administered as a single dose in Balb/c mice
Figure 6 (A) Red cell count, (B) platelet count, (C) leukocyte count, and (D) leukocyte differential count in mice after 24 hours of intravenous LDE-ddMTX or methotrexate (both at 180 mg/kg dose) administration and after intravenous LDE without methotrexate.
Notes: ns indicates p > 0.05; *** indicates p < 0.001.
Abbreviations: ddMTX, didodecyl methotrexate; LDE, lipid nanoemulsion; LYMPH, lymphocytes; NEUTRO, neutrophyls; EOSINO, eosinophyls; MONO, monocytes; LYMPHOBL, lymphoblast.
Discussion
The major achievement in this study was the manufacture of a formulation for methotrexate endowed with an ability to concentrate the drug into the cell at much greater rates than those of commercial methotrexate.
The excellent approximately 100% yield of the esterification reaction and the excellent approximately 100% association of ddMTX with the lipid nanoemulsion support the assumption that conditions for the manufacture of the LDE-ddMTX were near optimal. Esterification is a powerful strategy used in medicinal chemistry to improve drug features, such as passive transport, lipophilicity, and reduced toxicity. Esterification of both the α- and γ-carbonyl groups used here was a practical alternative to regioselective esterification of only the γ-carbonyl group, which is often a low-yield reaction. The choice of dodecyl group to attach to methotrexate was based on a study by Rosowsky et alCitation30 reporting that esters containing 10–12 carbon chains are more suited to maintain drug activity than long-chain methotrexate esters. The latter are less prone to bind to dihydrofolate reductase, which leads to decreased cytotoxicity. The higher distribution coefficient (n-octanol/water) showed by ddMTX compared with methotrexate, ie, 3.9 and −1.4, respectively, was key to achieving the high entrapment efficiency obtained here.
Thus, both the esterification reaction and the procedure for associating the drug with the lipid nanoemulsion involve methods that are easy to perform, do not require expensive reagents, and can be easily adapted to large-scale production conditions. Ultimately, the particles of average size 60 nm resemble previously obtained preparations of lipid nanoemulsion-loaded paclitaxel, carmustine, or etoposide derivatives. These previously described formulations showed the ability to produce a several-fold reduction in the toxicity of the associated chemotherapeutic agents, whereas the pharmacological action was preserved or even improved.Citation21–Citation23
A precondition for drug targeting is the stability of the drug-carrier in the circulation until final uptake by the targeted tissue. In the dialysis experiments, the fact that only a small fraction of the ddMTX associated with the lipid nanoemulsion was released from the bags over 48 hours supports the stability of the complex. A potential limitation of this experimental setting is that the interactions between plasma proteins and lipoproteins are more restricted, because LDE-ddMTX particles are in a solution inside the dialysis bag whereas the plasma proteins are outside. Passage of proteins through the membrane is difficult due to their size, so interactions occur mainly at the membrane pores. ddMTX release from the lipid nanoemulsion could also be evaluated by mixing LDE-ddMTX with plasma inside the dialysis bag, but the drug eventually released from the lipid nanoemulsion could bind to plasma proteins or lipoproteins and remain in the bag, which would prevent dissociation from the lipid nanoemulsion.
LDE-ddMTX was mostly stable for at least 45 days. This was indicated by both the constant particle size and preservation of the ddMTX chemical structure during the observation period. As already mentioned in respect to drug entrapment by the lipid nanoemulsion, the increase in the distribution coefficient also contributes to the stability of the complex. Coalescence is critical for the stability of emulsions and other particulate systems.Citation33 The lipid nanoemulsion is prepared with phosphatidylcholine, a positively charged phospholipid that produces more stable systems than negatively charged phospholipids.Citation34
The uptake of methotrexate by cells occurs via folate receptors. Methotrexate is a substrate for polyglutamylation performed by folypolyglutamyl synthase that enhances cell retention of the drug. The enzyme activity varies among tumor cell lines, and it has been suggested that defective polyglutamation may partially account for tumor resistance to methotrexate.Citation1 Therefore, it is remarkable that the cellular uptake of LDE-ddMTX was 75-fold higher than of methotrexate in HL60 cells and 90-fold higher in K562 cells. This finding can be ascribed to either the receptor-mediated endocytic pathway being furnished by the lipid nanoemulsion drug-carrier or to the lipophilic features of ddMTX. The biological activity of lipophilic derivatives of methotrexate can be either the result of a direct effect on cell enzymes, or being mediated by intracellular release of free drug, that will ultimately be responsible for the activity.Citation35
The 75-fold greater uptake of LDE-ddMTX than methotrexate by HL60 cells was matched by 100-fold greater cytotoxicity, whereas the 90-fold greater uptake by K562 cells was linked with 10-fold greater cytotoxicity to these cells. Our results suggest that uptake of methotrexate is an important limitation for cytotoxicity, and when cell internalization of the drug increases, the pharmacological action of the compound can be optimized. However, it is noteworthy that the greatest uptake did not correspond to the greatest cytotoxicity, because the cytotoxicity of K562 was not higher than for HL60. The mechanism of action of methotrexate involves inhibition of tetrahydrofolate synthesis through its bonding to dihydrofolate reductase, interfering with DNA synthesis and repair. The fact that there was no cytotoxicity when the lipid nanoemulsion was incubated with the two cell lines without methotrexate and demonstrates that this vehicle has no toxicity.
In the animal toxicity experiments, the marked differences created by the association of ddMTX and the lipid nanoemulsion were reflected in the fact that escalating doses resulted in lower survival rates for LDE-ddMTX compared with methotrexate at all dose levels. It is possible that the increased toxicity of LDE-ddMTX to animals was consequent to the marked increase in cell uptake and in vitro cytotoxicity. It is interesting that the hematological toxicity was lower for LDE-ddMTX, which suggests an as yet undetermined myeloprotective mechanism conferred by the lipid nanoemulsion.
The 10–90-fold greater cytotoxicity of LDE-ddMTX, which resulted in a four-fold increase in LD50, as estimated by animal survival, and a two-fold increase in the maximum tolerated dose, suggests that LDE-ddMTX can be used in smaller doses, and would still have superior pharmacological activity with low toxicity.
As a limitation of this study, it can be mentioned that a comparison between methotrexate and ddMTX both associated with the lipid nanoemulsion could not be made because methotrexate associates poorly with this nanoemulsion system.
The lipid nanoemulsion also has the ability to concentrate in atherosclerotic lesions, as shown in cholesterol-fed rabbits.Citation36 In a subsequent as yet unpublished study, we observed that treatment of atherosclerotic rabbits with LDE-ddMTX resulted in marked regression of the lesions. This finding widens the potential therapeutic applications of LDE-ddMTX.
Conclusion
In conclusion, comparing LDE-ddMTX with other methotrexate delivery systems described in the literature, such as gelatin/methotrexate conjugates,Citation37 polymers,Citation7,Citation8 liposomes,Citation4,Citation5 dendrimers,Citation6 solid lipid nanoparticles,Citation11,Citation12 and chylomicronmimicking carrier emulsions,Citation10 this novel formulation for intravenous use, in which the association of ddMTX with the vehicle is virtually complete and stable, showed equivalent or superior cytotoxicity. These findings warrant further preclinical investigation.
Acknowledgments
This study was supported by a State of Sao Paulo Research Foundation grant. Professor Maranhão has a research award from the National Council for Scientific and Technological Development. The authors are indebted to Sylvia M Carneiro for her help with the transmission electron microscopy experiments.
Disclosure
The authors report no conflicts of interest in this work.
References
- EstlinEJContinuing therapy for childhood acute lymphoblastic leukaemia: clinical and cellular pharmacology of methotrexate, 6-mercaptopurine and 6-thioguanineCancer Treat Rev20012735136311908928
- HuennekensFMThe methotrexate story: a paradigm for development of cancer chemotherapeutic agentsAdv Enzyme Regul1994343974197942284
- ChabnerBARyanDPPaz-AresLGarcia-CarboneroRCalabresiPAntineoplastic agentsHardmanJGLimbirdLEGilmanAGoodman and Gilman’s The Pharmacological Basis of Therapeutics10th edNew York, NYMcGraw-Hill2001
- KaasgaardTAdresenTLJensenSSHolteHOJensenLTJorgensenKLiposomes containing alkylated methotrexate analogues for phospholipase A2 mediated tumor targeted drug deliveryChem Phys Lipids20091579410319094974
- DoddoliCChezOBarlésiFIn vitro and in vivo methotrexate disposition in alveolar macrophages: Comparison of pharmacokinetic parameters of two formulationsInt J Pharm200529718018915869851
- GurdagSKhandareJStapelsSMetherlyLHKannanRMActivity of dendrimer-methotrexate conjugates on methotrexate-sensitive and -resistant cell linesBioconjug Chem20061727528316536456
- LiYKwonGSMicelle-like structures of poly(ethylene oxide)-block-poly(2-hydroxyethyl aspartamide)-methotrexate conjugatesColloids and Surfaces B: Biointerfaces199916217226
- SeoDHJeongYIKimDGJangMJJangMKNahJWMethotrexate-incorporated polymeric nanoparticles of methoxypoly(ethyleneglycol)- grafted chitosanColloids Surf B Biointerfaces20096915716319135342
- BaiKBLángOOrbánEDesign, synthesis, and in vitro activity of novel drug delivery systems containing tuftsin derivatives and methotrexateBioconjug Chem2008192260226918959436
- PaliwalRPaliwalSRMishraNMehtaAVyasSPEngineered chylomicron mimicking carrier emulsion for lymph targeted oral delivery of methotrexateInt J Pharm200938018118819576973
- PaliwalRRaiSVaidyaBEffect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic deliveryNanomedicine2009518419119095502
- RuckmaniKSivakumarMGaneshkumarPAMethotrexate loaded solid lipid nanoparticles (SLN) for effective treatment of carcinomaJ Nanosci Nanotechnol200662991299517048509
- HoYKSmithRGBrownMSGoldsteinJLLow-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cellsBlood19785210991114214187
- MasquelierMVitolsSPetersonCLow-density lipoprotein as a carrier of antitumoral drugs: in vivo of drug-human low-density lipoproteins complexes in miceCancer Res198646384238473731059
- MasquelierMVitolsSPalssonMMarsULarssonBSPetersonCOLow density lipoprotein as a carrier of cytostatics in cancer chemotherapy: study of stability of drug-carrier complexes in bloodJ Drug Target2000815516410938525
- LundbergBPreparation of drug-low density lipoprotein complexes for delivery of antitumoral drugs via the low density lipoprotein pathwayCancer Res198747410541083607752
- VitolsSAngelinBEricssonSGUptake of low density lipoproteins by human leukemic cells in vivo: relation to plasma lipoprotein levels and possible relevance for selective chemotherapyProc Natl Acad Sci U S A199087259826022320578
- MaranhãoRCGaricocheaBSilvaELLlacerPDPileggiFJCChamoneDAFIncreased plasma removal of microemulsions resembling the lipid phase of low-density lipoproteins (LDL) in patients with acute myeloid leukemia: a possible new strategy for the treatment of the diseaseBraz J Med Biol Res199225100310071342820
- MaranhãoRCGaricocheaBSilvaELPlasma kinetics and biodistribution of a lipid emulsion resembling low-density lipoprotein in patients with acute leukemiaCancer Res199454466046668062260
- MaranhãoRCCésarTBPedroso-MarianiSRHirataMHMesquitaCHMetabolic behavior in rats of a non-protein microemulsion resembling low density lipoproteinLipids1993286916968377582
- HungriaVTMLatrilhaMCRodriguesDGBydlowskySPChiattoneCSMaranhaoRCMetabolism of a cholesterol-rich microemulsion (LDE) in patients with multiple myeloma and preliminary clinical study of LDE as drug vehicle for the treatment of the diseaseCancer Chemother Pharmacol200453516014574458
- RodriguesDGMariaDAFernandesDCImprovement of paclitaxel therapeutic index by derivatization and association to a cholesterol-rich microemulsion: in vitro and in vivo studiesCancer Chemother Pharmacol20055556557615726368
- ValdugaCJFernandesDCLo PreteACAzevedoCHMRodriguesDGMaranhaoRCUse of a cholesterol-rich microemulsion that binds to low-density lipoprotein receptors as vehicle for etoposideJ Pharm Pharmacol2003551615162214738586
- Lo PreteACMariaDARodriguesDGValdugaCJIbanezOCMaranhãoRCEvaluation in melanoma-bearing mice of an etoposide derivative associated to a cholesterol-rich nano-emulsionJ Pharm Pharmacol20065880180816734981
- AdesACarvalhoJPGrazianiSRUptake of a cholesterol-rich emulsion by neoplastic ovarian tissuesGynecol Oncol200181848711426966
- GrazianiSRIgrejaFAFHeggRUptake of a cholesterol-rich emulsion by breast cancerGynecol Oncol20028549349712051880
- AzevedoCHCarvalhoJPValdugaCJMaranhãoRCPlasma kinetics and uptake by the tumor of a cholesterol-rich microemulsion (LDE) associated to etoposide oleate in patients with ovarian carcinomaGynecol Oncol20059717818215790455
- DiasMLCarvalhoJPRodriguesDGGrazianiSRMaranhãoRCPharmacokinetics and tumor uptake of a derivatized form of paclitaxel associated to a cholesterol-rich nanoemulsion (LDE) in patients with gynecologic cancersCancer Chemother Pharmacol20075910511116699792
- PinheiroKVHungriaVTFickerESValdugaCJMesquitaCHMaranhaoRCPlasma kinetics of a cholesterol-rich microemulsion (LDE) in patients with Hodgkin’s lymphoma and a preliminary study on the toxicity of etoposide associated with LDECancer Chemother Pharmacol20065762463016133527
- RosowskyAForschRAYuCSLazarusHBeardsleyGPMethotrexate analogues. 21. Divergent influence of alkyl chain on the dihydrofolate reductase affinity and cytotoxicity of methotrexate monoestersJ Med Chem1984276056096585550
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assayJ Immunol Methods19836555636606682
- LowryOHRosebroughNJFarrALRandallRProtein measurement with the Folin phenol reagentJ Biol Chem195119326527514907713
- SaitoHKawagishiATanakaMCoalescence of lipid emulsions in floating and freeze-thawing processes: examination of the coalescence transition state theoryJ Colloid Interface Sci199921912913410527578
- KabalnovATararaTArlauskasRWerrsJPhospholipids as emulsion stabilizers. 2. Phase behavior versus emulsion stabilityJ Colloid Interface Sci19961842272358954658
- PignatelloRSpampinatoGSorrentiVLipophilic methotrexate conjugates with antitumor activityEur J Pharm Sci20001023724510767601
- MaranhãoRCTavaresERPadovezeAFValdugaCJRodriguesDGPereiraMDPaclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbitAtherosclerosis200819795996618289548
- OfnerCM3rdPicaKBowmanBJChenCSGrowth inhibition, drug load, and degradation studies of gelatin/methotrexate conjugatesInt J Pharm2006308909916361072