Abstract
Background
Active, ligand-mediated, targeting of functionalized liposomes to folate receptors (FRs) overexpressed on cancer cells could potentially improve drug delivery and specificity. Studies on folate-targeting liposomes (FTLs) have, however, yielded varying results and generally fail to display a clear benefit of FR targeting.
Method
Tumor accumulating potential of FTLs and NTLs were investigated in a FR overex-pressing xenograft model by positron emission tomography/computed tomography imaging.
Results
Tumors displayed significantly lower activity of FTLs than NTLs. Furthermore, FTLs displayed worse circulating properties and increased liver-accumulation than NTLs.
Conclusion
This study underlines that long-circulating properties of liposomes must be achieved to take advantage of EPR-dependent tumor accumulation which may be lost by functionalization. FR-functionalization negatively affected both tumor accumulation and circulation properties.
Background
Since the first description of the enhanced penetration and retention (EPR) effect in solid cancers, nano-sized drug delivery systems have been the subject for extensive research following their passive accumulation in malignant tissue.Citation1 Liposome-based drug delivery systems is the most successful EPR-dependent nano-sized drug delivery systems due to highly flexible and versatile nature of liposomes.Citation2 Despite their ability to achieve high level of fat accumulation in the cancerous tissue, liposomal drug delivery systems relying on passive uncontrolled drug release mechanisms have not revolutionized cancer therapy. Based on this, functionalized liposomes, which combine the passive EPR accumulation with selective uptake in cancer cells displaying a specific cell receptor, have been subject for intense research. Several studies and reviews conclude and state that actively targeting liposomes can significantly increase the amount of drug delivered to the target cell in comparison to the free-drug and passively targeting liposomal drug delivery systems.Citation3 Even though these statements have been published extensively, very limited in vivo data support them. In vitro data provide extensive evidence of receptor targeting liposomes being taken up actively by cancer cells expressing the specific surface receptor.Citation4–Citation6 Notwithstanding these observations, we speculate that the tumor microenvironment creates an extensive barrier toward the trafficking and distribution of nano-sized molecules. This barrier may limit the access of active targeting liposomes to a very limited number of cancer cells in solid tumors and therefore reduce any therapeutic benefit relative to passively targeting liposome. The microregional intratumoral distribution of nano-sized liposomes has been illustrated to primarily be in the extracellular compartment adjacent to functional tumor blood vessels, which indicate that the proportion of cancer cells in direct contact with liposomes may be limited.Citation7 This observation is further complicated by the existence of disorganized tumor vasculature and intervascular distance and highly therapy-resistant hypoxic tumor regions.Citation8 Active targeting of liposome formulations may additionally make them more prone to recognition by the reticuloendothelial system (RES) thereby altering the circulating properties and reducing liposome availability for tumor accumulation.Citation3,Citation9 Despite the theoretical potential of active targeting liposomes the described obstacles may directly hinder the translation of in vitro efficacy to in vivo tumor models.
In the present study, we investigated the biodistribution and tumor-targeting potential of folate-targeting liposomes (FTLs). This was achieved using a highly flexible radiolabeled liposome platform, which was applied for somatostatin receptor targeting liposomes in human neuroendocrine cancer xenografts.Citation10 Folate receptors (FRs) are highly expressed on a wide spectrum of cancers, including ovarian, lung, brain, head and neck, renal cell, and breast cancers and display relatively low expression on nontumorous tissues with the main exception being inflamed tissue.Citation11,Citation12 Importantly, FRs mediate endocytosis to nonlysosomal endosomal vesicles and therefore provides an attractive therapeutic target for intracellular liposomal drug delivery.Citation13 The use of radiolabeled nontargeting liposomes (NTLs), radiolabeled FTLs, and functional positron emission tomography (PET) imaging combined with computed tomography (CT) allows for quantification of biodistribution and tumor accumulation levels of both formulations.
Previous studies evaluating the therapeutic potential of FTL drug delivery systems are limited and results are conflicting in regards to therapeutic advantage for intravenously administered formulations.Citation4,Citation6,Citation14–Citation16 In comparison, intraperitoneal administration to ascites cancer models have improved the therapeutic efficacy of FTLs in comparison to NTLs. Thus, FTLs have potential, but this may require very directly accessible cancer cells.Citation4,Citation17 FTLs has been shown to be selectively taken up in the liver via receptor-mediated endocytosis in hepatic macrophages and display increased blood clearance relative to NTLs.Citation9 Admistrating folic acid in mice intravenously or intraperitonealy has been shown to reduce hepatic uptake and increase circulating properties of liposomes, folate-targeting imaging agents, and folate-targeting liposomes.Citation9,Citation11
The current study was conducted in two stages; first, the biodistribution of copper-64 radiolabeled FTLs (64Cu-FTLs) was compared to copper-64 radiolabeled NTLs (64Cu-NTLs) in mice bearing subcutaneous folate expressing KB-cell xenografts, and second, the effect of intravenous excess folic acid or vehicle on tumor uptake and biodistribution was compared for radiolabeled FTLs and NTLs.
Methods
Preparation of 64Cu-radiolabeled FTLs and NTLs
Preparation of nontargeted liposomes
NTLs entrapping the high-affinity copper chelator, DOTA, were prepared. Briefly, 50 mg/mL freeze-dried lipid powder (HSPC:Chol:DSPE-PEG2k 56.5:38.2:5.3, Lipoid) was dispersed in a buffer containing 10 mM DOTA, 10 mM HEPES, and 150 mM NaCl (pH 7.4). The lipid suspension was hydrated for 60 minutes at 65°C and subsequently sized to 100 nm using a Lipex thermo barrel extruder (Northern lipids). The nonencapsulated DOTA was removed by tangential flow filtration (Minimate™, Pall Corporation).
Preparation of FTLs
FTLs were prepared by postinsertion of 0.5 mol% DSPE-PEG5k-folate into NTLs containing DOTA. Briefly, 1.7 mL NTLs (9.4 mM lipid) was added to 0.5 mg freeze-dried DSPE-PEG5k-Folate powder, incubated for 20 minutes at 45°C with gentle shaking, then cooled on an ice bath, and checked for precipitates. The folate concentration was determined by UV-vis. Samples were diluted 1:9 in 10% SDS solution and incubated for 30 minutes at 60°C. Absorbance was measured at 282 nm and the folate concentration was determined via the molar extinction coefficient of DSPE-PEG5k-Folate 27,500 M−1cm−1 using the absorbance of NTLs as a reference. The liposome size and zeta potential were verified by DLS (ZetaPALS Brookhaven) and the lipid concentration was determined by ICP-MS (iCAP Q, Thermo Scientific). NTLs were produced having an average size of 110 nm (polydispersity index=0.07) and a zeta potential of −5±1 mV. No change in size or zeta potential was determined for the FTLs upon post insertion of DSPE-PEG5k-Folate.
Remote loading of 64CuCl2 into NTLs and FTLs
A total of 3.3 mM liposomes were added to a vial containing dried 64CuCl2. The liposome sample was incubated for 75 minutes at 65°C using constant stirring and then cooled to room temperature. The 64Cu loading efficiency was determined by radio-HPLC and radio-thin-layer chromatography.Citation18 The activity concentration was set to 60–75 MBq/mL corresponding to 12–15 MBq/animal at the time of injection.
Animal tumor model
KB cells (human nasopharyngeal carcinoma cell line, CCL-17; known for overexpressing FRsCitation19,Citation20 were obtained from the American Type Culture Collection (ATCC, VA, USA). Cells were grown in monolayers in FFRPMI culture medium (modified RPMI without folic acid, vitamin B12, and phenol red) in a humidified atmosphere at 37°C containing 5% CO2. The media was supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyrovate, nonessential amino acids, 100 U/mL penicillin, and 100 µg/mL streptomycin.
Six-week-old female, athymic nude mice (NMRI nu/nu, Taconic Europe, Borup, Denmark) were allowed to acclimatize for 1 week before subcutaneous inoculation on both flanks with KB cancer cells (1.5×106 cells in 100 µL serum-free media and Matrigel).
Experimental setup
Tumor xenografts were allowed to grow for 10 (study part 1) and 14 days (study part 2) prior to imaging. In study part 1, animals were randomized into two groups to receive either; 64Cu-FTLs (n=7) or 64Cu-NTLs (n=7). In study part 2, animals were randomized into four groups: group 1, 64Cu-FTLs preinjected with folic acid (n=8); group 2, 64Cu-FTLs pre-injected with vehicle (n=8); group 3, 64Cu-NTLs preloaded pre-injected with folic acid (n=5); and group 4, 64Cu-NTLs preinjected with vehicle (n=7). All experimental procedures were approved by and conducted under the guidelines of The Danish Animal Experiments Inspectorate.
Imaging procedures
All animals were anesthetized with a sevoflurane gas mixture and anesthetized mice had a tail vein catheter position to ensure strict intravenous injection of all formulations. All mice received 3.3 mM of lipid. Two anesthetized mice were positioned side-by-side, separated by a 6 mm polystyrene block, on a heated platform for PET/CT imaging. PET/CT imaging was performed on a dedicated Inveon® small animal PET/CT system with CT-based PET image attenuation (Siemens Medical Systems, Malvern, PA, USA).
In study part 1, 64Cu-labelled FTLs (64Cu-FTLs) and 64Cu-labelled NTLs (64Cu-NTL) were administered as a bolus injection performed simultaneously with the start of a 10-minute dynamic PET acquisition. Three PET/CT sessions were conducted; a 10-minute dynamic acquisition commencing simultaneously with the bolus injection of liposomes, 5-minute static PET scan 3 hours after injection, and a 15-minute static PET scan 24 hours after injection. Following all PET scans, the imaging platform was moved automatically into the CT-scanner for anatomical imaging. The 10-minute dynamic PET scan was reconstructed into 10×3 seconds, 6×5 seconds, 4×30 seconds, and 7×1 minutes frames. PET scans were reconstructed using a maximum a posteriori (MAP) reconstruction algorithm (pixel size: 0.815×0.815×0.796 mm) and attenuation correction was performed based on the corresponding CT scan.
For study part 2, mice were injected with 100 µg of folic acid (Sigma-Aldrich, St Louis, MO, USA) suspended in 100 µL of isotonic bicarbonate solution or 100 µL of isotonic bicarbonate solution (vehicle) 2 minutes prior to infusing 64Cu-FTLs or 64Cu-NTLs. Animals were allowed to cage rest until performing PET/CT scans. Scans were performed using the same PET/CT scanner and set up as study part 1.
Dynamic PET imaging was performed on two mice from groups 1, 2, and 4 using a MicroPET® Focus 120 (Siemens Medical Systems, Malvern, PA, USA). Mice were subsequently CT imaged using a MicroCAT-II system (Siemens Medical Systems, Malvern, PA, USA). The PET scan commenced simultaneously with the injection of 64Cu-labelled liposomes and dynamic images were collected for 2 hours. Only the static 24-hour PET/CT scans were performed for these mice. The 2-hour dynamic PET scans were reconstructed into 10×3 seconds, 6×5 seconds, 4×30 seconds, 7×1 minutes, and 22×5 minutes frames using a similar MAP reconstruction.
Image analysis
Image analysis was performed using commercially available Inveon software (Siemens Medical Systems, Malvern, PA, USA). Regions of interests (ROIs) were manually constructed based on the coregistered PET/CT images. The following ROIs were constructed: tumors (complete volume delineated), liver (multiple slices taking care to avoid the hilar region), spleen, muscle, and blood. Blood activity was estimated from a constructed volume of interest ROI covering the left ventricular lumen of the heart, except for the dynamically scanned mice from study part 2, where an ROI was placed in the abdominal aorta. ROIs in the left ventricle and abdominal aorta were subsequently segmented to only include the voxels displaying above 80% of maximum activity with the original ROI. Time-activity curves (TACs) were constructed based on the dynamic images.
Statistical analysis
Statistical analysis was performed using Prism 5.0 software (GraphPad Software Inc., La Jolla, San Diego, CA, USA).
Comparison between activity of 64Cu-FTLs and 64Cu-NTLs in study part one was performed using unpaired t-test. For intergroup comparison between liposome activities in study part 2, one-way ANOVA with Tukey post hoc multiple comparison analysis was used. Data were tested to follow a normal distribution by Komolgorov-Smirnov test. Results reported mean±SEM (unless otherwise stated) and P<0.05 were considered statistically significant.
Results
Tumor uptake of FR-targeted and nontargeted radiolabeled liposomes
Tumor volume was compatible between groups displaying a mean size of 78.4±7.4 mm3. The PET 64Cu activity levels were determined as % injected dose per gram of tissue (%ID/g) for the PET scans performed 3 hours and 24 hours postinjection (pi.). No tumor uptake was reported for the initial 10-minute dynamic PET scan. There was no statistical difference between mean and maximum activity between 64Cu-FTLs (2.6%±0.3%ID/g and 5.6%±0.5%ID/g) and 64Cu-NTLs (2.5%±0.2%ID/g and 5.7%±0.4%ID/g) at 3 hours pi. (unpaired t-test) (). However, 64Cu-FTLs displayed a statistically significant lower tumor-to-liver ratio compared with 64Cu-NTLs (P=0.0052). No difference between mean tumor-to-blood or tumor-to-spleen ratios was observed between groups ().
Figure 1 Accumulation of FTL and NTL in tumors.
Notes: (A) PET tumor activity of 64Cu-FTLs and 64Cu-NTLs 3 hours pi and 24 hours pi in tumors. Asterisks indicate statistically different means between mean and maximum activity of 64Cu-FTLs and 64Cu-NTLs (*P=0.025, **P=0.011, unpaired Student’s t-test). (B) Tumor (T) to blood (B), liver (L), and spleen (S) activity ratios of 64Cu-FTLs and 64Cu-NTLs 3 hours pi. and 24 hours pi. (*P=0.0052, **P=0.005, ***P=0.001, unpaired Student’s t-test, bars represent mean %ID/g±SEM).
Abbreviations: NTL, nontargeting liposomes; FTL, folate-targeting liposomes; SEM, standard error of mean; pi., postinjection.
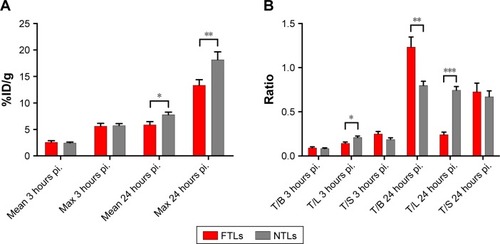
At the PET scan 24 hours pi., 64Cu-NTLs displayed a statistically significant higher mean (7.8%±0.5%ID/g) and maximum (18.2%±1.5%ID/g) tumor activity in comparison to the mean (5.9%±0.6%ID/g) and maximum (13.4%±1.0%ID/g) tumor activity of 64Cu-FTLs (P=0.025 and P=0.011) (). Mean tumor-to-blood ratio was statistically higher and tumor-to-liver ratio statistically lower for the 64Cu-NTLs compared to 64Cu-FTLs (P=0.005 and P=0.001), whereas tumor-to-spleen ratio was compatible between groups (). This reflects the increased liver uptake and the decreased circulating half-life of 64Cu-FTLs.
Biodistribution and uptake kinetics of FTLs and NTLs
Mean liver activity of 64Cu-FTLs was statistically higher than 64Cu-NTLs 3 hours pi. (18.2%±0.5%ID/g and 11.5%±0.7%ID/g, respectively, P<0.0001) and at 24 hours pi. (24.4%±1.2%ID/g and 10.3±0.7, respectively, P<0.0001). No difference was observed for mean activity in the blood between 64Cu-FTLs and 64Cu-NTLs 3 hours pi. (28.7%±1.3%ID/g and 29.6%±1.7%ID/g, respectively). However, 24 hours pi. activity of 64Cu-FTLs was significantly lower than 64Cu-NTLs (4.9%±0.3%ID/g and 9.1%±1.0%ID/g, P=0.001). On the contrary, splenic uptake of 64Cu-FTLs was significantly lower 24 hours pi. relative to 64Cu-NTLs (8.5%±0.6%ID/g and 12.3%±1.5%ID/g, respectively, P=0.032), whereas no significant difference was observed for mean activity 3 hours pi. (10.4%±0.4%ID/g and 13.2%±1.5%ID/g, respectively). However, the potential influence of blood volume in the spleen and the difference observed in blood activity 24 hours pi., must be taken into consideration and differences can probably not solely be attributed to splenic uptake. No difference was observed for uptake levels in muscle 3 in 24 hours pi. ( and ).
Figure 2 Biodistribution of FTLs and NTLs.
Notes: (A) Biodistribution of 64Cu-FTLs and 64Cu-NTLs 3 hours pi. and 24 hours pi. Asterisks indicate statistically different means between the mean and maximum activity of 64Cu-FTLs and 64Cu-NTLs (bars represent mean %ID/g±SEM). (B–D) TAC of blood (B), liver (C), and spleen. (D) activity of 64Cu-FTLs and 64Cu-NTLs during a 10-minute dynamic PET scan immediately after injection and static 3 hours and 24 hours pi. scans (mean±SEM). (E) TAC of spleen-to-blood ratios for 64Cu-FTLs and 64Cu-NTLs during the initial 10-minute dynamic PET scan and static 3 hours and 24 hours pi. scans (mean±SEM, *P=0.032, **P=0.001, ***P<0.00001, unpaired Student’s t-test).
Abbreviations: NTL, nontargeting liposomes; FTL, folate-targeting liposomes; TAC, time-activity curves; PET, positron emission tomography; SEM, standard error of mean.
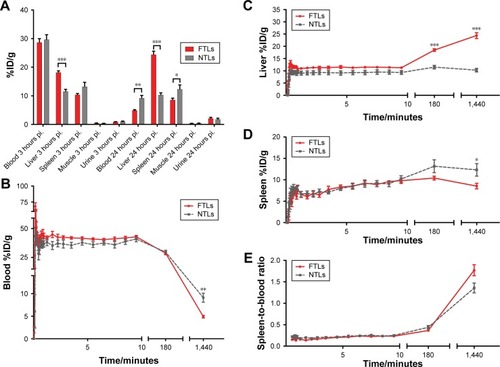
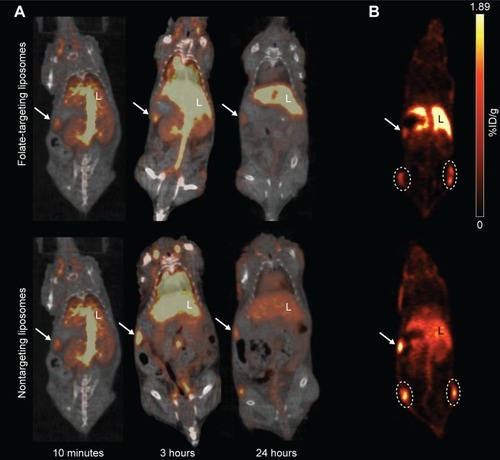
Figure 3 PET/CT of FTLs and NTLs in tumor-bearing mice.
Notes: (A) 64Cu-liposome PET/CT images of 64Cu-FTLs 10 minutes, 3 hours, and 24 hours pi. (top images) and 64Cu-NTLs 10 minutes, 3 hours, and 24 hours pi. (bottom images). Liver (L), spleen (arrow). (B) 64Cu-liposome PET images 24 hours pi. of tumor uptake of 64Cu-FTLs (top image) and 64Cu-NTLs (bottom image). Left and right flank tumors are encircled in white, liver (L), spleen (arrow).
Abbreviations: FTL, folate-targeting liposomes; NTL, nontargeting liposomes; PET/CT, positron emission tomography/computed tomography.
Dynamic uptake of the liposomal formulation was determined from the initial dynamic PET scan and scans 3 and 24 hours pi. (). TACs illustrate the increased blood clearance and hepatic accumulation of 64Cu-FTLs relative to 64Cu-NTLs (). Interestingly, splenic TAC illustrates the higher activity of 64Cu-NTLs rather 64Cu-FTLs 24 hours pi. (). However, constructing a TAC of the spleen-to-blood ratio reverses this observation, which could indicate that the observed difference could be attributed to the higher activity of 64Cu-NTLs in the blood ().
Tumor uptake of FTLs after folic acid coadministration
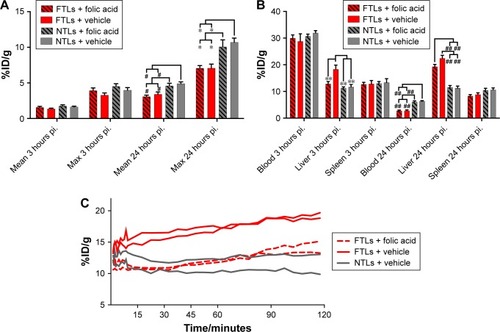
Tumor volume was compatible between the four groups of mice in the study part 2 (119.6±12.9 mm3), although slightly larger than the mean tumor volume of the tumors in study part 1 following a longer growth period. No difference in mean tumor activity between the group receiving excess folic acid or vehicle and 64Cu-FTLs or 64Cu-NTLs was observed 3 hours pi. (). However, 24 hours pi., tumors in both 64Cu-NTLs groups display a higher mean and maximum activity of radiolabeled liposomes relative to groups receiving 64Cu-FTLs (P<0.0001). Treatment with folic acid or vehicle mediated no change in tumor uptake between the 64Cu-FTLs groups or between the folic acid and vehicle 64Cu-NTLs mice ().
Figure 4 Biodistribution of FTLs and NTLs in competition with folic acid.
Notes: (A) PET tumor activity of 64Cu-FTLs and 64Cu-NTLs with folic acid or vehicle coadministration 3 hours pi. and 24 hours pi. Tumor mean and maximum activity was statistically higher for 64Cu-NTL in comparison to 64Cu-FTL independent of administration of folic acid (#P=0.0005 and *P<0.0001). (B) Biodistribution of 64Cu-FTLs and 64Cu-NTLs with folic acid or vehicle coadministration 3 hours pi. and 24 hours pi. Folic acid predosing was able to reduce early (3 hours pi.) mean liver activity of 64Cu-FTL to the level of 64Cu-NTL liver activity. The liver activity of vehicle administered 64Cu-FTL was significantly higher than 64Cu-NTL (vehicle and folic acid predosed) and folic acid predosed 64Cu-FTL (**P<0.01). Independent of folic acid or vehicle predosing the blood mean activity 24 hours pi. was significantly higher for 64Cu-NTL in comparison to the both 64Cu-FTL groups (##P<0.0001). At 24 hours pi. mean liver activity of both folic acid and vehicle predosed 64Cu-FTL groups was significantly higher than 64Cu-NTL groups independent of predosing (##P<0.0001). (C) 2-hour dynamic liver activity (%ID/g) of 64Cu-FTL and folic acid predosing (n=2) or vehicle (n=2) and 64Cu-NTL and vehicle (n=2) on PET scans commencing simultaneously with the injection of radiolabeled liposomes (bars represent mean %ID/g±SEM. All comparisons were performed using one-way ANOVA analysis and post hoc Tukey multiple comparison analysis).
Abbreviations: FTL, folate-targeting liposomes; NTL, nontargeting liposomes; PET, positron emission tomography; SEM, standard error of mean; pi., postinjection.
Effect of coadministration of excess folic acid on biodistribution and uptake kinetics of FTLs and NTLs
The coadministration of excess folic acid reduced the mean liver activity of 64Cu-FTLs 3 hours pi. to 12.85%±0.9%ID/g, which was not statistically higher than the folic acid (11.7%±0.9%ID/g) and vehicle (11.3%±0.4%ID/g) treated 64Cu-NTLs mice. Liver activity in 64Cu-FTLs mice receiving vehicle was significantly higher (18.3%±1.6%ID/g) compared to all other groups (P=0.0005). However, the effect of excess folic acid on liver uptake was no longer evident 24 hours pi., where mean liver activity of 64Cu-FTLs in the folic acid (19.3%±0.9%ID/g) and vehicle group (22.4%±1.1%ID/g) was no longer significantly different and both were significantly higher than 64Cu-NTLs in the folic acid (11.6%±0.6%ID/g) and vehicle group (11.2%±0.8%ID/g) (P<0.0001) (). Additionally, liver activities during the first 120 minutes pi. of FTLs coadministered with folic acid resembled NTLs administered with a vehicle while FTLs with vehicle displayed higher liver activity ().
Blood activity of radiolabeled liposomes was not statistically different between groups 3 hours pi. The blood activity levels 24 hours pi. were statistically lower in both 64Cu-FTLs groups (folic acid; 2.8%±0.3%ID/g and vehicle; 2.8%±0.3%ID/g) in comparison to 64Cu-NTLs (folic acid; 6.1%±0.4%ID/g and vehicle; 6.3%±0.2%ID/g) (P<0.0001). The activity of radiolabeled liposomes in the spleen was not statistically different between any of the groups 3 hours pi. nor 24 hours pi. ().
Dynamic PET scanning was performed to evaluate the influence of coadministration of excess folic acid on the initial liver uptake of mice receiving 64Cu-FTLs and excess folic acid or vehicle and 64Cu-NTLs plus vehicle. Comparison of the liver TAC indicates that coadministration of excess folic acid delays the initial liver activity of 64Cu-FTLs to a level comparable to 64Cu-NTLs. However, after the first-hour uptake rate increases to a level comparable to that of 64Cu-FTLs vehicle-treated mice. TACs are based on only two mice in each group and they must be carefully interpreted.
Discussion
Despite the generally positive effects of FTLs in vitro, studies conducted in vivo have failed to yield compatible improved efficacy. This limited success of intravenously administered liposome formulations targeting FRs was the basis for the present study, which aimed to provide direct quantitative information on tumor accumulation and circulating characteristics of FTLs by high sensitive and quantitative PET imaging.Citation4,Citation6,Citation21
The presented data illustrate the impact of the reduced circulating half-life of FTLs on tumor accumulation and that actively targeting FRs, highly expressed on cancer cells, does not improve overall delivery. FTLs were rapidly taken up in the liver which was already observable 3 hours pi. The high level of liver uptake has previously been reported for both FTLs and folate-targeting imaging agents.Citation9,Citation11
This study was conducted on relatively small solid tumors. This was based on the previously reported improved therapeutic efficacy of encapsulated doxorubicin in FTLs in comparison to NTLs in small KB xenografts inoculated in the footpad of mice,Citation4 which suggested a potential targeting possibility in small tumors. The larger tumors in the second part of the study displayed slightly lower mean tumor uptake, supporting previous reports of lower EPR-based liposome uptake with increasing tumor size.Citation22
Although a folate-restricted diet might be expected to boost FR expression, it has been shown not to influence folate binding of KB xenografts and M109 tumors in miceCitation9, and thus this study was conducted without feeding mice folate-restricted diets. Additionally, low-folate diets were shown to increase tissue retention of folate-targeted radiopharmaceuticalsCitation23,Citation24 which would further deplete circulating FTLs.
The effect of blocking uptake by liver by coadministering excess folic acid to improve the circulating properties of FTLs was investigated. This approach has been identified to decrease uptake in liver and other tissues expressing high levels of FRsCitation11 and a similar folate preinjection approach has been safely performed in human ovarian and endometrial cancer patients.Citation25 Thus, intravenous preinjection of excess folate had acceptable translational potential and was chosen over the intraperitoneal administration of very high doses of folic acid, which may result in a more prolonged blockade of hepatic receptors.Citation24 A preinjection of excess folic acid has, additionally, been shown to decrease tumor accumulation of folate-targeting radiotracersCitation11,Citation26 and may have no effect on liposome accumulation in tumor.Citation9 In this study, the results of folic acid coadministration demonstrate no difference between FTLs and NTLs uptake levels in tumors of mice pretreated with folic acid or vehicle. Liver uptake was only significantly reduced in folic acid injected mice in the 3 hours PET scan, indicating that the blockade is short lived and insufficient for liposomes with long-circulating half-lives. To increase the circulating half-life of FTLs, multiple doses or long-acting folic acid may be needed which, following the reported blockade of tumor cell receptors by the excess folic acid administration, could directly inhibit the cancer cell-targeting strategy. Importantly, the influence of folate-mediated liver uptake in humans injected with FTLs remains to be determined. However, the liver is the primary storage organ for folate and FRs are expressed by activated human macrophages. Macrophage-mediated uptake and elimination may therefore also be an important issue for circulatory properties in humans.Citation27–Citation29 Alternatively, administration of antifolates (eg, pemetrexed) has been performed in conjunction with FR-targeting agents and shown to decrease renal uptake without affecting tumor uptake.Citation30 Administration of pemetrexed may, however, be associated with unwanted side effects, especially in patients already undergoing chemotherapy.
The results of this study indicate that the EPR effect and circulating properties of liposome formulations determine the liposome levels that may be achieved, independently of active tumor cell receptor targeting. This statement is supported by the previously observed reduced, but more compatible, uptake levels of somatostatin receptor targeting liposomes relative to NTLs.Citation10
The results demonstrate that the active targeting of FRs on human cancer cells with known high expression decreases the overall liposome uptake within solid tumor xenografts. Thus, drug delivery by liposomes targeting FRs significantly decrease the total amount of liposomes delivered to solid tumors. It is therefore of key importance to include a nontargeting liposomal comparator when evaluating anticancer therapy based on targeting liposomes if statements on the improved efficiency of active targeting are to be valid. Despite the lack of valid comparators, FTLs have been purported to provide improved therapeutic efficacy based on their targeting properties although this effect is primarily attributable to an EPR-based improved drug delivery within tumors.Citation31
The presented data do not provide information on the uptake levels achieved in single cancer cells within the solid tumor mass between targeting and NTLs. Several studies and reviews highlight the increased cellular uptake by active targeting as a method to increase the amount of drug delivered and thus improve the therapeutic efficacy.Citation3
This consideration might be attractive if all cells within solid tumors were readily accessible for nano-sized particles. However this does not seem to be the case.Citation7 Active targeting to the more readily available neo-angiogenic tumor vasculature is far more appealing. Several attractive vascular targets are probably directly accessible to circulating liposomes and could allow accumulation and subsequent triggered drug release within the vasculature to increase loco-regional drug concentration.Citation32,Citation33 FTLs may have therapeutic potential beyond the direct targeting of FR expressing cancer cells as important associations between tumor-associated macrophages (TAMs) expressing FR-β and malignant characteristics has been identified. TAMs play prominent roles in angiogenesis, cancer cell invasion, and extravasation. FR-β expressing TAM has additionally been shown to mediate potent immunosuppressive functions.Citation34–Citation36 Importantly, with respect to the perivascular accumulation liposomes, the TAMs reside in the perivascular compartment thus making them potential targets that could be reached by FTLs.Citation34,Citation37 The majority FR-β expressing TAM in human pancreatic cancer has additionally been shown to express vascular endothelial growth factor which increases vascular permeability and therefore potentially improved liposome extravasation to perivascular areas harboring FR-β TAMs.Citation34,Citation38
Notwithstanding the challenges faced EPR-based liposome accumulation in solid cancer remains an attractive methodology for drug delivery, however, achieving improved targeting and triggered release within the accessible extracellular compartments remain an important goal.
Directly targeting specific cancer cell receptors, including FRs, has proven successful in numerous in vitro studies.Citation39–Citation42 Varying therapeutic effects of FR-targeting in tumor models has been observed in in vivo studies, ranging from promising to discouraging resultsCitation4,Citation6,Citation14,Citation15,Citation43,Citation44 However, other in vivo studies has also, in agreement with the current study, targeted FR with liposomes with poor tumor accumulation compared to controls.Citation9,Citation12,Citation45 Although results by previous studies are somewhat contradicting, it may be partly explained by differences in tumor models, formulations, and other experimental details and, as previously stated, be partly explained by differences in cellular uptake. Previous in vivo studies have mainly used 3H- or fluorophore-imaging to assess biodistribution while the current study included PET/CT imaging to obtain more dynamic and sensitive results. Furthermore, this study demonstrates the importance of in vivo studies to demonstrate the actual targeting potential.
In conclusion, targeting FRs provided no improvement of the overall liposome delivery to solid KB xenografts determined by PET. Although only FTLs were included in the present study. It clearly demonstrates that surface modifications to liposomes must be carefully evaluated as this may significantly influence the circulation and biodistribution. Any observed improved in vitro cytotoxic efficacy must, therefore, be carefully evaluated as this improved effect, in comparison to long-circulating controls, can easily be lost following significant difference in EPR-dependent tumor accumulation. Further studies are warranted to fully elucidate the potential of various ligands targeting cancer cell receptors and subsequent intratumoral microregional distribution. Importantly, the present study was conducted in a murine xenograft model, which may display a far less complicated tumor environment than clinical cancers and therefore potentially illustrate the problems targeted drug delivery systems faced in clinical cancers. The targeting and therapeutic potential of FTLs may benefit from investigations in additional animal models with a folate metabolism that better simulate human folate metabolism to elucidate their and therapeutic potential fully.
Disclosure
The authors report no conflicts of interest in this work.
References
- MatsumuraYMaedaHA new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancsCancer Res19864612 Pt 1638763922946403
- PetersenALHansenAEGabizonAAndresenTLLiposome imaging agents in personalized medicineAdv Drug Deliv Rev201264131417143522982406
- NobleGTStefanickJFAshleyJDKiziltepeTBilgicerBLigand-targeted liposome design: challenges and fundamental considerationsTrends Biotechnol2014321324524210498
- GabizonATzemachDGorinJImproved therapeutic activity of folate-targeted liposomal doxorubicin in folate receptor-expressing tumor modelsCancer Chemother Pharmacol2010661435219779718
- SaulJMAnnapragadaANatarajanJVBellamkondaRVControlled targeting of liposomal doxorubicin via the folate receptor in vitroJ Control Release2003921–2496714499185
- RiviereKHuangZJergerKMacaraegNSzokaFCAntitumor effect of folate-targeted liposomal doxorubicin in KB tumor-bearing mice after intravenous administrationJ Drug Target2011191142420353291
- YuanFLeunigMHuangSKBerkDAPapahadjopoulosDJainRKMicrovascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograftCancer Res19945413335233568012948
- VaupelPMayerAHypoxia in cancer: significance and impact on clinical outcomeCancer Metastasis Rev200726222523917440684
- GabizonAHorowitzATGorenDTzemachDShmeedaHZalipskySIn vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing miceClin Cancer Res20039176551655914695160
- PetersenALBinderupTJølckRIPositron emission tomography evaluation of somatostatin receptor targeted 64Cu-TATE-liposomes in a human neuroendocrine carcinoma mouse modelJ Control Release2012160225426322245688
- MüllerCForrerFSchibliRKrenningEPde JongMSPECT study of folate receptor-positive malignant and normal tissues in mice using a novel 99mTc-radiofolateJ Nucl Med200849231031718199624
- PohSChelvamVLowPSComparison of nanoparticle penetration into solid tumors and sites of inflammation: studies using targeted and nontargeted liposomesNanomedicine20151091439144925996118
- YangJChenHVlahovIRChengJXLowPSEvaluation of disulfide reduction during receptor-mediated endocytosis by using FRET imagingProc Natl Acad Sci U S A200610337138721387716950881
- PanXQZhengXShiGWangHRatnamMLeeRJStrategy for the treatment of acute myelogenous leukemia based on folate receptor beta-targeted liposomal doxorubicin combined with receptor induction using all-trans retinoic acidBlood2002100259460212091353
- PanXQWangHLeeRJAntitumor activity of folate receptor-targeted liposomal doxorubicin in a KB oral carcinoma murine xenograft modelPharm Res200320341742212669962
- YamadaATaniguchiYKawanoKHondaTHattoriYMaitaniYDesign of folate-linked liposomal doxorubicin to its antitumor effect in miceClin Cancer Res200814248161816819088031
- PanXQLeeRJIn vivo antitumor activity of folate receptor-targeted liposomal daunorubicin in a murine leukemia modelAnticancer Res2005251A34334615816557
- HenriksenJRPetersenALHansenAERemote Loading of (64) Cu(2+) into liposomes without the use of ion transport enhancersACS Appl Mater Interfaces2015741227962280626426093
- ForsterMDOrmerodMGAgarwalRKayeSBJackmanALFlow cytometric method for determining folate receptor expression on ovarian carcinoma cellsCytometry A2007711194595017712798
- SiwowskaKSchmidRMCohrsSSchibliRMüllerCFolate receptor-positive gynecological cancer cells: in vitro and in vivo characterizationPharmaceuticals2017103E7228809784
- TongLChenWWuJLiHFolic acid-coupled nano-paclitaxel liposome reverses drug resistance in SKOV3/TAX ovarian cancer cellsAnticancer Drugs201425324425424275314
- HarringtonKJMohammadtaghiSUsterPSEffective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomesClin Cancer Res20017224325411234875
- LeamonCPReddyJADortonRImpact of high and low folate diets on tissue folate receptor levels and antitumor responses toward folate-drug conjugatesJ Pharmacol Exp Ther2008327391892518791065
- GabizonAShmeedaHHorowitzATZalipskySTumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugatesAdv Drug Deliv Rev20045681177119215094214
- SiegelBADehdashtiFMutchDGEvaluation of 111In-DTPA-folate as a receptor-targeted diagnostic agent for ovarian cancer: initial clinical resultsJ Nucl Med200344570070712732670
- RossTLHonerMMüllerCGroehnVSchibliRAmetameySMA new 18F-labeled folic acid derivative with improved properties for the PET imaging of folate receptor-positive tumorsJ Nucl Med201051111756176220956469
- HoppnerKLampiBFolate levels in human liver from autopsies in CanadaAm J Clin Nutr19803348628647189090
- MüllerABeckKRancicZImaging atherosclerotic plaque inflammation via folate receptor targeting using a novel 18F-folate radiotracerMol Imaging2014132111
- van der HeijdenJWOerlemansRDijkmansBAFolate receptor beta as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patientsArthritis Rheum2009601122119116913
- MüllerCSchibliRProspects in folate receptor-targeted radionuclide therapyFront Oncol2013324924069581
- MortonSWLeeMJDengZJA nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathwaysSci Signal20147325ra4424825919
- PetersenALBinderupTRasmussenP64Cu loaded liposomes as positron emission tomography imaging agentsBiomaterials20113292334234121216003
- ChenQTongSDewhirstMWYuanFTargeting tumor microvessels using doxorubicin encapsulated in a novel thermosensitive liposomeMol Cancer Ther20043101311131715486198
- KuraharaHTakaoSKuwahataTClinical significance of folate receptor β-expressing tumor-associated macrophages in pancreatic cancerAnn Surg Oncol20121972264227122350599
- Puig-KrögerASierra-FilardiEDomínguez-SotoAFolate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophagesCancer Res200969249395940319951991
- QuailDFJoyceJAMicroenvironmental regulation of tumor progression and metastasisNat Med201319111423143724202395
- WyckoffJBWangYLinEYDirect visualization of macrophage-assisted tumor cell intravasation in mammary tumorsCancer Res20076762649265617363585
- ChenXLNamJOJeanCVEGF-induced vascular permeability is mediated by FAKDev Cell201222114615722264731
- PatilYShmeedaHAmitayYOhanaPKumarSGabizonATargeting of folate-conjugated liposomes with co-entrapped drugs to prostate cancer cells via prostate-specific membrane antigen (PSMA)Nanomedicine20181441407141629680672
- WatanabeKKanekoMMaitaniYFunctional coating of liposomes using a folate-polymer conjugate to target folate receptorsInt J Nanomedicine201273679368822888227
- GuoBXuDLiuXYiJEnzymatic synthesis and in vitro evaluation of folate-functionalized liposomesDrug Des Devel Ther20171118391847
- WangFChenYZhangDFolate-mediated targeted and intracellular delivery of paclitaxel using a novel deoxycholic acid-O-carboxymethylated chitosan-folic acid micellesInt J Nanomedicine2012732533722287842
- KeXLinWLiXWangHXiaoXGuoZSynergistic dual-modified liposome improves targeting and therapeutic efficacy of bone metastasis from breast cancerDrug Deliv20172411680168929092646
- SriramanSKSalzanoGSarisozenCTorchilinVAnti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acidEur J Pharm Biopharm2016105404927264717
- PatilYAmitayYOhanaPShmeedaHGabizonATargeting of pegylated liposomal mitomycin-C prodrug to the folate receptor of cancer cells: Intracellular activation and enhanced cytotoxicityJ Control Release2016225879526809007
