?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Treatment for melanoma is a challenging clinical problem, and some new strategies are worth exploring.
Purpose
The objective of this study was to investigate the in vitro and in vivo anti-melanoma effects of hydroxyapatite nanoparticles (HANPs) and discuss the involved material factors.
Materials and methods
Five types of HANPs, ie, HA-A, HA-B, HA-C, HA-D, and HA-E, were prepared by wet chemical method combining with polymer template and appropriate post-treatments. The in vitro effects of the as-prepared five HANPs on inhibiting the viability of A375 melanoma cells and inducing the apoptosis of the cells were evaluated by Cell Counting Kit-8 analysis, cell nucleus morphology observation, flow cytometer, and PCR analysis. The in vivo anti-melanoma effects of HANPs were studied in the tumor model of nude mice.
Results
The five HANPs had different physicochemical properties, including morphology, size, specific surface area (SSA), crystallinity, and so on. By the in vitro cell study, it was found that the material factors played important roles in the anti-melanoma effect of HANPs. Among the as-prepared five HANPs, HA-A with granular shape, smaller size, higher SSA, and lower crystallinity exhibited best effect on inhibiting the viability of A375 cells. At the concentration of 200 μg/mL, HA-A resulted in the lowest cell viability (34.90%) at day 3. All the HANPs could induce the apoptosis of A375 cells, and the relatively higher apoptosis rates of the cells were found in HA-A (20.10%) and HA-B (19.41%) at day 3. However, all the HANPs showed no inhibitory effect on the viability of the normal human epidermal fibroblasts. The preliminary in vivo evaluation showed that both HA-A and HA-C could delay the formation and growth speed of melanoma tissue significantly. Likely, HA-A exhibited better effect on inhibiting the growth of melanoma tissue than HA-C. The inhibition rate of HA-A for tumor tissue growth reached 49.1% at day 23.
Conclusion
The current study confirmed the anti-melanoma effect of HANPs and provided a new idea for the clinical treatment of melanoma.
Introduction
As the largest organ and outer shell of human body, skin mainly protects tissues and organs in the body from the attack of physical factor, chemical substance, mechanical stress, and pathogenic microorganism.Citation1,Citation2 In the epidermal layer of skin, there are five layers from inside to outside, in which the melanocytes in the basal layer are susceptible to lesions and then transform into melanoma.Citation3,Citation4 In recent years, melanoma took on the increasing incidence rate and can also be found in mucosa, choroid, and other tissues.Citation5–Citation9 So far, the general clinical treatment is still surgical resection, accompanied by chemotherapy and immunotherapy. However, melanoma has the characteristics of rapid proliferation, local invasion, long-distance migration, and strong resistance to currently clinical therapies.Citation1,Citation10 Except the thin primary skin melanoma (<1 mm), the clinical surgery for metastatic melanoma and deep primary malignant melanoma (>4 mm) still have a very high recurrence rate and mortality.Citation11,Citation12 Therefore, new strategies for improving the clinical treatment effect of melanoma are quite necessary.
Hydroxyapatite (HA) is a major inorganic component of human bone and teeth, and exhibits excellent biocompatibility, bioactivity, osteoconduction, and even osteoinduction in biomedical application.Citation13–Citation15 In 1990s, Aoki et al and Kano et al first reported the in vitro anti-tumor effect of HA nanoparticles (HANPs).Citation16,Citation17 They occasionally found that HANPs without loading doxorubicin still had the inhibitory effect on the proliferation for Ca-9 tumor cells. After that, the anti-tumor effects of HANPs were widely regarded and investigated. A large number of reports indicated that HANPs could inhibit the proliferation of various tumor cells, such as hepatoma cells,Citation18–Citation20 osteosarcoma cells,Citation21–Citation23 lung cancer cells,Citation24,Citation25 and gastric cancer cellsCitation26–Citation28 to some extent. Moreover, HANPs showed little or no inhibitory effect on the normal tissue cells, including osteoblasts,Citation23 hepatocytes,Citation18 lung fibroblasts,Citation25 etc. This was undoubtedly hopeful to overcome the drawbacks of some anti-tumor drugs, which could kill cancer cells as well as normal tissue cells.
In previous studies, Li et al reported that HANPs had certain anti-melanoma effect.Citation29 They found that for HANPs, the size had stronger influence on the proliferation of A875 melanoma cells than the morphology. However, the involved mechanism has not been well revealed. Besides, the correlation between the material factors of HANPs and proliferation inhibition or apoptosis of melanoma cells need be further investigated. Hence, in the present study, we prepared five different HANPs by wet chemical method combining with polymer template and different post-treatments, and investigated their anti-melanoma effects by in vitro and in vivo experiments. Besides, human fibroblasts were chosen as the control to investigate their impacts on normal tissue cells. The influences of various material factors on the anti-melanoma effects of the HANPs were studied systematically and discussed.
Materials and methods
Reagents
Ca(NO3)2·4H2O, (NH4)2HPO4, and NH3·H2O were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). PEG2000 was purchased from the Aladdin (Shanghai, China). Human melanoma cells (A375) and human epidermal fibroblasts (HSF) were purchased from iCell Bioscience Inc (Shanghai, China). FBS, DMEM medium, penicillin–streptomycin solution, PBS, and Trypsin 0.25% EDTA were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Fluorescein diacetate and propidium iodide (PI) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). DAPI was purchased from Beyotime (Shanghai, China). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumanoto, Japan). Fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit I was purchased from BD (Franklin Lakes, NJ, USA). The RNeasy Mini Kit was purchased from Qiagen (Hilden, Germany). The iScript™ gDNA Clear cDNA Synthesis kit and SsoFast™ EvaGreen Supermix were purchased from Bio-Rad Laboratories Inc. (Hercules, CA, USA). The Matrigel was purchased from Corning Incorporated (Corning, NY, USA).
Preparation of HANPs
HANPs were synthesized at room temperature by wet chemical method using reactive system of Ca(NO3)2·4H2O and (NH4)2HPO4, in which NH3·H2O was used for pH adjustment and PEG2000 as a template reagent. The molar ratio of Ca/P was maintained at 1.67. A certain amount of PEG2000 solution (8.0 wt%) was added to Ca(NO3)2·4H2O solution, and then (NH4)2HPO4 solution was added dropwise to the solution, whose pH was kept at ~10.0 by addition of NH3·H2O. After that, the stirring for mixing the solution continued for a period of time, and then the slurry was aged at room temperature for 24 hours. Next, the slurries were washed with ultrapure water to neutrality. With or without post-treatment, the slurries were dried at 60°C for 10 hours. Various preparing parameters for HANPs, including the concentration of reactants and the post-treatment of the slurries are summarized in . In total, five HANPs, ie, HA-A, HA-B, HA-C, HA-D, and HA-E, were prepared for subsequent experiments.
Table 1 The preparing parameters for the five HANPs
Characterization of HANPs
The phase composition of HANPs was determined by X-ray diffraction (XRD, Shimazu XRD-6100, Kyoto, Japan) using Cu Ka radiation (λ=1.5418 Å) with the test voltage at 40 kV and the operating current at 30 mA. The diffraction spectrum was scanned from 20° to 60° with a speed of 5°/min. Patterns were analyzed by using Jade 6.0 software. The crystallinity of HANPs was calculated using the following EquationEquation 1(1) , where β002 is the full width at half maximum of the (002).Citation30,Citation31
The chemical groups of HANPs were analyzed by Fourier-transform infrared spectroscopy (FTIR, Nicolet 6700; Thermo Fisher Scientific) with the range from 4,000 to 400 cm−1.
The morphology and particle size of HANPs were observed by Transmission Electron Microscope (TEM, FEI; Tecnai G2F20, Hillsboro, OR, USA) at the working voltage of 200 kV. The morphology of the NPs was observed by dropping HANPs dissolved onto a copper grid. The particle size of HANPs was measured by using Nano Measurer. When measuring the particle sizes, we selected at least three images of as-prepared five HANPs at the scale of 200 nm, and at least 70 particles in each image were evaluated for statistical analysis.
Zeta potentials of HANPs were measured by a Nano Zetasizer (ZS90; Malvern Instruments, Malvern, UK) based on dynamic light scattering theory. The specific surface areas (SSAs) of HANPs were determined by Brunauer–Emmett– Teller method using a surface area analyzer (GeminiVII 2390 t; Micromeritics Instrument Corporation, Norcross, GA, USA). The releases of Ca2+ from HANPs were measured by ICP-AES (ARCOS; Spectro Analytical Instruments GmbH, Kleve, Germany). Simply, the five HANPs were dispersed in Tris-HCl solution (pH7.4) at the concentration of 200 μg/mL and then placed in a 37°C incubator for 3 days. Next, the supernatants were collected and analyzed.
Cell culture
Both A375 and HSF cells were cultured in DMEM medium supplemented with 1% penicillin and streptomycin and 10% FBS at 37°C under 5% CO2 atmosphere. After the cells grew to 70%–80% confluence in tissue culture flasks, the cells were digested with 0.25% trypsin containing EDTA and counted, followed by seeding in a 24-well plate with a density of 104 cells per well. The HANPs suspensions with different concentrations (100, 200, and 400 μg/mL) were prepared by dispersing the NPs in DMEM. After cell attachment, the HANPs suspension was added into each well. Then, the cells were subjected to the subsequent analysis after culturing for 1, 2, and 3 days.
CCK-8 analysis
Cell viability was evaluated by CCK-8 method. After culturing A375 or HSF cells with various HANPs for the set times, the media were removed, followed by addition of fresh DMEM medium, including water-soluble tetrazolium (WST)-8 (WST-8/DMEM =1/9). After keeping in a dark place for 2 hours, the OD value in all wells were measured by a microplate reader (EON; BioTek, Winooski, VT, USA) at the wavelength of 450 nm. The cells without addition of HANPs were used as the control group. Three duplicates for each group were used in the test, and cell viability (%) was calculated according to EquationEquation 2(2) .
Confocal laser scanning microscopy (CLSM) observation
After culturing A375 or HSF cells with various HANPs for the set times, the media were removed, then the cells were washed with PBS (pH 7.4) and fixed with 4% paraformal-dehyde for 20 minutes, followed by staining with DAPI for 10 minutes in the dark. The nuclear morphology of the cells was examined by CLSM (Leica-TCS-SP5; Leica Microsys-tems, Wetzlar, German), and the cell nucleus was dyed blue.
Flow cytometer
The apoptosis of A375 cells was analyzed by flow cytometer (CytoFLEX; Beckman Coulter, Guangzhou, China). After culturing with the HANPs for the set times, the cells were double stained with Annexin V FITC/PI. The normal, apoptotic, and necrotic cells were examined. FITC and PI staining were performed according to the manufacturer’s instructions. A375 cells in different states were mapped in a bidirectional dot plot.
PCR analysis
After culturing with the HANPs for the set times, RNA of the cells (A375 or HSF) was isolated using RNeasy Mini Kit. The cells were fully lysed using Buffer RLT, and the centrifuged RNA samples were added to the DNase reagent to remove genomic DNA contamination. The extracted RNA was reversely transcribed into cDNA using the iScriptTM gDNA Clear cDNA kit. The resulting cDNA was then amplified using the SsoFastTM EvaGreen® kit. Three parallel samples were set for each target gene. The PCR reaction was performed using a CFX96™ system (Bio-Rad), and the data were processed and analyzed by CFX Manager software. The target gene expression level was calculated by 2−ΔΔCt method, and glyceraldehyde-3-phosphate dehydrogenase was used as the internal reference gene to normalize the result. Primer sequences in the present study are shown in .
Table 2 The primer sequences for the apoptotic-related genes
Animal study
In the present study, 15 BALB/C nude mice (female, 14–16 weeks old, weighing 20.0±1.0 g) purchased from Laboratory Animal Center of Sichuan University (Chengdu, China) were used for animal study. The animal experiments were approved by the Animal Care and Use Committee of Sichuan University and followed the guidelines of the Chinese Society of Laboratory Animals on animal welfare. The nude mice were randomly divided into three groups, ie, one control group and two experimental groups. Based on the aforementioned in vitro evaluation, HA-A and HA-C were selected as the experimental materials. According to 1 mg/mL concentration, the HANPs suspension was prepared by dispersing 50 mg HANPs into 50 mL DMEM. After A375 cells grew to 70%–80% confluence in tissue culture flasks, the cells were trypsinized and the cell suspension was collected. Then, the cell suspension containing 2×106 cells were transferred into a centrifuge tube and centrifuged at the speed of 1,000 rpm, followed by adding 1 mL HANPs suspension (or DMEM for control group) and 10 μL commercial Matrigel. The mixture was subcutaneously injected into the left side of the nude mouse after blending thoroughly. Therefore, the injection dose of HANPs in each mouse in the experimental groups was 50 mg/kg weight. The formation and volume change of tumor tissue in the animal was observed and recorded. The length and width of the formed tumor tissue in each animal and each time point were measured by caliper, and the tumor volume was calculated according to EquationEquation 3(3) :
Statistical analysis
Statistical analysis was performed with one-way ANOVA using SPSS13.0 software. All data were expressed as mean ± SD and obtained by at least three replicates for each data set. The level of P<0.05 was considered to be statistically significant.
Results
Characterization of HANPs
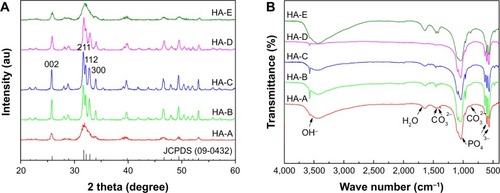
shows the XRD patterns of the as-prepared five HANPs. The characteristic peaks in the five HANPs were consistent with those in the HA standard (JCPDS: 09-0432), indicating that all of them were composed of pure HA phase. In the three samples with different post-treatments (HA-B, HA-C and HA-D), the three diffraction peaks, which respectively corresponds with (211), (112) and (300) crystal faces, were obviously sharp. However, in other two directly dried samples (HA-A and HA-E), they were partly fused. shows the infrared spectra of the as-prepared five HANPs. In all the samples, two characteristic peaks corresponding with OH− and PO43− groups in HA could be seen clearly. The vibration peaks of OH− appeared at about 3,560 cm−1.Citation33 There were two weak peaks ranging from 550 cm−1 to 650 cm−1, which could be the stretching vibration peaks of PO43− bond. The band between 1,120 cm−1 and 940 cm−1 belonged to the strong and broad peaks of PO43−.Citation33,Citation34 Except for HA-D, the other four HANPs showed the characteristic peak of CO32− at about 1,460 cm−1 and 870 cm−1. This could be attributed to CO2 in the air entering the solution and participating in the precipitation of HANPs, leading to formation of B-HA.Citation33–Citation35 As for HA-D, the calcination process could result in the loss of CO32−. Besides, there was a very weak tensile vibration peak at about 1,650 cm−1, indicating that the as-prepared HANPs could contain the crystal water.Citation33,Citation35
Figure 1 X-ray diffraction patterns (A) and Fourier-transform infrared spectra (B) of the as-prepared five HANPs.
Abbreviation: HANP, hydroxyapatite nanoparticle.
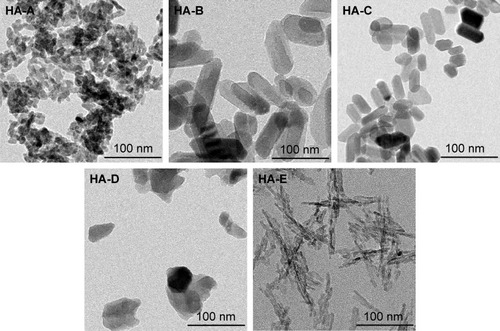
shows the TEM images of the as-prepared five HANPs, and summarizes their physicochemical properties. They all were nano-scaled particles but had different morphologies and sizes. Both HA-A and HA-D had granular shapes, but their average particle sizes were about 20 and 50 nm, respectively. HA-E was needle-like, and its average diameter and length were about 5 and 40 nm, respectively. Both HA-B and HA-C were rod-like, but HA-B had a little larger diameter and length than HA-C. All the five HANPs had negative zeta potentials, indicating that they had negative surface net charges. Based on the XRD analysis, HA-A and HA-E had far lower crystallinity than the other three HANPs, indicating that the hydrothermal or calcinating process could improve the crystallization of the synthesized HANPs. Likely, SSA of HANPs was also influenced by the post-treatments. HA-A and HA-E had far higher SSA than the other three HANPs. However, the calcinated HA-D had lower crystallinity and SSA than the hydrothermal treated HA-B and HA-C. Under two different hydrothermal treatments, no obvious difference of crystallinity and SSA was found between HA-B and HA-C.
Table 3 The physicochemical properties of the as-prepared five HANPs
Cell viability
Based on the CCK-8 analysis, cell viabilities of A375 cells after culturing with the HANPs are shown in . At three different HANPs concentration (100, 200, and 400 μg/mL), among the five HANPs, only HA-A presented the continuous inhibition on the viability of A375 cells, and the cell viability almost decreased with the increase of culturing time. For the other four HANPs, both HA-B and HA-C exhibited the continuous inhibition on the viability of A375 cells only at the concentration of 200 μg/mL. It should be noted that the inhibitory effect of HANPs was not always positively related with the concentration of the nanoparticles. At day 3, almost all the HANPs showed the strongest inhibitory effect on the cell viability at the concentration of 200 μg/mL.
Figure 3 Changes of cell viabilities of A375 (A) and HSF (B) cells with the culturing time at different concentrations of HANPs. (n=3; vs 100 μg/mL, *P<0.05, **P<0.01; vs 400 μg/mL, #P<0.05, ##P<0.01).
Abbreviations: HANP, hydroxyapatite nanoparticle; HSF, human epidermal fibroblasts.
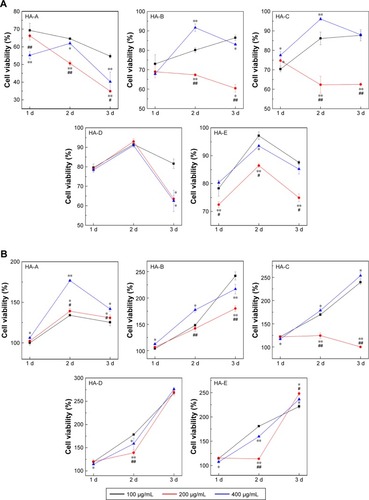
shows the cell viabilities of normal HSF cells after culturing with the HANPs. It could be seen that for all the five HANPs, the cell viability almost increased with the increase of culturing time at each concentration, indicating that HANPs showed no inhibitory effect on the viability of HSF cells.
gives the concrete cell viabilities of both types of cells after culturing with various HANPs at the concentration of 200 μg/mL for 3 days. It could be seen that HA-A led to the lowest cell viability of A375 cells (34.90%), and HA-E resulted in the highest cell viability (74.90%). The other three HANPs, ie, HA-B, HA-C, and HA-D, presented similar cell viabilities of A375 cells. On the contrary, all the HANPs except HA-C increased the viability of HSF cells significantly.
Table 4 Cell viabilities of A375 cells after culturing with various HANPs at the concentration of 200 μg/mL for 3 days (n=3)
Cell nucleus morphology
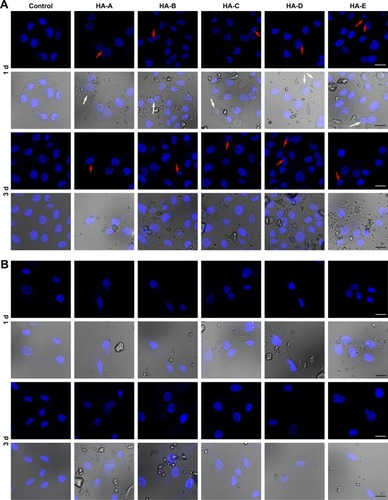
shows the CLSM images of A375 cells stained with DAPI after culturing with various HANPs at the concentration of 200 μg/mL for 1 and 3 days. Some morphological changes in the cell nucleus could be easily observed. In control group, the cells were not observed to have obvious morphology change of the nucleus. But in the experimental groups, after culturing with HANPs for 1 day, the vacuoles were found occurring on the nuclear membrane. Also, the nuclear shrinkage or fragmentation were observed in some cells. When the culturing time increased to 3 days, more cells presented the phenomena of nuclear shrinkage and fragmentation, tending to apoptosis.Citation36 shows the CLSM images of HSF cells stained with DAPI after culturing with various HANPs for 1 and 3 days. Either in the control or experimental groups, no obvious morphology change of cell nucleus could be found.
Figure 4 The typical CLSM images of A375 (A) and HSF (B) cells stained with DAPI (blue) after culturing with various HANPs at the concentration of 200 μg/mL for 1 and 3 days (both fluorescence field and superimposed pictures of the bright and fluorescence fields are given in the figure; magnification: ×100; scale bars: 20 μm; white arrow: vacuoles presented on nuclear membrane; red arrows: nuclear shrinkage or fragmentation; the dispersed HANPs are seen in the superimposed pictures; three duplicates for each experiment).
Abbreviations: CLSM, confocal laser scanning microscopy; HANP, hydroxyapatite nanoparticle; HSF, human epidermal fibroblasts.
Apoptosis rate of A375 cells
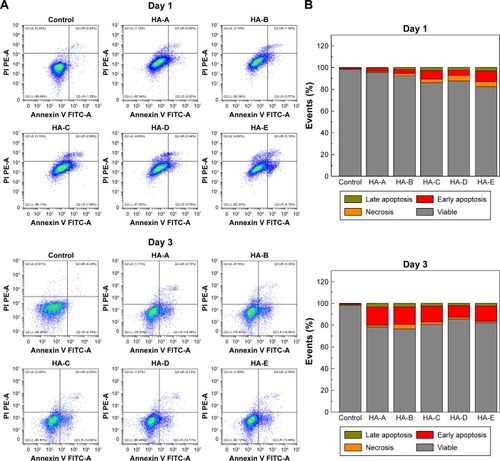
To quantify the cell apoptosis, A375 cells were double stained with Annexin V FITC/PI and analyzed by flow cytometer after culturing with various HANPs at the concentration of 200 μg/mL for 1 and 3 days. The results are shown in . For the control group, without addition of HANPs, only tiny apoptotic cells could be found, and the apoptosis rate of the cells was almost negligible. However, after culturing with HANPs, the apoptosis, especially early apoptosis of the cells was obvious and increased with the increase in culturing time in each experimental group. At day 3, the total apoptosis rates of A375 melanoma cells in HA-A, HA-B, HA-C, HA-D, and HA-E were 20.10%, 19.41%, 17.14%, 12.84%, and 16.39%, respectively.
Figure 5 (A) Density maps of FITC Annexin V apoptosis detection results and (B) the rates of apoptosis and necrosis obtained by double-staining with Annexin V-FITC/PI of A375 cells after culturing with various HANPs at the concentration of 200 μg/mL for 1 and 3 days (three duplicates for each experiment).
Abbreviations: FITC, fluorescein isothiocyanate; PI, propidium iodide; HANP, hydroxyapatite nanoparticle.
Apoptosis-related gene expressions
shows the apoptosis-related gene expressions in A375 cells after culturing with various HANPs at the concentration of 200 μg/mL for 1 and 3 days. Caspases are tightly related to cell apoptosis.Citation26,Citation37,Citation38 Compared with the control group, three caspase genes (caspase-3, caspase-8, and caspase-9) were significantly up-regulated at day 1 by all the HANPs. Relatively, HA-A showed weaker ability to up-regulate the caspase gene expressions than other HANPs. At day 3, their expressions decreased sharply, but caspase-3 and caspase-9 still had higher expressions in some experimental groups. Besides caspases, Bax, Bcl-2, and p53 also played the active roles in cell apoptosis.Citation39 Compared with the control group, Bax gene had slightly elevated expressions in some experimental groups at day 1. But at day 3, its expression in all the experimental groups, especially in HA-E was highly up-regulated. As for Bcl-2, it had much higher expression in the experimental groups except for HA-A at day 1. At day 3, its gene expression decreased sharply, but still had obvious up-regulation in the experimental groups, especially in HA-E. As for p53, it always showed highly up-regulated gene expressions in the experimental groups at day 1 and 3.
Figure 6 Apoptosis-related gene expressions in A375 (A) and HSF (B) cells after culturing with various HANPs at the concentration of 200 μg/mL for 1 and 3 days (n=3; vs control group, * P<0.05, ** P<0.01; vs HA-A, #P<0.05, ##P<0.01).
Abbreviations: HANP, hydroxyapatite nanoparticle; HSF, human epidermal fibroblasts.
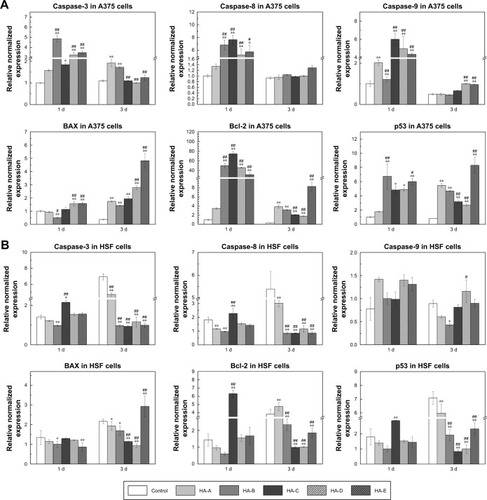
shows the apoptosis-related gene expressions in HSF cells after culturing with various HANPs at the concentration of 200 μg/mL for 1 and 3 days. It could be seen that compared with the control group, almost all the tested genes showed no significant up-regulation in the experimental groups except HA-C. At day 1, the expressions of caspase-3, caspase-8, Bcl-2, and p53 genes were up-regulated to various extents by HA-C.
Tumor tissue growth
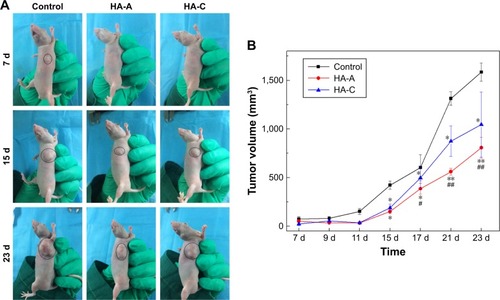
After in vivo injection of the suspensions of HANPs and A375 cells, the formation of tumor tissue was observed, and the volume changes with time prolongation were recorded. shows the volume change of the formed tumor tissue in nude mice with the prolongation of the feeding time. In the control group, the formed tumors could be observed clearly at day 7, and then grew sharply. However, in the two experimental groups, the formation of tumor tissue was delayed obviously. After day 11, the tumor tissue formed and then grew up at a relatively lower speed. At each time point, the volume of the formed tumor tissue in HA-A or HA-C group was always smaller than that in the control group, indicating that both HANPs could inhibit the growth of tumor tissue. At the same time, both HANPs showed the different inhibition effect on tumor tissue growth. HA-A presented better efficiency than HA-C. At day 23, the inhibition ratios of HA-A and HA-C for tumor tissue growth were 49.1% and 34.0%, respectively.
Discussion
So far, melanoma remains one of the world’s clinical treatment challenges. The conventional therapy is surgical resection supplemented with chemotherapy, which could lead to severe toxic and adverse effects on patients. Besides, some drugs, such as metformin,Citation40 were also used for treating melanoma in clinic, but the current study showed that melanoma could have high tolerance to these drugs. In recent years, the rapid development of nanotechnology and nanomedicine could provide a new idea for the treatment for melanoma.
The Se NPs prepared by Chen et al using the wakame polysaccharide solution could induce apoptosis of A375 cells.Citation41 Wang et al used CuO NPs to achieve good effect on inducing apoptosis of melanoma in vitro and in vivo.Citation42 Meanwhile, other treatments, such as photothermal therapyCitation43,Citation44 and heat shock proteins combined with magnetic NPsCitation45,Citation46 were also used for killing melanoma as well as healing the wounds. However, although achieving good results, the aforementioned methods would inevitably kill some normal cells during the treatments and thus have an adverse impact on the local tissue repair. Therefore, it is quite necessary to find out a safer method to treat melanoma.
In 1993, Aoki et al and Kano et al first reported the in vitro anti-tumor effect of HANPs by inhibiting the proliferation of Ca-9 tumor cells.Citation16,Citation17 Afterward, HANPs received much attention as an anti-tumor material. In the past two decades, there were lots of literatures that reported the positive effects of HANPs on the proliferation inhibition or apoptosis of various tumor cells, including hepatoma cells,Citation16–Citation18 osteosarcoma cells,Citation19–Citation21 lung cancer cells,Citation22,Citation23 gastric cancer cells,Citation24–Citation26 etc. We previously found that HANPs had certain anti-melanoma effect by inhibiting the proliferation of A875 cells.Citation29 In the present study, we further investigated the influences of the material factors on the anti-melanoma effects of HANPs by in vitro and in vivo evaluation.
Five HANPs were synthesized by using a wet chemical method in combination with polymer template and appropriate post-treatment. According to the results of XRD and FTIR tests (), the as-prepared five HANPs were all pure HA. However, they exhibited different material features (), including morphology, size, crystallinity, etc. The in vitro cellular experiments confirmed that all the five HANPs had certain toxicity for A375 cells, but they showed no adverse or even promoting effect on the proliferation of normal HSF cells (; ). This selective inhibition on the proliferation of tumor cells was also reported in some previous literatures.Citation27,Citation47,Citation48 For example, Sun et al found that compared with normal human bronchial epithelial cells (16HBE), the rod-shaped HANPs had the selective growth inhibition and apoptosis on human lung cancer cells (A549) by mitochondrial-targeted pathway.Citation25
In accordance with the previous studies, our results also showed that the cell viability was dependent on the concentration of HANPs.Citation18,Citation24,Citation25,Citation27 However, the viability of A375 melanoma cells did not always decrease with the increase in the concentration of HANPs. At day 3, compared with 100 and 400 μg/mL, 200 μg/mL of HANPs almost in each group exhibited higher inhibition effect on the viability of A375 cells (). This might be ascribed to the aggregation of HANPs in the media, which could be influenced by the concentration of the NPs. The increased particle aggregation in 400 μg/mL of HANPs could weaken the interactions between A375 cells and HANPs, leading to the decreased viability inhibition of the NPs on the cells. This phenomenon was also reported in some previous studies.Citation22,Citation49 However, in order to further verify it, a wider range of HANPs concentration should be selected to investigate the concentration-dependent inhibition on cell viability in future work. After treatment with 200 μg/mL of HANPs (), the as-prepared five HANPs showed different inhibitory effect on the viability of A375 cells, in the order of HA-A> HA-B≈HA-C≈HA-D> HA-E. The lowest cell viability was only 34.90%, indicating the excellent anti-melanoma effect of HA-A.
It is well known that cell death is caused by two different molecular mechanisms, ie, necrosis and apoptosis. Necrosis is a disorderly death of cells caused by strong physicochemical or biological factors.Citation50 Apoptosis is a programmed death of cells caused by DNA fragmentation.Citation51 After treating the cells with 200 μg/mL of HANPs, the nucleus of A375 cells had obvious morphological change and presented the apoptosis features, However, HSF cells were not observed to have obvious nuclear morphological change (), indicating that the HANPs did not induce the apoptosis of normal fibroblasts. The flow cytometry tests further verified that all the HANPs could induce the apoptosis of A375 cells to some extent, and the relatively higher apoptosis rates of A375 cells were found in HA-A (20.10%) and HA-B (19.41%) at day 3.
It is well known that the morphology of HANPs had a certain correlation with tumor cell apoptosis.Citation27,Citation52–Citation54 Laquerriere et al reported that needle-shaped HANPs had stronger cytotoxicity than other morphologies of HANPs.Citation52,Citation53 The influence of the morphology of HANPs on melanoma cells was also evaluated in the present study. The as-prepared five HANPs in the form of granules (HA-A and HA-D), rods (HA-B and HA-C), and needles (HA-E) all promoted the apoptosis of A375 cells to some extent (). Relatively, granular HANPs (HA-A) presented better effect on inducing the apoptosis of A375 cells. We speculated that other material factors could have an effect on the apoptosis of A375 cells. Comparing with other HANPs, granular HANPs (HA-A) had higher SSA and smaller particle size (), and could be more easily absorbed by tumor cells. Cui et al prepared three HANPs with different particle size, and they found that L200-HANPs with smallest size and highest SSA exhibited strongest toxicity to MGC80-3 tumor cells.Citation27 Qi et al reported that small size of chitosan NPs had strong apoptosis induction of Sarcoma-180 and H22 tumor cells.Citation54
Our results confirmed that the crystallinity of HANPs was also an important factor for inducing the apoptosis of tumor cells, as was seldom mentioned in previous studies. Among the as-prepared five HANPs, HA-A had the lowest crystallinity but the highest inhibitory effect on the viability of A375 cells ( and ). The possible reason was that low crystallinity of HANPs could release Ca2+ more efficiently. It is known that Ca2+ plays an important role in almost all cellular processes, especially cell proliferation and apoptosis.Citation55 The proliferation of tumor cells is greatly affected by the change in the Ca2+ concentration of the surrounding environment.Citation56 Generally, intracellular Ca2+ maintains a relatively low level. When penetrating into the cells, HANPs would release Ca2+ into the cytoplasm; this could disrupt intracellular Ca2+ homeostasis and have an adverse impact on cellular metabolism.Citation57,Citation58 The low crystallinity of HA-A released more Ca2+ and exhibited high cytotoxicity of A375 cells. However, it showed no inhibitory effect on the viability of normal HSF cells, same as other four HANPs. This could be attributed to the higher ability of normal cells to regulate Ca2+ balance than that of tumor cells. Tang et al evaluated the intracellular Ca2+ of MGC80-3, HepG2, HeLa, and normal hepatocytes (L-02) after cultured with HANPs, and the results showed that Ca2+ levels in tumor cells were unrecoverable, while normal cells could reduce Ca2+ to baseline level.Citation59 On the other hand, it should be noted that although having a little higher crystallinity and similar Ca2+ release, HA-E exhibited lower inhibitory effect on the viability of A375 melanoma cells. The needle-shaped HA-E could form bigger aggregation of NPs in the solution than granular HA-A. Therefore, comparing with HA-E, HA-A could be absorbed by tumor cells more easily, leading to its higher viability inhibition on the cells.
The previous studies revealed that HANPs could penetrate into the cells by endocytosis.Citation60–Citation62 Our results indicated that the as-prepared five HANPs were all negatively charged, with similar zeta potentials. It is known that cell membrane was also electronegative, which could impede the approach of HANPs through the electrostatic repulsion. But in fact, it did not hinder the uptake of electronegative NPs by tumor cells. Patil et al reported that the negatively charged cerium oxide NPs were preferentially devoured by adenocarcinoma lung cells (A549) compared with positively charged ones.Citation60 Villanueva et al reported that among the four differently charged iron oxide NPs, only the negatively charged ones were effectively absorbed by human cervical carcinoma cells (Hela) and showed some potential toxicity.Citation61 Yin et al reported that HANPs with surface electronegativity could be efficiently taken up by Bel-7402 cells.Citation62 Generally accepted, the endocytosis of negatively charged NPs by tumor cells was dependent on the local positively charged regions occurred on the cell membrane, which electrostatically attracts the NPs.Citation60
Previously, the molecular mechanism of HANPs inducing apoptosis of tumor cells selectively has been widely investigated.Citation63 Generally reported, after culturing with HANPs, apoptosis of tumor cells could be triggered by both the exogenous pathway from the death receptor and the endogenous pathway from mitochondria.Citation26,Citation63 The activation of caspase cascade plays an important role in the apoptosis of tumor cells.Citation26,Citation37,Citation38 The endogenous apoptotic pathway causes caspase-9 to be activated by mitochondrial damage, and the exogenous apoptotic pathway activates caspase-8 by recruiting a death-inducing signaling complex.Citation64 Both pathways subsequently activate the apoptosis-executing factor caspase-3, which ultimately leads to the apoptosis of tumor cells. Our results of PCR analysis () showed that after culturing with the as-prepared five HANPs for 1 day, the gene expressions of caspase-3, caspase-8, and caspase-9 genes in A375 cells were all up-regulated. This indicated that both exogenous and endogenous apoptotic pathways were simultaneously involved in the apoptosis mechanism of A375 cells induced by HANPs.
It is known that as a transcriptional regulation factor, p53 plays an important role in cell apoptosis. The DNA damage could induce the elevated protein level of p53 in the cells, which would then regulate the downstream genes and finally induce cell apoptosis.Citation39,Citation65 We examined the related gene expressions in A375 cells involving the p53-mediated apoptotic pathway, and the results () showed that after culturing with the as-prepared five HANPs, p53 always had significantly up-regulated gene expression in the cells, and the gene expression of the downstream Bax with pro-apoptotic function was also highly up-regulated at day 3. However, as an inhibitor of apoptosis, the gene expression of Bcl-2 was also up-regulated by the HANPs. Therefore, the p53-Bax signaling pathway was also initiated in the course of the apoptosis of A375 cells induced by HANPs.
For the expressions of apoptosis-related genes in A375 melanoma cells at day 1, HA-A showed relatively lower up-regulating effect than other HANPs groups. However, like other groups of HANPs, HA-A exhibited better proapoptosis effect on the cells. One possible reason could be due to the special morphology and size of HA-A. As aforementioned, the granular and smaller HA-A could have better membrane permeability than other HANPs and then cause the apoptosis of the cells. More importantly, comparing with the gene expressions, the synthesis and secretion of the apoptosis-related proteins in the cells regulated by the HANPs would play more decisive role in the cell apoptosis. Therefore, more evidences need be found to reveal the definite mechanism by which HANPs induce apoptosis of A375 melanoma cells.
Likely, the aforementioned apoptosis-related gene expressions in HSF cells were also evaluated by the PCR analysis (). Almost all the tested genes were not activated in HSF cells by HANPs, as could be the main reason that the HANPs only inhibited the viability of A375 melanoma cells and induced the apoptosis of the cells (–; ).
According to the in vitro evaluation, the as-prepared five HANPs exhibited different suppression effect on the viability of A375 melanoma cells. To further verify the in vivo anti-melanoma effect of HANPs, two HANPs (HA-A and HA-C) with different efficiency on inhibiting the viability of A375 cells were selected as the experimental materials for in vivo animal study. The results () confirmed that both HANPs delayed the formation of tumor tissue in the nude mice about 1 week. Moreover, the growth rate of the formed tumor tissue was also inhibited by the HANPs, and HA-A showed stronger inhibitory effect than HA-C. The result was in accordance with the in vitro evaluation, in which HA-A induced the lower viability of A375 melanoma cells than HA-C ().
For HANPs, their physicochemical properties are influenced by each other. Therefore, it is hard to reveal how each material factor inhibits the viability of the melanoma cells or induces the apoptosis of the cells. Therefore, finding out the optimized HANPs for tumor suppression needs much work and advanced technology, such as materials genome initiative based on big data analytics. But anyway, our in vitro and in vivo experiments verified the anti-melanoma effect of HANPs, as could provide a new idea for the clinical treatment of melanoma.
Conclusion
The present study verified the anti-melanoma effect of HANPs by the in vitro and in vivo experiments, while they did not inhibit and even promote the viability of the normal fibroblasts. The physicochemical properties of HANPs, including morphology, size, SSA, crystallinity, and so on, played important role in inhibiting the viability of A375 cells and inducing the apoptosis of the cells. Among the as-prepared five HANPs, HA-A with granular shape, smaller size, higher SSA, and lower crystallinity presented better anti-melanoma efficiency. The apoptosis-related genes were up-regulated in A375 cells but not in HSF cells by the HANPs; this could be the reason that HANPs had the selective inhibition on the viability of melanoma cells. Therefore, HANPs could be an excellent candidate for the clinical treatment of melanoma.
Acknowledgments
This work was financially supported by National Key Research and Development Program of China (2017YFB0702600, 2017YFB0702603), the National Natural Science Foundation of China (81801852) and the “111” Project of China (B16033).
Disclosure
The authors report no conflicts of interest in this work.
References
- KöllischGKalaliBNVoelckerVVarious members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytesImmunology2005114453154115804290
- GrimstadOPukstadBStenvikJEspevikTOligodeoxynucleotides inhibit Toll-like receptor 3 mediated cytotoxicity and CXCL8 release in keratinocytesExp Dermatol201221171222082188
- KonstantinovNKUlff-MøllerCJDimitrovSHistone variants and melanoma: facts and hypothesesPigment Cell Melanoma Res201629442643326909678
- PaluncicJKovacevicZJanssonPJRoads to melanoma: key pathways and emerging players in melanoma progression and oncogenic signalingBiochim Biophys Acta20161863477078426844774
- RigelDSRussakJFriedmanRThe evolution of melanoma diagnosis: 25 years beyond the ABCDsCA Cancer J Clin201060530131620671054
- LensMBDawesMGlobal perspectives of contemporary epidemiological trends of cutaneous malignant melanomaBr J Dermatol2004150217918514996086
- PrasadMLPatelSGHuvosAGShahJPBusamKJPrimary mucosal melanoma of the head and neckCancer200410081657166415073854
- FingerPTRadiation therapy for choroidal melanomaSurv Ophthalmol19974232152329406368
- HallHIMillerDRRogersJDBewerseBUpdate on the incidence and mortality from melanoma in the United StatesJ Am Acad Dermatol199940135429922010
- WeyersWEulerMDiaz-CascajoCSchillWBBonczkowitzMClassification of cutaneous malignant melanomaCancer199986228829910421265
- MukherjiBChakrabortyNGImmunobiology and immunotherapy of melanomaCurr Opin Oncol1995721751847756383
- EttinghausenSERosenbergSAImmunotherapy and gene therapy of cancerAdvances in surgery1995282232547879680
- DorozhkinSCalcium orthophosphates in nature, biology and medicineMaterials200922399498
- TangZLiXTanYFanHZhangXThe material and biological characteristics of osteoinductive calcium phosphate ceramicsRegen Biomater201851435929423267
- HongYFanHLiBGuoBLiuMZhangXFabrication, biological effects, and medical applications of calcium phosphate nanoceramicsMate Sci Eng R Rep2010703–6225242
- AokiHOhgakiMKanoSEffects of Adriacin-absorbing hydroxyap-atite-sol on Ca-9 cell growthRep Inst Med Dent Eng1993273944
- KanoSYamazakiAOtsukaROhgakiMAkaoMAokiHApplication of hydroxyapatite-sol as drug carrierBiomed Mater Eng1994442837950876
- YuanYLiuCQianJWangJZhangYSize-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cellsBiomaterials201031473074019836072
- BauerIWLiSPHanYCYuanLYinMZInternalization of hydroxyapatite nanoparticles in liver cancer cellsJ Mater Sci Mater Med20081931091109517701307
- LiSHuSYanYWangYInvestigation of Hap nanoparticles absorbed by hepatoma cells in vitroJ Wuhan Univ Technol2007222288290
- CaiYLiuYYanWRole of hydroxyapatite nanoparticle size in bone cell proliferationJ Mater Chem200717363780
- QingFWangZHongYSelective effects of hydroxyapatite nanoparticles on osteosarcoma cells and osteoblastsJ Mater Sci Mater Med20122392245225122903597
- HengartnerMOThe biochemistry of apoptosisNature2000407680577077611048727
- ChenMHHanagataNIkomaTHafnium-doped hydroxyapatite nanoparticles with ionizing radiation for lung cancer treatmentActa Biomater20163716517327060620
- SunYChenYMaXMitochondria-targeted hydroxyapatite nanoparticles for selective growth inhibition of lung cancer in vitro and in vivoACS Appl Mater Interfaces2016839256802569027602785
- ChenXDengCTangSZhangMMitochondria-dependent apoptosis induced by nanoscale hydroxyapatite in human gastric cancer SGC-7901 cellsBiol Pharm Bull200730112813217202672
- CuiXLiangTLiuCYuanYQianJCorrelation of particle properties with cytotoxicity and cellular uptake of hydroxyapatite nanoparticles in human gastric cancer cellsMater Sci Eng C Mater Biol Appl20166745346027287142
- LiJYinYYaoFZhangLYaoKEffect of nano- and micro-hydroxyapatite/chitosan-gelatin network film on human gastric cancer cellsMater Lett20086217–1832203223
- LiBGuoBFanHZhangXPreparation of nano-hydroxyapatite particles with different morphology and their response to highly malignant melanoma cells in vitroAppl Surf Sci20082552357360
- StojanovićZVeselinovićLMarkovićSIgnjatovićNUskokovićDHydrothermal synthesis of nanosized pure and cobalt-exchanged hydroxyapatiteMater Manuf Processes20092410–1110961103
- UtaraSKlinkaewnarongJPreparation of nano-hydroxyapatite particles by ultrasonic method at 25 kHz using natural rubber latex as a templating agentChiang Mai J Sci2015432320328
- TomaykoMMReynoldsCPDetermination of subcutaneous tumor size in athymic (nude) miceCancer Chemother Pharmacol19892431481542544306
- ThamaraiselviKRajeswariSSynthesis of hydroxyapatite that mimic bone mineralogyTrends Biomater Artif Organs200619281
- FeatherstoneJDMayerIDriessensFCVerbeeckRMHeijligersHJSynthetic apatites containing Na, Mg, and CO3 and their comparison with tooth enamel mineralCalcif Tissue Int19833521691716850399
- BayraktarDTasACChemical preparation of carbonated calcium hydroxyapatite powders at 37°C in urea-containing synthetic body fluidsJ Eur Ceram Soc19991913–1425732579
- EmanueleSLauricellaMCarlisiDSAHA induces apoptosis in hepatoma cells and synergistically interacts with the proteasome inhibitor bortezomibApoptosis20071271327133817351739
- BudihardjoIOliverHLutterMLuoXWangXBiochemical pathways of caspase activation during apoptosisAnnu Rev Cell Dev Biol151269
- BoatrightKMSalvesenGSMechanisms of caspase activationCurr Opin Cell Biol200315672573114644197
- TayCYFangWSetyawatiMINano-hydroxyapatite and nano-titanium dioxide exhibit different subcellular distribution and apoptotic profile in human oral epitheliumACS Appl Mater Interfaces2014696248625624734929
- TomicTBottonTCerezoMMetformin inhibits melanoma development through autophagy and apoptosis mechanismsCell Death Dis201129e19921881601
- ChenTWongYSZhengWBaiYHuangLSelenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cellsColloids Surf B Biointerfaces2008671263118805679
- WangYYangFZhangHXCuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondriaCell Death Dis201348e78323990023
- YuQHanYWangXCopper silicate hollow Microspheres-Incorporated scaffolds for chemo-photothermal therapy of melanoma and tissue healingACS Nano20181232695270729518321
- WangXLvFLiTElectrospun micropatterned nanocomposites incorporated with Cu2S Nanoflowers for skin tumor therapy and wound healingACS Nano20171111113371134929059516
- ItoASaitoHMitobeKInhibition of heat shock protein 90 sensitizes melanoma cells to thermosensitive ferromagnetic particle-mediated hyperthermia with low Curie temperatureCancer Sci2009100355856419154416
- ItoAMatsuokaFHondaHKobayashiTAntitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanomaCancer Immunol Immunother2004531263214551746
- KramerEPodurgielJWeiMControl of hydroxyapatite nanoparticle morphology using wet synthesis techniques: reactant addition rate effectsMater Lett2014131145147
- ZhangHDarvellBWMorphology and structural characteristics of hydroxyapatite whiskers: effect of the initial Ca concentration, Ca/P ratio and pHActa Biomater2011772960296821421085
- ZhaoHWuCGaoDAntitumor effect by hydroxyapatite nano-spheres: activation of mitochondria-dependent apoptosis and negative regulation of phosphatidylinositol-3-kinase/protein kinase B pathwayACS Nano20181287838785430059628
- WangLZhouGLiuHNano-hydroxyapatite particles induce apoptosis on MC3T3-E1 cells and tissue cells in SD ratsNanoscale2012492894289922450902
- KerrJFRHistory of the events leading to the formulation of the apoptosis conceptToxicology2002181–18224471474
- LaquerrierePGrandjean-LaquerriereAJallotEBalossierGFrayssinetPGuenounouMImportance of hydroxyapatite particles characteristics on cytokines production by human monocytes in vitroBiomaterials200324162739274712711520
- Grandjean-LaquerriereALaquerrierePLaurent-MaquinDGuenounouMPhillipsTMThe effect of the physical characteristics of hydroxyapatite particles on human monocytes IL-18 production in vitroBiomaterials200425285921592715183606
- QiLXuZIn vivo antitumor activity of chitosan nanoparticlesBioorg Med Chem Lett200616164243424516759859
- ClaphamDECalcium signalingCell200713161047105818083096
- SchullerHMCorreaEOrloffMReznikGKSuccessful chemotherapy of experimental neuroendocrine lung tumors in hamsters with an antagonist of Ca2+/calmodulinCancer Res199050516452302720
- MattsonMPChanSLCalcium orchestrates apoptosisNat Cell Biol20035121041104314647298
- MotskinMWrightDMMullerKHydroxyapatite nano and microparticles: correlation of particle properties with cytotoxicity and biostabilityBiomaterials200930193307331719304317
- TangWYuanYLiuCWuYLuXQianJDifferential cytotoxicity and particle action of hydroxyapatite nanoparticles in human cancer cellsNanomedicine20149339741223614636
- PatilSSandbergAHeckertESelfWSealSProtein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potentialBiomaterials200728314600460717675227
- VillanuevaACañeteMRocaAGThe influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cellsNanotechnology2009201111510319420433
- YinMYinYHanYDaiHLiSEffects of uptake of hydroxyapatite nanoparticles into hepatoma cells on cell adhesion and proliferationJ Nanomater20142014117
- WangYWangJHaoHIn vitro and in vivo mechanism of bone tumor inhibition by selenium-doped bone mineral nanoparticlesACS Nano201610119927993727797178
- FuchsYStellerHProgrammed cell death in animal development and diseaseCell2011147474275822078876
- SunJDingTP53 reaction to apoptosis induced by hydroxyapatite nanoparticles in rat macrophagesJ Biomed Mater Res A200988367367918335527