Abstract
Objectives
Acute kidney injury (AKI) is a growing global health concern, and is associated with high rates of mortality and morbidity in intensive care units. Se is a trace element with antioxidant properties. This study aimed to determine whether porous Se@SiO2 nanospheres could relieve oxidative stress and inflammation in ischemia/reperfusion (I/R)-induced AKI.
Methods
Male 6- to 8-week-old C57bl/6 mice were divided into four groups: sham + saline, sham + Se@SiO2, I/R + saline, and I/R + Se@SiO2. Mice in the I/R groups experienced 30 minutes of bilateral renal I/R to induce an AKI. Porous Se@SiO2 nanospheres (1 mg/kg) were intraperitoneally injected into mice in the I/R + Se@SiO2 group 2 hours before I/R, and the same dose was injected every 12 hours thereafter. Hypoxia/reoxygenation (H/R) was used to mimic I/R in vitro. PBS was used as a control treatment. Human kidney 2 cells were seeded into 12-well plates (5×105 cells/well) and divided into four groups: control + PBS group, control + Se@SiO2 group, H/R + PBS group, and H/R + Se@SiO2 group (n=3 wells). We then determined the expression levels of ROS, glutathione, inflammatory cytokines and proteins, fibrosis proteins, and carried out histological analysis upon kidney tissues.
Results
In vitro, intervention with porous Se@SiO2 nanospheres significantly reduced levels of ROS (P<0.05), inflammatory cytokines (P<0.05), and inflammation-associated proteins (P<0.05). In vivo, tubular damage, cell apoptosis, and interstitial inflammation during AKI were reduced significantly following treatment with porous Se@SiO2 nanospheres. Moreover, the occurrence of fibrosis and tubular atrophy after AKI was attenuated by porous Se@SiO2 nanospheres.
Conclusion
Porous Se@SiO2 nanospheres exhibited a protective effect in I/R-induced AKI by resisting oxidative stress and inflammation. This suggests that porous Se@SiO2 nanospheres may represent a new therapeutic method for AKI.
Introduction
Acute kidney injury (AKI) is estimated to occur in 20–200 patients per million of the global population; 7%–18% of these patients remain in hospital and ~50% of patients are admitted to intensive care units (ICUs).Citation1–Citation3 Furthermore, AKI has been widely recognized as being an important risk factor which can lead to the occurrence and progression of chronic kidney disease (CKD).Citation4–Citation6 Ischemic injury is the main cause of AKI, although at present, there is a significant lack of therapeutic options for treatment.Citation7 However, research has provided strong evidence that oxidative stress and inflammation are major contributors to the pathogenesis of ischemic AKI.Citation8–Citation11 Ischemia/reperfusion (I/R) injury can lead to the production of large amounts of reactive oxygen species (ROS) in tubular epithelial cells (TECs), thus triggering mitochondrial damage and lipid peroxidation and causing devastating cell damage. The inflammatory factors produced by TECs cause a large number of inflammatory cells to migrate and infiltrate, further aggravating renal damage, and subsequently, inflammation amplification.Citation12–Citation14
Recent studies have provided evidence that TEC-associated inflammation aggravates kidney injury and impairs tissue repair after I/R injury.Citation15 It has also been demonstrated that nuclear factor-κB (NF-κB) and NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) are involved in mediating injury and inflammation associated with ischemic AKI.Citation16–Citation18 Activation of NF-κB, and the accumulation of NLRP3, can cause levels of the effector molecule caspase-1 to increase, which can then promote the production of interleukin-1β (IL-1β), thus amplifying inflammation and aggravating damage. Therefore, it is particularly important to explore the early damage mechanisms underlying AKI and to intervene and treat this condition, particularly if we wish to prevent the transition from AKI to CKD.
Se is a natural trace element and an ingredient of glutathione peroxidase. Porous Se@SiO2 nanospheres are a new material synthesized by the use of nanotechnology. These nanospheres can directly or indirectly scavenge intracellular free radicals and ROS, thus inhibiting oxidative stress.Citation19 Our previous studies showed that porous Se@SiO2 nanospheres can effectively relieve acute stress damage in the mice heart, and rats’ femoral head and lungs.Citation20–Citation22 However, this has not yet been investigated for potential applications in kidney disease. Therefore, we hypothesized that porous Se@SiO2 nanospheres may have therapeutic significance for I/R-induced AKI. To test this hypothesis, we studied the pattern and dynamics of ROS production, and the expression of inflammation-associated proteins in severe AKI models induced by I/R injury.
Materials and methods
Synthesis and characterization of porous Se@SiO2 nanospheres
Porous Se@SiO2 nanospheres (College of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, Shanghai, China) used herein were synthesized as described in our previous study.Citation21,Citation23 Firstly, Cu2−xSe nanocrystals were prepared and mixed with n-hexanol, n-hexane, deionized water, Triton X-100, and tetraethyl orthosilicate. [Cu(NH3)4]2+ was developed by adding ammonium hydroxide to the mixture. Oxygen was used to oxidize Se2− to develop Se quantum dots. In an alkaline environment, the silica was coated upon the Se quantum dots by orthosilicate hydrolysis, thus forming solid Se@SiO2 nanospheres. Then, the solid Se@SiO2 nanospheres were coated with polyvinylpyrrolidone. After treatment with hot water, the Se@SiO2 nanospheres formed porous structures. Porous Se@SiO2 nanospheres were characterized by means of a D/max-2550 PC X-ray diffractometer (XRD; Rigaku Corporation, Tokyo, Japan; Cu-Kα radiation) and transmission electron microscopy (TEM; JEM-2100F; JEOL, Tokyo, Japan). Synthesized porous Se@SiO2 nanospheres were then dispersed in deionized water for subsequent experiments.
Animal experiments
Male gender confers a greater susceptibility to I/R renal injury and avoids the interference of estrogen in experimental results.Citation24–Citation27 Thus, our experiments involved 6- to 8-week-old male C57bl/6 mice, weighing 20–22 g (SLAC Laboratory Animal Center, Shanghai, China). In total, 60 mice were randomly divided equally (n=15) into four groups: sham + saline, sham + Se@SiO2, I/R + saline, and I/R + Se@SiO2 (). The I/R group mice were exposed to 30 minutes of bilateral renal I/R to induce an AKI. In the I/R + Se@SiO2 group, 1 mg/kg of porous Se@SiO2 nanospheres was intraperitoneally injected 2 hours before I/R, and the same dose of porous Se@SiO2 nanospheres was injected every 12 hours thereafter. The sham groups were injected with the same amount of saline and Se@SiO2, respectively. Each group was randomly divided into three subgroups (), which were sacrificed at 24 hours, 72 hours, and 2 weeks after the initial surgery. Serum samples were then collected and stored at −80°C for detection and analysis. Both kidneys were immediately removed from the culled animals and processed for histological evaluation and protein extraction. The animal experimental protocol used herein was in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Ethics Committee of Shanghai General Hospital.
Table 1 Treatments and survivals of different experimental mice groups
Renal function
In order to evaluate renal function, we measured blood urea nitrogen (BUN) and creatinine (Cr) levels in the serum using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China).
ELISA
ELISA kits, including mouse IL-1β, monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), and human glutathione (GSH), were purchased from Jiancheng Bioengineering Institute. Cell culture supernatants were collected and centrifuged for 10 minutes at a speed of 1,500 rpm. Levels of cytokines and GSH in the cell supernatant were subsequently determined according to the relevant manufacturer’s protocol. All test indicators were performed in triplicate.
Histology and apoptosis evaluation
Renal histology was subjected to a blind examination after H&E staining. Severe injury, restricted to the highly susceptible outer medullary regions, is known to result in significant tubular damage and atrophy over the long term.Citation15,Citation28 Tissue damage was then scored according to the proportion (%) of damaged tubules: 0, no damage; 1, <25% damage; 2, 25%–50% damage; 3, 50%–75% damage; and 4, >75% damage.Citation29 The criteria for tubular damage included the loss of brush border, tubular dilation, cast formation, and cell lysis. For quantification, 10 fields per section were randomly selected at a magnification of ×200 for evaluation and scoring. Tubular atrophy was also scored in the same way: 0, no atrophy; 1, <25% atrophy; 2, 25%–50% atrophy; 3, 50%–75% atrophy; and 4, >75% atrophy. Ten fields per section were randomly selected at a magnification of ×200 for evaluation and scoring. The TUNEL assay was also carried out according to the manufacturer’s protocol (In Situ Cell Death Detection Kit, POD; Hoffman-La Roche Ltd, Basel, Switzerland). Ten fields per section were randomly selected from each tissue section at a magnification of ×200 for evaluation.
Renal immunohistochemistry
The kidneys were removed and fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 3-µm sections. Sirius Red, Masson’s trichrome, and monoclonal mouse anti-α-smooth muscle actin (α-SMA; 1:200 dilution; Cell Signaling Technology, Danvers, MA, USA) stains were used to estimate fibrosis. Immunohistochemistry was performed to assess renal tubular injury and macrophage infiltration. For immunohistochemistry, kidney tissues sections were first deparaffinized and then incubated with 0.1 M sodium citrate (pH 6.0) at 98°C for antigen retrieval for 15 minutes. After incubation with blocking buffers, tissue sections were exposed sequentially to the primary antibody, and then a biotinylated secondary antibody (Dako REAL™ EnVision™; Dako Denmark A/S, Glostrup, Denmark). Immunohistochemical positive staining was consecutively revealed by the 3,3′-Diaminobenzidine Peroxidase Substrate Kit (Dako REAL™ EnVision™; Dako Denmark A/S) in accordance with the manufacturer’s instructions. The following antibodies were used: monoclonal rabbit anti-neutrophil gelatinase-associated lipocalin (NGAL; 1:200 dilution; Abcam, Cambridge, UK) and monoclonal rabbit anti-F4/80 (1:200 dilution; Cell Signaling Technology). We randomly chose 10 fields at ×200 magnification in each slide to score the severity of interstitial fibrosis (Masson, Sirius Red, and α-SMA staining): 0, no evidence of interstitial fibrosis; 1, <10% involvement; 2, 10% to <25% involvement; 3, 25% to <50% involvement; and 4, 50% to <75% involvement; and 5, >75% involvement. NGAL staining was then scored according to the proportion (%) of positive areas: 0, no positive; 1, <10% positive; 2, 10%–20% positive; 3, 20%–30% positive; 4, 30%–40% positive; and 5, >40% positive. For quantification, 10 fields per section were randomly selected at a magnification of ×200 for evaluation and scoring.
Cell culture and hypoxia/reoxygenation (H/R)
Human kidney 2 (HK-2; FuHeng Cell Center, Shanghai, China) cells, a proximal tubular cell line derived from normal kidneys, were grown in DMEM/nutrient mixture F-12 (DMEM/F-12; HyClone Laboratories Inc, Logan, UT, USA) with 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA), 100 µg/mL streptomycin, and 100 U/mL penicillin (Thermo Fisher Scientific). The cells were cultured at 37°C in a 5% CO2 incubator, and the culture medium was changed every 2–3 days. The cells were digested with 0.25% trypsin (Thermo Fisher Scientific) and passaged when they reached 80%–90% confluency. The cells were then exposed to an anaerobic medium (serum- and glucose-free) in a hypoxia incubator chamber (STEMCELL Technologies, Vancouver, BC, Canada) with an anoxic mixture gas (95% N2 and 5% CO2) for 12 hours at 37°C followed by reoxygenation for 1.5 hours with fresh culture medium (95% air and 5% CO2) to simulate I/R injury.
Cell viability assays
The influence of porous Se@SiO2 nanospheres on cell viability was detected with a cell counting kit-8 (CCK-8; Yeasen Biological Technology, Shanghai, China). HK-2 cells (8×103/well) were grown and treated with porous Se@SiO2 nanospheres for 24 hours in 96-well plates. The concentrations of porous Se@SiO2 nanospheres applied to HK-2 cells were 0, 10, 20, 40, 80, 100, 150, 200, 400, and 600 µg/mL. After 24 hours, 10 µL of CCK-8 solution was added to each well, and the plates were incubated for 2 hours. The absorbance at 450 nm was then measured using a multimode reader (Multiskan GO; Thermo Fisher Scientific).
Assay for intracellular ROS production
H/R was used to mimic I/R in vitro with PBS as a control treatment. HK-2 cells were seeded into 12-well plates (5×105 cells/well) and divided into four groups: control + PBS group, control + Se@SiO2 group, H/R + PBS group, and H/R + Se@SiO2 group (n=3 wells). The H/R + Se@SiO2 group was pre-stimulated using porous Se@SiO2 nano-spheres at a final concentration of 40 µg/mL for 6 hours. H/R groups were exposed to H/R. The levels of intracellular ROS were subsequently detected with a ROS assay kit (Jiancheng Bioengineering Institute). Fluorescent signals were then determined with a fluorescence microscope (Zeiss LSM 800; Carl Zeiss Meditec AG, Jena, Germany). The production of ROS was quantified by using ImageJ software (National Institutes of Health, Bethesda, MD, USA). ROS ratio (%) = (each group mean OD value/normal control (NC) + PBS group mean OD value) ×100%. For quantification, 10 fields from each group were randomly selected at a magnification of ×400. In the assessment of all fields, identical analysis settings and thresholds were employed.
Western blotting
HK-2 cells were cultured in six-well plates. Two wells were treated as one treatment group and divided into three groups (n=2 wells): control + PBS group, H/R + PBS group, and H/R + Se@SiO2 group. HK-2 cells and renal tissue from the outer medulla area were lysed and denatured at 100°C for 5 minutes in SDS buffer and separated by 10% PAGE. Proteins were then transferred onto 0.45-µm polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA), blocked for 1 hour with 5% dried nonfat skimmed milk powder in TBS-Tween-20 (TBS containing 0.1% Tween 20), and probed with appropriate antibodies. The following primary antibodies were used: monoclonal rabbit antibodies anti-phospho-NF-κB (1:1,000 dilution; Cell Signaling Technology), anti-NLRP3 (1:1,000 dilution; Cell Signaling Technology), anti-collagen type I, anti-collagen type III (1:1,000 dilution; Abcam), anti-NGAL (1:1,000 dilution; Abcam), and caspase-1 (1:1,000 dilution; Proteintech, Wuhan, China) and monoclonal rabbit antibodies anti-glyceraldehyde-3-phosphate dehydrogenase and anti-β-actin (1:2,000 dilution; Proteintech). Positive protein binding was visualized by a horseradish peroxidase-conjugated secondary antibody followed by the use of an enhanced chemiluminescence kit (New Cell Molecular Biotech, Suzhou, China).
Statistical analysis
Qualitative data, including histological tissue images, were representative of at least three experiments. Quantitative data were expressed as means ± SD. Statistical analysis was conducted using GraphPad Prism 5 (Graphpad Software Inc, La Jolla, CA, USA). Multiple groups were compared by ANOVA followed by Tukey’s posttests. Statistical differences between two groups were determined by two-tailed unpaired or paired t-tests. P<0.05 was considered to be significantly different.
Results
Structure, characterization, and toxicity of porous Se@SiO2 nanospheres
Porous Se@SiO2 nanospheres were prepared according to a previous methodology.Citation23 The phase structure of the porous Se@SiO2 nanospheres was detected by an XRD pattern (). There were several well-defined characteristic peaks, such as (100), (011), (110), and (012), indicating the hexagonal phase and referenced as the standard Se phase (Joint Committee on Powder Diffraction Standards card no 65-1876). In addition, due to the amorphous silica coating, the XRD pattern of porous Se@SiO2 nanospheres showed a steady increase in the low angle region. TEM () showed that the porous Se@SiO2 nanospheres had a diameter of ~55 nm, and that many very small nanoparticles (<5 nm) were interspersed from the center to the surface ().
Figure 1 Structure, characterization, and toxicity of porous Se@SiO2 nanocomposites.
Notes: (A) Characterization of porous Se@SiO2 nanospheres: (a) XRD pattern of the porous Se@SiO2 nanospheres and standard hexagonal phase of Se (Joint Committee on Powder Diffraction Standards and no 65-1876). (b) TEM of porous Se@SiO2 nanospheres. (c) Low magnification and (d) high magnification images of porous Se@SiO2 nanospheres. (B) The influence of porous Se@SiO2 nanospheres on the viability of HK-2 cells was detected by a cell counting kit-8. Data were expressed as means ± SDs (n=3). Scr changes (C, D) and ALT changes (E, F) caused by different doses of porous Se@SiO2 nanospheres to mice were detected during 24 hours and 2 weeks. Data were expressed as means ± SDs (n=3).
Abbreviations: TEM, transmission electron microscopy; XRD, X-ray diffractometer; Scr, serum creatinine; ALT, alanine aminotransferase; ip, intraperitoneal dose.
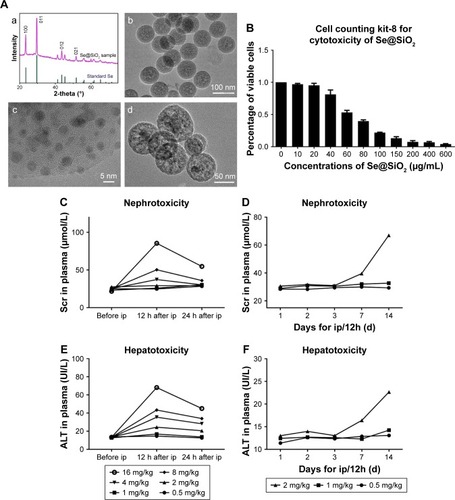
In order to use porous Se@SiO2 nanospheres safely and effectively, we first determined their toxicity in vivo and in vitro. As shown in , a concentration of 40 µg/mL was reasonable with only minimal toxicity in vitro. When the concentration exceeds 40 µg/mL, side effects could significantly inhibit cell viability and proliferation. As in our previous research,Citation30 1 mg/kg of porous Se@SiO2 nanospheres appeared to be the appropriate dose for in vivo experiments because this concentration was associated with very little toxic damage to the liver and kidney (). We determined that this dose would not affect liver and kidney function even if administered to mice per 12 hours until 2 weeks of duration (). An excessive concentration of Se@SiO2 would cause significant damages to both the liver and kidney, thereby elevating serum levels of alanine aminotransferase and Cr.
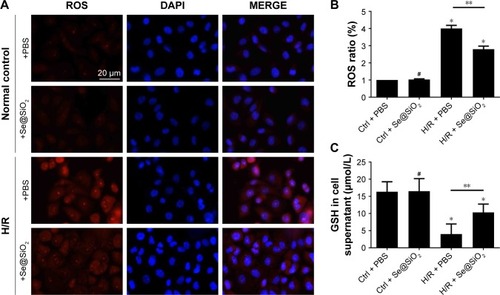
Se@SiO2 reduced the production of ROS in HK-2 cells after H/R
In vitro, considerable levels of ROS were produced in HK-2 cells exposed to H/R (P<0.05 vs control + PBS) (). However, there was no difference in ROS production when compared between control groups with PBS or Se@SiO2 treatment (P>0.05) (). Compared with the H/R + PBS group, the H/R + Se@SiO2 group pretreated by Se@SiO2 showed a significant reduction of ROS (P<0.05) (), indicating that Se@SiO2 can suppress the hypoxia-induced production of ROS in order to protect cells against oxidative stress damage. In addition, we found that the production of GSH in the H/R groups was much lower than that in control groups, and that GSH in the H/R + Se@SiO2 group was significantly higher than in the H/R + PBS group (P<0.05) (). GSH is the main antioxidant present in living organisms and its deficiency may result in insufficient antioxidant capacity against I/R-induced AKI. These data proved that Se@SiO2 can not only reduce ROS levels but also preserve GSH levels in H/R.
Figure 2 The level changes of ROS and GSH in HK-2 cells with different treatments.
Notes: (A) Staining of HK-2 cells with ROS, DAPI. Scale bars =20 µm. (B) Changes of ROS levels in HK-2 cells after different treatments of PBS or porous Se@SiO2 nanospheres. Data were expressed as means ± SDs (n=10). #P>0.05, *P<0.05 vs Ctrl + PBS group, **P<0.05, H/R + PBS vs H/R + Se@SiO2. (C) Changes of GSH levels in HK-2 cell supernatant after different treatments of PBS or porous Se@SiO2 nanospheres. #P>0.05, *P<0.05 vs Ctrl + PBS group, **P<0.05, H/R + PBS vs H/R + Se@SiO2. Data were expressed as means ± SDs (n=3).
Abbreviations: Ctrl, control; GSH, glutathione; H/R, hypoxia/reoxygenation.
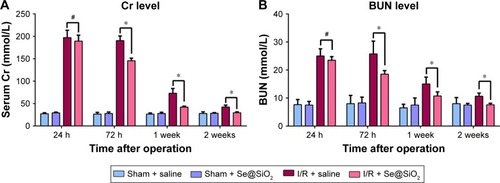
Se@SiO2 attenuated tubular injury and protected renal function
To determine the effect of Se@SiO2 upon renal function in vivo, we induced renal I/R injury in experimental mice. Then, we measured levels of Cr and BUN, which are the most representative clinical indicators of renal function, at 24 hours, 72 hours, 1 week, and 2 weeks. We also evaluated renal function and found that plasma Cr and BUN levels in I/R mice were more than two times higher than those in sham mice at 24 hours (P<0.05) (), which corresponded with the clinical diagnostic criteria for AKI.Citation31 Interestingly, there was no statistical difference in the levels of Cr and BUN when compared between the I/R + Se@SiO2 group and the I/R + saline group at 24 hours (P>0.05). However, Se@SiO2 treatment alone resulted in a significant reduction in Cr and BUN levels from 3 days to 2 weeks after I/R (P<0.05 vs I/R + saline), whereas these groups showed very little difference when tested 24 hours after I/R (P>0.05) (). This indicated that Se@SiO2 had a certain protective effect upon renal function.
Figure 3 Cr/BUN levels in different groups of mice treated with saline or porous Se@SiO2 nanospheres.
Notes: (A, B) There was no significant difference of Cr/BUN levels between I/R + Se@SiO2 group and I/R + Se@SiO2 group 24 hours after I/R. #P>0.05. But the levels of Cr/BUN in I/R + Se@SiO2 group were decreased significantly compared to those in I/R + saline group from 3 days to 2 weeks after I/R. *P<0.05. Data were expressed as means ± SDs (n≥4).
Abbreviations: Cr, creatinine; BUN, blood urea nitrogen; I/R, ischemia/reperfusion.
Morphologically, renal TECs located in the outer medulla are extremely sensitive to intrinsic oxidative stress, particularly during the reperfusion phase.Citation32,Citation33 Therefore, we further evaluated the damage caused to the outer medullary regions in each experimental group. Upon histological examination, the I/R group showed features typical of severe acute tubular damage, including extensive tubular necrosis, tubular dilatation, and loss of the brush border. Mice treated with Se@SiO2 had significantly lower H&E scores for tubular damage after I/R (P<0.05 vs I/R + saline) (). Moreover, as shown in the results, the I/R + Se@SiO2 group exhibited less cellular apoptosis (P<0.05 vs I/R + saline), as detected by TUNEL staining (). NGAL protein, a biomarker of AKI, is known to accumulate in the blood and urine and can be detected in patients with AKI after only a few hours.Citation34 We found that the proportion of NGAL-positive tissue in samples from the I/R + saline group was significantly greater than that in the I/R + Se@SiO2 group (P<0.05), while the sham group was normal (). Compared to groups without Se@SiO2 intervention, Western blotting results also showed that the expression of NGAL was inhibited in the Se@SiO2 intervention group, both in vivo (P<0.05) () and in vitro (P<0.05) (). Hence, these results show that intervention with Se@SiO2 was favorable for the renal tubules and had a certain protective effect during I/R-induced AKI.
Figure 4 Histological analysis and apoptosis assessment for kidney tissues.
Notes: (A) Mouse kidney tissues stained with H&E. Scale bars =0.2 mm. (B) Tubular damage in outer medullary tissues from sham groups and I/R groups was semi-quantified using pathological scores. *P<0.05. Data were expressed as means ± SDs (n=10). (C) NGAL was detected in kidney tissues by immunohistochemistry. Scale bars =0.2 mm. (D) Positive NGAL area in outer medullary tissues from sham groups and I/R groups was semi-quantified using scores. *P<0.05. Data were expressed as means ± SDs (n=10). (E) Apoptosis cells in tissue were detected using a TUNEL kit. Scale bars =0.2 mm. (F) Statistical analysis of TUNEL staining. The numbers of TUNEL- positive cells of all cells in the field was calculated under a fluorescent microscope. *P<0.05. Data were expressed as means ± SDs (n=10).
Abbreviations: NGAL, neutrophil gelatinase-associated lipocalin; I/R, ischemia/reperfusion.
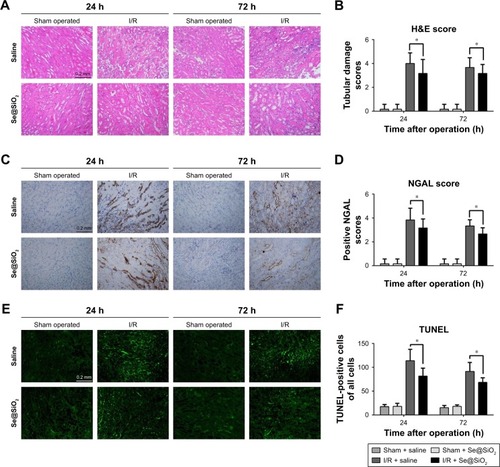
Figure 5 Expression changes of p-NF-κB/NLRP3/caspase-1 pathway and NGAL proteins with porous Se@SiO2 nanospheres intervention in vivo and in vitro.
Notes: (A, B) Expression of p-NF-κB/NLRP3/caspase-1 and NGAL protein in HK-2 cells was determined by Western blotting. *P<0.05, H/R + Se@SiO2 group vs H/R + PBS group. Data were expressed as means ± SDs (n=3). (C, D) Expression of p-NF-κB/NLRP3/caspase-1 and NGAL proteins in mouse kidney tissues was determined by Western blotting. *P<0.05, I/R + Se@SiO2 group vs sham + saline group. Data were expressed as means ± SDs (n=3).
Abbreviations: p-NF-κB, phosphorylated nuclear factor-κB; NLRP3, NACHT, LRR, and PYD domains-containing protein 3; NGAL, neutrophil gelatinase-associated lipocalin; I/R, ischemia/reperfusion; H/R, hypoxia/reoxygenation.
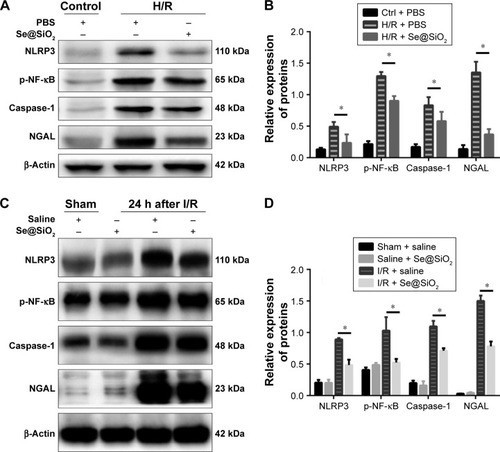
The NF-κB/NLRP3/caspase-1 pathway was suppressed by Se@SiO2 treatment
In vivo, NF-κB, NLRP3, and caspase-1 are the main effectors of the inflammasome signaling interactive pathway, which regulates inflammatory responses. As experiments performed in vitro showed, the expression of phospho-NF-κB, NLRP3, and caspase-1 were significantly increased in the H/R groups compared with the control + PBS group (P<0.01) (). However, the expression of phospho-NF-κB, NLRP3, and caspase-1 in the H/R group pretreated with Se@SiO2 were reduced significantly compared to the H/R + PBS group (P<0.05) (). Our in vivo experiments further showed that the increase in phospho-NF-κB, NLRP3, and caspase-1 expression in response to I/R injury was inhibited by treatment with Se@SiO2 (P<0.05 vs I/R + saline group) (). Collectively, these results demonstrated that Se@SiO2 can inhibit activation of the NF-κB/NLRP3/caspase-1 pathway.
Se@SiO2 relieved I/R-induced inflammation both in vitro and in vivo
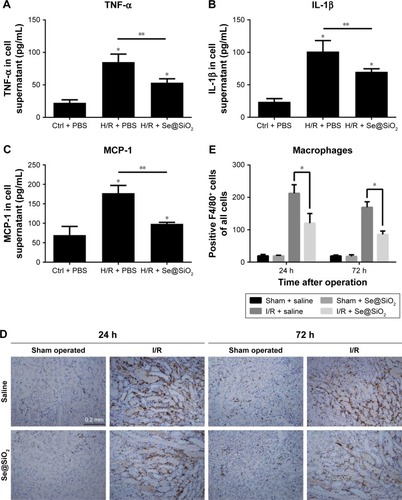
Next, we decided to assess whether inflammation could be reduced by Se@SiO2 as well. Damaged TECs produce pro-inflammatory cytokines and chemokine, including TNF-α, IL-1β, and MCP-1, which are secreted outside of cells.Citation12 In vitro we found that porous Se@SiO2 nanospheres significantly reduced pro-inflammatory cytokine production from HK-2 cells exposed to H/R (P<0.05 vs H/R + PBS group) (). F4/80 is a surface antigen preferentially expressed on monocytes/macrophages in mice. Macrophages are known to migrate into the outer medulla of the rat kidney following ischemia/reperfusion injury (IRI).Citation35 Animal models demonstrate that macrophages are a major contributor to the inflammatory response and fibrosis to AKI.Citation11 To assess renal inflammation after IR, we examined the number of F4/80+ cells in each group. Interestingly, with Se@SiO2 intervention, the quantity of macrophages in kidneys exposed to I/R was significantly reduced compared to the nonintervention group, indicating that inflammatory infiltration of the kidneys was suppressed (P<0.05) (). These data indicate that porous Se@SiO2 nanospheres can relieve inflammation in I/R-induced AKI.
Figure 6 Changes of inflammation involving cytokines and macrophages after porous Se@SiO2 nanospheres treatment.
Notes: (A–C) The secretion levels of TNF-α, IL-1β, and MCP-1 in HK-2 cells supernatant as determined by Western blotting. *P<0.05 vs Ctrl + PBS group, **P<0.05, H/R + Se@SiO2 group vs H/R + PBS group. Data were expressed as means ± SDs (n=3). (D, E) Macrophage infiltration level in mouse kidney tissues was determined by immunohistochemistry for F4/80. *P<0.05, I/R + Se@SiO2 group vs I/R + saline group. Scale bars =0.2 mm. Data were expressed as means ± SDs (n=10).
Abbreviations: TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; MCP-1, monocyte chemotactic protein-1; Ctrl, control; I/R, ischemia/reperfusion; H/R, hypoxia/reoxygenation.
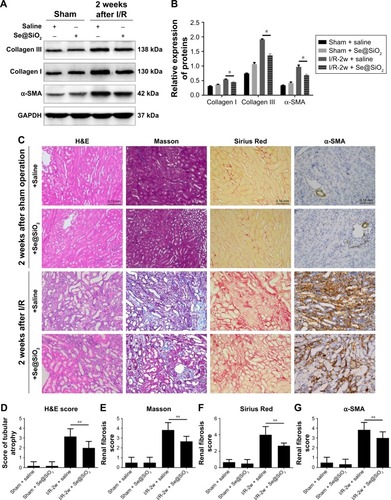
Se@SiO2 attenuated tubular atrophy and interstitial fibrosis
Severe ischemic AKI may progress to CKD, and is mainly characterized by renal parenchymal atrophy and interstitial fibrosis.Citation28 Renal interstitial fibrosis and tubular atrophy are the main pathological features of chronic progression after ischemic AKI.Citation36,Citation37 To further investigate the effect of Se@SiO2 on the prognosis of kidneys after AKI, we performed morphological and histological comparisons in the kidneys of mice 2 weeks after reperfusion. Masson and Sirius Red staining can specifically label collagen fibers, and can thus be used to evaluate renal fibrosis. Collagen type I, collagen type III, and α-SMA are commonly used markers used to evaluate fibrosis. Renal tubular atrophy was assessed using H&E scores (). Results showed that kidneys treated with Se@SiO2 for 2 weeks after AKI exhibited less tubular atrophy and interstitial fibrosis (P<0.05 vs I/R + saline) (). Masson and Sirius Red staining showed appreciably increased renal fibrosis in I/R mice experiencing Se@SiO2 treatment but a robust increase of fibrosis in I/R mice with saline treatment compared with sham groups. Renal fibrosis in I/R mice experiencing Se@SiO2 treatment was significantly reduced compared to the I/R + saline group (P<0.05). Consistent with the increase in histological fibrosis, the expression of fibrotic proteins, including collagen type I, collagen type III, and α-SMA, were all increased in the I/R + Se@SiO2 group compared with sham mice and further increased in the I/R + saline group (). In other words, early treatment with Se@SiO2 can effectively attenuate tubular atrophy and renal fibrosis following AKI.
Figure 7 The effect of porous Se@SiO2 nanospheres on renal prognosis after AKI.
Notes: (A, B) The expression levels of collagen I and III and α-SMA protein in mouse kidney tissues 2 weeks after I/R-induced AKI as detected by Western blotting. *P<0.05, I/R-2w + Se@SiO2 group vs I/R-2w + saline group. Data were expressed as means ± SDs (n=3). (C) Renal histology H&E and fibrosis staining in 2 weeks after AKI. Scale bars =0.15 mm. Data were expressed as means ± SDs (n=10). (D) H&E tubule atrophy score was used to assess the severity of tubule atrophy after AKI. **P<0.05. Data were expressed as means ± SDs (n=10). (E–G) Masson trichrome staining, Sirius Red staining, and α-SMA immunohistochemical staining were used to assess the severity of renal fibrosis by proportion score. **P<0.05, I/R-2w + Se@SiO2 group vs I/R-2w + saline group. Data were expressed as means ± SDs (n=10).
Abbreviations: α-SMA, α-smooth muscle actin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; 2w, 2 weeks; I/R, ischemia/reperfusion; AKI, acute kidney injury.
Discussion
AKI is a common and important diagnostic and therapeutic challenge for clinicians. Currently, it is the most frequent cause of organ dysfunction in ICUs and the occurrence of even mild AKI is associated with a 50% higher risk of death.Citation38 Furthermore, incomplete recovery from severe AKI can lead to long-term functional deficits and greater risk of progression to CKD. The kidneys of patients recovering from AKI exhibit chronic dysfunction, tubular atrophy, and interstitial fibrosis.Citation28 Therefore, the fundamental principle of preventing AKI is to treat the cause or trigger.Citation31
However, the mechanism underlying I/R-induced AKI is yet to be fully elucidated. Most previous studies have demonstrated that large amounts of ROS produced by H/R in I/R-induced AKI lead to TEC damage, thus activating the inflammatory signaling pathway and triggering a cascade of inflammatory responses.Citation8,Citation9,Citation39,Citation40 TNF-α and IL-1β are regarded as important inflammatory mediators in the early stages of acute tissue injury.Citation41 TNF-α and IL-1β have been confirmed to regulate the activity of helper T lymphocytes, the accumulation of neutrophils, macrophages, and lymphocytes, and as mediators of the inflammatory response. MCP-1 is a small cytokine, which recruits monocytes, memory T cells, and dendritic cells to the sites of inflammation produced by either tissue injury or infection.Citation42,Citation43 Ischemic AKI involves complement activation, the generation of cytokines and chemokine, including TNF-α, IL-1β, MCP-1, and infiltration of the kidney by macrophages.Citation12,Citation44 Many researchers have reported that macrophages are involved in I/R-induced AKI damage and inflammation, which may stimulate fibroblasts and accelerate kidney fibrosis after AKI.Citation45,Citation46
Our current research found that proteins, including phosphorylated NF-κB (p-NF-κB), NLRP3, and caspase-1, were highly expressed in kidney tissue experiencing IRI. It was previously reported that NF-κB and NLRP3 are involved in AKI.Citation16–Citation18,Citation47,Citation48 NF-κB regulates the expression of numerous genes, including cytokines/chemokines, cell adhesion molecules, and stress response genes. p-NF-κB is trafficked into the nucleus to activate the expression of inflammatory cytokines (IL-1β, TNF-α, IL-6, etc) and then forms an “inflammatory cascade” to initiate renal interstitial inflammation.Citation49 NLRP3−/− mice are protected against ischemic AKI, thus confirming that the role of the NLRP3 inflammasome is unfavorable in ischemic AKI.Citation17,Citation18 Interestingly, our results showed that increased levels of cytokines (TNF-α, IL-1β, MCP-1) and proteins (p-NF-κB, NLRP3, caspase-1) were not significant owing to the intervention of porous Se@SiO2 nanospheres. Thus, these results indicated that porous Se@SiO2 nanospheres may alleviate I/R-induced inflammation by resisting ROS.
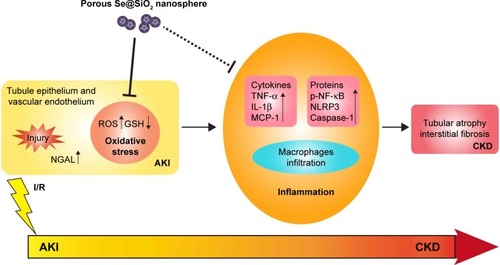
In terms of the long-term outcome after AKI, more and more studies have found that ischemic AKI is associated with tubular interstitial inflammation, which may contribute to renal atrophy and sclerosis in the future.Citation37,Citation50 The combination of tubular atrophy and tubular interstitial fibrosis (TIF) is an important hallmark of CKD because tubular atrophy has repeatedly been shown to be superior to glomerular pathology as a predictor of CKD progression.Citation51,Citation52 Based on existing research and data, we can hypothesize that the kidney develops a strong oxidative stress response while experiencing I/R injury. Subsequently, there is a long-term inflammatory reaction in the kidney which includes the infiltration of monocytes and lymphocytes, which contribute to the proliferation of renal interstitial fibroblasts. Renal tubules exposed to severe injury gradually undergo atrophy, which makes the structure of the kidney change irreversibly, thus leading to abnormal functionality. The outcome of TIF and tubular atrophy appears to be responsible for the progression from AKI to CKD (). Reducing the prevalence of CKD is therefore of utmost importance. Therefore, reducing the severity of injury in early AKI, attenuating oxidative stress, and restricting or preventing further enhanced inflammatory responses are the key to preventing AKI–CKD transformation ().
Figure 8 The description of porous Se@SiO2 nanospheres attenuating ischemic AKI injury and improving prognosis.
Note: Porous Se@SiO2 nanospheres attenuate ischemic AKI injury and improve prognosis by reducing the severity of injury in early AKI, resisting oxidative stress, relieving inflammation, and preventing further transition of AKI to CKD.
Abbreviations: AKI, acute kidney injury; GSH, glutathione; CKD, chronic kidney disease; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; MCP-1, monocyte chemotactic protein-1; p-NF-κB, phosphorylated nuclear factor-κB; NLRP3, NACHT, LRR, and PYD domains-containing protein 3; NGAL, neutrophil gelatinase-associated lipocalin.
As a trace element, Se exerts strong antioxidant properties and participates in regulating the activity of GSH.Citation53 However, Se also has cytotoxic properties, and a relatively narrow dose range separates physiological effects from toxicity.Citation54 The porous structure of Se@SiO2 nanospheres means that they are slow-releasing and provide favorable biocompatibility. In vitro, intervention with porous Se@SiO2 nanospheres could significantly reduce the levels of ROS () and inflammatory cytokines (TNF-α, IL-1β, MCP-1) () produced by HK-2 cells after H/R treatment. In vivo, porous Se@SiO2 nanospheres significantly attenuated renal tubular damage () and reduced cell apoptosis () and macrophage infiltration () during AKI. Moreover, the occurrence of fibrosis and tubular atrophy after AKI was reduced significantly following the injection of porous Se@SiO2 nanospheres (). Results showed that porous Se@SiO2 nanospheres effectively inhibited the increased expression of phospho-NF-κB, NLRP3, and caspase-1 when AKI occurred in vivo and in vitro (). However, the specific mechanism underlying how the porous Se@SiO2 nanospheres can inhibit the inflammatory signaling pathway effector molecules p-NF-κB, NLRP3, and caspase-1, and whether the anti-inflammatory effect of Se@SiO2 occurs directly through the inhibition of the inflammatory pathway or indirectly by combatting oxidative stress remain unclear and require further investigation.
In addition, whether Se@SiO2 can play the same role in human beings is still uncertain. Moreover, the optimal administration method and administration time for porous Se@SiO2 nanospheres for I/R-induced AKI have still to be defined. It is therefore important that future work addresses the specific therapeutic mechanisms of Se@SiO2.
Conclusion
In this study, we verified that Se@SiO2 reduced the production of ROS, preserved GSH, and diminished the expression of inflammation-associated proteins and cytokines. Consequently, Se@SiO2 could alleviate oxidative stress and inflammation, exhibiting a protective effect for the kidney in AKI. Subsequently, tubular atrophy and fibrosis were attenuated by Se@SiO2 intervention. Hence, porous Se@SiO2 nanospheres were effective agents which may represent a new therapeutic method for the clinical therapy of AKI and prevent the transition from AKI to CKD.
Author contributions
ZZheng, GD, CQ, YX and ZZhao performed the studies and statistical analyses, interpreted the data, and wrote the manuscript. XL and YC collected the materials and managed references. ZZhang, HW, and JL conducted the experiments. ZZheng, GD, CQ, YX, YC, ZZhang, HW and JL have revised the manuscript critically and prepared the final version of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no 21476136), Natural Science Foundation of Shanghai (grant no 15ZR1433500), Science and Technology Commission of Shanghai Municipality (grant no 15140902900), China and medical-engineering funding of Shanghai Jiao Tong University (grant no ZH2018QNA20).
Disclosure
The authors report no conflicts of interest in this work.
References
- ChawlaLSBellomoRBihoracAAcute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 WorkgroupNat Rev Nephrol201713424125728239173
- HosteEABagshawSMBellomoREpidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI studyIntensive Care Med20154181411142326162677
- LewingtonAJCerdáJMehtaRLRaising awareness of acute kidney injury: a global perspective of a silent killerKidney Int201384345746723636171
- HsuCYYes, AKI truly leads to CKDJ Am Soc Nephrol201223696796922499588
- WaikarSSWinkelmayerWCChronic on acute renal failure: long-term implications of severe acute kidney injuryJAMA2009302111227122919755705
- Kam Tao LiPBurdmannEAMehtaRLWorld Kidney Day Steering Committee 2013Acute kidney injury: Global health alertJ Nephropathol201322909724475433
- ZhangYLQiaoSKWangRYGuoXNNGAL attenuates renal ischemia/reperfusion injury through autophagy activation and apoptosis inhibition in ratsChem Biol Interact2018289404629704511
- WinterbergPDWangYLinKMReactive oxygen species and IRF1 stimulate IFNα production by proximal tubules during ischemic AKIAm J Physiol Renal Physiol20133052F164F17223657854
- GuYHuangFWangYConnexin 32 plays a crucial role in ROS-mediated endoplasmic reticulum stress apoptosis signaling pathway in ischemia reperfusion-induced acute kidney injuryJ Transl Med201816111729728112
- Andres-HernandoALiNCicerchiCProtective role of fructokinase blockade in the pathogenesis of acute kidney injury in miceNat Commun201781418128194018
- HuenSCCantleyLGMacrophages in Renal Injury and RepairAnnu Rev Physiol201779144946928192060
- RabbHGriffinMDMckayDBInflammation in AKI: Current Understanding, Key Questions, and Knowledge GapsJ Am Soc Nephrol201627237137926561643
- FriedewaldJJRabbHInflammatory cells in ischemic acute renal failureKidney Int200466248649115253694
- MulaySRLinkermannAAndersHJNecroinflammation in Kidney DiseaseJ Am Soc Nephrol2016271273926334031
- DevarajanPUpdate on mechanisms of ischemic acute kidney injuryJ Am Soc Nephrol20061761503152016707563
- SzetoHHLiuSSoongYMitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1β and IL-18 and Arrests CKDJ Am Soc Nephrol20172851437144927881606
- KimHJLeeDWRavichandranKNLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injuryJ Pharmacol Exp Ther2013346346547223833276
- MarkóLVigoloEHinzeCTubular Epithelial NF-κB Activity Regulates Ischemic AKIJ Am Soc Nephrol20162792658266926823548
- EbertRUlmerMZeckSSelenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitroStem Cells20062451226123516424399
- DengGChenCZhangJSe@SiO2 nanocomposites attenuate doxorubicin-induced cardiotoxicity through combatting oxidative damageArtif Cells Nanomed Biotechnol2018150110
- DengGDaiCChenJPorous Se@SiO2 nanocomposites protect the femoral head from methylprednisolone-induced osteonecrosisInt J Nanomedicine2018131809181829606872
- ZhuYDengGJiAPorous Se@SiO2 nanospheres treated paraquat-induced acute lung injury by resisting oxidative stressInt J Nanomedicine2017127143715229026307
- LiuXDengGWangYA novel and facile synthesis of porous SiO2-coated ultrasmall Se particles as a drug delivery nanoplatform for efficient synergistic treatment of cancer cellsNanoscale20168168536854127072410
- WuCCChangCYChangSTChenSH17β-Estradiol Accelerated Renal Tubule Regeneration in Male Rats After Ischemia/Reperfusion-Induced Acute Kidney InjuryShock201646215816326849629
- TanakaRYazawaMMorikawaYSex differences in ischaemia/reperfusion-induced acute kidney injury depends on the degradation of noradrenaline by monoamine oxidaseClin Exp Pharmacol Physiol201744337137727998005
- KangKPLeeJELeeASLeeAESINEffect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in miceMol Med Rep2014962061206824682292
- IkedaMSwideTVaylALahmTAndersonSHutchensMPEstrogen administered after cardiac arrest and cardiopulmonary resuscitation ameliorates acute kidney injury in a sex- and age-specific mannerCrit Care201519133226384003
- VenkatachalamMAWeinbergJMKrizWBidaniAKFailed Tubule Recovery, AKI-CKD Transition, and Kidney Disease ProgressionJ Am Soc Nephrol20152681765177625810494
- ZhangDLiuYWeiQTubular p53 regulates multiple genes to mediate AKIJ Am Soc Nephrol201425102278228924700871
- DengGNiuKZhouFTreatment of steroid-induced osteonecrosis of the femoral head using porous Se@SiO2 nanocomposites to suppress reactive oxygen speciesSci Rep2017714391428256626
- BellomoRKellumJARoncoCAcute kidney injuryLancet2012380984375676622617274
- BrezisMRosenSHypoxia of the renal medulla – its implications for diseaseN Engl J Med1995332106476557845430
- JiangMWeiQDongGKomatsuMSuYDongZAutophagy in proximal tubules protects against acute kidney injuryKidney Int201282121271128322854643
- BennettMDentCLMaQUrine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective studyClin J Am Soc Nephrol20083366567318337554
- YsebaertDKde GreefKEVercauterenSRIdentification and kinetics of leukocytes after severe ischaemia/reperfusion renal injuryNephrol Dial Transplant200015101562157411007823
- WangWWangALuoGMaFWeiXBiYS1P1 receptor inhibits kidney epithelial mesenchymal transition triggered by ischemia/reperfusion injury via the PI3K/Akt pathwayActa Biochim Biophys Sin201850765165729901713
- LiuYEpithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic interventionJ Am Soc Nephrol200415111214694152
- LinderAFjellCLevinAWalleyKRRussellJABoydJHSmall acute increases in serum creatinine are associated with decreased long-term survival in the critically illAm J Respir Crit Care Med201418991075108124601781
- LiuLWuXXuHMyocardin-related transcription factor A (MRTF-A) contributes to acute kidney injury by regulating macrophage ROS productionBiochim Biophys Acta201818641031093121
- MengXMRenGLGaoLNADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammationLab Invest2018981637829106395
- Prieto-MoureBLloris-CarsíJMBelda-AntolíMToledo-PereyraLHCejalvo-LapeñaDAllopurinol Protective Effect of Renal Ischemia by Downregulating TNF-α, IL-1β, and IL-6 ResponseJ Invest Surg201730314315127690698
- GuraTImmunology: Chemokines Take Center Stage in Inflammatory IllsScience199627252649549568638140
- CarrMWRothSJLutherERoseSSSpringerTAMonocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractantProc Natl Acad Sci U S A1994919365236568170963
- ThurmanJMTriggers of inflammation after renal ischemia/reperfusionClin Immunol2007123171317064966
- LeeSHuenSNishioHDistinct macrophage phenotypes contribute to kidney injury and repairJ Am Soc Nephrol201122231732621289217
- CaoQHarrisDCWangYMacrophages in kidney injury, inflammation, and fibrosisPhysiology201530318319425933819
- HuLChenCZhangJIL-35 Pretreatment Alleviates Lipopolysaccharide-Induced Acute Kidney Injury in Mice by Inhibiting NF-κB ActivationInflammation20174041393140028497278
- WenYLiuYRTangTTmROS-TXNIP axis activates NLRP3 inflammasome to mediate renal injury during ischemic AKIInt J Biochem Cell Biol201898435329477360
- AksentijevichIZhouQNF-κB Pathway in Autoinflammatory Diseases: Dysregulation of Protein Modifications by Ubiquitin Defines a New Category of Autoinflammatory DiseasesFront Immunol2017839928469620
- ZeisbergMNeilsonEGMechanisms of tubulointerstitial fibrosisJ Am Soc Nephrol201021111819183420864689
- RosenbaumJLMikailMWiedmannFFurther correlation of renal function with kidney biopsy in chronic renal diseaseAm J Med Sci196725421561604951793
- BohleAMackensen-HaenSvon GiseHSignificance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contributionAm J Nephrol1987764214333439552
- BirringerMPilawaSFlohéLTrends in selenium biochemistryNat Prod Rep200219669371812521265
- WangYChenPZhaoGInverse relationship between elemental selenium nanoparticle size and inhibition of cancer cell growth in vitro and in vivoFood Chem Toxicol201585717726260751
