Abstract
Background
Developing new methods to deliver cells to the injured tissue is a critical factor in translating cell therapeutics research into clinical use; therefore, there is a need for improved cell homing capabilities.
Materials and methods
In this study, we demonstrated the effects of labeling rat bone marrow-derived mesenchymal stem cells (MSCs) with fabricated polydopamine (PDA)-capped Fe3O4 (Fe3O4@PDA) superparticles employing preassembled Fe3O4 nanoparticles as the cores.
Results
We found that the Fe3O4@PDA composite superparticles exhibited no adverse effects on MSC characteristics. Moreover, iron oxide nanoparticles increased the number of MSCs in the S-phase, their proliferation index and migration ability, and their secretion of vascular endothelial growth factor relative to unlabeled MSCs. Interestingly, nanoparticles not only promoted the expression of C-X-C chemokine receptor 4 but also increased the expression of the migration-related proteins c-Met and C-C motif chemokine receptor 1, which has not been reported previously. Furthermore, the MSC-loaded nanoparticles exhibited improved homing and anti-inflammatory abilities in the absence of external magnetic fields in vivo.
Conclusion
These results indicated that iron oxide nanoparticles rendered MSCs more favorable for use in injury treatment with no negative effects on MSC properties, suggesting their potential clinical efficacy.
Introduction
Mesenchymal stem cells (MSCs) hold great promise for the treatment of multiple diseases and disorders.Citation1,Citation2 Although MSCs are capable of high levels of ex vivo expansion and are already in clinical use for numerous applications,Citation3–Citation5 a significant challenge remains in their efficient delivery to target locations in order to enhance successful engraftment.Citation6 This limitation is mainly evident when the therapeutic application requires MSCs to secrete factors immediately following injection.Citation7 MSCs exhibit low efficiency in targeting injury or inflamed tissues.Citation8 Targeting of MSCs to sites of inflammation/injury is mediated by crucial adhesion or homing ligands that bind to intercellular adhesion molecules or chemokine receptors (eg, C-X-C chemokine receptor 4 [CXCR4] and C-C motif receptor 1 [CCR1]).Citation9,Citation10 Additionally, expression of the hepatocyte growth factor (HGF)-receptor, c-Met, in MSCs promotes migration via the HGF/c-Met axis,Citation11,Citation12 however, the expression levels of these receptors are low or inconsistent in MSCs.Citation13 Culture conditions can affect the expression of these receptors, and long-term culture can affect the homing ability of MSCs in vitro;Citation14 therefore, several studies have suggested ways to transiently enhance MSC homing toward sites of inflammation.Citation15 Previous studies reported enhanced homing efficiency by altering the cell surface through conjugation of specific antibodies or proteins;Citation16,Citation17 whereas others achieved similar results using retroviral vectors encoding homing receptors.Citation18,Citation19 However, the experimental procedures associated with these findings are very complicated. Iron oxide nanoparticles are conventional magnetic resonance imaging (MRI) contrast agents widely studied in recent years for their potential application in MSC labeling.Citation20,Citation21 Although studies suggest that labeling with iron oxide nanoparticles does not alter MSC viability, proliferation, or functional properties, the effect of such labeling remains controversial.Citation22,Citation23 Even though a previous study reported that iron oxide nanoparticles increased MSC migration,Citation24 the associated mechanism remains unknown. Moreover, in vivo investigations are needed to clarify the effect of iron oxide nanoparticles on the specific migration of MSCs to inflammation sites. In addition, inconsistencies in the findings of several studies might be related to the sources of the MSCs used, nanoparticles size, surface-coating material, incubation time, transfection agent, and/or labeling methods.Citation25
The Fe3O4 form of iron oxide nanoparticles is approved for use in clinical applications due to its pronounced bio-compatibility.Citation26 These nanoparticles have garnered increased attention because of their unique response features to external magnetic fields (EMFs), making them suitable for use in MRI.Citation27 Polydopamine (PDA) is highly biocompatible and biodegradable,Citation28 and, therefore, widely used to coat nanoparticles for numerous biomedical applications.Citation29,Citation30 PDA-capped Fe3O4 (Fe3O4@PDA) and their composites are among the safest nanomaterials used for clinical diagnosis and therapy.Citation31 Given the cell uptake necessary for cell therapeutics, the size and shape of the spherical, self-assembled structures comprising hundreds of nanoparticles (also known as superparticles) make them highly suitable for such applications.Citation32 Additionally, PDA can spontaneously form a conformal layer through in situ polymerization in an alkaline dopamine solution.Citation33
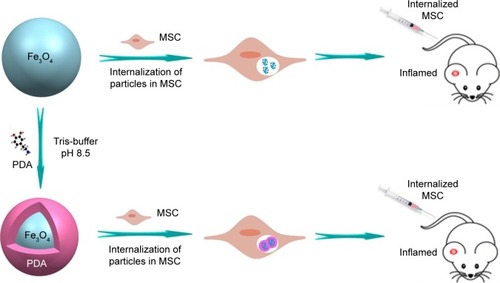
In this study, we fabricated Fe3O4@PDA composite super-particles by employing preassembled Fe3O4 nanoparticles as the core and PDA as the shell, with the PDA coating further improving the physiological stability and biocompatibility of the Fe3O4 superparticles. In vitro experiments indicated that the Fe3O4@PDA composite superparticles exhibited no adverse effects on MSC characteristics and increased the number of MSCs in the S-phase, their proliferation index and migration ability, and their secretion of vascular endothelial growth factor (VEGF). Additionally, these represent the first findings of nanoparticles not only promoting CXCR4 expression,Citation24 but also increasing levels of the migration-related proteins c-Met and CCR1 in MSCs. Although some studies showed that noninvasive EMFs can increase nanoparticle-internalized MSC homing following an intra-arterial or intravenous injection,Citation34–Citation36 there have been no reports showing a similar effect in the absence of EMFs in vivo. However, static magnetic fields can affect MSC viability,Citation34 proliferation,Citation37 differentiation capacity,Citation38 colony formation,Citation39 and extracellular vesicle secretion.Citation40 Our results validated the hypothesis that iron oxide nanoparticles can improve MSC homing and internalization, and promote rapid directional movements following intravenous injection and in the absence of EMFs (), suggesting their potential clinical efficacy.
Materials and methods
Preparation and characterization of iron oxide nanoparticles
Nanoparticles were prepared as previously described.Citation31 Briefly, SDS-capped Fe3O4 nanoparticles were fabricated by emulsification.Citation32,Citation41 Under mechanical stirring and nitrogen atmosphere at room temperature, 4.0 mL of 7.0 mg/mL oleic acid-stabilized Fe3O4 nanoparticles were injected into SDS aqueous solution (12.5 mL; 14 mg/mL). After ultrasonic treatment, the emulsion was heated at 60°C to evaporate toluene and obtain SDS-capped Fe3O4 superparticles. Tris-buffer (12 mL) was added to the prepared Fe3O4 nanoparticle and superparticle solution, and the pH was adjusted to 8.5, followed by the addition of different volumes of 0.03 M dopamine solution. The reaction mixture was incubated at room temperature (22°C–26°C) for 3 hours while the solution color gradually turned dark brown, indicating in situ polymerization of dopamine. The Fe3O4@PDA nanoparticles were obtained by centrifugation at 10,000 rpm for 10 minutes and washed with deionized water.
MSC isolation and expansion
Bone marrow-derived MSCs from rats were obtained by their adherence to the culture plate. MSCs were cultured in DMEM/nutrient mixture F-12 Ham (DMEM/F12) supplemented with 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA), and maintained at 37°C with saturated humidity and 5% CO2. After 48 hours, non-adherent cells were removed by washing, and the medium was changed twice weekly. MSCs were sub-cultured at 80% confluence following the treatment with 0.05% trypsin and 0.02% EDTA (Sigma-Aldrich Co., St Louis, MO, USA) for 3 minutes at 37°C. The cells were washed and harvested by centrifugation at 1,000 rpm for 5 minutes, followed by replating at a lower density (1,000 cells/cm2).
Flow cytometric characterization of MSCs
Passage 3 MSCs were trypsinized and washed twice with PBS, followed by incubation with antibodies against CD44-phycoerythrin (PE), CD34-PE, CD90-fluorescein isothiocyanate (FITC), and CD45-FITC (all from BD Bio-sciences, San Jose, CA, USA) in the dark for 30 minutes at 4°C. After incubation, the cells were washed twice with PBS, fluorescence intensity was measured using flow cytometry (FC500; Beckman Coulter Inc., Brea, CA, USA), and the data were analyzed with CXP software (Beckman Coulter Inc.).
MSC differentiation potential
Adipogenesis
MSCs (5×104 cells/cm2) were seeded onto a 12-well plate with DMEM/F12 supplemented with 10% FBS, followed by the addition of adipogenic differentiation medium (StemPro adipogenesis differentiation kit; Thermo Fisher Scientific) on the second day. Unstimulated MSCs cultured in DMEM/F12 supplemented with 10% FBS were used as staining control. The cultures were refed every 3–4 days, and after 21 days, the cells were stained with Oil Red O (Sigma-Aldrich Co.) and evaluated using an optical microscope (X51; Olympus Corporation, Tokyo, Japan).
Osteogenesis
MSCs (5×104 cells/cm2) were seeded onto a 12-well plate with DMEM/F12 supplemented with 10% FBS. Osteogenic differentiation medium (StemPro osteogenesis differentiation kit; Thermo Fisher Scientific) was added to the cells on the second day after attachment to the bottom of the petri dish. Unstimulated MSCs were cultured in DMEM/F12 supplemented with 10% FBS and used as staining control. The cultures were refed every 3–4 days and after 35 days, cells were stained with 2% Alizarin Red S solution (Alizarin staining kit; Genmed, Shanghai, China) and subjected to examination using an optical microscope (X51; Olympus Corporation).
MSC labeling with Fe3O4@PDA or Fe3O4
Prussian blue staining
Iron oxide nanoparticles were added to the MSCs in DMEM/F12 expansion media (without FBS) supplemented with 1% penicillin-streptomycin solution and incubated for 2 hours, after which the cells were allowed to recover in fresh media (10% FBS) for 14 hours. Cells were then washed twice with PBS, labeled with Fe3O4@PDA or Fe3O4, and stained with a Prussian blue iron stain kit (Beijing Solarbio Science and Technology, Co. Ltd., Beijing, China) according to manufacturer instructions.
Fe quantification in MSCs
For a typical sample, 0.1 mL of the cell suspension was digested overnight using 0.3 mL of concentrated nitric acid (~70%) and 0.1 mL hydrogen peroxide (30%). Samples were then diluted to a volume of 10 mL with deionized water, yielding a final nitric acid concentration of 2%. Iron concentration was determined by inductively coupled plasma optical emission spectrometer (ICP-OES) using a PerkinElmer Optima 3300DV system (PerkinElmer Inc., Waltham, MA, USA).
Transmission electron microscopy (TEM)
Iron oxide nanoparticles (100 µg/mL) were detected using a Hitachi H-800 electron microscope (Hitachi Ltd., Tokyo, Japan) at an acceleration voltage of 200 kV with a charge-coupled device camera. MSCs were labeled with Fe3O4@PDA or Fe3O4 (50 µg/mL) for 16 hours and washed twice with PBS. MSCs were then fixed in phosphate-buffered Karnofsky’s solution, followed by staining with 2% osmium tetroxide at 4°C overnight, dehydration using graded ethanol, and embedding in Epon 812 resin. Resin blocks were sectioned using a microtome, doubly stained with uranyl acetate and lead hydroxide, and imaged using a Tecnai Spirit system (120 kV; FEI, Hillsboro, OR, USA).
Effect of nanoparticle labeling on MSC properties
Viability study
MSCs (~5×104) were seeded in a 96-well plate 24 hours before the experiment, after which iron oxide nanoparticles (0, 25, 50, 75, 100, 150, or 200 µg/mL) dispersed in media were added to the plates and incubated for 24 hours at 37°C. The cells were collected using 0.05% trypsin and counted, with each concentration determined in triplicate.
Proliferation assessment
MSCs (~2×106) were labeled with 50 µg/mL of iron oxide nanoparticles as described for the viability study. MSCs were then seeded on plates at 5,000 cells/cm2, and at each time point (days 1, 3, and 5) MSCs in the plates were estimated by measuring the OD at 450 nm (OD450) using a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). Data are shown as mean of three independent biological replicates. Data obtained from MSCs grown under standard conditions were used for comparison.
Differentiation and surface marker assays
Cells labeled with iron oxide nanoparticles were detected and imaged for differentiation potential. Fluctuations in MSC-associated surface markers following treatment with the magnetic particle for 24 hours were assessed by flow cytometry as described.
Colony-forming unit (CFU) assay
MSCs (60/well) were seeded in 6-well plates in medium supplemented with 10% FBS for 21 days, after which cells were fixed using 4% paraformaldehyde for 30 minutes and stained with Giemsa (Sigma-Aldrich Co.) for 10 minutes at room temperature. Excess stain was removed with distilled water. Colonies were observed and counted, with a colony defined as an aggregate of >40 cells.
In vitro migration assay
In a 24-well Transwell plate (FluoroBlok, 8.0 µm colored polyester membrane; BD Biosciences), complete medium supplemented with 10% FBS was added to the (bottom) wells, and ~30,000 MSCs labeled with Fe3O4@PDA or Fe3O4 and purified by Ficoll-Paque (Sigma-Aldrich Co.) isolation were seeded into the insert in media containing 1% FBS. After 16 hours of incubation at 5% CO2 and 37°C, inserts were washed twice with PBS, fixed with 4% paraformaldehyde for 15 minutes, stained with hematoxylin and eosin (H&E; Sigma-Aldrich Co.) for 10 minutes, and counted.
Quantitative reverse transcription PCR (qRT-PCR)
Total cellular RNA was extracted using Trizol reagent (Thermo Fisher Scientific) according to manufacturer’s instructions. RNA integrity was verified by electrophoresis using ethidium bromide staining and determining the OD260:OD280 ratio. Total RNA (500 ng) was reverse transcribed using 2.5 U avian myeoloblastosis virus reverse transcriptase extra large, 10 U RNase inhibitor, 1 mM deoxy-ribonucleotide triphosphate mixture, 1.25 pmoL Oligo dT-Adaptor primer, 5 mM MgCl2, and 1× reverse transcription buffer (TaKaRa Bio Inc., Shiga Prefecture, Japan) according to manufacturer’s instructions. A no amplification control lacking reverse transcriptase was included. Reactions were incubated at 42°C for 30 minutes, followed by 5 minutes at 95°C, and 5 minutes at 5°C. Approximately 25 ng of cDNA was used in qRT-PCR with SYBR Green PCR master mix (Thermo Fisher Scientific) and 0.2 µM of gene-specific forward and reverse primers (Sangon Biotech Co., Ltd., Shanghai, China). The thermo-cycler parameters used for amplifying these genes were as follows: one cycle at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds, −55°C for 15 seconds, and 72°C for 30 seconds. A list of the primers used is presented in . qRT-PCRs were performed in triplicate in the ABI StepOnePlus real-time PCR system (Thermo Fisher Scientific). Quantitative measurements from all data were obtained using the 2−ΔΔCt method with normalization against β-actin mRNA levels.
Table 1 Primer sequences and product sizes
Western blot
After treatment with iron oxide nanoparticles, MSCs were washed with PBS and lysed in ice-cold radioimmunoprecipitation assay buffer containing a complete protease inhibitor cocktail (Sigma-Aldrich Co.). Primary antibodies against CXCR4, CCR1, c-Met, and histone H3 (Cell Signaling Technology, Danvers, MA, USA) were purchased and used according to manufacturer’s recommendations, with histone H3 used as an internal control. An equal amount of protein (50 µg) for each sample was loaded onto a 12% SDS gel subjected to electrophoresis and transferred to a polyvinylidene fluoride membrane (Merck Millipore, Billerica, MA, USA), which was blocked for 1 hour at room temperature with 5% nonfat dry milk in PBS containing 1% Tween-20 and incubated with primary antibodies for CCR1, c-Met, CXCR4, and histone H3 (1:1,000, 1:1,000, 1:500, and 1:2,000, respectively) overnight at 4°C. After washing, the membrane was incubated with a fluorescently labeled secondary antibody (1:5,000; Thermo Fisher Scientific) at room temperature for 1 hour and visualized using the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Cell cycle assay
MSCs incubated with iron oxide nanoparticles were collected, washed, and suspended in cold 75% ethanol overnight at 4°C. After fixation, cells were pelleted, washed with PBS, and stained using 50 µg/mL propidium iodide (PI) and 50 µg/mL RNase A (Beyotime Institute of Biotechnology, Jiangsu, China) dissolved in 0.5 mL PBS. The suspension was incubated in the dark for 30 minutes at 37°C, after which the cells were analyzed by flow cytometry. The S-phase fraction (SPF) was calculated as follows: SPF = S/(G0/G1 + S + G2/M)×100%. The proliferation index (PIndex) was calculated as follows: PIndex = (S + G2/M)/(G0/G1 + S = G2/M)×100%.Citation42
Cell apoptosis assay
After incubation with iron oxide nanoparticles, MSCs were harvested, rinsed twice with PBS, and suspended in 500 µL binding buffer. The suspended MSCs were incubated for 15 minutes at 4°C with 5 µL annexin V-FITC solution, followed by incubation for another 5 minutes at 4°C after adding 10 µL of PI solution. The emitted green fluorescence of annexin-V and red fluorescence of PI were detected by flow cytometry at an excitation wavelength of 488 nm and emission wavelengths of 525 nm and 575 nm, respectively. For each sample, 10,000 events were recorded. The amount of early apoptosis, late apoptosis, and necrosis was determined as the percentage of annexin-V+/PI−, annexin-V+/PI+, and annexin-V-/PI+ cells, respectively.
ELISA
The supernatants from MSCs incubated with iron oxide nanoparticles and MSCs alone were collected at 24, 72, and 120 hours, with each sample assayed in triplicate. Soluble VEGF and TGF-β were measured using a rat VEGF and TGF-β ELISA kit (Elabscience, Wuhan, China), according to manufacturer’s instructions. Photometric measurements were conducted at 450 nm using a microplate reader (Bio-Rad Laboratories Inc.).
Animals
Pathogen-free male Wistar rats (6 weeks old) were purchased from the Institute of Laboratory Animal Sciences (Beijing, China) and maintained under specific pathogen-free conditions. Rats were allowed to adapt to the experimental conditions for 1 week before starting the experiment. All experimental animal protocols were approved by the Welfare and Research Ethics Committee on Animal Experiments of Jilin University. The animal experiments were carried out following the internationally accepted animal care guidelines (EEC Directive of 1986; 86/609/EEC).
In vivo ear inflammation model
Hairs from the ears of rats were removed using depilatory cream, and the right ears were inflamed using Escherichia coli lipopolysaccharide (LPS; Sigma-Aldrich Co.) as previously described.Citation43 Briefly, rats were sedated and administered a single injection of 240 µg LPS in PBS (8 mg/mL) into the base of the right ear. Similarly, 30 µL of PBS was injected into the base of the left ear as a control. Before injection, MSCs were incubated with iron oxide nanoparticles and cultured for 24 hours. Untreated and nanoparticle-treated MSCs were stained using 5 µM 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine (DiD) tracer (Sigma-Aldrich Co.) according to manufacturer instructions. A one-time, tail vein injection of 1×106 MSCs (ie, untreated or nanoparticle-treated) per rat was administered. The right ears of the rats were imaged 24 hours after MSC injection using a CellviZio intravital microscope (Mauna Kea Technologies, Paris, France).
Histology and Prussian blue analysis
Ear-tissue samples from all groups were excised and fixed in 4% formalin until use. All samples were embedded at the hedge in optimal cutting temperature (OCT) (OCT compound; Tissue-Tek®; Sakura Finetek USA, Inc., Torrance, CA, USA) and instantly frozen in liquid nitrogen. Slides were obtained by cutting the ear block with a cryostat at −20°C. For H&E staining, slides were thawed, hydrated, washed, and stained with an H&E staining kit (Sigma-Aldrich Co.). Images were captured with a Nikon Eclipse Ti inverted fluorescence microscope (Nikon, Tokyo, Japan). The slides were stained with Prussian blue iron stain kit (Beijing Solarbio Science and Technology, Co. Ltd) according to manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using SPSS software (v 17.0; SPSS Inc., Chicago, IL, USA). Nonparametric tests (Wilcoxon and Mann–Whitney U tests) were used for statistical analysis. Parametric data are expressed as the mean ± SD, (n=3). Statistical significance was defined as a P<0.05.
Results and discussion
Preparation and characterization of iron oxide nanoparticles
Fe3O4@PDA nanoparticles were prepared by coating preassembled Fe3O4 nanoparticles with a PDA shell. shows a typical TEM image of the Fe3O4@PDA and Fe3O4 nanoparticles. Because nanoparticles larger than 100 nm can scarcely penetrate cells by cellular phagocytosis, Fe3O4 nanoparticles with an average diameter of 48.3 nm were designated as the cores used to prepare Fe3O4@PDA nanoparticles.Citation44 shows the size distributions of Fe3O4@PDA and Fe3O4 nanoparticles that were <100 nm. These results of electron microscopy analysis allowed synthesis of appropriately sized nanoparticles for labeling cells.
Figure 2 TEM image and size distribution of iron oxide nanoparticles.
Notes: (A and C) are TEM images of representative 100 µg/mL Fe3O4@PDA and Fe3O4 nanoparticles, respectively. (B and D) are the size distribution of Fe3O4@PDA and Fe3O4 nanoparticles, respectively.
Abbreviations: Fe3O4@PDA, PDA-capped Fe3O4; PDA, polydopamine; TEM, transmission electron microscopy.

Due to the collective effect of the preassembled Fe3O4 nanoparticles, utilization of a PDA shell greatly enhanced the superparamagnetism, physiological stability, and biocompatibility of the Fe3O4 core.Citation31 The high degree of aggregation of Fe3O4 nanoparticles frequently reduces Brownian motion and precludes cellular uptake of the aggregates due to their size.Citation45 Accordingly, PDA encapsulation reduced Fe3O4 aggregation and increased their uptake by cells. These results implied the potential efficacy of the Fe3O4@PDA complex for use in biological applications.
MSC labeling with Fe3O4@PDA or Fe3O4
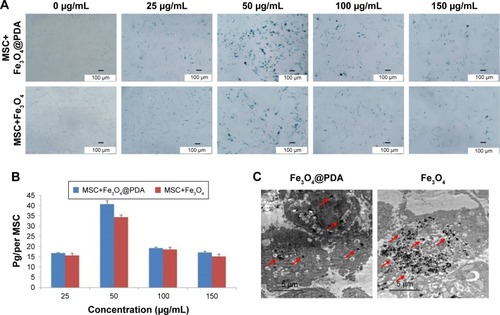
To investigate the efficiency of nanoparticle entry into MSCs, we performed Prussian blue staining of MSCs (). ICP-OES results indicated that 50 mg/mL nanoparticles resulted in the highest cellular uptake efficiency among the four different concentrations tested () and that the labeling efficiency of 50 µg/mL Fe3O4@PDA was higher than that of Fe3O4. shows TEM images of the MSCs after 16 hours of incubation with Fe3O4@PDA or Fe3O4 nanoparticles. These results suggested that PDA-encapsulated Fe3O4 nanoparticles exhibited an increased capacity for uptake by MSCs, as well as better biocompatibility than Fe3O4 based on morphological assessment of the MSCs following uptake.
Figure 3 Iron oxide nanoparticle internalization by MSCs.
Notes: Different concentrations of nanoparticles (0, 25, 50, 100, and 150 µg/mL) were labeled MSCs for 16 hours to determine the optimal labeling efficiency. (A) Then MSCs were stained with a Prussian blue iron stain kit. The scale bar is 100 µm. (B) Iron concentration was determined by ICP-OES. (C) TEM image of nanoparticles (50 µg/mL) internalized in a MSC. The scale bar is 5 µm. The red arrows indicate that nanoparticles were observed in the cell cytoplasm.
Abbreviations: Fe3O4@PDA, PDA-capped Fe3O4; PDA, polydopamine; ICP-OES, inductively coupled plasma optical emission spectrometer; MSC, mesenchymal stem cell; TEM, transmission electron microscopy.
Characterization of MSCs loaded with iron oxide nanoparticles
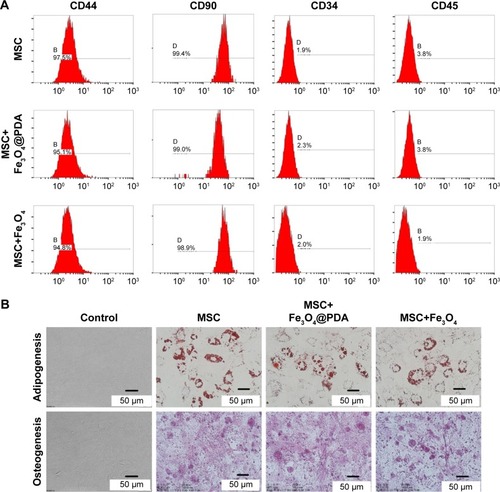
Most cultured adherent cells exhibited a fibroblastic morphology characteristic of MSCs. Fluorescence-activated cell sorting of MSCs loaded with iron oxide nanoparticles indicated that they were negative for CD45 and CD34 surface markers while strongly expressing typical surface antigens, such as CD44 and CD90 (). To confirm whether nanoparticle-labeled MSCs could differentiate into two lineages (ie, osteogenic and adipogenic), we performed differentiation assays. When cultured in the appropriate medium, the cells differentiated into adipocytes or osteoblasts (), respectively, with no differences observed between their respective controls. Numerous studies have described flow cytometric evaluation of MSC surface markers in order to assess the differentiation potential of MSCs following nanoparticle internalization.Citation35,Citation45–Citation49 In agreement with our findings, their results consistently reported no effects on surface-marker expression or osteogenic and adipogenic differentiation ability. These results showed that nanoparticle-labeling did not alter MSC differentiation or characteristics.
Figure 4 Characterization of MSCs was not influenced by iron oxide nanoparticles.
Notes: A total of 50 µg/mL nanoparticles were labeled MSCs for 16 hours. (A) FACS analysis of MSC-labeled iron oxide nanoparticles showed that they were negative for CD45 and CD34, and strongly expressed typical surface antigens, such as CD44 and CD90. (B) Differentiation of MSC-labeled iron oxide nanoparticles: cultured in appropriate differentiation media, MSCs differentiated into adipocytes or osteoblasts. All exhibited adipogenic and osteogenic differentiation potential similar to that of control MSCs.
Abbreviations: FACS, fluorescence-activated cell sorting; Fe3O4@PDA, PDA-capped Fe3O4; PDA, polydopamine; MSC, mesenchymal stem cell.
Effect of iron oxide nanoparticles on MSC viability and proliferation
Nanoparticles used to label MSCs for cell-based therapeutic approaches should not compromise MSC survival and proliferation; therefore, we investigated the viability and proliferation of nanoparticle-labeled MSCs. We found that MSC uptake of 50 µg/mL nanoparticles did not affect cell viability (). Additionally, although concentrations higher than 50 µg/mL did not enhance cell-uptake efficiency, at 100 µg/mL, the nanoparticles tended to aggregate in the culture medium and adhere to the plate. Moreover, we generally observed gradually reduced cell viability at >50 µg/mL Fe3O4 nanoparticles, whereas MSCs viability in the presence of Fe3O4@PDA was less strongly affected according to concentration. These findings suggested that Fe3O4@PDA nanoparticles displayed better biocompatibility relative to that observed in the presence of Fe3O4 nanoparticles. To assess potential effects on MSC proliferation, we evaluated this characteristic at 5 days post-labeling with both nanoparticles. A cell counting kit-8 assay demonstrated that MSCs labeled with both types of nanoparticles showed similar proliferation rates as compared to the control (). Moreover, CFU assay is based on the principle that a single MSC when cultured in medium will undergo proliferation to produce distinct colonies. The CFU assay results indicated that labeled MSCs plated at low density were able to form colonies (). These results demonstrated that MSCs labeled with nanoparticles at a concentration of 50 µg/mL exhibited normal proliferative activity.
Figure 5 Proliferation and viability of MSCs are not affected by nanoparticle labeling.
Notes: Cell viability assay was observed in MSCs labeled with different concentrations (0, 25, 50, 75, 100, 150, or 200 µg/mL) of nanoparticles for 24 hours. (A) Proliferation of 50 µg/mL nanoparticle-labeled MSCs was assessed after 5 days by CCK-8. (B) In vitro CFU assays are usually used to detect the proliferation and differentiation features of MSCs. A colony was defined as an aggregate of >40 cells. (C) CFU colonies of MSC-labeled nanoparticles (50 µg/mL) were stained with Giemsa stain, (D) and the number of colonies was counted.
Abbreviations: CCK-8, cell counting kit-8; CFU, colony-forming unit; Fe3O4@PDA, PDA-capped Fe3O4; PDA, polydopamine; MSC, mesenchymal stem cell.
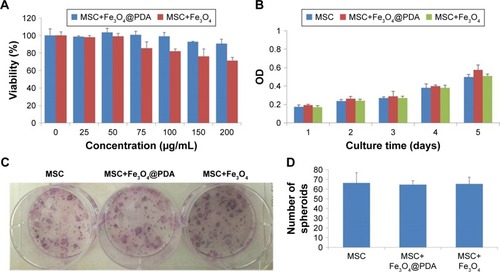
Previous reports indicated that labeling with Fe3O4 nanohybrids increases MSC viability,Citation42 whereas use of commercial iron oxide nanoparticles (ie, magnetic iron oxide beads) reduces viability.Citation45 In the present study, we observed similar rates of MSC proliferation as those of unlabeled control MSCs, which agreed with previously reported results,Citation50–Citation53 however, some previous studies indicated that uptake of iron oxide nanoparticles stimulated in vitro MSC proliferation but reduced CFU.Citation54,Citation55 These conflicting results might be associated with differences in nanoparticles size, shape, surface modification, incubation concentration, and time.
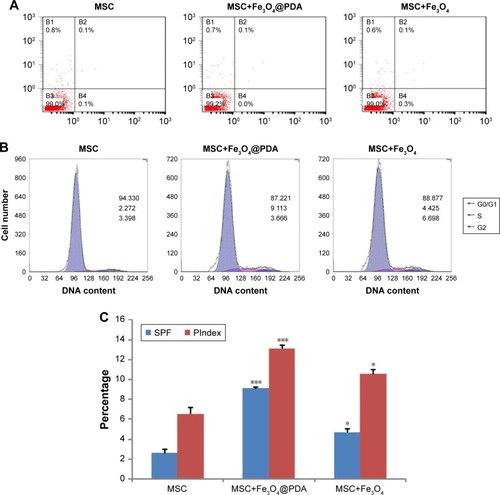
Uptake of iron oxide nanoparticles increase MSC SPF and PIndex
To determine whether nanoparticle labeling induces MSC apoptosis, we examined labeled MSCs using annexin V and PI double staining. As shown in , we observed no increases in the percentage of apoptotic nanoparticle-labeled MSCs relative to control MSCs. Additionally, cell cycle analysis showed a higher percentage of Fe3O4@PDA-labeled MSCs in the S-phase relative to controls and that this percentage was slightly higher in MSCs loaded with Fe3O4 (). Moreover, nanoparticle labeling significantly increased the SPF and PIndex of MSCs relative to unlabeled MSCs (), with these values larger for Fe3O4@PDA-labeled MSCs than those for Fe3O4-labeled MSCs. The results of our cell cycle analyses agreed with those reported previously,Citation54,Citation56 and indicated that cell cycle progression was unaffected by nanoparticle labeling.
Figure 6 Uptake of iron oxide nanoparticles did not induce apoptosis but increased MSC SPF and PIndex.
Notes: MSCs were cultured for 16 hours in the presence or absence of 50 µg/mL iron oxide nanoparticles. At harvest, the MSCs were stained with antibodies to annexin V and PI, and analyzed by flow cytometry. (A) The FACS plots were representative of one of the three experiments. The DNA content of the MSCs was measured by PI staining using flow cytometry. (B) The FACS plots were representative of one of the three experiments. (C) The SPF and PIndex of three independent experiments were averaged. Bar represent the SD. *P<0.05 and ***P<0.001, vs the MSCs group.
Abbreviations: FACS, fluorescence-activated cell sorting; Fe3O4@PDA, PDA-capped Fe3O4; PDA, polydopamine; PI, propidium iodide; PIndex, proliferation index; MSC, mesenchymal stem cell; SPF, S-phase fraction.
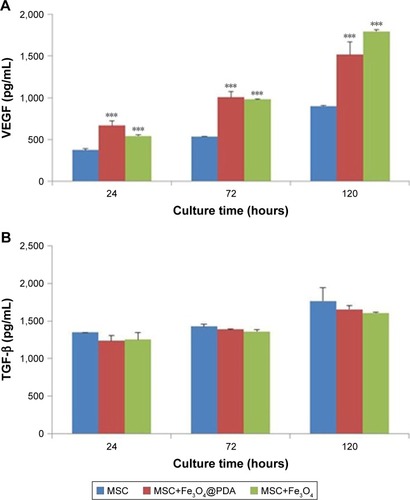
Cytokine release from MSCs labeled with iron oxide nanoparticles
To examine cytokine release from MSCs labeled with iron oxide nanoparticles, we analyzed the release of VEGF and TGF-β. Cells were incubated with 50 µg/mL of iron oxide nanoparticles, and ELISA was used to measure cytokine release after 24, 72, and 120 hours. We found no clear differences in the TGF-β release between MSCs labeled with iron oxide nanoparticles and control MSCs, however, there was a significant and time-dependent increase in VEGF release in MSCs labeled with the nanoparticles as compared to controls (). Cytokines such as VEGF can promote neovascularization of injured tissue.Citation32 VEGF-mediated angiogenesis also is a critical determinant of the increased cellular tumorigenicity.Citation57 However, VEGF alone is not sufficient to promote cell tumorigenicity, suggesting that additional angiogenic factors and cytokines (such as TGF-β, tumor necrosis factor-α [TNF-α], interleukin-6 [IL-6]) regulated in their expression also are critical for an effective tumor initiation.Citation58–Citation60 Additionally, MSC-specific VEGF expression appears to be tightly regulated based on physiological need, and may represent a superior means of inducing therapeutic angiogenesis.Citation61,Citation62 Therefore, MSCs labeled with iron oxide nanoparticles might be advantageous to repairing damaged tissues.
Figure 7 Cytokine release of MSCs labeled with iron oxide nanoparticles.
Notes: The MSCs were labeled with 50 µg/mL iron oxide nanoparticles for 16 hours. And MSC alone cultures were collected at the time points of 24, 72, and 120 hours. Standard cell culture medium served as a control. (A) VEGF and (B) TGF-β concentrations were measured by ELISA. Bar represent the SD. ***P<0.001, vs the MSCs group.
Abbreviations: Fe3O4@PDA, PDA-capped Fe3O4; PDA, polydopamine; MSC, mesenchymal stem cell; VEGF, vascular endothelial growth factor.
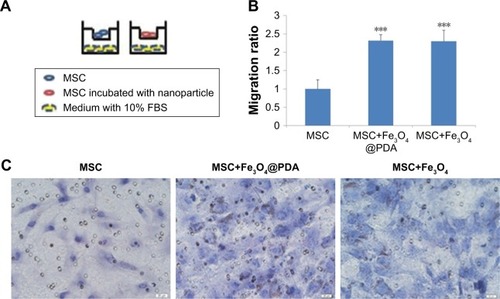
Labeling with iron oxide nanoparticles promotes MSC migration in vitro
We examined MSC migration in vitro using a Transwell migration assay. MSCs in the presence or absence of nanoparticles in media supplemented with 1% FBS were seeded on an insert adjacent to chambers containing complete media with 10% FBS (). Evaluation of migration potential revealed a significant difference in the number of migrated cells between control MSCs (22±5 cells) and MSCs labeled with iron oxide nanoparticles (50±3 and 49±6) (; P<0.001). These results showed that labeling with iron oxide nanoparticles increased MSC migration in agreement with previous reports,Citation63 suggesting that these labeled MSCs might be suitable for promoting rapid migration to injury sites.
Figure 8 Effect of nanoparticle exposure on MSC migration in vitro.
Notes: The migration of MSCs labeled with 50 µg/mL nanoparticles for 16 hours. (A) Schematic illustration of the Transwell assay. (B) The migratory capacity after nanoparticle labeling is displayed as the histogram of the ratio of migrated cells. Bar represent the SD. ***P<0.001, vs the MSCs group. (C) MSCs passed through the inserts were dyed by H&E. Scale bar =20 µm.
Abbreviations: Fe3O4@PDA, PDA-capped Fe3O4; H&E, hematoxylin and eosin; PDA, polydopamine; MSC, mesenchymal stem cell.
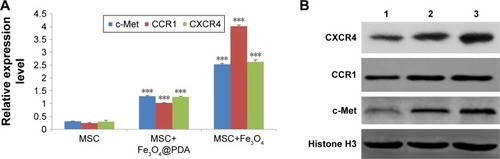
Labeling with iron oxide nanoparticles increases c-Met, CCR1, and CXCR4 expressions in MSCs
Homing capacity is a critical characteristic of therapeutic MSCs, with homing ability mediated by specific chemokine receptors, including CXCR4, CCR1,Citation64 and the tyrosine kinase receptor c-Met.Citation65 In the present study, we determined whether iron oxide nanoparticles could regulate the expression of these receptors by qRT-PCR analysis of c-Met, CCR1, and CXCR4 mRNA levels, finding that they were significantly increased in MSCs following iron oxide nanoparticle uptake (, P<0.001). Western blot subsequently confirmed significant elevations in the levels of the cell-surface receptors c-Met and CCR1 in these MSCs (). These results were consistent with studies showing that labeling with iron oxide nanoparticles improved CXCR4 expression in MSCs,Citation24,Citation66 demonstrating the efficacy for the enhanced homing ability of nanoparticle-labeled MSCs in the absence of an EMF. Moreover, increased CXCR4 levels in nanoparticle-labeled MSCs might promote the CXCR4/stromal-cell-derived-1 signaling pathway at the injured area.Citation50 Furthermore, CCR1 reportedly mediate MSC homing,Citation64 and CCR1 overexpression enhances MSC migration, survival, and engraftment.Citation19 Additionally, upregulated c-Met expression enhanced MSC migration to injury sites.Citation12 It is the first finding that nanoparticles increased the levels of the migration-related proteins c-Met and CCR1 in MSCs. Meanwhile, our results suggested that nanoparticle-labeled MSCs might display improved homing to inflammatory sites.
Figure 9 Expression of migration-related genes analyzed by qRT-PCR and Western blot.
Notes: The mRNA level of migration-related genes from the MSCs labeled with 50 µg/mL iron oxide nanoparticles for 16 hours was measured by qRT-PCR. (A) The column charts represent the qRT-PCR data. The data are presented as the mean ± SD from three independent experiments. ***P<0.001 when compared to MSCs. (B) MSCs which were labeled with 50 µg/mL iron oxide nanoparticles for 16 hours expressed protein level of migration-related genes. 1 represents MSCs group; 2 represents MSCs labeled with Fe3O4@PDA group; 3 represents MSCs labeled with Fe3O4 group.
Abbreviations: CCR1, C-C motif receptor 1; c-Met, cellular-mesenchymal to epithelial transition factor; CXCR4, C-X-C chemokine receptor 4; Fe3O4@PDA, PDA-capped Fe3O4; PDA, polydopamine; MSC, mesenchymal stem cell; qRT, quantitative reverse transcription.
In vivo homing ability of labeled MSCs in an inflammatory ear model
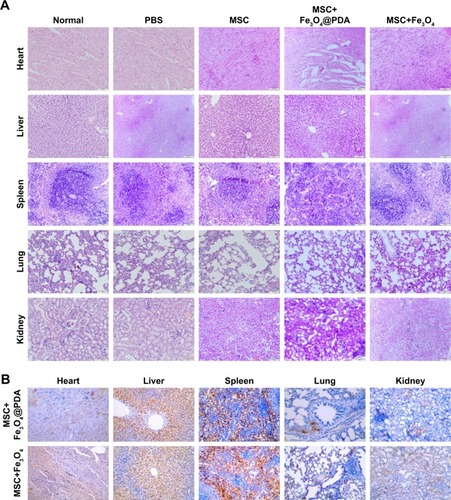
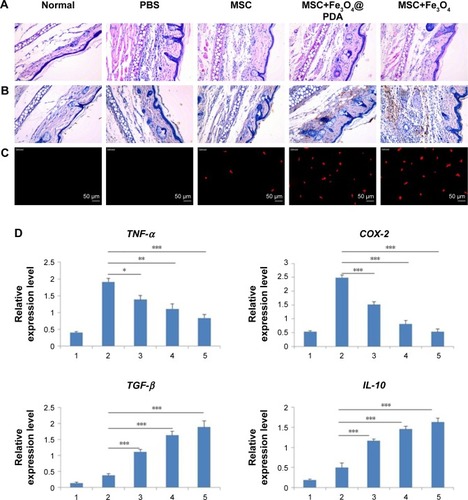
In vitro migration assays () indicated that nanoparticle labeling affected MSC migration. Because homing of systemically administered MSCs can be influenced by factors not accounted for in our in vitro assay, including shear stress, immune system interference, and endothelial barriers,Citation67 we investigated the influence of iron oxide nanoparticles on the translocation ability of MSCs in vivo to a distant site of inflammation in a rat injury model. To facilitate cell imaging, unlabeled MSCs and MSCs labeled with iron oxide nanoparticles (>97% labeling efficiency) were infused with the cell-tracker dye DiD via the tail vein. Macroscopic observations of the ear were performed using H&E staining, which showed gross signs of improvement in rats treated with nanoparticle-labeled MSCs relative to the unlabeled MSCs and PBS-treated control groups, and the PBS-treated control group showed greater neutrophil infiltration and edema as compared to the groups treated with MSCs (). Prussian blue staining showed minimal levels of iron deposition in the inflamed ears () and shows that increased MSC translocation was observed in the inflamed ears of rats treated with nanoparticle-labeled MSCs relative to MSCs. Consistent with other studies,Citation68 MSCs were detectable at inflamed sites as quickly as 24 hours following injection. Our results suggested that the iron oxide nanoparticles could also increase the migratory capacity of the MSCs in the absence of EMFs in vivo. Furthermore, H&E staining of major organs at 24 hours after MSC injection showed undamaged organs () and Prussian blue staining showed minimal iron deposition in the heart, lung, and kidney as well as positive staining for iron deposition in the liver and spleen ().
Figure 10 Reduction of inflammation following nanoparticle-treated MSC administration.
Notes: (A) Histological analysis of ear tissues (scale bars: 100 µm). The sections showed an alteration of tissue architecture in the inflamed ears and in the ones treated with MSCs. (B) Prussian blue analysis of ear sections at 24 hours reveals that iron oxide nanoparticles stayed at ear tissues. Scale bars: 100 µm. Moreover, nanoparticles treatment enhanced the recruitment of MSCs in the inflamed ear. (C) Nanoparticles labeled MSCs exhibited a significant addition in ear compared to the other groups. Scale bars: 20 µm. (D) qRT-PCR for the expressions of pro-(TNF-α and COX-2) and anti-(TGF-β and IL-10) inflammatory genes on explants 24 hours following the injection of MSCs labeled with iron oxide nanoparticles and unlabeled MSCs. The expression levels of pro-inflammatory molecules found in the inflamed ears are also shown for control ear with no inflammation. (n=3; bar represent the SD. *P<0.05, **P<0.01, and ***P<0.001). 1 represents normal ear with no inflammation; 2 represents inflamed ears injected with PBS; 3 represents inflamed ears injected with MSC; 4 represents inflamed ears injected with MSCs labeled with Fe3O4@PDA; and 5 represents inflamed ears injected with MSCs labeled with Fe3O4.
Abbreviations: COX-2, cyclooxygenase-2; Fe3O4@PDA, PDA-capped Fe3O4; IL-10, interleukin-10; PDA, polydopamine; MSC, mesenchymal stem cell; qRT, quantitative reverse transcription; TNF-α, tumor necrosis factor-α.
In vivo molecular analysis
Concomitant with MSC retention at sites of inflammation, reduced expressions of the pro-inflammatory genes TNF-α and cyclooxygenase-2 (COX-2) were observed according to qRT-PCR analysis of explanted ears at 24 hours postinjection (). Data showed that the expression of such genes decreased in MSCs labeled with iron oxide nanoparticles but was comparable between Fe3O4- and Fe3O4@PDA-labeled groups. Moreover, the expression of such genes in tissues injected with control MSCs was higher than that observed in tissues injected with nanoparticle-labeled MSCs. Concurrently, a statistically significant increase in the expression of anti-inflammatory genes (TGF-β and IL-10) was observed in tissues injected with MSCs (), with TGF-β levels higher in nanoparticle-labeled MSCs and IL-10 expression 25- and 12-fold higher relative to levels in control-treated ears.
Additionally, MSC recruitment and accumulation within the inflamed ear led to a decreased expression of pro-inflammatory markers, agreeing with previous finding.Citation69 H&E staining following systemic administration demonstrated that nanoparticle-treated MSCs produced anti-inflammatory effects in vivo. Furthermore, increased IL-10 and TGF-β expressions in the inflamed ears following treatment validated these findings at the molecular level. It is likely that the significant reduction in local inflammation following systemic administration of nanoparticle-labeled MSCs was dependent upon rapid localization of MSCs to the inflamed site. These data confirmed that the uptake of iron oxide nanoparticles improved the homing capacity and therapeutic efficacy of MSC-based therapies.
Conclusion
In this study, in vitro and in vivo experiments demonstrated that MSCs internalize iron oxide nanoparticles with no adverse effects on MSC viability and proliferation, suggesting their biocompatibility with the MSCs. Moreover, MSC labeling with these nanoparticles increased MSCs SPF and PIndex, as well as VEGF secretion, and promoted MSC migration through the upregulation of c-Met, CCR1, and CXCR4 levels, thereby increasing their ability to systemically translocate to sites of inflammation in the absence of EMFs. These results demonstrated that iron oxide nanoparticles promoted MSC migration to sites of inflammation, thereby suggesting the potential clinical efficacy of this method.
Acknowledgments
This work was supported by grants from Jilin Provincial Department of Finance Project of China (No 3D516P803430).
Disclosure
The authors report no conflicts of interest in this work.
References
- Česen MazičMGirandonLKneževičMAvčinSLJazbecJTreatment of severe steroid-refractory acute-graft-vs.-host disease with mesenchymal stem cells–single center experienceFront Bioeng Biotechnol201869330087891
- DaneshmandiSKarimiMHPourfathollahAATGF-β engineered mesenchymal stem cells (TGF-β/MSCs) for treatment of Type 1 diabetes (T1D) mice modelInt Immunopharmacol20174419119628110219
- BarnhoornMde Jonge-MullerEMolendijkIEndoscopic administration of mesenchymal stromal cells reduces inflammation in experimental colitisInflamm Bowel Dis20182481755176729796655
- AhnSYChangYSSungDKSungSIParkWSHypothermia broadens the therapeutic time window of mesenchymal stem cell transplantation for severe neonatal hypoxic ischemic encephalopathySci Rep201881766529769612
- XiaHLiangCLuoPPericellular collagen I coating for enhanced homing and chondrogenic differentiation of mesenchymal stem cells in direct intra-articular injectionStem Cell Res Ther20189117429945671
- KarpJMLeng TeoGSMesenchymal stem cell homing: the devil is in the detailsCell Stem Cell20094320621619265660
- LevyOZhaoWMortensenLJmRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammationBlood201312214e23e3223980067
- von BahrLBatsisIMollGAnalysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formationStem Cells20123071575157822553154
- WuYZhaoRCThe role of chemokines in mesenchymal stem cell homing to myocardiumStem Cell Rev20128124325021706142
- HockingAMThe role of chemokines in mesenchymal stem cell homing to woundsAdv Wound Care2015411623630
- VogelSTrappTBörgerVHepatocyte growth factor-mediated attraction of mesenchymal stem cells for apoptotic neuronal and car-diomyocytic cellsCell Mol Life Sci201067229530319888551
- LiuJPanGLiangTHuangPHGF/c-Met signaling mediated mesenchymal stem cell-induced liver recovery in intestinal ischemia reperfusion modelInt J Med Sci201411662663324782653
- de BeckerARietIVHoming and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy?World J Stem Cells201683738727022438
- ZhouPLiuZLiXMigration ability and Toll-like receptor expression of human mesenchymal stem cells improves significantly after three-dimensional cultureBiochem Biophys Res Commun2017491232332828734835
- Naderi-MeshkinHBahramiARBidkhoriHRMirahmadiMAhmadiankiaNStrategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapyCell Biol Int2015391233425231104
- LevyOMortensenLJBoquetGA small-molecule screen for enhanced homing of systemically infused cellsCell Rep20151081261126825732817
- DykstraBLeeJMortensenLJGlycoengineering of E-selectin ligands by intracellular versus extracellular fucosylation differentially affects osteotropism of human mesenchymal stem cellsStem Cells201634102501251127335219
- BrennerSWhiting-TheobaldNKawaiTCXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cellsStem Cells20042271128113315579633
- HuangJZhangZGuoJGenetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardiumCirc Res2010106111753176220378860
- WangYXuFZhangCHigh MR sensitive fluorescent magnetite nanocluster for stem cell tracking in ischemic mouse brainNanomedicine2011761009101921530678
- RieglerJLiewAHynesSOSuperparamagnetic iron oxide nanoparticle targeting of MSCs in vascular injuryBiomaterials20133481987199423237516
- ChenCHLinYSFuYCElectromagnetic fields enhance chondrogenesis of human adipose-derived stem cells in a chondrogenic microenvironment in vitroJ Appl Physiol2013114564765523239875
- SahaSYangXBTannerSCurranSWoodDKirkhamJThe effects of iron oxide incorporation on the chondrogenic potential of three human cell typesJ Tissue Eng Regen Med20137646146922396122
- HuangXZhangFWangYDesign considerations of iron-based nanoclusters for noninvasive tracking of mesenchymal stem cell homingACS Nano2014854403441424754735
- QiYFengGHuangZYanWThe application of super paramagnetic iron oxide-labeled mesenchymal stem cells in cell-based therapyMol Biol Rep20134032733274023269616
- LeeNHyeonTDesigned synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agentsChem Soc Rev20124172575258922138852
- SundstrømTDaphuIWendelboIAutomated tracking of nanoparticle-labeled melanoma cells improves the predictive power of a brain metastasis modelCancer Res20137382445245623423977
- DavisMEChenZGShinDMNanoparticle therapeutics: an emerging treatment modality for cancerNat Rev Drug Discov20087977178218758474
- ZhangRWangSYangYModification of polydopamine-coated Fe3O4 nanoparticles with multi-walled carbon nanotubes for magnetic-µ-dispersive solid-phase extraction of antiepileptic drugs in biological matricesAnal Bioanal Chem2018410163779378829658094
- ChenYZhangFWangQThe synthesis of LA-Fe3O4@PDA-PEG-DOX for photothermal therapy-chemotherapyDalton Trans20184772435244329379913
- GeRLiXLinMFe3O4@polydopamine Composite Theranostic Superparticles Employing Preassembled Fe3O4 Nanoparticles as the CoreACS Appl Mater Interfaces2016835229422295227560801
- ZhangXXuXLiTComposite photothermal platform of polypyrrole-enveloped Fe3O4 nanoparticle self-assembled superstructuresACS Appl Mater Interfaces2014616145521456125134068
- BiDZhaoLYuRSurface modification of doxorubicin-loaded nanoparticles based on polydopamine with pH-sensitive property for tumor targeting therapyDrug Deliv201825156457529457518
- WangJXiangBDengJFreedDHAroraRCTianGInhibition of viability, proliferation, cytokines secretion, surface antigen expression, and adipogenic and osteogenic differentiation of adipose-derived stem cells by seven-day exposure to 0.5 T static magnetic fieldsStem Cells International201620164112
- MengYShiCHuBExternal magnetic field promotes homing of magnetized stem cells following subcutaneous injectionBMC Cell Biol20171812428549413
- QiYYangZDingQZhaoTHuangZFengGTargeted transplantation of iron oxide-labeled, adipose-derived mesenchymal stem cells in promoting meniscus regeneration following a rabbit massive meniscal defectExp Ther Med201611245846626893631
- KimECLeesungbokRLeeSWEffects of moderate intensity static magnetic fields on human bone marrow-derived mesenchymal stem cellsBioelectromagnetics201536426727625808160
- AminHDBradyMASt-PierreJPStevensMMOverbyDREthierCRStimulation of chondrogenic differentiation of adult human bone marrow-derived stromal cells by a moderate-strength static magnetic fieldTissue Eng Part A20142011–121612162024506272
- SchäferRBantleonRKehlbachRFunctional investigations on human mesenchymal stem cells exposed to magnetic fields and labeled with clinically approved iron nanoparticlesBMC Cell Biol20101112220370915
- MarędziakMMaryczKŚmieszekALewandowskiDTokerNYThe influence of static magnetic fields on canine and equine mesenchymal stem cells derived from adipose tissueIn Vitro Cell Dev Biol Anim201450656257124477562
- BaiFWangDHuoZA versatile bottom-up assembly approach to colloidal spheres from nanocrystalsAngew Chem Int Ed Engl200746356650665317661295
- ZhangHQuSLiSSilencing SATB1 inhibits proliferation of human osteosarcoma U2OS cellsMol Cell Biochem20133781–2394523516037
- CorradettiBTaraballiFMartinezJOHyaluronic acid coatings as a simple and efficient approach to improve MSC homing toward the site of inflammationSci Rep201771799128801676
- TangLYangXYinQInvestigating the optimal size of anticancer nanomedicineProc Natl Acad Sci U S A201411143153441534925316794
- PernalSWuVMUskokovićVHydroxyapatite as a vehicle for the selective effect of superparamagnetic iron oxide nanoparticles against human glioblastoma cellsACS Appl Mater Interfaces2017945392833930229058880
- HuangZLiCYangSMagnetic resonance hypointensive signal primarily originates from extracellular iron particles in the long-term tracking of mesenchymal stem cells transplanted in the infarcted myocardiumInt J Nanomedicine2015101679169025767388
- SilvaLHASilvaSMLimaECDEffects of static magnetic fields on natural or magnetized mesenchymal stromal cells: repercussions for magnetic targetingNanomedicine20181472075208529933023
- QiaoYGuminJMaclellanCJMagnetic resonance and photo-acoustic imaging of brain tumor mediated by mesenchymal stem cell labeled with multifunctional nanoparticle introduced via carotid artery injectionNanotechnology2018291616510129438105
- RosenbergJTYuanXGrantSMaTTracking mesenchymal stem cells using magnetic resonance imagingBrain Circ20162310811330276283
- YunWChoiJJuHEnhanced homing technique of mesenchymal stem cells using iron oxide nanoparticles by magnetic attraction in olfactory-injured mouse modelsInt J Mol Sci20181951376
- ShenWBPlachezCTsymbalyukOCell-based therapy in TBI: magnetic retention of neural stem cells in vivoCell Transplant20162561085109926395573
- KhanMRDudhiaJDavidFHBone marrow mesenchymal stem cells do not enhance intra-synovial tendon healing despite engraftment and homing to niches within the synoviumStem Cell Res Ther20189116929921317
- YudintcevaNMBogolyubovaIOMuraviovANApplication of the allogenic mesenchymal stem cells in the therapy of the bladder tuberculosisJ Tissue Eng Regen Med2018123e1580e159328990734
- HuangDMHsiaoJKChenYCThe promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticlesBiomaterials200930223645365119359036
- SchäferRKehlbachRMüllerMLabeling of human mesenchymal stromal cells with superparamagnetic iron oxide leads to a decrease in migration capacity and colony formation abilityCytotherapy2009111687819191056
- Zhe-ZhenYQing-HuaWZhangS-LMiaoJ-YZhaoB-XSuLTwo novel amino acid-coated super paramagnetic nanoparticles at low concentrations label and promote the proliferation of mesenchymal stem cellsRSC Advances20166121015910161
- BeckBDriessensGGoossensSA vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumoursNature2011478736939940322012397
- ZhangCWangNTanHYGuoWLiSFengYTargeting VEGF/VEGFRs pathway in the antiangiogenic treatment of human cancers by traditional chinese medicineIntegr Cancer Ther201817358260129807443
- QuXTangYHuaSImmunological approaches towards cancer and inflammation: a cross talkFront Immunol2018956329662489
- VillalbaMEvansSRVidal-VanaclochaFCalvoARole of TGF-β in metastatic colon cancer: it is finally time for targeted therapyCell Tissue Res20173701293928560691
- KagiwadaHYashikiTOhshimaATadokoroMNagayaNOhgushiHHuman mesenchymal stem cells as a stable source of VEGF-producing cellsJ Tissue Eng Regen Med20082418418918452238
- ArutyunyanIFatkhudinovTKananykhinaERole of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: in vitro studyStem Cell Res Ther2016714627001300
- ÖksüzSAlagözMŞKaragözHComparison of treatments with local mesenchymal stem cells and mesenchymal stem cells with increased vascular endothelial growth factor expression on irradiation injury of expanded skinAnn Plast Surg201575221923026165573
- PonteALMaraisEGallayNThe in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activitiesStem Cells20072571737174517395768
- AenlleKKCurtisKMRoosBAHowardGAHepatocyte growth factor and p38 promote osteogenic differentiation of human mesenchymal stem cellsMol Endocrinol201428572273024673557
- LuYWeiLZhangXThe Regulation of Mesenchymal stem cell therapy through magnetic resonance imaging agents-based cellular condition and oxygen environmentJ Biomed Nanotechnol201814111906192030165927
- ChamberlainGSmithHRaingerGEMiddletonJMesenchymal stem cells exhibit firm adhesion, crawling, spreading and transmigration across aortic endothelial cells: effects of chemokines and shearPLoS One201169e2566321980522
- KurtzAMesenchymal stem cell delivery routes and fateInt J Stem Cells2008111724855503
- HerreraMBBussolatiBBrunoSExogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injuryKidney Int200772443044117507906