Abstract
Purpose
Resistant strains of Acinetobacter baumannii (AB) that can form biofilms are resistant to polymyxin. Therefore, effective and safe polymyxin preparations against biofilm-producing AB are urgently needed. This study aims to prepare chitosan-modified polymyxin B-loaded liposomes (CLPs) and ultrasound microbubbles (USMBs) and then explore the synergistic antibacterial effects of USMBs combined with CLPs in vitro.
Methods
CLPs were prepared using a modified injection method, and microbubbles were prepared using a simple mechanical vibration method. Minimal biofilm inhibitory concentration (MBIC) of CLPs against resistant biofilm-producing AB was determined. Antibacterial activities of CLPs with or without USMBs were analyzed by crystal violet staining and resazurin assays to evaluate biofilm mass and viable counts, respectively. Then, the anti-biofilm effects of CLPs with or without USMBs on biofilm-producing AB were confirmed via scanning electron microscopy (SEM) analysis.
Results
We prepared CLPs that were 225.17±17.85 nm in size and carried positive charges of 12.64±1.44 mV. These CLPs, with higher encapsulation efficiency and drug loading, could exhibit a sustained release effect. We prepared microbubbles that were 2.391±0.052 µm in size and carried negative charges of −4.32±0.43 mV. The MBICs of the CLPs on the biofilm-producing AB was 8±2 µg/mL, while that of polymyxin B was 32±2 µg/mL. USMBs in combination with 2 µg/mL of polymyxin B could completely eliminate the biofilm-producing AB and achieve the maximum antimicrobial effects (P>0.05 vs sterile blank control). SEM imaging revealed some scattered bacteria without a biofilm structure in the USMB combined with the CLP group, confirming that this combination has the greatest anti-biofilm effects.
Conclusion
In this research, we successfully prepared USMBs and CLPs that have a more significant antibacterial effect on biofilm-forming AB than polymyxin B alone. Experiments in vitro indicate that the synergistic antibacterial effect of combining USMBs with CLPs containing as little as 2 µg/mL of polymyxin B is sufficient to almost eliminate drug-resistant biofilm-producing AB.
Introduction
Acinetobacter baumannii (AB) is one of the most serious opportunistic pathogens in nosocomial infections. It can persist and form biofilms on various abiotic materials in a hospital environment, thereby coming into contact with susceptible patients and causing outbreaks of ventilator-associated pneumonia, meningitis, septicemia, urinary tract infections, and skin and soft tissue infections (SSTIs).Citation1 A biofilm is an aggregate of microbial cells embedded in a self-produced matrix on living or non-living surfaces.Citation2 It can be viewed as a protected mode of microbial growth that can provide protection from hostile environments, eg, in cases involving Acinetobacter, biofilm-forming isolates can survive longer than their non-biofilm-forming counterparts.Citation3 Biofilms have significantly higher antibiotic resistance than their planktonic counterparts and thus have serious consequences for the treatment of biofilm-associated infections.Citation4
Reports from various parts of the world have indicated a growing concern regarding multi-, extensive-, and pan-drug-resistant (MDR, XDR, and PDR) strains of AB, some of which are resistant to even polymyxin.Citation5–Citation7 Polymyxin comprises a class of cyclic polypeptide antibiotics that include polymyxins A–E, of which only polymyxins B and E are used in the clinic. Polymyxin B or E is applied to treat severe infections caused by Gram-negative bacteria. However, due to their severe renal toxicity, neurotoxicity, and narrow therapeutic window, their clinical applications are limited to use as a last resort for treating MDR-AB or other MDR Gram-negative bacterial infections.Citation8 Therefore, effective and safe polymyxin B or E preparations against biofilm-producing AB are urgently needed.
Liposomes, which are a type of a drug delivery system (DDS), are spherical vesicles consisting of one or more phospholipid bilayers surrounding a drug and thus affect pharmacokinetics, pharmacodynamics, toxicity, immunogenicity, and biological identification.Citation9 They can protect antimicrobial agents from binding to matrix material and from enzymatic inactivation, thus making chemical treatments more effective, reducing the toxicity of antimicrobials, and increasing the safety of chemical treatments.Citation10 However, liposomes still have some shortcomings, such as the chemical instability due to the hydrolysis of ester bonds in structures, the oxidation of unsaturated acyl chains in lipids, and the physical instability caused by the leakage of encapsulated drugs.Citation11 Chitosan as a polycationic heteropolysaccharide has attracted the attention of researchers due to its low toxicity, bacteriostasis, biocompatibility, and moisture-retention properties. Using chitosan to modify liposomes can improve the stability of preparations.Citation12,Citation13 Ultrasound microbubbles (USMBs) are a new type of DDS for the treatment of bacterial infection.Citation14–Citation17 A number of publications have indicated that ultrasound with cavitation can enhance the inhibitory effects of antimicrobial agents on bacterial biofilms, which can be amplified by microbubbles.Citation18,Citation19 As a result, USMBs can promote the bacterial uptake of antimicrobials and improve the antibacterial efficacy of drugs.Citation20–Citation22 This study aims to explore the synergistic antibacterial effects of combining USMBs with chitosan-modified polymyxin B-loaded liposomes (CLPs) in vitro to assess the feasibility of employing this combined DDS in systemic or topical antibacterial treatment of biofilm-producing AB infections.
Materials and methods
Bacterial strains and culture conditions
In this study, the bacterial strain AB W1340, the strain of AB that had been clinically isolated from the sputum of a pneumonia patient in the First Affiliated Hospital of Chongqing Medical University as part of a routine hospital laboratory procedure, was used. The minimum inhibitory concentration (MIC) values of the antibacterial agent against this clinical isolate had been determined, and the results showed that this strain is resistant to multiple antibiotics, including polymyxin B (). The strain forms dense biofilms on polystyrene and glass surfaces, and the mature biofilms are resistant to polymyxin B. The strain was inoculated onto blood agar plates (Jiangmen Caring Trading Company, Jiangmen, China) and cultivated for 18 hours at 37°C. Ninety-six-well polystyrene microtiter plates were used for the cultivation of AB biofilms. Briefly, the organisms were grown in Luria-Bertani broth (LB; Qingdao Haibo Biochemistry Instrument, Qingdao, China) overnight at 37°C with agitation, and bacterial suspensions were adjusted to contain an equivalence of the McFarland standard of 0.5. Then, 200 µL of bacterial suspensions was added to 96-well plates and incubated at 37°C for 4 days, and the LB was replaced every 24 hours. Biofilms were cultivated in 24-well polystyrene microtiter plates in a similar manner. In short, sterilized 12 mm coverslip disks were placed into the bottoms of 24-well plates. Then, 200 µL of bacterial suspensions with a McFarland standard of 0.5 and 1.5 mL of LB were added to each well. The 24-well plates were incubated at 37°C for 4 days, and the LB was replaced every 24 hours.
Preparation of CLPs and chitosan-modified liposomes
CLPs were prepared using a modified injection method.Citation23 Briefly, the components dipalmitoylphosphatidylcholine (DPPC), distearoylphosphatidylcholine (DSPE), and cholesterol (CHOL) (Corden Pharma Switzerland LLC, Liestal, Switzerland) (at a weight ratio of 3:1:1) were completely dissolved in 10 mL of chloroform as the lipid phase. Polymyxin B (Sangon Biotech, Shanghai, China) was dissolved in 2 mL of PBS (pH 7.4) at 50°C as the aqueous phase. Then, the lipid phase was dropped into either the aqueous phase or PBS (pH 7.4) under gentle magnetic stirring, followed by evaporation via rotary evaporator to remove the organic solvent (Shanghai Yarong Biochemistry Instrument, Shanghai, China). Next, the liposomes and 0.1% chitosan (Sangon Biotech) solution (chitosan was dissolved in a 0.1 M acetic acid glacial solution) were mixed at equal volumes, followed by 10 minutes of sonication in an ice bath using an ultrasonic processor (175 W; Sonics & Material, Newtown, CT, USA). Then, CLPs and chitosan-modified liposomes were prepared. Finally, ultrafiltration was used to remove the unencapsulated polymyxin B using the liposome solution in a 15 mL ultrafiltration tube and centrifuging at 5,000 rpm for 20 minutes at 4°C. Then, the CLPs were collected. All CLP dispersions were stored at 4°C for further analysis. The particle size and potential of the final liposomes were analyzed using a laser particle size analyzer system (Zetasizer 3000 HS; Malvern Instruments, Malvern, UK). For morphological analysis, the liposomes were stained with 0.1% phosphotungstic acid for 10 minutes, washed with ultrapure water, and subjected to transmission electron microscopy (TEM, TECNAI-10; Philips, Eindhoven, the Netherlands).
Preparation of microbubbles
Microbubbles were prepared using a simple mechanical vibration method.Citation16 Briefly, 5 mg of DPPC, 2 mg of DSPE, and 0.5 mg of CHOL were dissolved in 500 µL of 10% glycerol solution in a vial. After heating in a water bath at 50°C for 30 minutes, the air in the vial was exchanged with perfluoropropane (C3F8; Research Institute of Physical and Chemical Engineering of Nuclear Industry, Tianjin, China), followed by vigorous shaking for 45 seconds via dental amalgamator (YJT; Shanghai Medical Apparatus and Instruments, Shanghai, China). Finally, the microbubbles were washed with PBS twice to obtain supernatant microbubbles via centrifugation at 500 rpm, and the concentration was then adjusted to 108 per mL. The particle size and potential of the microbubbles were analyzed using a laser particle size analyzer system (Zetasizer 3000 HS; Malvern Instruments). The morphological characteristics of microbubbles were analyzed using an inverted optical microscope (IX71; Olympus, Tokyo, Japan).
Encapsulation and drug loading efficiency of CLPs
Free polymyxin B was separated by ultrafiltration– centrifugation technique using 10-kDa MWCO Amicon centrifugal filters (EMD Millipore, Billerica, CA, USA) at 5,000 rpm for 20 minutes at 4°C. Then, the free polymyxin B content in the filtrate was determined by measuring the absorbance at 215 nm using ultraviolet spectrophotometry (UV 2600; Shimadzu, Kyoto, Japan). Blank liposomes were used as controls.
In vitro drug release study of CLPs
The release kinetics of CLPs was monitored as previously described.Citation24 In short, a dialysis bag containing 2 mL of CLPs was placed in an opaque bottle filled with 100 mL of PBS as the release medium. The medium was then incubated at 37°C under mild continuous agitation. At 1, 2, 3, 6, 9, 12, 24, and 48 hours, 2 mL of the release medium was withdrawn and replaced with 2 mL of fresh PBS, and the amount of polymyxin B released was determined by measuring absorbance at 215 nm. Blank liposomes were used as controls.
Minimal biofilm inhibitory concentration (MBIC) determinations
First, 96-well plates were used to cultivate AB W1340 biofilms. The biofilms were gently washed three times using sterile water to remove planktonic bacteria and then incubated at 37°C in 200 µL of LB containing twofold serial dilutions of polymyxin B or CLPs. After 24 hours, the plates were gently washed and supplemented with 200 µL of fresh LB. The lowest concentration of polymyxin B that prevented the appearance of visible growth within the inoculation area after 24 hours at 37°C was defined as the MBIC.
Ultrasound experiments
Ultrasound was applied using a gene ultrasonic transfer machine (UGT 1025; CQMU, Chongqing, China). The frequency of this unfocused ultrasonic transducer was 1.0 MHz, and the acoustic intensity was set as a continuous ultrasonic intensity of 3 W/cm2. The duration of the intervention was 5 minutes. During the experiment, we placed an ultrasonic probe underneath the bottom of a well using coupling gel.
In vitro antimicrobial activity
Biofilms were cultivated on 24- or 96-well polystyrene microtiter plates, and planktonic bacteria were removed by washing the plates with sterile water. Then, the biofilms were randomly divided into eight treatment groups and treated as follows: biofilm control (BF control), ultrasound (US), chitosan-modified liposome (CL), USMB, polymyxin B (PMB), CLP, USMB and polymyxin B (USMB + PMB), and USMB and CLP (USMB + CLP); a sterile blank control group (SB control) was used as the background control. The biofilms of each group were treated with LB supplemented with various preparation solutions or the mixture of various preparation solutions and microbubbles (1:1 vol) with various concentrations of polymyxin B (2 µg/mL, 4 µg/mL, 8 µg/mL, and 16 µg/mL). The final concentration of microbubbles was 4% (v/v). After ultrasound treatment, the plates were incubated at 37°C for 24 hours. Then, crystal violet staining assays were applied to evaluate the biofilm mass, and resazurin assays were used to estimate viable counts, as described in the following section.
Crystal violet staining assay
The crystal violet staining assay used in this study was performed according to a previously described method with some modifications.Citation25 After biofilms in 24-well plates were gently washed to remove planktonic bacteria, plates were air-dried at room temperature, and each well was stained with 1 mL of 1% crystal violet solution for 10 minutes. Then, the plates were washed with sterile distilled water for five times. The whole process was performed without light. One milliliter of 95% ethanol was added to destain the wells and 200 µL of destaining solution from each well was transferred to a 96-well plate. The destaining solution was measured by the absorbance at 570 nm using a Varioskan Flash Microplate Reader (Thermo Fisher Scientific, Waltham, MA, USA).
Resazurin assay
The resazurin assay was performed as previously described.Citation16 After biofilms in 96-well plates were gently washed to remove planktonic bacteria, 100 µL of LB was added to each well. The LB was then mixed with 10 µL of resazurin solution (Alamar blue, Yeasen, Shanghai, China), followed by shaking and incubation at 37°C for 1 hour. The percent reduction in Alamar blue was calculated by measuring absorbances at 570 and 600 nm.
Scanning electron microscopy (SEM) analysis
AB biofilms were cultivated using 24-well plates and treated according to the different groups. Then, the AB biofilms were fixed with 2.5% glutaraldehyde for 2 hours and rinsed twice with PBS. They were dehydrated with 30%, 50%, 70%, 90%, and 100% concentrations of ethanol in series, and then replaced with 50%, 70%, 90%, and 100% concentrations of tertiary butyl alcohol in series. Finally, each sample was coated with gold by a sputter coater and imaged via SEM (S-3000N; Hitachi, Tokyo, Japan).
Statistical analysis
Statistical analysis was conducted using SPSS 19.0. One-way analysis of variance (ANOVA) was used for multiple comparisons, and the least-significant difference (LSD) test was used for comparisons between two groups. Statistical significance was determined at P<0.05.
Results
Characterization of CLPs and microbubbles
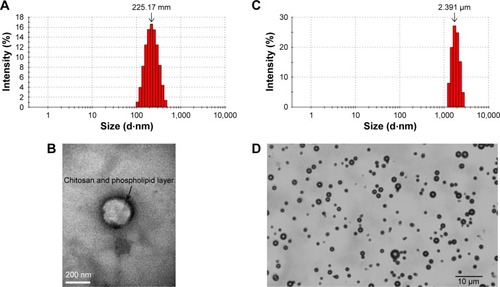
First, we prepared CLPs composed of DPPC, DSPE, and CHOL at a mass ratio of 3:1:1. The sizes of the CLPs were 225.17±17.85 nm () and CLPs had positive charges of 12.64±1.44 mV, as their outer layer was coated with chitosan. The morphology of the CLPs revealed that the liposomes were regularly spherical, and the outer layer was composed of chitosan and phospholipid (). A modified injection method was used to prepare the CLPs, yielding an encapsulation efficiency as high as 90.31%±2.84% and a drug loading of 15.62%±1.97%. Next, we prepared microbubbles containing DPPC, DSPE, and CHOL at a mass ratio of 10:4:1. The microbubbles had an average diameter of 2.391±0.052 µm () and showed negative charges of −4.32±0.43, as the surfaces were composed of phospholipid shells and the cores were filled with C3F8. As shown in , phospholipid-coated microbubbles were annular and uniformly dispersed without aggregation.
Figure 1 Characterizations of CLPs and microbubbles.
Notes: (A) The size distribution of CLPs. (B) Transmission electron microscopic image of CLP (×100,000). (C) The size distribution of microbubbles. (D) Optical microscopic image of microbubbles (×400).
Abbreviation: CLP, chitosan-modified polymyxin B liposome.
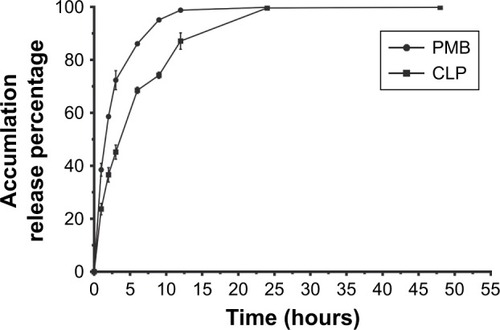
In vitro drug release of CLPs
The dialysis method was adopted to determine the accumulated release percentages of polymyxin B and CLPs in vitro over time (). Polymyxin B showed sudden release and was completely released from the dialysis medium within 12 hours, while polymyxin B in CLPs was released more slowly, with nearly all of the drug released in 24 hours. These data suggest that the polymyxin B preparation of CLPs prolonged the release time of polymyxin B.
MBIC of CLPs
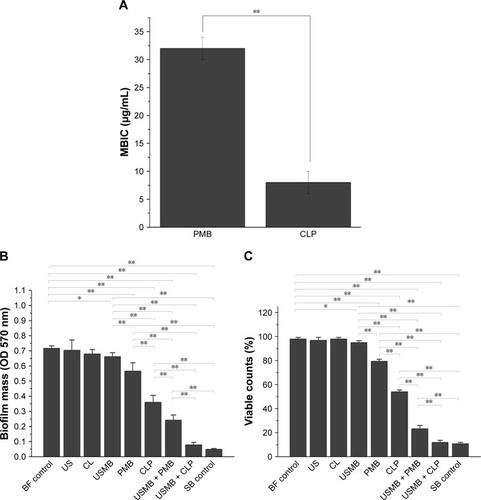
MBIC is defined as the lowest concentration that inhibits visible biofilm cell growth. The MBIC of CLPs on the biofilm-producing AB was 8±2 µg/mL, while that of polymyxin B alone was 32±2 µg/mL (). Compared with polymyxin B alone, CLPs induced a significant antibacterial effect on the biofilm-producing AB (P<0.01).
Figure 3 Antibacterial effects of different treatments on biofilm-producing AB in vitro.
Notes: (A) MBIC of PMB and CLPs to biofilm-producing AB (n=3). (B) Crystal violet staining assays and (C) resazurin assays were used to evaluate the effects of different treatments on the biofilm biomass (n=6). The different preparations contained 2 µg/mL of polymyxin B. The frequency of this unfocused ultrasonic transducer was 1.0 MHz, and the acoustic intensity was set as a continuous ultrasonic intensity of 3 W/cm2. The duration of the US intervention was 5 minutes. *P<0.05, **P<0.01.
Abbreviations: AB, Acinetobacter baumannii; MBIC, minimal biofilm inhibitory concentration; BF, biofilm; US, ultrasound; CL, chitosan-modified liposome; USMB, ultrasound microbubble; PMB, polymyxin B; CLP, chitosan-modified polymyxin B-loaded liposome; USMB + PMB, ultrasound microbubble and polymyxin B; USMB + CLP, ultrasound microbubble and chitosan-modified polymyxin B-loaded liposome; SB, sterile blank.
Synergistic antibacterial effects of USMBs and CLPs against biofilm-producing AB
We further explored the synergistic antibacterial effects of USMBs combined with different preparations of polymyxin B. The crystal violet staining assay was applied to evaluate biofilm mass, and the resazurin assay was used to estimate viable counts. As shown in , compared with the biofilm control, USMBs induced an inhibitory effect on both biofilm mass and viable bacteria against biofilm-producing AB (P<0.05), while ultrasound alone and chitosan-modified liposomes exerted no antibacterial effects (P>0.05). When the polymyxin B concentration of each preparation was 2 µg/mL, which is the susceptible breakpoint for polymyxin B in the Clinical and Laboratory Standards Institute (CLSI),Citation26 the inhibitory effects of CLPs or polymyxin B alone were enhanced compared with those of USMBs (P<0.01). Furthermore, CLPs inhibited biofilm mass and viable bacteria more obviously than polymyxin B alone (P<0.01). Compared with polymyxin B or CLPs alone, USMBs combined with polymyxin B or CLPs induced significant inhibitory effects on the biofilm mass and viable bacteria (P<0.01), suggesting that USMBs enhanced the antibacterial effects of polymyxin B preparations on biofilm-producing AB. Among these treatments, USMBs combined with CLPs induced significantly greater inhibition of the biofilm-producing AB than that of USMBs combined with polymyxin B (P<0.01). There was no significant difference in the biofilm mass and viable counts of USMBs combined with CLPs and the sterile blank control (P>0.05). These results indicate that the combination of USMBs and CLPs yielded the most significant synergistic antibacterial effect, which could almost completely eliminate biofilm-producing AB.
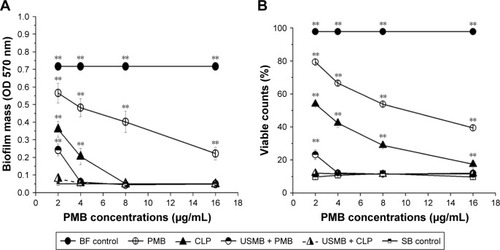
Dose–effect relationship of antibacterial effects against biofilm-producing AB
In this section, we further illuminated the dose–effect relationship of antibacterial effects of polymyxin B or CLPs with or without USMBs. As shown in the biofilm mass () and the viable count () results, with increasing polymyxin B concentrations (2, 4, 8, and 16 µg/mL), the scavenging effect of each treatment on bacterial biofilms was gradually enhanced within a certain dose range. Though the antibacterial activity of polymyxin B alone showed a dose–effect relationship within the range from 2 to 16 µg/mL, there were significant differences in biofilm mass and viable counts between 16 µg/mL polymyxin B and the sterile blank control (P<0.05), suggesting that 16 µg/mL polymyxin B, which was the maximum dose in this study, could not eliminate the biofilm-producing AB. The antibacterial activity of CLPs also showed a dose–effect relationship between 2 and 16 µg/mL concentrations. Furthermore, between CLPs with 8 µg/mL polymyxin B and the sterile blank control, there was no significant difference in the biofilm mass (P>0.05), while between CLPs with 16 µg/mL polymyxin B and the sterile blank control, there was a significant difference in the viable counts (P<0.05), suggesting that CLPs even with 16 µg/mL polymyxin B could not eliminate the biofilm-producing AB. The antibacterial activity of USMBs combined with polymyxin B showed a dose–effect relationship within the range from 2 µg/mL to 4 µg/mL polymyxin B, and USMBs combined with 4 µg/mL polymyxin B could effectively remove the biofilm-producing AB (P>0.05 vs sterile blank control). However, USMBs in combination with 2 µg/mL polymyxin B could completely eliminate the biofilm-producing AB and achieved the maximum antimicrobial effects (P>0.05 vs sterile blank control).
Figure 4 Dose–effect relationships of different treatments on biofilm-producing AB were measured by crystal violet staining assays (A) and resazurin assays (B) (n=6).
Note: **P<0.01 vs SB control.
Abbreviations: AB, Acinetobacter baumannii; BF, biofilm; PMB, polymyxin B; CLP, chitosan-modified polymyxin B-loaded liposome; USMB + PMB, ultrasound microbubble and polymyxin B; USMB + CLP, ultrasound microbubble and chitosan-modified polymyxin B-loaded liposome; SB, sterile blank.
Morphological evaluation of the biofilm
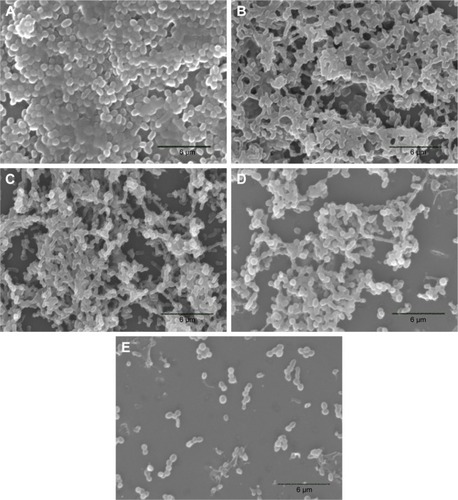
The anti-biofilm effects of different treatments against biofilm-producing AB were then confirmed by SEM analysis. The results of the biofilm control () showed that AB adhered to the coverslip surface to form dense biofilms, which were rod shaped with a cluster distribution, closely linked with the extracellular matrix. USMBs () had sparser biofilms than the biofilm control. Although the density was sparser than that of USMBs, most of the biofilms were still present in the polymyxin B group (). Compared with those of the 2 µg/mL polymyxin B group, the biofilms of the CLPs with 2 µg/mL polymyxin B group were obviously sparser, and some planktonic bacteria were present (). However, USMBs combined with CLPs showed the greatest anti-biofilm effects. A few scattered bacteria without biofilm structure were observed, as shown in .
Figure 5 Scanning electron microscopy images of AB biofilms.
Notes: (A) BF control; (B) USMB; (C) PMB; (D) CLP; and (E) USMB + CLP. The images A–E are at 3,000× magnification.
Abbreviations: AB, Acinetobacter baumannii; BF, biofilm; USMB, ultrasound microbubble; PMB, polymyxin B; CLP, chitosan-modified polymyxin B-loaded liposome; USMB + CLP, ultrasound microbubble and chitosan-modified polymyxin B-loaded liposome.
Discussion
AB is one of the most serious opportunistic pathogens in clinical settings, which can largely be attributed to the following characteristics. First, AB has such a strong adhesion capacity that it can easily adhere to living or non-living surfaces and form biofilms both in vitro and in vivo.Citation27,Citation28 The formation of biofilms makes it difficult for antibacterial drugs to act directly on bacteria. Furthermore, biofilm bacteria have slower growth rates than planktonic bacteria, resulting in slower responses to antibiotics and increased drug resistance, as there is a lack of oxygen and nutrients in biofilms.Citation4 Next, AB induces a poor inflammatory response in human cells.Citation29 The effective treatment of MDR, XDR, and PDR AB remains a great medical challenge. In this study, we prepared CLPs and microbubbles and then explored the synergistic antibacterial effects of the combination of USMBs and CLPs against biofilm-producing AB.
Compared with liposomes, besides improving the stability of preparations, chitosan-modified liposomes might have the following advantages. First, the surface charge of liposomes is generally neutral or slightly negative, which is unfavorable for the electrostatic interaction with membranes.Citation9 However, the positive charge of chitosan could facilitate electrostatic cross-linking with bacterial membranes and drug delivery into cells, thus enhancing antibacterial activity.Citation23 Second, the hydrophilicity of chitosan may prolong the cycle time of drugs encapsulated in liposomes and enhance the drugs’ interactions with bacteria. Third, chitosan has the pharmacological capacity to promote wound healing and prevent scarring.Citation30 To increase the antibacterial effectiveness of polymyxin B and reduce its toxicity, we prepared CLPs that were 225.17±17.85 nm in size and carried a positive charge of 12.64±1.44 mV (). These CLPs, which had higher encapsulation efficiency and drug loading, could have a sustained release effect, as shown in . Next, we discovered that the MBIC of CLPs on the biofilm-producing AB was 8±2 µg/mL (), but the CLSI susceptible breakpoint for polymyxin B was ≤2 µg/mL, suggesting that CLPs could still not effectively eliminate the polymyxin B-resistant biofilm-producing AB, though it had four times more anti-biofilm efficacy than polymyxin B. As the study assumed, CLPs could significantly enhance the anti-biofilm effects of polymyxin B (). These results were reconfirmed by morphological assays ().
In this study, ultrasound alone could not inhibit biofilm bacteria (). However, USMBs can obviously inhibit biofilm-producing AB (). The morphological analysis via SEM confirmed the inhibitory effect of USMBs on biofilms (). Previous reports indicated that high-intensity ultrasound could induce cavitation to cause tiny air bubbles in liquid culture medium to expand and break, and thus opening temporary channels in biofilms. However, when no antibiotics were present, these temporary channels only promoted oxygen and nutrient penetration into biofilms and bacterial metabolite discharge.Citation31 However, in our study, microbubbles filled with C3F8 were large, stable, and present in high concentrations, and microbubbles were affected by high-intensity ultrasound at 3 W/cm2. All of these parameters were enough to make the microbubbles instantly cavitate and release energy, causing the formation of temporary channels in the biofilms. As a result, the planktonic bacteria were freed from biofilms, and the continuous ultrasound disintegrated these planktonic bacteria.Citation22,Citation32,Citation33 However, USMBs had the less anti-biofilm effectivity than 2 µg/mL of polymyxin B (). Moreover, as the study assumed, CLPs could significantly enhance the anti-biofilm effects of polymyxin B (). These results were reconfirmed via morphological assay ().
Though USMBs combined with 2 µg/mL of polymyxin B had significant synergistic inhibitory effects on biofilm-producing AB, this combination could still not completely inhibit biofilm-producing AB ( and ), while USMBs combined with CLPs containing 2 µg/mL of polymyxin B could almost eliminate AB ( and ), as confirmed by the morphological assay of USMBs combined with CLPs (). The possible mechanisms of these significant synergistic anti-biofilm effects might be the formation of temporary channels caused by cavitation, which induced CLPs penetration into biofilms and the disintegration of planktonic bacteria dissociated from biofilms. In addition, this study revealed that polymyxin B or CLPs alone and in combination with USMBs had obvious dose–effect relationships, suggesting that each treatment specifically inhibited biofilm-producing AB ().
Conclusion
In this research, we successfully prepared USMBs and CLPs, which had a more significant antibacterial effect on biofilm-forming AB than polymyxin B alone. Experiments in vitro indicate that the synergistic antibacterial effect of USMBs in combination with CLPs containing as little as 2 µg/mL of polymyxin B is sufficient to almost eliminate drug-resistant biofilm-producing AB. In subsequent studies, we will further verify the biological toxicity of CLPs, and develop AB bio-film lung infection animal models or SSTI animal models to further evaluate whether USMBs combined with CLPs can enhance the antibacterial effect of polymyxin B and reduce its toxicity for treating AB infection in systemic or topical antibacterial treatment conditions.
Acknowledgments
This work was supported by a grant from the Health Commission of Chongqing, China (2016ZDXM010).
Supplementary material
Table S1 MIC values of antibacterial agents against AB W1340
Disclosure
The authors report no conflicts of interest in this work.
References
- KrzyściakPChmielarczykAPobiegaMRomaniszynDWójkowska-MachJAcinetobacter baumannii isolated from hospital-acquired infection: biofilm production and drug susceptibilityAPMIS2017125111017102628913903
- FlemmingH-CWingenderJThe biofilm matrixNat Rev Microbiol20108962363320676145
- GreeneCVadlamudiGNewtonDFoxmanBXiCThe influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumanniiAm J Infect Control2016445e65e7126851196
- SunFQuFLingYBiofilm-associated infections: Antibiotic resistance and novel therapeutic strategiesFuture Microbiol20138787788623841634
- MoradiJHashemiFBBahadorAAntibiotic resistance of Acinetobacter baumannii in Iran: a systemic review of the published literatureOsong Public Health Res Perspect201562798625938016
- InchaiJLiwsrisakunCTheerakittikulTRisk factors of multidrug-resistant, extensively drug-resistant and pandrug-resistant Acinetobacter baumannii ventilator-associated pneumonia in a medical intensive care unit of university hospital in ThailandJ Infect Chemother201521857057426026660
- GuptaMLakhinaKKamathAColistin-resistant Acinetobacter baumannii ventilator-associated pneumonia in a tertiary care hospital: an evolving threatJ Hosp Infect2016941727327238611
- NationRLLiJCarsOFramework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensusLancet Infect Dis201515222523425459221
- Drulis-KawaZDorotkiewicz-JachALiposomes as delivery systems for antibioticsInt J Pharm20103871–218719819969054
- ForierKRaemdonckKDe SmedtSCLipid and polymer nanoparticles for drug delivery to bacterial biofilmsJ Control Release201419060762324794896
- SharmaASharmaUSLiposomes in drug delivery: progress and limitationsInt J Pharm19971542123140
- YangZLiuJGaoJChenSHuangGChitosan coated vancomycin hydrochloride liposomes: characterizations and evaluationInt J Pharm2015495150851526325316
- ManconiMMancaMLValentiDChitosan and hyaluronan coated liposomes for pulmonary administration of curcuminInt J Pharm2017525120321028438698
- ZhuCHeNChengTUltrasound-targeted microbubble destruction enhances human β-defensin 3 activity against antibiotic-resistant Staphylococcus biofilmsInflammation201336598399623519963
- LiaoA-HHungC-RChenH-KChiangC-PUltrasound-Mediated EGF-Coated-Microbubble cavitation in dressings for wound-healing applicationsSci Rep201881832729844469
- GuoHWangZduQStimulated phase-shift acoustic nanodroplets enhance vancomycin efficacy against methicillin-resistant Staphylococcus aureus biofilmsInt J Nanomedicine2017124679469028721044
- LiSZhuCFangSUltrasound microbubbles enhance human β-defensin 3 against biofilmsJ Surg Res2015199245846926119274
- HuJZhangNLiLThe synergistic bactericidal effect of vancomycin on UTMD treated biofilm involves damage to bacterial cells and enhancement of metabolic activitiesSci Rep20188119229317687
- OhlCDAroraMIkinkRSonoporation from jetting cavitation bubblesBiophysical Journal200691114285429516950843
- DongYChenSWangZPengNYuJSynergy of ultrasound micro-bubbles and vancomycin against Staphylococcus epidermidis biofilmJ Antimicrob Chemother201368481682623248238
- RunyanCMCarmenJCBecksteadBLLow-frequency ultrasound increases outer membrane permeability of Pseudomonas aeruginosaJ Gen Appl Microbiol200652529530117310073
- HeNHuJLiuHEnhancement of vancomycin activity against biofilms by using ultrasound-targeted microbubble destructionAntimicrob Agents Chemother201155115331533721844319
- WangMZhaoTLiuYUrsolic acid liposomes with chitosan modification: promising antitumor drug delivery and efficacyMat Sci Eng C20177112311240
- AlipourMHalwaniMOmriASuntresZEAntimicrobial effectiveness of liposomal polymyxin B against resistant gram-negative bacterial strainsInt J Pharm20083551–229329818164881
- DjordjevicDWiedmannMMclandsboroughLAMicrotiter plate assay for assessment of Listeria monocytogenes biofilm formationAppl Environ Microbiol20026862950295812039754
- EllingtonMJEkelundOAarestrupFMThe role of whole genome sequencing in antimicrobial susceptibility testing of Bacteria: report from the EUCAST SubcommitteeClin Microbiol Infect201723122227890457
- SivaranjaniMSrinivasanRAravindrajaCKarutha PandianSVeera RaviAInhibitory effect of α-mangostin on Acinetobacter baumannii biofilms-an in vitro studyBiofouling201834557959329869541
- PakharukovaNTuittilaMPaavilainenSStructural basis for Acinetobacter baumannii biofilm formationProc Natl Acad Sci U S A2018115215558556329735695
- de BreijADijkshoornLLagendijkEDo biofilm formation and interactions with human cells explain the clinical success of Acinetobacter baumannii?PLoS One201055e1073220505779
- ThakurKSharmaGSinghBChitosan-tailored lipidic nano-constructs of fusidic acid as promising vehicle for wound infections: an explorative studyInt J Biol Macromol20181151012102529680503
- WaltersMCRoeFBugnicourtAFranklinMJStewartPSContributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycinAntimicrob Agents Chemother200347131732312499208
- DongYXuYLiPAntibiofilm effect of ultrasound combined with microbubbles against Staphylococcus epidermidis biofilmInt J Med Microbiol2017307632132828610835
- HernotSKlibanovALMicrobubbles in ultrasound-triggered drug and gene deliveryAdv Drug Deliv Rev200860101153116618486268