?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Biocompatibility and stability of zinc oxide nanoparticles (ZnO NPs) synthesized using plants is an interesting research area of study in nanotechnology, due to its wide applications in biomedical, industrial, cell imaging, and biosensor fields. The present study reports the novel green synthesis of stable ZnO NPs using various concentrations of zinc nitrate (0.01M, 0.05M, 0.1M) and Albizia lebbeck stem bark extracts as an efficient chelating agent. Antimicrobial, antioxidant, cytotoxic, and antiproliferative activities of the synthesized NPs on human breast cancer cell lines were evaluated using different assays.
Methods
Characterization of the synthesized ZnO NPs were carried out using various spectroscopic and microscopic techniques. Antimicrobial activity evaluation using disc diffusion method, antioxidant activity using hydrogen peroxide (H2O2) free radical scavenging assay and cytotoxic activity on MDA-MB 231 and MCF-7 using tryphan blue dye exclusion and MTT assay.
Results
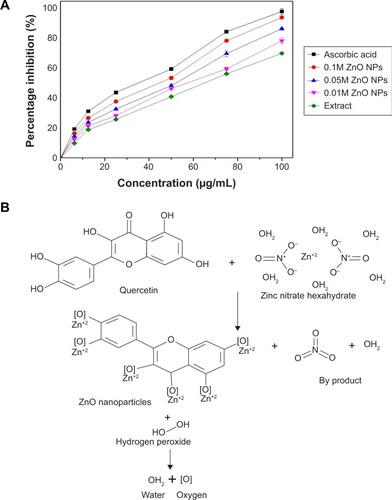
The UV–vis spectroscopy result revealed an absorption peak in the range of 370 nm. The involvements of A. lebbeck bioactive compounds in the stabilization of the ZnO NPs were confirmed by X-ray diffraction and Fourier transform infrared analysis. Zeta sizer studies showed an average size of 66.25 nm with a polydisparity index of 0.262. Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) analyses results revealed irregular spherical morphology and the presence of primarily Zn, C, O, Na, P, and K, respectively. The biosynthesized ZnO NPs revealed strong antimicrobial potentials against various gram-negative and gram-positive bacterial pathogens. Antioxidant activities carried out using H2O2 free radical scavenging assay revealed higher IC50 values of 48.5, 48.7, and 60.2 µg/mL for 0.1M, 0.05M, and 0.01M ZnO NPs, respectively. Moreover, the biosynthesized ZnO NPs showed significant cytotoxic effects on MDA-MB 231 and MCF-7 breast cancer cell lines (P< 0.001, n≥3) in a concentration-dependent manner.
Conclusion
Overall, various concentrations of ZnO NPs were synthesized through a stable, simple, and eco-friendly green route via the use of A. lebbeck stem bark extract. The biosynthesized ZnO NPs showed strong antimicrobial, antioxidant and cytotoxic activity against strongly and weakly metastatic breast cancer cell lines.
Introduction
Nanotechnology is a rapidly growing area of studies that utilizes biosynthetic and environmental-friendly technology for the purpose of synthesis of zinc oxide nanoparticles (ZnO NPs), which are known to be nontoxic, chemically stable, biocompatible, and could be used as drug carriers,Citation1 cell imaging agents,Citation2 anticancer agents,Citation3 antimicrobials,Citation4 biosensors,Citation5 antidiabetics,Citation6 and cosmetics,Citation7 because of their novel physicochemical properties. Biocompatibility of zinc has been attributed to its presence in human system as the second most common and essential trace elements after iron with diverse role in body metabolic activities, and it is the fourth most commonly consumed metals worldwide.Citation8 The physical and chemical methods that are used for the synthesis of metal oxide NPs are expensive and toxic, and antagonistic chemicals are used as a stabilizing agent.Citation9 Metals oxide NPs modifications could be achieved by substitution with specific atoms, which then enhance optical, mechanical, and electrical properties of materials by changing their chemical surface properties.Citation10 ZnO NPs have recently gained special attention due to their hexagonal phase, n-type semiconductor, and wurzite structure.Citation11 Green biosynthesis of ZnO NPs is simple, viable, and cost-effective and provides high yield with novel physical appearance, compared with other NPs such as silver, gold, titanium, and nickel.Citation12
Recent studies have demonstrated the antibacterial potential of ZnO NPs through disruption of the cell membrane integrity, against extended-spectrum beta-lactamase-producing bacteria Klebsiella pneumonia and Escherichia coli.Citation13 It has been recommended that ZnO NPs could retard E. coli growth through disorganizing the bacterial cell membrane, which increases permeability of the NPs through the membrane, leading to its accumulation in the cytoplasm and cell disruption.Citation14 However, some microorganisms appear to show strong resistance against ZnO, but ZnO NPs showed strong antimicrobial activities on certain pathogenic bacteria such as Salmonella enteritidis, Listeria monocytogenes, Bacillus subtilis, Staphylococcus aureus, and E. coli.Citation15–Citation17 Medicinal plants are known to possess vitamins, terpenoids, and phenolic compounds that have antioxidant potentials,Citation18 but literature showed that synthesized NPs using medicinal plants as dispersing agents display more antioxidant activity in vitro.Citation19 Suresh et al reported ZnO NPs synthesized using Cassia fistula to exhibit significant antioxidant activities through scavenging of 1, 1-diphenyl-2-picrylhydrazil (DPPH) radicals.Citation20 Investigations on the cytotoxic activity of ZnO NPs demonstrate cytotoxic effect against cancerous cells and normal cells including lung epithelial cellsCitation21 and human lens epithelial cells.Citation22 Green synthesis of ZnO NPs using various medicinal plants including Costus pictus D. Don,Citation23 Pongamia pinnata,Citation24 Vitex negundo,Citation25 and Cassia auriculataCitation26 has been reported to exhibit cytotoxic activity.
Albizia lebbeck is a tropical species that belongs to Albizia genus, mostly growing to an average height of 24 m, trunk width of 50 cm, and seed pods that contain 6–12 seeds. The plant is widely distributed in Australia, Asia, Africa, and Southern America.Citation27 The seeds of the plant demonstrate anti-tumor, antifungal, and antibacterial activities against HepG2 hepatoma cells, fungi Rhizoctonia solani, and E. coli.Citation28 The leaves of the plant contain phytochemicals such as flavonoids, tannins, alkaloids, triterpenoid saponins, and cardiac glycosides that have therapeutic value.Citation29
In the present study, cost-effective green synthesis of ZnO NPs using A. lebbeck aqueous extract as a capping agent and their characterization using various spectroscopic techniques are reported. The antibacterial activity of the synthesized NPs was studied against five pathogenic microorganisms: two gram-positive (Bacillus cereus and S. aureus) and three gram-negative (E. coli, K. pneumoniae, and Salmonella typhi). The in vitro free radical scavenging activity of ZnO NPs was assessed by hydrogen peroxide (H2O2) free radical scavenging assay. Furthermore, we investigated antiproliferative and cytotoxic activities of the NPs against human breast cancer lines MDA-MB 231 and MCF-7. The results are reported and images are presented.
Materials and methods
Preparation of A. lebbeck extract
Fresh stem barks of A. lebbeck were collected from Gaya Local Government Area, Kano State (Nigeria: 11° 52′ 5″ N and 9° 0′ 40″ E) and authenticated by a botanist at the herbarium of the Department of Plant Biology, Bayero University Kano, Nigeria. The specimen was given a voucher number BUKHAN187 and was deposited at the herbarium of the institute. The barks were washed properly with deionized water, shade dried, and then pulverized into coarse powder using mortar and pestle. A. lebbeck aqueous extract was prepared by slight modifications of the method described by Suresh et al.Citation20 Briefly, the extraction was performed using water as a solvent in which 20 g of the coarse powder was soaked in 100 mL deionized water in a conical flask and was heated in a water bath under constant shaking at 45°C for 24 hours. The extract was then filtered using Whatman No 1 filter paper and the filtrate was stored at 4°C until used.
Synthesis of ZnO nanoparticles
ZnO NPs were prepared successfully using A. lebbeck aqueous extract by the method of Elham Zare et al with slight modifications.Citation30 The ZnO NPs were synthesized using 0.01M, 0.05M, and 0.1M Zn(NO3)2⋅6H2O solution in 90 mL distilled water; then, 10 mL of the prepared A. lebbeck extract was added dropwise into the zinc nitrate solutions under constant stirring at 60°C for 5 hours to achieve a complex formation, and NaOH (5M) was added to the solution during stirring process to adjust the pH. Both the extract (control) and the mixture (zinc nitrate + extract) were then calcined at 350°C±10°C for 2 hours in a muffle furnace to obtain ZnO NPs.
Characterization
The synthesized ZnO NPs were characterized using various spectroscopic and microscopic techniques. UV–visible spectrum was evaluated using UV–Visible spectrophotometer (Shimadzu UV-2450) and the spectrum was recorded between 300 and 800 nm. Hydrodynamic (Z-average) size and polydispersity index (PDI) of the synthesized ZnO NPs were evaluated by Zeta sizer instrument (Malvern Zetasizer Nano ZS90), and the results were acquired by the Malvern ZS nano software. Fourier transform infrared (FTIR) analysis of the NPs was carried out with Fourier transform spectrometer (Shimadzu FT-IR Prestige-21 Model) at a frequency range of 4,000–500 cm−1. Crystalline structure was analyzed using X-ray diffractometer (Rigaku ZSX Primus II). Morphological analysis of the synthesized ZnO NPs coated with platinum was carried out using scanning electron microscope (SEM) (JOEL JSM 6335-F) equipped with 150 kV acceleration voltage, and energy-dispersive X-ray spectroscopy (EDS) (Oxford Instruments AZTEC EDS) attached to the same instrument was used to ascertain the elemental composition and purity of the synthesized ZnO NPs.
Antimicrobial activity
Evaluation of antimicrobial activity of A. lebbeck ZnO NPs against five pathogenic microorganisms – two gram-positive, B. cereus (ATCC 7064) and S. aureus (6538 P), and three gram-negative, E. coli (O157:H7), K. pneumoniae (ATCC 27738), and S. typhi (B-4420) – was carried out on Muller–Hilton agar dishes using disc diffusion method.Citation31 Ciprofloxacin (10 µg/disc) was used as a standard and sterile blank disc with 5 mm diameter infused with known concentration of ZnO NPs, extracts, and dimethylsulfoxide (DMSO) was used to ascertained the antibacterial activity. Pure culture of the microorganisms was provided by the Department of Food Engineering Laboratory, Faculty of Engineering, Ege University, Turkey.
Antioxidant activity
Antioxidant activity was carried out by hydrogen peroxide (H2O2) free radical scavenging assay using the method of Pick and Mizel with some modifications.Citation32 Briefly, various concentrations (100, 75, 50, 25, 12.5, and 6.25 µg/mL) of the synthesized ZnO NPs, extract, and ascorbic acid as standard, were mixed with 100 µL H2O2 solution (5 mM), and the absorbance was read at 230 nm after 20 minutes of incubation. PBS without hydrogen peroxide was used as a blank solution. The percentage inhibition of H2O2 scavenging was calculated using the following equation:
Cell culture
Breast cancer cell lines MDA-MB 231 and MCF-7 were obtained from Professor Dr Mustafa Djamgoz (Imperial College London, UK), and the use of the cell lines was approved by Biotechnology Research Center Ethical Committee (BRCEC2011-01). Cells were cultured in DMEM (Gibco by Life Technology, Carlsbad, CA) supplemented with 10% 2 mM l-glutamine, and penicillin/streptomycin. The cells were constantly maintained under cell culture conditions of 37°C and 5% CO2 in a humidified chamber. All the chemicals used for the cell culture are of analytical grades.
Cytotoxicity and proliferation assay
Cytotoxic activity of the ZnO NPs was evaluated by tryphan blue dye exclusion assay on breast cancer cell lines MDA-MB 231 and MCF-7.Citation33 The assay was performed to determine whether the ZnO NPs have toxic effect on the cell lines. The cells were plated in 35 mm dishes, incubated overnight, and treated with various concentrations (100, 50, 25, and 5 µg/mL) of the synthesized ZnO NPs. After the treatment, tryphan blue dye (4%) was added into the 35 mm cell culture dishes and incubated for 10 minutes. Thirty random fields of view with at least 20 cells in each field were viewed at 100× using an inverted microscope (Leica DFC295) and the number of live vs dead cells was determined.
Antiproliferative activity of the synthesized ZnO NPs was analyzed using MTT reagent (Sigma-Aldrich) as previously described by Fraser et al with slight modifications.Citation34 Briefly, cells (MDA-MB 231 and MCF-7) were plated in 12-well Falcon tissue plates at a density of 3×104 cells/well in 1 mL DMEM and allowed to settle overnight. After that, the DMEM was removed and replaced with various concentrations of ZnO NPs viz., 5, 25, 50, and 100 µg/mL (minimum three wells per each concentration), and then incubated for 24 hours. After the treatment periods, the medium was removed, and 0.15 mL MTT was added to 0.6 mL fresh medium. The plates were then wrapped in foil and incubated at 37°C for 3 hours. The medium containing the MTT was substituted with 0.89 mL DMSO and 0.11 mL glycine buffer. Absorbance was measured using Absorbance Microplate Reader (ELX 800™) at a wavelength of 570 nm after 10 minutes.
Statistical analysis
Data are given as means ± standard errors of the mean (SEM). Graphical representations and statistical analysis were done using OriginPro (version 2016) and InStatGraphPad software (version 3). Statistical comparisons were determined using one-way ANOVA followed by Tukey–Kramer multiple comparison test, where necessary. All the experiments were carried out in triplicate and repeated at least three times (n≥3). P<0.05 was considered significant or P>0.05 insignificant and P<0.0001 was considered highly significant.
Results and discussion
In this present study, ZnO NPs were rapidly synthesized using A. lebbeck stem bark extract as bioreductant.
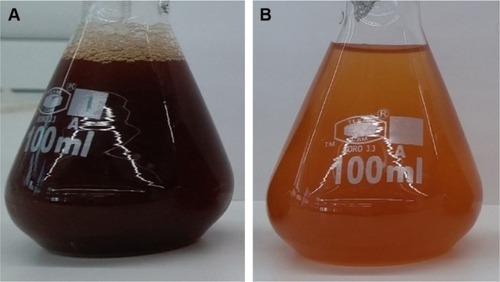
UV–vis spectroscopy was employed to analyze the formation of ZnO NPs from zinc nitrate solution, since there is a visible color change (from dark brown to light brown) that occurred, which indicates the formation of ZnO NPs (). Intensity of the light brown color of the NPs increased with increase in the concentration of zinc nitrate, which could be due to the excitation of surface plasmon vibrations.Citation35
Figure 1 Synthesis of ZnO NPs: (A) Albizia lebbeck aqueous extract; (B) ZnO NPs.
Abbreviation: ZnO NPs, zinc oxide nanoparticles.
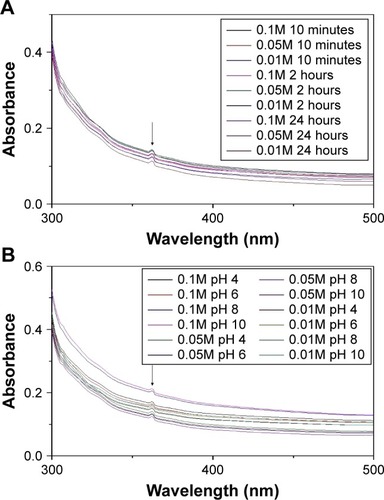
UV–visible absorption of the synthesized ZnO NPs with various concentrations of zinc nitrate and pH at different incubation periods are shown in . The results of our study show that the absorption peak for the synthesized ZnO NPs is 368 nm, and is in conformity with the range of light absorption of ZnO NPs, which is 360–380 nm.Citation36 Elham Zare et al reported the biosynthesis of ZnO NPs using cumin seeds and zinc nitrate, with an average size of 7 nm and UV– vis absorption peak at 370 nm.Citation30 Additionally, Singh et al also reported the green synthesis of ZnO NPs using Pseudomonas aeruginosa and zinc nitrate, with a size between 35 and 80 nm and UV–vis absorption at 360 nm.Citation37 Also, some physicochemical parameters such as concentration of metal ions, pH, and incubation period were studied to determine the suitable condition for the synthesis of ZnO NPs (). Increasing the concentrations of the metal ions (zinc nitrate) from 0.01 to 0.1M did not directly show any significant effect on the absorption peak but it revealed effect on the intensity of the synthesized ZnO NPs with increased metal ion concentrations. The pH was regulated using 0.1M NaOH and 0.1 HCl. At pH 4, 6, and 8, the nanoparticle was synthesized but there was a slight shift in the absorption intensity of the synthesized ZnO NPs; however, at pH 10, no effect on both the intensity and the absorption peak of the nanoparticle was observed. Nagarajan et al revealed that, at lower pH 4–5 and higher pH 10, ZnO NPs synthesized using seaweed did not show absorption.Citation36 The presence of absorption peaks at those pH in our study could be a result of high carboxylic acid content present in A. lebbeck stem bark extract.Citation29 Similarly, measuring the UV–vis spectra of the synthesized ZnO NPs at different time intervals after the synthesis did not show any effect on the absorption peak and intensity (), and it reflects the stability of the synthesized ZnO NPs as suspension after 24 hours.
Figure 2 UV–visible spectra of ZnO NPs prepared with various concentrations of zinc nitrate at different (A) incubation time and (B) pH.
Abbreviation: ZnO NPs, zinc oxide nanoparticles.
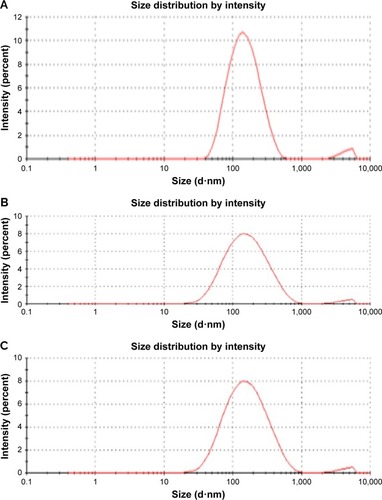
Hydrodynamic size distribution results of the ZnO NPs were obtained by the dynamic light scattering analysis of the particles to confirm the synthesis of the NPs; the Z-average size of the 0.05M and 0.1M ZnO NPs was 82.31 nm and average PDI was 0.262 (). The Z-average of 0.01M synthesized NPs was 110 nm, and PDI was 0.326 (). Our zeta sizer results clearly reveal that the synthesized ZnO NPs are monodispersed in nature due to their broad size distribution and show PDI values of <0.7, and this confirmed the monodispersity of ZnO NPs.Citation38 Particle size can be elucidated more with transmission electron microscope or SEM.Citation39 The result obtained was almost similar to our SEM data.
Figure 3 Particle size distribution of synthesized ZnO NPs using Albizia lebbeck stem bark extract: (A) 0.1M, (B) 0.05M, and (C) 0.01M.
Abbreviation: ZnO NPs, zinc oxide nanoparticles.
FTIR analysis of the synthesized ZnO NPs was carried out at room temperature and a frequency range between 400 and 4,500 cm−1. The FTIR spectra show the composition of the bioactive molecules of A. lebbeck and their distribution on the surface of the ZnO NPs. Synthesized ZnO NPs and A. lebbeck spectra, shown in , revealed the different absorption bands of the NPs and the plant extract, respectively. Wide absorption bands around 3,356 cm−1 were associated with O–H bending of the water molecules, which were adsorbed on the sample.Citation40 FTIR spectrum of the ZnO NPs (0.01M, 0.05M, and 0.1M) prepared with the A. lebbeck extract absorbed at 1,402, 1,115, 1,030, and 618 cm−1. The absorption peak at 1,402 cm−1 correspond to C=CH stretching of methyl group, and 1,030 cm−1 implies the presence of C=O amide band of aliphatic carboxylic acid. The absorption peak observed at 1,030 cm−1 can be attributed to C–O of primary saturated alcohol. The bands observed at 618 cm−1 correspond to Zn–O bond, which confirms that the synthesized nanoparticle is a ZnO NP.Citation41 Our FTIR analysis revealed that carboxylic acid and alcoholic compounds are capable of binding metals, and may form metal NPs through stabilizing the medium and preventing agglomeration.Citation23
Figure 4 FTIR spectra of Albizia lebbeck extract and synthesized ZnO NPs using various concentrations of zinc nitrate.
Abbreviations: FTIR, Fourier transform infrared; ZnO NPs, zinc oxide nanoparticles.
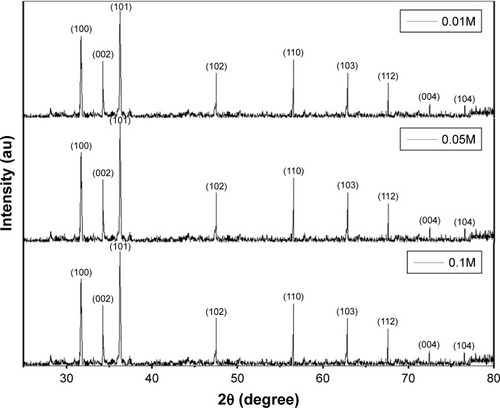
Phase purity of the various concentrations of the biosynthesized ZnO NPs using A. lebbeck extract was studied using X-ray diffraction (XRD). shows the XRD spectrum of the biosynthesized ZnO NPs. The main peaks obtained at 100, 002, 101, 102, 110, 103, 112, 004, and 104 correspond to Bragg reflections with 2θ values of 31.70°, 34.34°, 36.16°, 47.54°, 56.48°, 62.78°, 67.66°, 72.53°, and 76.58° respectively. The presence of the peaks confirms the formation of highly purified ZnO NPs. Synthesized ZnO NPs using various concentrations of 0.01M, 0.05M, and 0.1M reveal similar XRD spectrum pattern. The XRD spectrum obtained indicates the absence of impurities. Furthermore, the pattern shows that the ZnO NPs were synthesized using natural source.Citation30
Figure 5 XRD pattern of 0.01M, 0.05M, and 0.1M synthesized ZnO NPs.
Abbreviations: XRD, X-ray diffraction; ZnO NPs, zinc oxide nanoparticles.
The surface morphology of synthesized ZnO NPs was explored using SEM. Typical SEM micrographs display many agglomerated particles with irregular spherical morphology, and the various concentrations of 0.1M, 0.05M, and 0.01M ZnO NPs reveal average diameter sizes of 66.25, 82.52, and 112.87 nm, respectively (). The ZnO NPs appear to be more agglomerated, and some of the crystals are more visible in . In , the particles reveal slight agglomeration of zinc oxides and appear to be elongated and hexagonal shaped, and some particles in appear to be elongated and rod-like shaped. Raut et al synthesized ZnO NPs using Ocimum tenuiflorum extract, which revealed hexagonal and rod-shaped NPs with size ranging between 11 and 25 nm.Citation42
Figure 6 SEM images of ZnO NPs synthesized using Albizia lebbeck stem bark extract: (A) 0.1M, (B) 0.05M, and (C) 0.01M. EDX spectra of the ZnO NPs: (D) 0.1M, (E) 0.05M, and (F) 0.01M.
Abbreviations: EDX, energy-dispersive X-ray spectroscopy; SEM, scanning electron microscope; ZnO NPs, zinc oxide nanoparticles; cps/eV, counts per second per electron-volt.
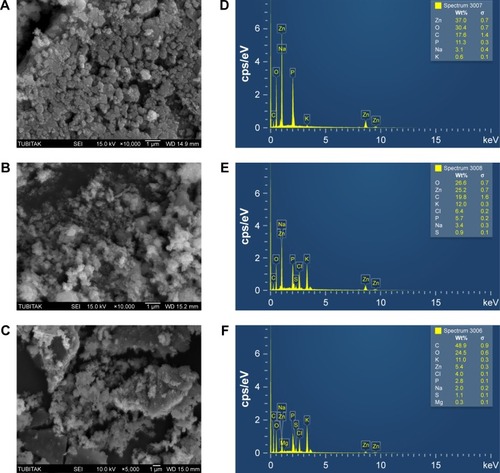
Energy-dispersive X-ray (EDX) spectra revealed the surface chemical composition of the synthesized ZnO NPs. Three clear signals of Zn and one signal of C, O, Na, P, and K were observed in both particles, but there is a presence of an additional signal of S in 0.05M and two signals of Mg and Cl in 0.01 ZnO NPs (). The presence of small signals of C, O, P, K, S, Mg, and Cl in the EDX spectrum confirmed the presence of the bioactive compounds of A. lebbeck stem bark on the surface of the synthesized ZnO NPs, and the Na signal in both particles could as a result of pH regulation during the synthesis of the NPs. Elemental mapping analysis of the synthesized ZnO NPs revealed 37% distribution of zinc in 0.1M, 25.2% in 0.05M, and 5.4% in 0.01M. The EDS results revealed the highest proportion of zinc in 0.1M and 0.05M, and could ascertain the green synthesis of ZnO NPs. Additionally, the optical absorption signals of zinc shown by the synthesized ZnO NPs were due to plasmon resonance of ZnO NPs.Citation36
Antimicrobial activity
The results of the antimicrobial activities of the synthesized ZnO NPs, extract, zinc nitrate solution, DMSO, and antibiotics evaluated using agar disc diffusion method against five pathogenic bacterial strains are shown in .
Table 1 Antimicrobial activity of ZnO NPs in comparison with extract, zinc nitrate solution, DMSO, and antibiotics
While analyzing the antimicrobial activities of the synthesized ZnO NPs, we observed that the inhibitory effects of 0.1M ZnO NPs against B. cereus and S. typhi revealed no significant difference when compared with those of ciprofloxacin (standard), and significant differences were observed against S. aureus, E. coli, and K. pneumoniae ( and ). The lower concentrations of ZnO NPs demonstrated moderate inhibitory effect against both gram-negative and gram-positive bacteria tested. The A. lebbeck aqueous extract showed mild activity, and zinc nitrate solution revealed moderate to mild activity against all the bacterial strains tested. DMSO used a solvent did not demonstrate any antibacterial activity ().
Figure 7 Zone of inhibition of 0.1M synthesized ZnO NPs compared with extract, zinc nitrate solution, DMSO, and antibiotics: (a) extract, (b) zinc nitrate solution, (c) ZnO NPs, (d) DMSO, and (e) antibiotic (ciprofloxacin).
Abbreviations: DMSO, dimethylsulfoxide; ZnO NPs, zinc oxide nanoparticles.

The results revealed that gram-negative bacteria are less resistant to ZnO NPs treatments than gram-positive bacteria, and this could attributed to the presence of a thick layer in the cell walls (peptidoglycan) of the latter group. Sinha et al studied the effect of ZnO NPs on halophilic and mesophilic bacterial species and revealed that Enterobacteria, which is gram-negative, is more sensitive to these NPs than B. subtilis, which is gram-positive bacteria; They concluded that the resistance in gram-positive bacteria is due to the presence of a thick layer of peptidoglycan in their cell wall.Citation43 Elham Zare et al also found a strong antibacterial potential against gram-negative bacteria using ZnO NPs synthesized from cumin seeds, and concluded that the slight resistance of gram-positive bacteria is due the presence of a thick layer of peptidoglycan in their cell wall,Citation30 which is similar to the results achieved in this study. It has been reported that binding of zinc ion to the bacterial cell membrane and production of reactive oxygen species (ROS) inside the cell result in the disruption of the cell.Citation30
Antioxidant activity
Antioxidant activity of the nanoparticles was studied using H2O2 free radical scavenging assay.
The results of the antioxidant activities of the ZnO NPs analyzed spectrophotometrically using H2O2 free radical scavenging assay are shown in . The H2O2 activity of ascorbic acid, 0.1M ZnO NPs, 0.05M ZnO NPs, 0.01M ZnO NPs, and extract revealed IC50 values of 45.7, 48.5, 48.7, 60.2, and 66.6 µg/mL, respectively. Additionally, 0.1M ZnO NPs revealed the highest percentage inhibition among the NPs, followed by 0.05M ZnO NPs. The percentage inhibition shown by 0.1M ZnO NPs is close to that of ascorbic acid (standard), which could be due to increase in zinc ion concentration. The synthesized ZnO NPs revealed a concentration-dependent effect in H2O2 free radical scavenging activity. The extract revealed the lowest antioxidant activity as shown in . Increase in the antioxidant activity of the biosynthesized ZnO NPs when compared with the plant might be a result of metal ions present in the particles; the possible predicted mechanism is illustrated in . It has been reported that enzymes utilizing metal ion (Zn) as a cofactor scavenge H2O2 free radicals, and the presence of Zn ion in particles might be responsible for higher H2O2 free radical scavenging activity when compared with the plant.Citation44 Parashant et al revealed that phenolic compounds present in plant extracts always demonstrated high antioxidant activity and they plays an important role in the green synthesis of nanoparticles.Citation45
In vitro cytotoxic and antiproliferative activity
ZnO NPs were screened for cytotoxic activity against strongly metastatic and weakly metastatic breast cancer (BCa) cell lines, MDA-MB 231 and MCF-7 cells, respectively. Tryphan blue exclusion assay was carried out to ascertain the effects of different concentrations (5, 25, 50, and 100 µg/mL) of 0.1M, 0.05M, and 0.01M ZnO NPs on the viability of MDA-MB 231 and MCF-7 cells after 24-hour incubation period. The results showed that synthesized ZnO NPs significantly inhibited the viability of MDA-MB 231 cells with increased concentration when compared with control (P<0.001, n≥3, ). Typical phase-contrast light-microscopy images of ZnO NPs-treated MDA-MB 231 cells and controls obtained from tryphan blue assays are shown in . Higher concentration of 100 µg/mL for 0.1M, 0.05M, and 0.01M ZnO NPs decreased MDA-MB 231 viability to 53.6%, 60.1%, and 66.2%, respectively.
Figure 9 Effect of 0.1M, 0.05M, and 0.01M synthesized ZnO NPs on the (A) viability of MDa-MB 231 cells, (B) viability of McF-7 cells, (C) proliferation of MDa-MB 231 cells, (D) proliferation of McF-7 cells. (E) Typical phase-contrast light-microscopy images obtained from tryphan blue exclusion assay of MDa-MB 231 and (F) McF-7 cell lines. Typical phase-contrast light-microscopy images (20×) of plasma membrane blebs (directed by an arrow) induced by synthesized ZnO NPs on (G) MDA-MB 231 and (H) MCF-7 cell lines (I) Predicted mechanism behind cytotoxic activity of biosynthesized ZnO NPs using A. lebbeck stem bark against breast cancer lines. *P<0.05, **P<0.01, ***P<0.0001.
Abbreviation: ZnO NPs, zinc oxide nanoparticles.
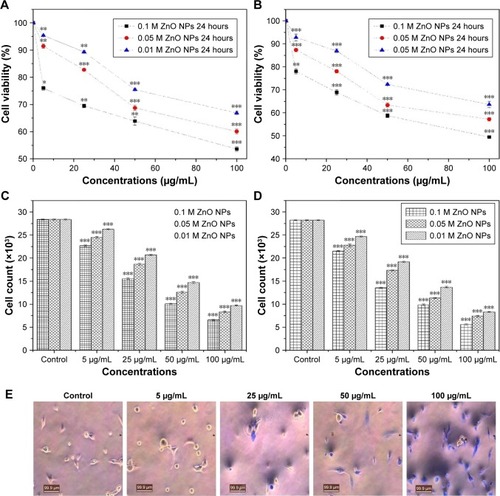
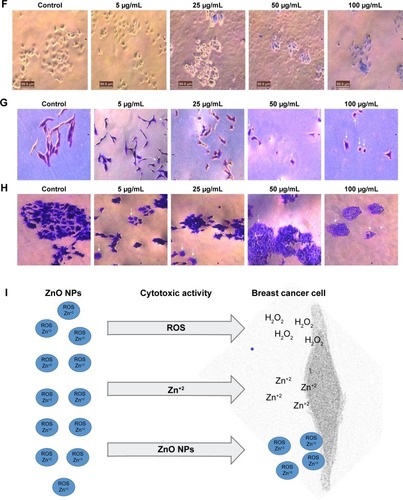
Synthesized ZnO NPs similarly revealed cytotoxic effects on the viability of MCF-7 cells in a concentration-dependent manner as shown in . The 0.1M ZnO NPs revealed higher cytotoxic effects than 0.05M and 0.01M, which might be due to higher percentage of zinc in the nanoparticles. The 5 µg/mL dose of 0.05M and 0.01M synthesized ZnO NPs showed extremely significant difference when compared with control (P<0.001, n≥3, ), but a similar concentration of 0.1M ZnO NPs revealed significant difference upon comparison with the control (P<0.001, n≥3, ). Additionally, all the concentrations of ZnO NPs used in our study showed significant activity against MCF-7 cells when compared with control (P<0.5, n≥3, ). Typical phase-contrast light-microscopy images of ZnO NPs-treated MCF-7 cells and controls obtained from tryphan blue assays are shown in . ZnO NPs showed a cytotoxic effect against colon cancer cell lines HT29, and the effect was found to increase with increased particle concentration.Citation46 Prashant et al reported the cytotoxic effect of ZnO nanopowders synthesized using Punica granatum and Tamarindus indica L. by combustion-assisted facile process against MCF-7 Bca cell line; the cytotoxic effects were found to be concentration dependent,Citation45 and the results were similar to those obtained in our studies.
The study also revealed effect on cell number as a confirmation of the cytotoxic effect of synthesized ZnO NPs on MDA-MB 231 and MCF-7 human Bca cell lines. Synthesized ZnO NPs of 0.1M, 0.05M, and 0.01M showed a significant decrease in the proliferation of both cell lines when compared with control (P<0.001, n≥3, ). The effects of the NPs on cell number following 24 hours incubation period were also found to be concentration dependent. High reduction in the cell number of both cell lines was observed with 0.1M ZnO NPs in a concentration-dependent manner and 100 µg/mL inhibit the cell number of MDA-MB 231 and MCF-7 with 76.8% and 80.2%, respectively; this finding served as a confirmation for the cytotoxic effect as determined by tryphan blue assay. Metal ion NPs and their peptide conjugates inhibit endothelial cell angiogenesis and proliferation through binding to endothelial growth factor, mitogens, and mediators of angiogenesis.Citation32
Membrane blebs were also observed on MDA-MB 231 and MCF-7 cells treated with ZnO NPs synthesized using A. lebbeck stem bark, following 48 hours of incubation (). This may possibly indicate an alternative apoptotic mechanism experienced by MDA-MB 231 and MCF-7 cells as membrane blebs were observed in the cells upon exposure to ZnO NPs (). It has been reported that plasma membrane blebs are among the basic sign that indicate apoptosis in cells.Citation47 The plasma membrane blebs in our studies were also found to occur in a concentration-dependent manner as membrane blebs were not observed with the untreated cells (control) but were observed with treated cells in a concentration-dependent manner. Membrane blebbing and a decrease in cell viability observed in both cells might be a result of ROS, metal ions (Zn+2), and other molecules released in the culture medium by the synthesized ZnO NPs.Citation48 Hanley et al revealed that the increase in intracellular level of Zn+2 ions and other molecules released from ZnO NPs are correlated with an increase in ROS generation, and this will lead to apoptosis.Citation49 The summary of the whole process involved can be seen in .
Conclusion
Overall, various concentrations of ZnO NPs were synthesized through a stable, simple, and eco-friendly green route via the use of A. lebbeck stem bark extract. The extract acts as both reducing and stabilizing agent, which was confirmed by FTIR analysis. The successful synthesis of 0.1M, 0.05M, and 0.01M ZnO NPs was confirmed by Zeta sizer, UV–vis, FTIR, XRD, SEM, and EDX analyses. The UV–vis spectroscopy revealed an absorption peak in the range of 370 nm. SEM results revealed many agglomerated particles with irregular hexagonal morphology and an average size of 66.25 nm. XRD spectrum revealed that the synthesized ZnO NPs have hexagonal wurzite structure. The biosynthesized ZnO NPs showed strong antimicrobial activity against B. cereus, S. aureus, E. coli, K. pneumoniae, and S. typhi. Various concentrations of the ZnO NPs demonstrated antioxidant activity. Additionally, the ZnO NPs showed significant cytotoxic effects and induction of membrane blebs on MDA-MB 231 and MCF-7 breast cancer cell lines in a concentration-dependent manner. The 0.1M ZnO NPs revealed the best biological activity compared with 0.05M and 0.01M nanoparticles and the whole process is illustrated in .
Acknowledgments
The authors acknowledge the support of Prof. Dr. Mustapha Djamgoz, Department of Life Sciences, Faculty of Natural Sciences, Imperial College London.
Disclosure
The authors report no conflicts of interest in this work.
References
- AlbrechtMAEvansCWRastonCLGreen chemistry and the health implications of nanoparticlesGreen Chem200685417432
- XuDLiuMZouHA new strategy for fabrication of water dispersible and biodegradable fluorescent organic nanoparticles with AIE and ESIPT characteristics and their utilization for bioimagingTalanta201717480380828738657
- HassanHFMansourAMAbo-YoussefAMElsadekBEMessihaBAZinc oxide nanoparticles as a novel anticancer approach; in vitro and in vivo evidenceClin Exp Pharmacol Physiol201744223524327718258
- NirmalaMAnukalianiASynthesis and characterization of undoped and TM (Co, Mn) doped ZnO nanoparticlesMater Lett20116517–1826452648
- ThapaASoaresACSoaresJCCarbon nanotube matrix for highly sensitive biosensors to detect pancreatic cancer biomarker CA19-9ACS Appl Mater Interfaces2017931258782588628696659
- El-GharbawyRMEmaraAMAbu-RishaSEZinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in Type-2 diabetesBiomed Pharmacother20168481082027723572
- RosiNLMirkinCANanostructures in biodiagnosticsChem Rev200510541547156215826019
- JansenJKargesWRinkLZinc and diabetes – clinical links and molecular mechanismsJ Nutr Biochem200920639941719442898
- LiHCarterJDLabeanTHNanofabrication by DNA self-assemblyMater Today20091252432
- AltıntaşYOUnalanHEDurucanCHighly efficient room temperature synthesis of silver-doped zinc oxide (ZnO:Ag) nanoparticles: structural, optical, and photocatalytic propertiesJ Am Ceram Soc2013963766773
- ChauhanRKumarAChaudharyRPStructures and optical properties of Zn1−xNiOx nanoparticles by co-precipitation methodRes Chem Intermid201238714831493
- VigneshwaranNKumarSKatheAAVaradarajanPVPrasadVFunctional finishing of cotton fabrics using zinc oxide–soluble starch nanocompositesNanotechnology2006172050875095
- HameedASHChandrasekaranKAbdulazeesPAIn vitro antibacterial activity of ZnO and Nd doped ZnO nanoparticles against ESBL producing Escherichia coli and Klebsiella pneumoniaSci Rep201662431227071382
- BraynerRFerrari-IlliouRBrivoisNDjediatSBenedettiMFFievetFToxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal mediumNano Lett20066486687016608300
- AdamsLKLyonDYAlvarezPJComparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensionsWater Res200640193527353217011015
- HuangZZhengXYanDToxicological effect of ZnO nanoparticles based on bacteriaLangmuir20082484140414418341364
- JonesNRayBRanjitKTMannaACAntibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganismsFEMS Microbiol Lett20082791717618081843
- KolevaIIvan BeekTALinssenJPde GrootAEvstatievaLNScreening of plant extracts for antioxidant activity: a comparative study on three testing methodsPhytochem Anal200213181711899609
- BalanKQingWWangYAntidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extractRSC Adv20166464016240168
- SureshDNethravathiPCUdayabhanuCGRajanaikaHNagabhushanaHSharmaSCGreen synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activitiesMater Sci Semicond Process201531446454
- ZhangJQinXWangBZinc oxide nanoparticles harness autophagy to induce cell death in lung epithelial cellsCell Death Dis201787e295428749469
- WangDGuoDBiHWuQTianQDuYZinc oxide nanoparticles inhibit Ca2+-ATPase expression in human lens epithelial cells under UVB irradiationToxicol In Vitro20132782117212624060544
- SureshJPradheeshGAlexramaniVSundrarajanMHongSIGreen synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activitiesAdv Nat Sci Nanosci Nanotechnol201891015008
- SundrarajanMAmbikaSBharathiKPlant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteriaAdv Powder Technol201526512941299
- AmbikaSSundrarajanMAntibacterial behaviour of Vitex negundo extract assisted ZnO nanoparticles against pathogenic bacteriaJ Photochem Photobiol B2015146525725817218
- PadaliaHMoteriyaPChandaSSynergistic antimicrobial and cytotoxic potential of zinc oxide nanoparticles synthesized using Cassia auriculata leaf extractBionanoscience201881196206
- LowryJTrees for Wood and Animal Production in Northern AustraliaIndooroopilly, QueenslandRural Industries Research and Development Corporation200889
- LamSKNgTBSze KwanLTzi BunNFirst report of an anti-tumor, anti-fungal, anti-yeast and anti-bacterial hemolysin from Albizia lebbeck seedsPhytomedicine201118760160820850957
- NotéOPJihuDAntheaumeCTriterpenoid saponins from Albizia lebbeck (L.) Benth and their inhibitory effect on the survival of high grade human brain tumor cellsCarbohydr Res2015404263325662738
- ZareEPourseyediSKhatamiMDarezereshkiESimple biosynthesis of zinc oxide nanoparticles using nature’s source, and it’s in vitro bio-activityJ Mol Struct2017114696103
- BauerAWKirbyWMSherrisJCTurckMAntibiotic susceptibility testing by a standardized single disk methodAm J Clin Pathol19664544934965325707
- PickEMizelDRapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay readerJ Immunol Methods19814622112266273471
- FraserSPDingYLiuAFosterCSDjamgozMBTetrodotoxin suppresses morphological enhancement of the metastatic MAT-LyLu rat prostate cancer cell lineCell Tissue Res1999295350551210022970
- FraserSPDissJKChioniAMVoltage-gated sodium channel expression and potentiation of human breast cancer metastasisClin Cancer Res200511155381538916061851
- KokilaTRameshPSGeethaDBiosynthesis of AgNPs using Carica papaya peel extract and evaluation of its antioxidant and antimicrobial activitiesEcotoxicol Environ Saf2016134Pt 246747327156649
- NagarajanSArumugam KuppusamyKExtracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, IndiaJ Nanobiotechnol20131139
- SinghBNRawatAKKhanWNaqviAHSinghBRBiosynthesis of stable antioxidant ZnO nanoparticles by Pseudomonas aeruginosa rhamnolipidsPLoS One201499e10693725187953
- AnilreddyBPreparation and characterization of iron oxide nanoparticles on disaccharide templatesB JPRHC200912172183
- KumarBSmitaKCumbalLDebutASynthesis of silver nanoparticles using Sacha inchi (Plukenetia volubilis L.) leaf extractsSaudi J Biol Sci201421660560925473370
- KadamADhabbeRGophaneASatheTGaradkarKTemplate free synthesis of ZnO/Ag2O nanocomposites as a highly efficient visible active photocatalyst for detoxification of methyl orangeJ Photochem Photobiol B2016154243326658629
- YuvakkumarRSureshJSaravanakumarBJoseph NathanaelAHongSIRajendranVRambutan peels promoted biomimetic synthesis of bioinspired zinc oxide nanochains for biomedical applicationsSpectrochim Acta A Mol Biomol Spectrosc201513725025825228035
- RautSThoratPVThakareRGreen synthesis of zinc oxide (ZnO) nanoparticles using Ocimum tenuiflorum leavesInt J Sci Res2013412251228
- SinhaRKaranRSinhaAKhareSKInteraction and nanotoxic effect of ZnO and Ag nanoparticles against ESBL and Amp-C producing gram negative isolates from superficial wound infectionsInt J Curr Microbiol Appl Sci201513847
- GoughDRCotterTGHydrogen peroxide: a Jekyll and Hyde signalling moleculeCell Death Dis20112e21321975295
- ParashantGKPrashantPAUtpalBIn vitro antibacterial and cytotoxicity studies of ZnO nanopowders prepared by combustion assisted facile green synthesisKarbala Int J Mod Sci2015126777
- Bai AswathanarayanJRai VittalRMuddegowdaUJamunaBARavishankarAVUmashankarMAnticancer activity of metal nanoparticles and their peptide conjugates against human colon adenorectal carcinoma cellsArtif Cells Nanomed Biotechnol20184671444145128884587
- MukherjeePBhattacharyaRWangPAntiangiogenic properties of gold nanoparticlesClin Cancer Res20051193530353415867256
- FacklerOTGrosseRCell motility through plasma membrane blebbingJ Cell Biol200581879884
- HanleyCLayneJPunnooseAPreferential killing of cancer cells and activated human T cells using ZnO nanoparticlesNanotechnology2008192929510318836572