Abstract
Purpose
PB is one of the most severe complications of late stage prostate cancer and negatively impacts patient quality of life. A major challenge for the treatment of cancer bone metastasis is the management of efficient drug delivery to metastatic bone lesion. We aimed to explore the use of aptamers as promising tools to develop a targeted drug delivery system for PBs.
Materials and methods
In vivo SELEX was applied to identify bone targeting aptamer in a mouse model with PBs.
Results
The aptamer (designated as “PB”) with the highest bone targeting frequency in mice bearing PC3 PB was selected for further analysis. The PB aptamer specifically targeted modulated endothelial cells in response to cancer cells in the bones of mice bearing PC3 PBs. The targeting efficiency of the PB aptamer conjugated to gold particles was verified in vivo.
Conclusion
This investigation highlights the promise of in vivo SELEX for the discovery of bone targeting aptamers for use in drug delivery.
Introduction
Prostate cancer is the most common noncutaneous cancer in men in the USA. In 2017, ~160,000 men were diagnosed with prostate cancer, adding to 3.3 million existing survivors.Citation1 Most cancer-related deaths are caused by metastases in vital organs,Citation2 with bones among the most frequent sites of prostate cancer metastases. Therefore, the identification of effective treatments for bone metastases is an ongoing important topic in clinical practice related to prostate cancer.
Current treatments for PB are limited. Typically, radiation is used to inhibit tumor growth within bone; however, it is insufficiently effective. Bisphosphonates and denosumab are the two major clinical drugs used for palliative treatment to avoid bone-related events.Citation3,Citation4 Although such palliative treatment drugs delay cancer progression by inhibiting osteolysis in metastatic prostate cancer, they are not effective in killing cancer cells and do not confer patient any survival benefit. To survive in the bone marrow, cancer cells modulate the local environment to support tumor growth by stimulating stromal cells to secrete copious tumor growth factors, which accelerate tumor progression.Citation5–Citation7 The eradication of cancer cells in bone metastases is crucial for the treatment of PB, and precise and efficient delivery of cancer therapeutics to bone is the essential issue.
To resolve this problem, different synthetic carriers, based on cationic polymers, peptides, and lipids, have been developed to form compact nano-sized complexes carrying cancer therapeutics to specific tumor lesion.Citation8 However, the side effects caused by off-target effects have resulted in low therapeutic index and poor safety profiles, which cannot be ignored.Citation9 To improve the therapeutic index and safety of potential therapeutics, one promising approach is to transport drugs to their specific target destinations and shield the drugs from elimination and degradation in the process of overcoming biological barriers.Citation10
Aptamer is a specialized type of small single-stranded RNA or DNA nucleic acid. In a manner analogous to the function of antibodies, the particular spatial structure of aptamer can identify and bind to unique ligand ranging from a small organic molecule to a whole cell with high specificity and affinity. The unique properties of nucleotide acids provide aptamers with numerous advantages relative to protein antibodies, including fast tissue penetration, nonimmunogenicity, and thermal stability.Citation11 More significantly, the convenience ability of nucleotides in conjugation with diverse functional moieties and the extensive scope of their potential biological ligands make aptamer a promising targeting tool in the development of targeted delivery system.
In this research, we applied in vivo SELEX to identify bone targeting aptamer, with the aim of achieving targeted drug delivery using a nude mouse model bearing PC3 PBs. A thioaptamer library was intravenously injected into nude mouse with PC3 PBs, and residual bone marrow aptamers were amplified for subsequent rounds of SELEX. After ten rounds, the aptamer with the highest hitting point (referred as the “PB” aptamer) for bone in the diseased mice was obtained and further characterized in vitro and in vivo. The PB aptamer could not only exhibit high levels of accumulation in the bone of diseased mice through targeting endothelial cells but also specifically target bone in diseased mice, based on recognition of endothelial cells modulated by the local tumor microenvironment. Lastly, the PB aptamer-conjugated gold nanoparticle as targeted delivery system turned out to be of high efficiency in bone accumulation in mice with PBs in a local endothelial cell targeting manner, which validated the original purpose of our study.
Materials and methods
Cell culture
The luciferase-labeled PC3 prostate cancer cell line, DU145 cells, and LNCaP cells were from Nanjing COBIOER BIO-SCIENCES. PC3, DU145, and LNCaP cells were cultured in RPMI-1640 (Invitrogen) with 10% FBS (Invitrogen) and 1% penicillin–streptomycin (Invitrogen). Human umbilical vein endothelial cell (HUVEC) was also from Nanjing COBIOER BIOSCIENCES and was cultured in M199 (HyClone) added with 15% FBS (Invitrogen), 1% endothelial cell growth supplement, and heparin. Cells were incubated at 37°C, 5% CO2, and 100% humidity.
Murine model of human PB
The use of animals was conformed and approved by the Guiding Principles in the Care and Use of Animals for Xiangya Hospital. Animal work was processed following animal experiment regulation of Xiangya Hospital. Six-week-old male athymic nude mice were purchased from the Shanghai SLAC Laboratory Animal. They were housed in a pathogen-free facility and fed with a pathogen-free diet and water. To build up murine models bearing prostate cancer bone metastasis, 1×105 luciferase-labeled PC3 cells were inoculated into mice by intracardiac inoculation. Bone metastasis development was observed under a Xenogen IVIS 200 imaging system. Mice with extensive tumor signal (at least one strong luminescence signal over 60,000 in both spine and limbs) in bone were picked for experiments.
Thioaptamer library
A library of random ssDNA oligonucleotides was from BD Biosciences. This library consisted of a 30 base random region with a flanker of a 21 base 5′-primer (5′-CGCTCGATCGATCTAGATTCG-3′) and a 21 base 3′-primer (5′-GTCATCACGCTCAGATCACTG-3′). PCR amplification was repeated using the extracted ssDNA oligonucleotides after each selection round. First, the ssDNA random library was incubated with Taq DNA polymerase in a mixture of dATP, dTTP, dCTP, and dGTP (500 μM each) for 5 hours at 37°C. After reverse strand was synthesized, the dsDNA library (400 nM) went through PCR amplification. Then PCR products were filtered by a Millipore YM-30 filter to purify dsDNA product. The product was incubated with streptavidin-coated magnetic beads in buffer (10 mM Tris–HCl, 2 M NaCl, and 1 mM EDTA [pH 7.5]) for 20 minutes. Biotin on the reverse strand of the dsDNA binds to the streptavidin beads, followed by separation of the two DNA strands in melting solution (0.1 M NaOH). The forward strands were collected after filter with Millipore YM-10 filter for the next round of selection.
In vivo SELEX
Nude mouse bearing PC3 PBs was chosen to perform aptamer SELEX by a library composed of different ssDNA sequences. When the tumor models were ready about 4–6 weeks after inoculation (nude mouse at the middle stage of PC3 PB development before body paralysis), 10 μg thioaptamer library was intravenously injected to mice. After 4 hours circulation, mice were sacrificed, and bone marrow was collected and frozen in liquid nitrogen. Tissues were homogenized, and DNA was extracted for PCR amplification with aptamer library-specific primers. PCR products were recovered and purified for next round of screening. After ten rounds of screening, high-throughput sequencing was applied, and high-frequency sequences were validated. Non-relevant aptamer (5′-CTACTGGACTTCATCGGAGCTAGGTCATCGCTTGCATGCA-3′) was chosen from reference paper as a negative control.Citation12
Accumulation of PB aptamer in bone
Biophotonic imaging technology was used to examine aptamer distribution. To test bone targeting efficiency of PB aptamer, 0.5 nmol Cy5-PB or Cy5-Control aptamer was injected in nude mice with PC3 PBs (n=6). Four hours after injection, mice were sacrificed and Cy5 signal intensity of major skeletal organs (spine, femur, and tibia) was measured by IVIS-200 system ex vivo. To test whether PB aptamer has targeting specificity for bone with tumor, 0.5 nmol PB aptamer was also injected in healthy nude mice as a contrast.
PB aptamer bone targeting characterization
To see the biodistribution of PB aptamer in vivo, Cy5-PB aptamer-injected nude mouse bearing PC3 PB were sacrificed and major skeletal organs were gathered. After fixation in 10% formalin, decalcification in 10% EDTA, and embedding in paraffin, the skeletal organs were cut into 4 μm sections, which were processed with H&E staining or stained with rat anti-mouse CD31 antibody (1:100 diluted, BD Biosciences) and second Alexa Fluor® 488 donkey anti-rat antibody (1:100 diluted, Life Technologies). Sections were observed under a Nikon Eclipse 80i microscope.
To test whether PB aptamer can bind with bone endothelial cell of nude mice bearing PC3 PB, tumor mouse was sacrificed and major skeletal organs (spine, femur, and tibia) were gathered and smashed. Followed by digestion in DMEM with 1 mg/mL collagenase III for 1 hour, single cells were collected and incubated with PB aptamer or control aptamer (25, 50, and 100 nM) in aptamer binding buffer (4.5 mg/mL glucose, 1 mg/mL BSA, 0.1 mg/mL tRNA, 5 nM MgSO4) on ice for 0.5 hour. Cells were washed by washing buffer, and cells were suspended in washing buffer for flow cytometry analysis and aptamer binding with endothelial cell was measured by flow cytometry.
To test binding affinity of PB aptamer for HUVEC grown in different conditioned medium, medium conditioned with bone marrow was prepared by culturing healthy nude mouse bone marrow with RPMI-1960 for 2 days followed by recollection of the cultured medium. Similarly, medium conditioned with PC3 prostate cancer cell and bone marrow containing PC3 prostate cancer cells were also prepared. Then, HUVEC was cultured with primary RPMI-1960, bone marrow-conditioned RPMI-1960, PC3 prostate cancer cell-conditioned RPMI-1960, or bone marrow with prostate cancer cell-conditioned RPMI-1960 for 8 hours, respectively. After that, PB aptamer binding affinity with HUVEC grown in different medium was tested. PB aptamer uptake in HUVEC grown in conditioned medium was also visualized under fluorescence microscopy briefly. HUVEC grown in conditioned medium was fed with 50 nM Cy5-PB aptamer for 0.5 hour, and then stained with Alexa Fluor 488 Phalloidin (1:100 diluted, Thermo Fisher Scientific) and DAPI (1 μg/mL, Thermo Fisher Scientific). Effect of bone marrow containing LNCaP or DU145 prostate cancer cells conditioned medium on PB aptamer uptake in HUVEC was also measured.
Cellular internalization pathways of PB aptamer
To identify cellular internalization pathways of PB aptamer, uptake of PB aptamer by HUVEC at the presence of internalization pathway inhibitors was tested. The internalization pathway inhibitors include cytochalasin D (80 μM, Abcam), amiloride (400 μM, Sigma-Aldrich Co.), chlorpromazine (50 μM, MP Biomedicals), genistein (80 μM, Sigma-Aldrich Co.), and dynasore (400 μM, Abcam). HUVECs were preincubated with internalization pathway inhibitors for 30 minutes and then 25 nM Cy5-PB aptamer was added. After 30 minutes incubation, live cells were collected for cytometry analysis.
PB aptamer tracking in HUVEC
To test whether PB aptamer can escape from lysosome after cellular internalization, HUVEC was cultured in chamber slides and incubated with 100 nM PB aptamer for 1 hour, 4 hours, and 6 hours, respectively. Lysotracker (1:100 diluted, Cell Signaling) was added 0.5 hour prior to the end. After the incubation, medium was removed and cells were washed by PBS, followed by nuclear staining with DAPI. Cells were observed under a Nikon Eclipse 80i microscope.
PB aptamer-conjugated gold particle bone targeting
To test whether PB aptamer can be used as a bone targeting moiety for bone-targeted drug delivery, Cy5-PB thioaptamer was conjugated onto gold particles with a diameter of 1 μm by modifying gold particle with 3-aminopropyltriethoxysilane and using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride as a poly linker for conjugation of PB aptamer. One billion Cy5-PB-Au, Cy5-Au particles were injected into mouse bearing PB (n=6). After 4 hours, mice were sacrificed and major skeletal organs were collected. Tissues were processed as described above. Gold particles with bright fluorescence were counted under Nikon Eclipse 80i microscope at 40× magnitude, and average particle numbers were counted from ten views of each sample.
Statistical analysis
For statistical comparisons, Student’s t-test was applied (two-tailed distribution, two-sample equal variance) in two-group comparison. In three-group comparison, ANOVA test was performed. Data were presented as mean ± SD. A value of P<0.05 was considered statistically significant.
Results
Selection of the aptamer most strongly targeting bone
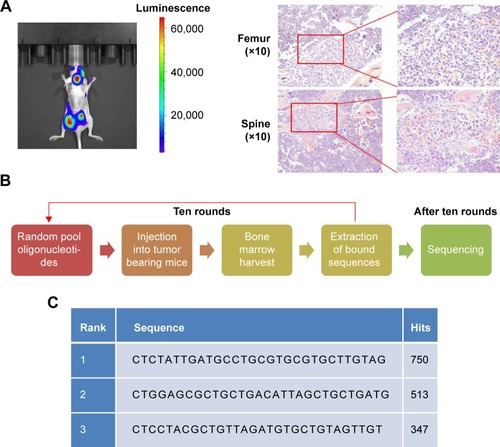
After ten continuous rounds of in vivo SELEX in nude mouse bearing PC3 PB (), three aptamer candidates with the strongest bone marrow targeting capability were identified. The aptamer with the highest hit frequency (sequence: 5′-CTCTATTGATGCCTGCGTGCGTGCTTGTAG-3′) was named by PB (prostate cancer bone metastasis) and selected for further analysis ().
Figure 1 In vivo SELEX of bone targeting aptamer in nude mice bearing PC3 PB.
Notes: (A) Bioluminescence produced by luciferase in nude mice with PC3 PB (left) and bone marrow tumor sites stained using H&E (right). (B) In vivo SELEX procedures. (C) The three aptamers with the highest hitting points after SELEX.
Bone targeting of the PB aptamer in tumor-bearing mice
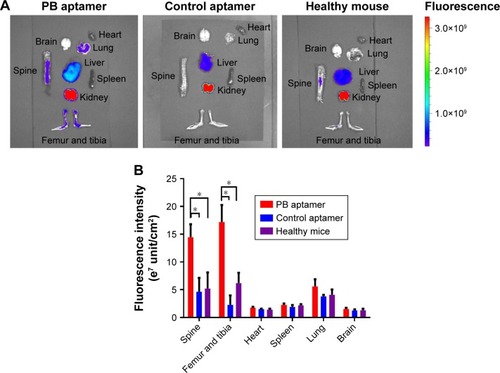
Because PB aptamer had the highest hitting frequency among those in the library, it was anticipated to target bone in tumor-bearing mice. Therefore, we analyzed the distribution of Cy5-PB aptamer and Cy5-Control aptamer in different organs after vein injection ex vivo. The PB aptamer exhibited much higher levels of accumulation in bone compared with the control aptamer (), consistent with in vivo SELEX outcome. In addition, the bone targeting efficiency of PB aptamer was compared in tumor-bearing and healthy mice. The results showed that the PB aptamer exhibited much higher levels of accumulation in bone of tumor-bearing mice than healthy controls, indicating that the PB aptamer specifically targeted bone in tumor-bearing mice (). These properties of bone-specific accumulation in tumor-bearing mice indicated that the PB aptamer had potential as a bone targeting moiety for use in drug delivery.
Figure 2 Higher accumulation of the PB aptamer in bone of tumor mouse compared with the control aptamer, more accumulation of the PB aptamer in bone of tumor mice compared with healthy mice (*P<0.05).
Notes: (A) Distribution of the PB aptamer and control aptamer in different organs ex vivo. (B) Fluorescence intensity of aptamer in different organs.
PB aptamer targets bone marrow endothelial cell
In bone marrow, the endothelium features in the thin capillary walls, no muscle layer, and sparse basement membrane, which facilitates free exchanges between bone interstitial fluid and the vascular fluids.Citation13 Hence, endothelial cell represents a barrier to aptamer entry into the bone marrow and preferred binding to endothelial cell can achieve effective accumulation in the bone. Microscopy analysis revealed that the Cy5-PB aptamer showed an endothelium-associated distribution in bone of tumor mouse, suggesting that endothelial cells may be the target of the PB aptamer (). Immunofluorescence staining of endothelial cell marker CD31 demonstrated that Cy5-PB aptamer colocalized with endothelial cells, not only in bone marrow () but also within tumor in bone (), verifying that bone marrow endothelial cells are the target of PB aptamer.
Figure 3 (A) Distribution of the PB aptamer relative to bone marrow vascular endothelium (red: Cy5-PB aptamer; blue: nuclei). (B) Colocalization of PB aptamer with a vascular endothelial cell marker in bone marrow (red: Cy5-PB aptamer; green: CD31; blue: nuclei). (C) Colocalization of PB aptamer with a vascular endothelial cell marker within tumor in bone marrow (red: Cy5-PB aptamer; green: CD31; blue: nuclei). (D) Higher binding affinity of PB aptamer to bone marrow endothelial cell compared with control aptamer ex vivo (*P<0.05).
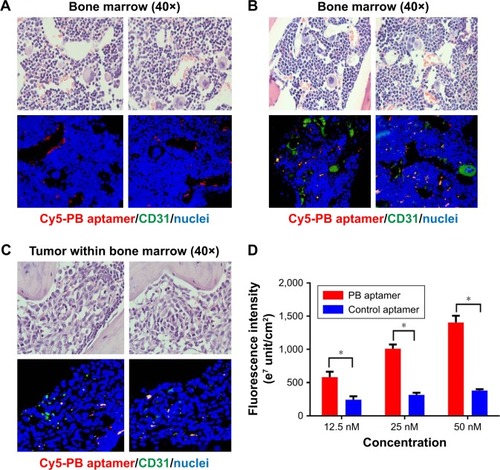
The binding affinities of the PB and control aptamer for bone marrow endothelial cells from tumor mice were compared in vitro. The results showed that the PB aptamer had a higher binding affinity with endothelial cells than control aptamer (), consistent with specificity of the PB aptamer for bone marrow endothelial cells in tumor-bearing mice.
Binding affinity of PB aptamer with HUVEC
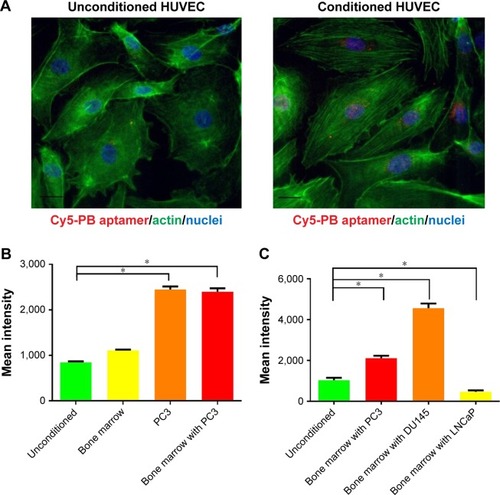
To investigate the bone targeting property of PB aptamer, HUVEC was utilized to test the interaction between PB aptamer and bone vascular endothelial cell in vivo. The PB aptamer showed specificity for targeting bone in tumor-bearing mice; however, the underlying mechanism required further elucidation. We hypothesized that prostate cancer cells from bone could stimulate large amount of PB aptamer affinity molecules on endothelial cell surface as binding ligands. To mimic endothelial cell in vivo, we cultured HUVECs in medium conditioned with bone marrow, or bone marrow containing PC3 prostate cancer, and compared their uptake of the PB aptamer. HUVECs grown in medium conditioned with bone marrow containing PC3 prostate cancer cells exhibited markedly higher Cy5-PB aptamer uptake (). Cytometry analysis further reinforced these findings, as PB aptamer uptake increased by almost three times in HUVECs grown in medium conditioned with bone marrow containing PC3 prostate cancer cells, while remaining unchanged in HUVECs grown in medium conditioned with bone marrow (). These results strongly support the hypothesis that the targeting specificity of PB aptamer for bone marrow endothelial cell is attributable to modulation of the local bone marrow environment by PC3 prostate cancer cells.
Figure 4 (A) Increased PB aptamer uptake in HUVEC grown in PC3 prostate cancer cell-conditioned medium under fluorescence microscopy (red: Cy5-PB aptamer; green: actin; blue: nuclei). Scale bar: 20 μm. (B) Increased PB aptamer uptake in HUVECs grown in PC3 prostate cancer cell-conditioned medium (P<0.05). (C) Increased PB aptamer uptake in HUVECs grown in bone marrow with PC3 or DU145 prostate cancer cell-conditioned medium, while decreased PB aptamer uptake in HUVECs grown in bone marrow with LNCaP prostate cancer cell-conditioned medium (*P<0.05).
Abbreviation: HUVECs, human umbilical vein endothelial cells.
The effect of LNCaP or DU145 prostate cancer cell on PB aptamer uptake in HUVEC was also explored. The results showed that conditioned medium with bone marrow containing LNCaP prostate cancer cells greatly reduced PB aptamer uptake by HUVECs, while conditioned medium with bone marrow containing DU145 prostate cancer cells stimulated a more significant increase in PB aptamer uptake by HUVECs (). This result suggested that different tumor cells have remarkably diverse effects on bone marrow vascular endothelial cells and that these effects determine aptamer targeting specificity.
PB aptamer cellular internalization pathway and lysosome escape
Since PB aptamer could be efficiently taken up by PC3 prostate cancer cell-conditioned HUVEC, we investigated the cellular internalization pathway for the PB aptamer. We hypothesized that the high binding affinity of PB aptamer with HUVEC corresponded to a strong affinity of the aptamer for specific receptors on the surface of HUVEC and that ligand-mediated endocytosis was, therefore, likely to play a major role in the cellular internalization of PB aptamer. To test this hypothesis, we investigated the uptake of PB aptamer by HUVECs treated with different cell endocytosis pathway inhibitors. The results showed that cytochalasin D and genistein (both phagocytosis inhibitors) had no effect on PB aptamer uptake by HUVEC, and amiloride (pinocytosis inhibitor) led to an ~30% decrease in uptake, while chlorpromazine (clathrin-mediated endocytosis inhibitor) and dynasore (dynamin-mediated endocytosis inhibitor), both belonging to ligand-mediated endocytosis inhibitors, inhibited around 70%–80% (). These data indicate that the PB aptamer enters HUVEC mainly via ligand-mediated endocytosis and that the receptors for the PB aptamer are widely expressed on the surface of endothelial cell.
Figure 5 (A) Decreased PB aptamer uptake by chlorpromazine (clathrin-mediated endocytosis inhibitor) and dynasore (dynamin-mediated endocytosis inhibitor) treated HUVEC (*P<0.05). (B) Escape of the PB aptamer from lysosome 6 hours after HUVEC internalization (red: Cy5-PB aptamer; green: lysosome; blue: nuclei). Scale bar: 20 μm.
Abbreviation: HUVEC, human umbilical vein endothelial cell.
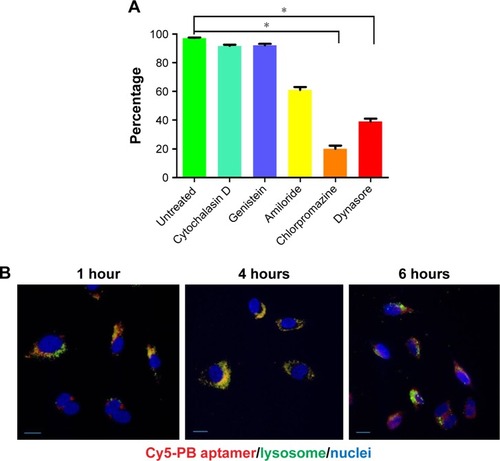
Nanoparticles taken up by endocytosis eventually fused with lysosome in which the highly acidic environment and rich with enzymes promote the degradation of organic nanoparticles.Citation14 To track the fate of PB aptamer after cellular internalization, endosomes in HUVEC were stained at several time points after PB aptamer internalization. The results revealed that PB aptamer escaped lysosome 6 hours after cellular internalization (). This lysosome escape property indicated that PB aptamer could avoid degeneration after vascular endothelial cell internalization, highlighting its potential to function as a protective carrier of conjugated drugs.
Ability of the PB aptamer to target the endothelium and deliver active drugs to bone
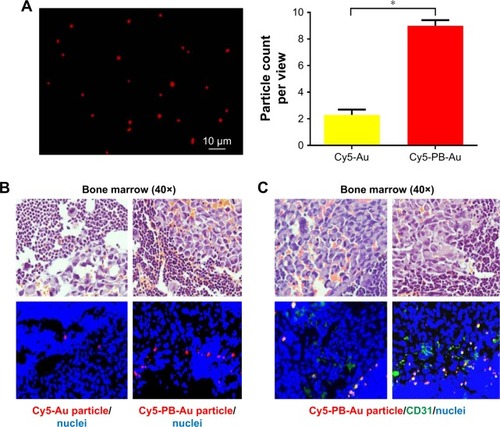
To evaluate whether the PB aptamer could efficiently deliver therapeutics to bone, we prepared Cy5-PB aptamer-conjugated gold particles () and administered them to nude mouse bearing PC3 prostate bone metastasis. Four hours after injection, much higher levels of Cy5-PB-Au particles were observed in bone marrow compared with control Cy5-Au particle (), demonstrating that the PB aptamer could actively deliver drugs to the bone of mice with PC3 prostate bone metastasis. By immunofluorescence staining of endothelium in bone, we detected the colocalization of Cy5-PB-Au particle with bone marrow endothelial cell, confirming the endothelium-targeted accumulation of Cy5-PB-Au aptamer particle consistent with the targeting characteristics of the PB aptamer ().
Figure 6 (A) Cy5-PB-Au particle under fluorescence microscopy. (B) Accumulation of a few Cy5-Au particles within tumor in bone marrow and marked accumulation of Cy5-PB-Au particle within tumor in bone marrow (red: Cy5-Au/Cy5-PB-Au particle; blue: nuclei) (*P<0.05). (C) Colocalization of Cy5-PB-Au particle with a vascular endothelial cell marker within tumor in bone marrow (red: Cy5-PB-Au particle; green: CD31; blue: nuclei).
Discussion
Nanotechnology is the manipulation of matter on a nanometric scale. Through this technology, it is possible to develop innovative devices with distinct physicochemical properties and enormous therapeutic and diagnostic potential.Citation9 As tumor vessels are frequently abnormal, exhibiting increased permeability, nanomedicines with a tendency to enhanced accumulation at the tumor site can improve therapeutic index and reduce side effects.Citation15,Citation16
Tumor environment is characterized by profound changes in molecular and protein expression profile compared with normal tissue. An appropriate aptamer can specifically bind to unique molecule or cell within the tumor environment and conjugation of such aptamer to drugs can facilitate accumulation of the cargo at a targeted area and achieve targeted drug delivery. Therefore, aptamers function as targeting moieties with important roles in drug delivery. Targeted molecules should be distributed on the surface of target cells, where they represent accessible motifs for their corresponding aptamer.
Our purpose in conducting in vivo SELEX was to obtain aptamers with high binding affinity and specificity for mouse bone with PC3 PB. After ten rounds of in vivo SELEX, the PB aptamer with the highest hitting frequency was attained and analyzed in vitro and in vivo. Compared with control aptamer, the PB aptamer exhibited high levels of accumulation in tumor mouse bone mediated by binding with bone marrow endothelial cell. These conclusions were supported by observations of bone sections under microscopy, as well as high binding affinity of the PB aptamer for mouse tumor-exposed bone marrow endothelial cell ex vivo. Targeting specificity of the PB aptamer was reflected by increased PB aptamer uptake in HUVECs grown in PC3 prostate cancer cell-conditioned medium. In addition, the decreased PB aptamer uptake in LNCaP prostate cancer-conditioned HUVEC and increased PB aptamer uptake in DU145 prostate cancer cell-conditioned HUVEC imply that different tumor cells exert huge different effects to reshape local endothelial cell phenotype, thereby determining the targeting specificity of aptamer. We also demonstrated that the PB aptamer underwent ligand-mediated endocytosis into HUVECs, reflecting the abundance of ligands bound by the PB aptamer on the HUVEC surface and consistent with an active targeting strategy. The ability of the PB aptamer to escape from lysosomes indicates that this molecule is a promising choice for application in targeted drug delivery, as it could potentially facilitate its conjugated drugs in avoidance from highly acidic environment and lytic enzymes, thus preserving drugs’ bioactivity to achieve desired therapeutic effect. The specificity and efficiency of PB aptamer conjugated with gold particles was highlighted by its ability to achieve targeted drug delivery.
Targeting of the tumor microenvironment represents a promising therapeutic approach. In vivo SELEX is a direct and reliable technique for the identification of aptamers that selectively recognize diseased organs or tissues in animal models.Citation17 Using selected aptamers, we can develop targeting moiety-equipped nano-based delivery systems with high efficiency and specificity to overcome particularly robust biological barrier, such as bone and brain, and realize targeted drug delivery. The tumor endothelial cell-based active targeting strategy developed in this study can deliver therapeutics to tumor adjacent area to kill cancer cells and has great value in targeting tumor endothelial cells. Targeting tumor endothelial cell directly can contribute to curing cancer by inhibiting angiogenesis, as cancer development is an angiogenesis-dependent process.Citation18–Citation20 Inhibition of tumor angiogenesis has been shown as an effective anticancer treatment.Citation21–Citation25 Moreover, tumor endothelial cells are unlikely to develop resistance to therapies.Citation26 Finally, active aptamer targeting may also improve the therapeutic efficacy of traditional chemotherapy drugs, thereby providing further benefits.
Conclusion
Tumor cells within bone marrow have a profound influence on local environment, especially on endothelial cells, which provides an opportunity to develop active targeting aptamer for directed drug delivery. In vivo SELEX conducted in this study facilitated the acquisition of an aptamer with high targeting efficiency that can function as a moiety for bone-targeted drug delivery in PB. Aptamer-mediated endothelium targeting could be an alternatively promising strategy for the eradication of cancer growth. Overall, this investigation highlights a potentially bright future for the application of aptamers in the clinic.
Abbreviations
| SELEX | = | Systematic Evolution of Ligands by Exponential Enrichment |
| PB | = | prostate cancer bone metastasis |
Acknowledgments
This work was supported by a Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education (No CQKLBST-2018-005).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- BonapaceLCoissieuxMMWyckoffJMertzKDVargaZJuntTBentires-AljMCessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesisNature2014515752513013325337873
- LluchACuevaJRuiz-BorregoMPonceJPérez-FidalgoJAZoledronic acid in the treatment of metastatic breast cancerAnticancer Drugs20142511724100278
- CasasALlombartAMartínMDenosumab for the treatment of bone metastases in advanced breast cancerBreast201322558559223759273
- KangYSiegelPMShuWA multigenic program mediating breast cancer metastasis to boneCancer Cell20033653754912842083
- YinJJSelanderKChirgwinJMTGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases developmentJ Clin Invest199910321972069916131
- EckhardtBLFrancisPAParkerBSAndersonRLStrategies for the discovery and development of therapies for metastatic breast cancerNat Rev Drug Discov201211647949722653217
- HongCANamYSFunctional nanostructures for effective delivery of small interfering RNA therapeuticsTheranostics20144121211123225285170
- MieleESpinelliGPMieleEDi FabrizioEFerrettiETomaoSGulinoANanoparticle-based delivery of small interfering RNA: challenges for cancer therapyInt J Nanomedicine201273637365722915840
- DangLLiuJLiFTargeted delivery systems for molecular therapy in skeletal disordersInt J Mol Sci201617342844327011176
- SunHZuYAptamers and their applications in nanomedicineSmall201511202352236425677591
- LiangCGuoBWuHAptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategyNat Med201521328829425665179
- CowinSCCardosoLBlood and interstitial flow in the hierarchical pore space architecture of bone tissueJ Biomech201548584285425666410
- BlancoEShenHFerrariMPrinciples of nanoparticle design for overcoming biological barriers to drug deliveryNat Biotechnol201533994195126348965
- CarmelietPAngiogenesis in life, disease and medicineNature2005438707093293616355210
- FukumuraDJainRKTumor microvasculature and microenvironment: targets for anti-angiogenesis and normalizationMicrovasc Res2007742–3728417560615
- MiJRayPLiuJIn vivo selection against human colorectal cancer xenografts identifies an aptamer that targets RNA helicase protein DHX9Mol Ther Nucleic Acids20165e31527115840
- FolkmanJToward an understanding of angiogenesis: search and discoveryPerspect Biol Med198529110362415913
- KerbelRSInhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agentsBioessays199113131361722975
- FolkmanJAngiogenesis in cancer, vascular, rheumatoid and other diseaseNat Med19951127307584949
- IngberDFujitaTKishimotoSSynthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growthNature199034863015555571701033
- KimKJLiBWinerJArmaniniMGillettNPhillipsHSFerraraNInhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivoNature199336264238418447683111
- O’ReillyMSHolmgrenLShingYAngiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinomaCell19947923153287525077
- BrekkenRAOverholserJPStastnyVAWaltenbergerJMinnaJDThorpePESelective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-1) activity by a monoclonal anti-VEGF antibody blocks tumor growth in miceCancer Res200060185117512411016638
- IshidaTKunduRKYangEHirataKHoYDQuertermousTTargeted disruption of endothelial cell-selective adhesion molecule inhibits angiogenic processes in vitro and in vivoJ Biol Chem200327836345983460412819200
- AirdWCEndothelial cell heterogeneityCold Spring Harb Perspect Med201221a00642922315715
