Abstract
Compared with traditional in-vitro cell culture materials, three-dimensional nanofibrous scaffolds provide a superior environment for promoting cell functions. Since nanofibrous scaffolds have nanometer pore sizes, cells are unable to penetrate on their own, so must be incorporated into the scaffold during fabrication to ensure proper cell distribution. In this study, biodegradable and cytocompatible poly(DL-lactide-co-glycolide) (PLGA) nanofibers were produced using an electrospinning process. As a model cell line, fibroblasts were periodically sprayed from a pump-action spray bottle onto the developing scaffold. The viability of cells before and after spraying, and also after incorporation into the scaffold, was compared. Results indicated that cell spraying and the scaffold fabrication process did not significantly reduce cell viability. These findings, thus, contribute to the understanding of how to produce more physiological relevant cell-seeded nanofibrous scaffolds, an important element for the future of nanotechnology and tissue engineering.
Introduction
The desire to produce small-diameter (ie, nanometer) fiber scaffolds for tissue engineering applications is derived, in part, from the resemblance of such scaffolds to naturally occurring extracellular matrix (ECM) proteins. Collagen fibrils, with diameters in the nanometer and submicron range, are a primary component of the ECM. Positive cell responses to scaffolds have been correlated to surface nanotopographies with biomimetic features. While some studies have found that the smallest fibers produced by electrospinning (close to 100 nm) are superior,Citation1,Citation2 others have concluded that slightly larger, submicron fibers (near 400 nm) offer the best performance.Citation3 However, in both cases, small-diameter fiber scaffolds provide a significant increase in functional surface area compared with conventional materials with no roughness at the nanoscale. Consequently, more proteins in a more ideal conformation adsorb to nanostructured material surfaces to facilitate enhanced cell attachment.Citation4
In addition, compared with cell culture on two-dimensional surfaces, three-dimensional structures allow for a more natural cell attachment and focal adhesion in all directions, a process necessary for proper cell function and survival. The more physiologically relevant cell morphology one can attain on and in three-dimensional scaffolds will provide the best structural cues to regulate cell function.Citation5–Citation7 The development of new methods for producing three-dimensional cell scaffolds is, therefore, of great interest. “Organ printing” systems improve two-dimensional cell culture through the deposition of collagen or other ECM-mimicking polymers with cells to build a three-dimensional tissue.Citation8 A number of studies have examined such cell responses to those cells simply seeded on top of electrospun scaffolds of significant thickness.Citation1,Citation3,Citation9 Although some migration into the scaffold has been reported, the pore size of nanometer fiber scaffolds is generally small enough to prevent substantial migration of cells throughout the scaffold. In an effort to improve cell distribution through such scaffolds, in one study, a parallel electrospinning apparatus drew small droplets of cell suspensions onto the polymer fiber scaffolds to improve cell distribution.Citation10
As a continuation of such efforts, the present study investigated the feasibility of producing a cell-seeded, three-dimensional scaffold of fibroblasts and electrospun poly(DL-lactide-co-glycolide) (PLGA) nanofibers. This scaffold was produced via a method of spraying a cell suspension at regular intervals over a continuously produced network of electrospun polymer fibers. In contrast to previous studies in which cells and polymer fibers were deposited simultaneously using a coaxial needle arrangement,Citation11,Citation12 here, cells were intermittently sprayed onto a developing scaffold to produce a layered tissue rather than being incorporated into the fibers themselves. Results showed that cells survived the scaffold production process and maintained viability and, thus, should be further studied for nanotechnology-related tissue-engineering applications.
Methods
Cell culture
Murine f ibroblasts (embryo 3T3; CRL-1658; ATCC, Manassas, VA) were used for cell experiments without further characterization. Fibroblasts were subcultured on tissue culture polystyrene in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) at 37°C in a humidified environment of 5% CO2/95% air. Upon reaching 90% confluency, flasks of fibroblasts (population numbers 3–5) were trypsinized, counted, and used for experimentation.
Cell spray viability
To investigate potential cell death due to the spraying process, cell viability of a cell suspension was evaluated with and without spraying. After counting and diluting cells to produce a suspension with a density of 2.5 × 105 cells/mL, the suspension was sprayed into a conical tube with a Bel-Art Spray Pump Bottle (Fisher Scientific, Saint Louis, MO). A 200 μL volume of the sprayed cell suspension was then transferred to a well plate and incubated for 1 hour. A comparable, unsprayed volume of the same cell suspensions was also pipetted into a well plate to serve as a control group. After 1 hour, 40 μL of an MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) reagent (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega Corporation, Madison, WI) was added to the wells of cell suspension and incubated for 1 hour. The optical density of the formazan produced was read with a spectrophotometer at 490 nm. To further investigate potential cell death due to spraying, an additional cell suspension with the same cell density was sprayed from the collection tube five consecutive times before being sampled and tested for cell viability with the same MTS assay.
Electrospinning setup
The electrospinning apparatus (previously described in greater detailCitation13) was set up according to standard operating procedures. PLGA (MW 40,000–70,000, Sigma-Aldrich, St. Louis, MO) was dissolved in a 1:1 mixture of tetrahydrofuran and dimethylformamide at a concentration of 0.25 g/mL. The polymer solution was then loaded into a glass syringe with a 20-gauge metal needle tip. The negative lead of a high-voltage source was connected to the needle tip, while an aluminum foil-covered collector plate was connected to ground. Parameters were adjusted to produce polymer fibers with an approximate diameter of 100 nm (distance between the needle tip and collector plate = 15 cm, voltage = 18 kV, flow rate = 0.5 mL/h). To facilitate removal of the scaffold after the electrospinning process, a glass slide was placed on the collector plate. Polymer nanofibers were produced for 90 minutes with cell suspensions added at 10-minute intervals. For this, cell suspensions were sprayed from a distance of 15 cm above the collector plate. Polymer nanofiber deposition continued for 10 minutes following the final application of a cell suspension to provide a top layer of polymer nanofibers. After the experiment, the glass slide was carefully excised from the collector plate with a razor blade and transferred to a Petri dish of complete media.
Scanning electron microscopy
The surface morphology of PLGA fibers was analyzed using a scanning electron microscope (Hitachi 2700, Hitachi High-Technologies, Berkshire, UK) at magnifications of 6000× and 30,000× with an accelerating voltage of 7 kV. Images were captured and analyzed with image analysis software (Quartz PCI, Quartz Imaging Corporation, Vancouver, Canada). Polymer nanofiber samples of 1 cm2 were attached to aluminum stubs (Electron Microscopy Sciences, Hatfield, PA) using carbon tape and coated with a 15 nm layer of AuPd by sputter coating (Emitech K-550, Quorum Technologies, East Sussex, UK) for 2 minutes at 20 mA from a height of 45 mm.
Scaffold viability
The viability of cells incorporated into polymer fiber scaffolds was determined using an MTS assay. Following the scaffold production, 1 cm2 pieces of the scaffolds were cut from the collector plate and placed in the well of a 24-well plate containing 500 μL of complete DMEM culture media. As a control group, cell suspensions were sprayed eight times from a height of 15 cm into a 1 cm2 well of a well plate in order to collect approximately the same number of cells that were theoretically deposited on the 1 cm2 sample of scaffold. 500 μL of complete DMEM culture media was added to the collected cell suspension. Both the scaffold sample and the collected cell suspension were incubated for 2 hours, along with the polymer nanofiber scaffold that did not contain cells and a well of complete media that did not contain cells. Following incubation, 100 μL of the MTS assay solution was added to each well and incubated for 1 hour at 37°C. A 200 μL volume of each resulting solution (with the colored formazan product) was transferred to a 96-well plate, and the optical density was measured with a spectrophotometer at 490 nm. Optical density values were converted to the approximate number of viable cells present.
Statistics
Numerical data were analyzed for significance using the student’s t-test. Experiments were performed in triplicate (N = 3). Values are reported as the mean ± SEM (standard error of the mean). The threshold for significance was set at P < 0.05.
Results and discussion
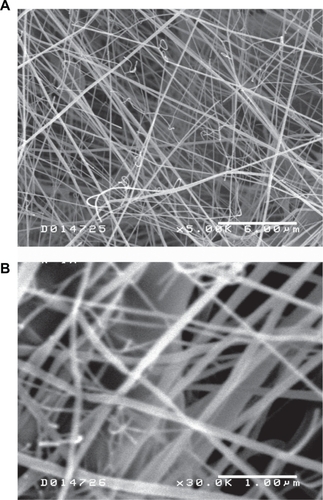
The electrospinning process produced polymer fibers in the nanometer range with an approximate diameter of 100 nm (). This fiber diameter was comparable to dimensions reported in previous literature reports that demonstrated enhanced cellular responses to nanoscale or submicron polymer topographies.
Figure 1 Scanning electron microscope images of poly(DL-lactide-co-glycolide) nanofibers at 5000× magnification (A) and 30,000× magnification (B). The polymer fiber diameter was slightly variable with a mean diameter of approximately 100 nm.
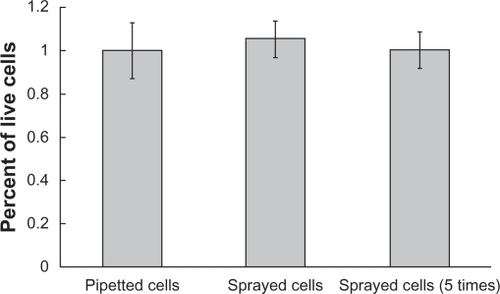
Cell viability assays confirmed that the process of spraying did not kill cells (). Although the spraying process subjected cells to increased pressure and shear stress, cell viability was not affected. However, due to the effect that mechanical forces may have on gene expression and cell function, an investigation into the role of these mechanical factors on subsequent cell behavior would need to be conducted to rule out an inhibitory influence of the spraying process. Five consecutive sprays also did not significantly reduce cell populations below that of control groups, further establishing cell survival of the spraying process.
Figure 2 Viability of cells before spraying and after spraying one or five times. No significant difference was found between the viability of cells in these three groups.
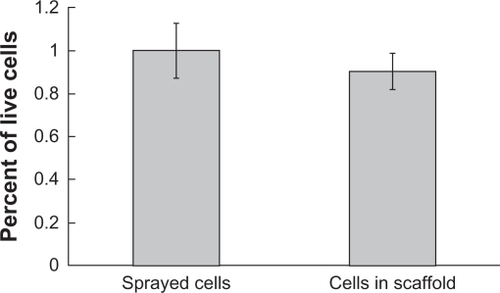
Fibroblasts also survived the scaffold production process. Two hours after the scaffolds were produced, cell viability levels were comparable to that of the approximate number of cells deposited over an area the size of the scaffold sample (). This suggests that short-term survival of nearly all incorporated cells is achievable. The porosity of the scaffold was great enough to allow nutrient and cellular waste diffusion; thus, cells will likely be viable for extended periods of time.
Figure 3 Viability of cells sprayed into polymer nanofiber scaffolds compared with a similar number of cells sprayed into well plates. No significant reduction in cell viability was observed for cells incorporated into scaffolds (P = 0.086).
This study was built on the premise that to place cells in a more physiological nanofiber scaffold, cells must be incorporated as the scaffold is made. The pore size of small fiber scaffolds is too small to allow for migration of cells after production. Substantial penetration of cells throughout the thickness of the scaffold is very unlikely.
However, one problem associated with incorporating live cells into an electrospun scaffold is the difficulty following aseptic technique. Due to undesirable paths to electrically ground the electrospinning system, a laminar flow hood could not be used here. Consequently, contamination (which would have become evident after extended periods of incubation) may occur during scaffold production and, thus, needs to be further addressed in future studies.
Furthermore, natural tissue has a much more homogenous distribution of cells throughout a tissue, rather than layers of cells between layers of ECM. While a more convenient, automated, and elegant system could be designed to spray smaller volumes of cell suspensions at more frequent intervals, this preliminary study showed promise for such approaches as it maintained cytocompatibility properties after the spraying process.
While other varieties of live cells could be incorporated into such polymer nanofiber scaffolds, 3T3 fibroblast precursor cells incorporated into a thin, three-dimensional structure may produce an ideal material for the promotion of wound healing. Degradation of the scaffold will take at least several weeks and allow for natural tissue formation.Citation14 There is also the potential to use this technology to produce novel cellular co-culture systems in which one type of cell would be encapsulated in the scaffold and a second cell type would be seeded on the outer surface of the scaffold. In this way, the effect of the signaling molecules produced by one cell type could be investigated while physically separating the two cell populations.
Conclusions
A method of fabricating three-dimensional scaffolds of live cells and polymer nanofibers was developed. In contrast to previous three-dimensional tissue production using electrospinning techniques, the cells were layered throughout the thickness of the scaffold, but not incorporated into individual polymer nanofibers. The production of live-cell scaffolds was confirmed with cell viability assays following fabrication, and, thus, represents a technique that should be further explored for nanotechnology-based tissue engineering applications.
Acknowledgements
The authors would like to acknowledge the Indo-US Center for Biomaterials for Healthcare for funding and Dr Bikramjit Basu, Dr Dhirendra Katti, Poonam Sharma, and Neha Arya for electrospinning training and project support.
Disclosure
The authors report no conflicts of interest in this work.
References
- YangFMuruganRWangSRamakrishnaSElectrospinning of nano/ micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineeringBiomaterials200426152603261015585263
- MatthewsJAWnekGESimpsonDGBowlinGLElectrospinning of collagen nanofibersBiomacromolecules20023223223811888306
- ChenMPatraPKWarnerSBBhowmickSRole of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffoldsTissue Eng200713357958717518604
- WebsterTJSchadlerLSSiegelRWBiziosRMechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectinTissue Eng20017329130111429149
- AlbrechtDRUnderhillGHWassermannTBSahRLBhatiaSNProbing the role of multicellular organization in three-dimensional microenvironmentsNat Methods20063536937516628207
- GrinnellFFibroblast biology in three-dimensional collagen matricesTrends Cell Biol200313526426912742170
- LiWDanielsonKGAlexanderPGTuanRSBiological response of chondrocytes cultured in three-dimensional nanof ibrous poly(ɛ-caprolactone) scaffoldsJ Biomed Mater Res A.200367A41105111414624495
- MironovVBolandTTruskTForgacsGMarkwaldRROrgan printing: computer-aided jet-based 3D tissue engineeringTrends Biotechnol200321415716112679063
- XuCInaiRKotakiMRamakrishnaSElectrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineeringTissue Eng2004107–81160116815363172
- StankusJJGuanJFujimotoKWagnerWRMicrointegrating smooth muscle cells into a biodegradable, elastomeric fiber matrixBiomaterials200627573574416095685
- Townsend-NicholsonAJayasingheSNCell electrospinning: a unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffoldsBiomacromolecules20067123364336917154464
- SandersEHKloefkornRBowlinGLSimpsonDGWnekGETwo-phase electrospinning from a single electrified jet: microencapsulation of aqueous reservoirs in poly(ethylene-co-vinyl acetate) fibersMacromolecules2003361138033805
- PhamQPSharmaUMikosAGElectrospinning of polymeric nanofibers for tissue engineering applications: a reviewTissue Eng20061251197121116771634
- ShinHJLeeCHChoIHPLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular response under mechanical stimulation in vitroJ Biomat Sci-Polym E.2006171–2103119