Abstract
Background
Biosurfactants are amphipathic molecules of microbial origin that reduce surface and interfacial tension at gas–liquid–solid interfaces. Earlier, the biosurfactant was isolated and characterized in our laboratory from Candida parapsilosis. The property of the biosurfactant is further explored in this study by using quantum dots (QDs) as nanocarrier.
Materials and methods
Graphene quantum dots (GQDs) were synthesized by bottom-up approach through pyrolysis of citric acid. GQDs were conjugated with both biosurfactant and folic acid (FA) using carbodiimide chemistry. The prepared GQD bioconjugate was studied for diagnostic and therapeutic effects against cancer cells.
Results and discussion
Photoluminescence quantum yield (QY) of plain GQDs was measured as 12.8%. QY for biosurfactant conjugated GQDs and FA-biosurfactant conjugated GQDs was measured as 10.4% and 9.02%, respectively, and it was sufficient for targeting cancer cells. MTT assay showed that more than 90% of cells remained viable at concentration of 1 mg/mL, hence GQDs seemed to be non-toxic to cells. Biosurfactant conjugated GQDs caused 50% reduction in cellular viability within 24 hours. FA conjugation further increased the specificity of bioconjugated GQDs toward tumor cells, which is clearly evident from the drug internalization studies using confocal laser scanning microscopy. A higher amount of drug uptake was observed when bioconjugated GQDs were decorated with FA.
Conclusion
The ability of GQD bioconjugate could be used as a theranostic tool for cancer. It is foreseen that in near future cancer can be detected and/or treated at an early stage by utilizing biosurfactant conjugated GQDs. Therefore, the proposed study would provide a stepping stone to improve the life of cancer patients.
Introduction
The application of quantum dots (QDs) has increased with the advent of nanotechnology. QDs are integrated with various biomolecules for specific targeting and purposes in the field of biological and medical sciences.Citation1 This is because of their small size, narrow emission spectra over broad excitation spectra, high fluorescent quantum yields, and resistance to photo-bleaching.Citation2,Citation3 Earlier, metallic QDs such as CdS, CdSe, and CdTe were the most investigated QDs due to their excellent optical and electrochemical properties.Citation4 However, cytotoxicity caused by metallic QDs hampers their practical applications in biology. Therefore, demand for biocompatible QDs has led to the discovery of carbon-based nanomaterials.Citation3–Citation5
Carbon nanodots (CNDs) are one of the popular nano-molecules in scientific communities.Citation6,Citation7 Fluorescent graphene quantum dots (GQDs), a class of CNDs, with tunable emissions are considered to be the next-generation nanomaterials.Citation3,Citation7–Citation9 Their properties include stable photoluminescence (PL), high solvent dispersibility, low cytotoxicity, and excellent biocompatibility,Citation7,Citation10 which make GQDs an attractive tool for diagnosis and therapy.Citation5,Citation7,Citation10
Graphene is an allotrope of carbon, and consists of thin sheet of carbon atoms arranged in a hexagonal lattice.Citation7,Citation8 Briefly, GQDs can be fabricated using top-down and bottom-up approaches.Citation6–Citation8,Citation11,Citation12 Pyrolysis of citric acid is one of the well-established method for the preparation of GQDs. It is a convenient and economical method in terms of precursors used and method followed for its synthesis.Citation13–Citation15 GQDs are formed by varying the carbonization degree of citric acid.Citation6,Citation13 Lately, the interest in bioconjugated GQDs has been increasing at an outstanding rate.Citation5 Biomolecules conjugated with GQDs can be used as theranostic tool against deadly diseases like cancer. These nanoconjugates exhibit targetability and have imaging properties enabling them to be used in downstream screening purposes.Citation1 Very recently, by utilizing unique physicochemical properties of GQDs, Ding et al had developed a novel type of theranostic agent by loading anticancer drug doxorubicin (DOX) to GQD’s surface and conjugating Cy5.5 (Cy) dye to GQD through a cathepsin D-responsive (P) peptide. Authors reported superior therapeutic performance of this type of agents along with functioning as probes for tracking the delivery and release of anticancer drug as well as drug-induced cancer cell apoptosis.Citation16 Recent review by Iannazzo et al discussed the application of GQDs as nanoplatforms for anticancer therapy.Citation17 Authors covered the methodology for synthesis and functionalization procedures of this agent, and discussed how it can affect their biocompatibility and physicochemical features. Wang et al showed efficient delivery of DOX to the nucleus through DOX/GQD conjugates. Authors also added that conjugates could markedly enhance DNA cleavage activity of DOX. DOX/GQD conjugates improved cytotoxicity of DOX dramatically. Furthermore, the DOX/ GQD conjugates could also increase the nuclear uptake and cytotoxicity of DOX toward drug-resistant cancer cells, indicating that the conjugates were capable of increasing chemotherapeutic efficacy of anticancer drugs.Citation18
Biosurfactants are amphipathic molecules of microbial and plant origin. Owing to their structural diversity, biodegradability, and non-toxicity, they are used in biomedical field.Citation19 Biosurfactants are proposed as suitable drug candidates against many diseases including cancer.Citation20,Citation21 Literature showed cytotoxicity of these molecules against different cancer cell lines. Huang et al reported that surfactin-based nanocarrier might be a promising platform to reverse multi-drug resistance in cancer chemotherapy.Citation22 In cancerous cells, various receptors like folic acid (FA), arginine–glycine– aspartic acid, hyaluronic acid, and antibodies are overexpressed.Citation23 On the contrary, expression of these receptors is significantly low in normal cells.Citation23,Citation24 FA can be used to increase internalization of nanoconjugates by targeting cells via receptor-mediated endocytosis.Citation23,Citation25,Citation26 Therefore, FA receptors would be a targeting marker for selective imaging and therapy of cancer.Citation27
Chemotherapeutic drugs/GQD-based nanosystem is developed to enhance efficiency of drug to kill cancerous cells.Citation28 It is observed that this nanosystem can increase the chemotherapeutic efficacy of anticancer drugs.Citation29 Until now, most of the studies related to the development of theranostic tool for cancer using GQDs was based on the use of standard chemotherapeutic drugs. Our present study focuses on the use of a biosurfactant which is supposed to be less toxic, having minimum side effects and more biodegradability compared to standard chemotherapeutic drugs used for cancer therapy. This biosurfactant was isolated from Candida parapsilosis in our laboratory. This study was directed toward the synthesis and characterization of GQDs through pyrolysis of citric acid and its conjugation with biosurfactant. There have been no previous reports regarding conjugation of biosurfactant with GQDs for the development of theranostic tool against cancer. Hence, this study is an initial step to explore a novel bioconjugate for the diagnosis and therapy of cancer.
Materials and methods
Chemicals and materials
Dimethyl sulfoxide (DMSO), DAPI, N-(3 dimethylaminopropyl)-N-ethylcarbodiimidehydrochloride (EDC), and N-hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Sodium hydroxide pellets, FA, and citric acid monohydrate were purchased from SRL, New Delhi, India. RPMI-1640, PBS, FBS, trypsin, penicillin, streptomycin, and MTT reagent were purchased from Himedia (Mumbai, India).
Microorganism and culture conditions
The fungal culture of C. parapsilosis was isolated from environmental source in our laboratory. Culture was grown in potato dextrose broth media at 37°C for 48 hours.Citation30
Production of biosurfactant
Biosurfactant was isolated from C. parapsilosis using acid precipitation approach. Details of the methodology for extraction of biosurfactant have been reported previously.Citation30
Synthesis of GQDs
GQDs were prepared by bottom-up approach with minor modifications of previously reported methodologies.Citation14,Citation15,Citation31 Briefly, 2 g of citric acid was heated upto 160°C for about 15 minutes under continuous mixing, until the color of citric acid changed to orange. Further, 100 mL of 1.5M NaOH solutionCitation14,Citation15,Citation31 was added dropwise into pyrolyzed citric acid under vigorous stirring. The pH of pale yellow GQD solution was adjusted to 7.0 with 1N HCl solution. The product was subsequently freeze-dried and stored in vials for further use.
Preparation of GQDs-biosurfactant conjugates
GQDs were activated by mixing 25 mL of GQDs (85 mg/mL in PBS, pH 7.4) solution with EDC (2 mg) and NHS (3 mg) at room temperature (RT) for 1 hours. EDC and NHS were mixed in the ratio 2:3. Then, 300 µL of biosurfactant (1 mg/mL in PBS, pH 7.4) was conjugated to GQDs through amine-carboxyl coupling reaction for 1 hours at RT with stirring. GQDs-biosurfactant conjugates (biosurfactant-GQDs) were separated from free cross-linkers by centrifugation (6,000 rpm, 5 minutes) and kept at 4°C.Citation1,Citation23
Preparation of GQDs-biosurfactant-FA conjugate
For folate conjugation, 3 mL of biosurfactant conjugated GQDs were mixed with 30 µL of FA (1 mM in PBS) and incubated for 1 hour at RT with stirring. Folate decorated GQDs were separated from excess free FA by centrifugation (6,000 rpm for 15 minutes). Finally, purified folate conjugated GQDs (FA-biosurfactant-GQDs) were stored at 4°C till further use.Citation1,Citation23,Citation32
Characterization
GQDs and their bioconjugates were characterized by spectrophotometeric techniques such as transmission electron microscopy (TEM), Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and zeta potential. The optical studies were investigated by PL and UV–visible spectroscopy. PL was recorded on F-2500 Fluorescence spectrometer (Hitachi Ltd., Tokyo, Japan). UV/Vis absorption spectra were obtained on Lambda 35 (PerkinElmer Inc., Waltham, MA, USA) spectrometer. TEM (JEOL, Tokyo, Japan) was employed for analysis of morphology of the prepared samples. Powdered XRD (PANalytical, Almelo, the Netherlands) was performed for analyzing surface characterstics of the particles. Zeta potential was measured for size and charge determination using Malvern Zetasizer. FTIR (Perkin Elmer) demonstrated the chemical nature of novel compounds. Confocal laser scanning microscopy (CLSM) using NIKON C2+ instrument was performed to determine cellular internalization of drugs by cancerous cells.
Cytotoxicity assay
MCF-7 (human breast cancer) cell line was procured from National Center for Cell Science (NCCS), Pune, India. Cells were grown in a RPMI-1640 medium containing 10% FBS, 100 U⋅mL−1 penicillin and streptomycin at 37°C, and 5% CO2. The cells were grown in abovementioned conditions for 2–3 days.Citation5 The cytotoxicity study was performed using MTT reduction assay. Cells were grown on a 96-well plate at a density of 1×104 cells/well. After overnight incubation, GQD solutions (500, 1,000, and 2,000 µg/mL) were added to respective wells and incubated for 24 and 48 hours in 5% CO2 at 37°C. Afterward, 50 µL of MTT solution (1 mg/mL in PBS) was added in each well and incubated for 4 hours followed by DMSO addition. The absorbance of produced formazon was monitored on ELISA reader at 460 nm.Citation5,Citation33 Moreover, MTT assays for folate decorated bioconjugated GQDs (1,000 µg/mL of GQDs) were also performed using different dosage concentrations of biosurfactant (2.5 and 5 µg/mL).
Cellular uptake studies of GQDs and GQDs bioconjugates
In cellular uptake studies, MCF-7 cells were seeded on coverslips in six-well plate (1×104 cells/well). After 48 hours, 200 µL of GQDs and GQDs conjugates were added in respective wells. After incubation for 1, 3, and 6 hours, nuclei of MCF-7 cells were stained using DAPI (5 µg/mL).Citation10 Cells were washed with PBS twice to remove unattached cells. Glass coverslips were fixed with 4% formaldehyde for 20 minutes. Imaging studies were recorded in the wavelength regime of 475–575 nm on CLSM.Citation23,Citation32
Statistical analyses
The values of results were expressed as mean with SD (mean ± SD). Statistical significance was set at P<0.05 for all comparisons and was analyzed using Student’s t-test.
Results and discussion
Synthesis of GQDs
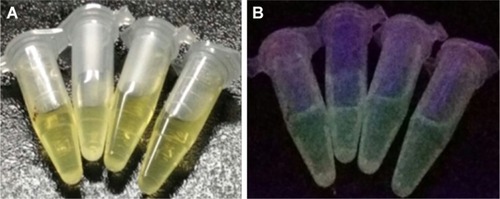
GQDs were synthesized with slight modification of pyrolysis method proposed by He et al.Citation31 It is a direct, one-step aqueous synthesis method involving components NaOH, HCl, and citric acid as the main precursors. Heating of the citric acid and subsequent addition of 1.5M NaOH resulted in the formation of nanocrystals of GQDs. The prepared nanocrystals of GQDs were highly stable and dispersible in water at pH 7.4. Nanocrystals emitted green fluorescence in the UV range, when excited at 365 nm. Prepared GQDs were dialyzed for 24 hours before further applications. After dialysis, GQDs were stored at 4°C until further use. GQDs exhibit a light yellow color under visible light while under UV light, they exhibit a strong fluorescence emission of green color.Citation33,Citation34 This could be visualized in .
Characterization
Optical characteristics of GQDs
The prepared GQDs have shown strong absorbance at 340 nm under UV light as shown in .Citation35 GQDs after conjugation with biosurfactant showed absorption maxima at 338 nm, whereas FA-biosurfactant-GQDs conjugate showed absorption maxima at 362 nm (). The shift of peak in bioconjugated GQDs might be due to the strong interaction and attachment of biosurfactant and FA with GODs.Citation5 Biosurfactant showed UV–vis absorbance at 263 nm as observed in .Citation36
Figure 2 Characterization of biosurfactant, GQDs, biosurfactant-GQDs, and FA-biosurfactant-GQDs conjugates.
Notes: (A) UV–vis absorption spectra. (B) Fluorescence spectra (under different excitation wavelengths ranging from 360 to 370 nm). (C) PL decay curves of GQDs and their conjugates under a UV beam of 365 nm.
Abbreviations: FA, folic acid; GQDs, graphene quantum dots; PL, photoluminescence; UV–vis, ultraviolet–visible.
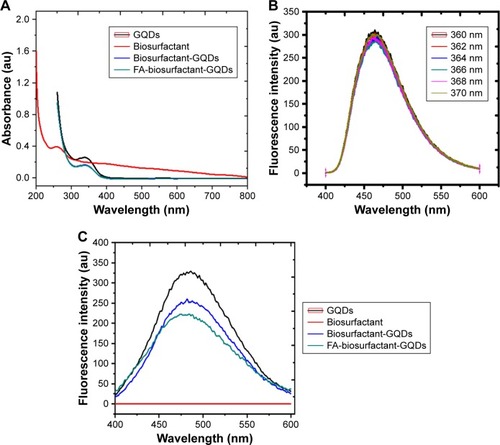
represents the PL spectra of GQDs, when excited at wavelengths ranging from 360 to 370 nm. It was observed that GQDs exhibited a slight excitation-dependent emission spectra. The maximum emission was obtained at 360 nm. This particular wavelength helped us in imaging the cells and tracking GQDs clearly. manifests the PL intensity of GQDs. Upon excitation at 365 nm, GQDs exhibited a prominent emission peak at 486 nm. It showed high QY (12.8%), due to the layered structure and better crystallinity as it was confirmed by XRD.Citation37 This characteristic emission peak of GQDs did not shift after surface functionalization of GQDs with biosurfactant and FA, as shown in . However, fluorescence peak intensity was diminished by 24% upon conjugation of bio-surfactant with GQDs. Further, decrease in fluorescence intensity by 15% was observed upon decoration with FA (10 µM FA) (). Decreased fluorescence intensity of bioconjugated GQDs was attributed to change in the QDs electronic energy. Chemical interactions, or local or electric or stress field occurred on QDs surface during bioconjugation process.Citation1 However, biosurfactant itself did not show fluorescence under UV light. This was further demonstrated by PL spectra, where no fluorescence emission peak corresponding to biosurfactant was observed upon excitation at 365 nm. Finally, bioconjugated GQDs exhibited excellent photostability and relatively narrow spectral width under UV illumination. The QY for biosurfactant conjugated GQDs and FA-biosurfactant conjugated GQDs was measured as 10.4% and 9.02%, respectively. Thus, it provided sufficient spectral resolution for quantitative detection of the fluorescence intensity.Citation37
TEM
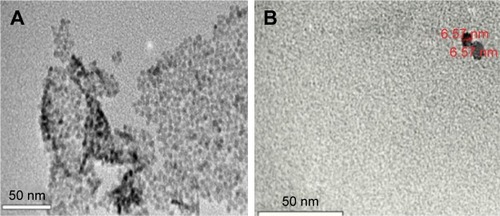
The prepared GQDs were found to be round shaped and homogenously dispersed, with depicted size range of 3.5–8 nm. This could be visualized from the TEM images shown in . GQDs were nearly identical in shape and well isolated from each other, confirming no aggregation during synthesis of GQDs. The obtained results were in line with the findings of previous studies.Citation31,Citation34,Citation38
XRD
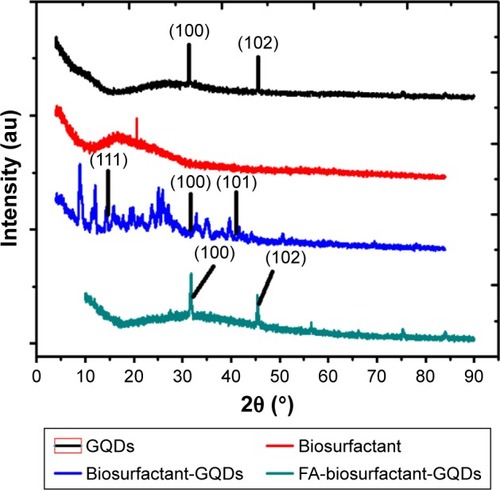
XRD measurement was carried out to study crystallinity and structure of GQDs. XRD pattern of GQDs showed two peaks near 2θ value of 31.7° and 45.8°, which corresponded to (100) and (102) planes of hexagonal carbon, respectively ().Citation39 The XRD pattern of bioconjugated GQDs (biosurfactant conjugated) showed the peaks near 2θ value of 14.87°, 31.92°, and 41.1°, respectively. These peaks correlated to (111),Citation40 (100),Citation39 and (101)Citation41 miller indices, confirming the crystalline nature as well as hexagonal shape of biosurfactant conjugated GQDs. The slight change in peaks of crystal structure of the GQDs upon conjugation to biosurfactant indicated the modification of the GQDs with biosurfactant.Citation42 Moreover, presence of peak at 31.92° suggested that GQDs have retained their hexagonal shape upon conjugation. Similar consistency was observed by XRD spectra of FA-biosurfactant-GQDs which showed the peaks near 2θ value of 31.7° (100)Citation39 and 45.5° (102).Citation39 The obtained planes of (100)Citation39 and (102)Citation39 further confirms the hexagonal shape of GQDs even after FA conjugation.
FTIR
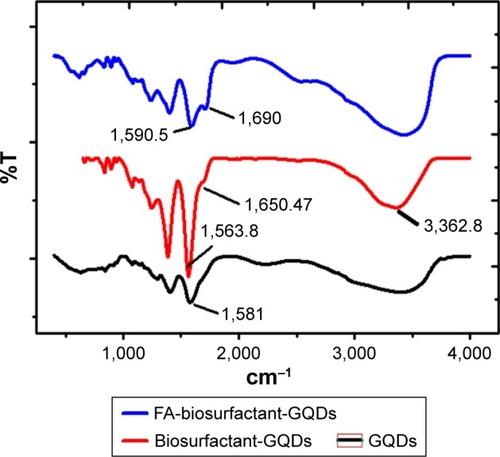
GQDs, biosurfactant conjugated GQDs, and FA-biosurfactant conjugated GQDs were characterized by FTIR spectroscopy and the spectra are shown in . The FTIR spectrum of GQDs showed −C=O stretch of −COONa+ at 1,581 cm−1 () confirming the presence of carbonyl functional group.Citation1 Presence of N−H stretch of amine group in biosurfactant was observed at 3,375.8 cm−1 in our previous report.Citation30 Various other functional groups such as O−H group, C=O bond stretching, and C=C were also observed.Citation30 Biosurfactant conjugated GQDs showed appearance of N−H stretch of CONH at 3,362.8 cm−1. Moreover, appearance of −C=O stretch of CONH of biosurfactant conjugated GQDs at 1,650.47 cm−1 (amide I) and 1,563.8 cm−1 (amide II) was observed, as shown in .Citation42 It confirmed the successful bioconjugation of GQDs with biosurfactant.Citation42 FTIR spectra of conjugated GQDs showed appearance of −C=O stretch of CONH of FA-biosurfactant conjugated GQDs at 1,690 cm−1 (amide I) and 1,590.5 cm−1 (amide II) ().Citation43 The abovementioned results indicated the presence of FA on the surface of biosurfactant conjugated GQDs.
Zeta potential
The zeta potential of biosurfactant was −0.81 mV, which indicated that the stability of biosurfactant is less in suspension.Citation44 GQDs had a surface charge of −18mV, which signified that they were highly stable and anionic in nature. Our result was well supported by the reports from other groups.Citation15,Citation35 The zeta potential of GQDs-biosurfactant conjugates was measured to be −13.25 mV, which manifested satisfactory level of stability of conjugates in dispersion form.
In vitro cell culture studies
Cell viability assay
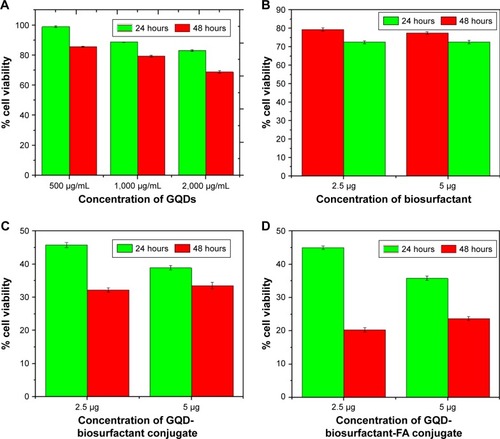
shows the graphical representation of percent viability of MCF-7 (breast cancer cells) cells after treatment with GQDs and its conjugate with increasing time and dose. It could be seen from graphs () that GQDs had no significant toxic effects on cells in concentrations ranging from 500 µg/mL to 2,000 µg/mL. There was cell viability of 98% for 500 µg/mL and decreased to 83% upto 2,000 µg/mL within 24 hours of GQDs exposure. During 48 hours of exposure of 500 µg/mL and 2,000 µg/mL of GQDs, cell viability decreased to 85% and 70%, respectively. This result was well corroborated by reports of other groups.Citation11,Citation33 However, cells treated with biosurfactant alone () had shown less cytotoxic effect in comparison to cells treated with biosurfactant conjugated GQDs (). Treatment with biosurfactant conjugated GQDs resulted in 50% reduction of cell viability within 24 hours when 2.5 µg/mL of biosurfactant was used in bio-conjugation. This viability further decreased by 20% as time increased to 48 hours. This change could be seen in . It was inferred that biosurfactant conjugated GQDs showed the potential to reduce significant number of viable cancerous cells. Further, conjugation with FA decreased cell viability by more than 60% within 24 hours of incubation and 75% after 48 hours of incubation (). This could be due to receptor-mediated targeting of FA-biosurfactant conjugated GQDs in MCF-7 cells. These observations showed that functionalized GQDs have ample potential to act as therapeutic agent against cancer cells.
Cellular uptake studies using CLSM
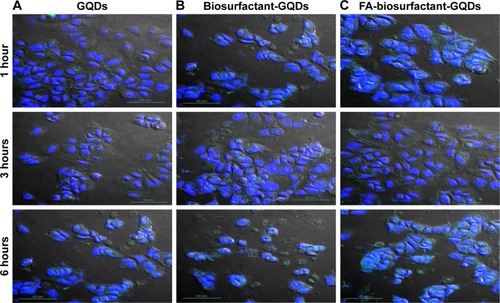
Time-dependent cellular uptake study of GQDs and bio-conjugated GQDs was performed for 1, 3, and 6 hours on MCF-7 cells. In order to determine and visualize the internalization of plain as well as conjugated QDs, the nuclei of MCF-7 cells were stained with DAPI as could be seen in . The cells were observed at 40× objective using CLSM (). Drug internalization was higher in cells treated with FA conjugated GQDs compared to biosurfactant conjugated GQDs. Cellular uptake of FA-biosurfactant-GQDs conjugate increased with time period from 1 hour to 6 hours. Better drug internalization with FA conjugated GQDs was due to receptor-mediated endocytosis.Citation32
Conclusion
The present work highlighted the development of a theranostic tool for cancer therapy using GQDs in conjugation with biosurfactant. This tool could be used for diagnosis as well as for therapy of cancer. These bioconjugated GQDs had formed homogenous dispersion and had shown stable PL intensity. Folate decoration of bioconjugated GQDs showed enhanced uptake in cancerous cells in comparison to plain GQDs. MTT assay results indicated that conjugation of biosurfactant with GQDs dramatically reduced the viability of cells with increasing time and dose. FA decoration reduced the cell viability further. MTT assay and CLSM results endorsed each other. Moreover, mechanism of action of this bioconjugate at cellular level needs to be unraveled for better understanding of diagnostics and development of cost-effective alternative tools for cancer. Our in vitro results are yet to be extrapolated to in vivo model. In future, this novel nanoconjugate system can open enormous opportunities in biomedical research for using quantum dot-based bioconjugates in cancer diagnosis as well as treatment.
Acknowledgments
Authors like to thank University Grants Commission (UGC), Basic Scientific Research, Government of India (sanction no. F.30-301/2016 [BSR] dt.16.02.2017) for providing funds to carry out this research endeavor. The authors gratefully acknowledge the Director of the University Institute of Engineering & Technology, Panjab University, for providing support for this work to be conducted. We acknowledge University Institute of Pharmaceutical Sciences, Central Instrumentation Laboratory/Sophisticated Analytical Instrumentation Facility, Panjab University, and the Institute of Microbial Technology, Chandigarh, for sample analysis and Dr Naveen Gupta for his technical support.
Disclosure
RPB and GS are supported by the UGC faculty recharge program and Department of Biotechnology (BT/PR27444/ BRB/10/1645/2018). The authors report no other conflicts of interest in this work.
References
- SinghGZaidiNHSoniUDetection of bioconjugated quantum dots passivated with different ligands for bio-applicationsJ Nanosci Nanotechnol20111153834384221780375
- RosenthalSJChangJCKovtunOMcBrideJRTomlinsonIDBiocompatible quantum dots for biological applicationsChem Biol2011181102421276935
- QianZSShanXYChaiLJMaJJChenJRFengHDNA nanosensor based on biocompatible graphene quantum dots and carbon nanotubesBiosens Bioelectron201460647024768864
- ZhuJJLiJJHuangHPInQuantum Dots for DNA BiosensingBerlin, HeidelbergSpringer2013924
- ChenMLHeYJChenXWWangJHQuantum-dot-conjugated graphene as a probe for simultaneous cancer-targeted fluorescent imaging, tracking, and monitoring drug deliveryBioconjug Chem201324338739723425155
- QuDZhengMLiJXieZSunZTailoring color emissions from N-doped graphene quantum dots for bioimaging applicationsLight Sci Appl2015412e364
- LiKLiuWNiYTechnical synthesis and biomedical applications of graphene quantum dotsJ Mater Chem B201752548114826
- KumawatMKThakurMGurungRBSrivastavaRGraphene Quantum Dots from Mangifera indica : Application in Near-Infrared Bio-imaging and Intracellular NanothermometryACS Sustain Chem Eng20175213821391
- LeeJChoiYKimJParkESongRPositively charged compact quantum Dot-DNA complexes for detection of nucleic acidsChemphyschem200910580681119253931
- ThakurMKumawatMKSrivastavaRMultifunctional graphene quantum dots for combined photothermal and photodynamic therapy coupled with cancer cell tracking applicationsRSC Advances20177952515261
- ZhangJMaY-QiangLiNPreparation of graphene quantum dots and their application in cell imagingJ Nanomater20162016519
- SunHWuLWeiWQuXRecent advances in graphene quantum dots for sensingMater Today20131611433442
- NaikJPSutradharPSahaMMolecular scale rapid synthesis of graphene quantum dots (GQDs)J Nanostructure Chem2017718589
- RoushaniMMavaeiMRajabiHRGraphene quantum dots as novel and green nano-materials for the visible-light-driven photocatalytic degradation of cationic dyeJ Mol Catalysis A Chem2015409102109
- JavanbakhtSNamaziHSolid state photoluminescence thermoplastic starch film containing graphene quantum dotsCarbohydr Polym201717622022628927602
- DingHZhangFZhaoCBeyond a Carrier: Graphene Quantum Dots as a Probe for Programmatically Monitoring Anti-Cancer Drug Delivery, Release, and ResponseACS Appl Mater Interfaces2017933273962740128782357
- IannazzoDZiccarelliIPistoneAGraphene quantum dots: multifunctional nanoplatforms for anticancer therapyJ Mater Chem B201753264716489
- WangCWuCZhouXEnhancing cell nucleus accumulation and DNA cleavage activity of anti-cancer drug via graphene quantum dotsSci Rep201331285224092333
- HabibaKBracho-RinconDPGonzalez-FelicianoJASynergistic antibacterial activity of PEGylated silver–graphene quantum dots nanocompositesAppl Mater Today2015128087
- FracchiaLCavalloMMartinottiMGBanatIMBiosurfactants and bioemulsifiers biomedical and related applications–present status and future potentialsGhistaGNBiomedical Science Engineering TechnologyRijeka, CroatiaInTech2012325370
- KarlapudiAPVenkateswaruluTCSriramaKKotaRKMikkiliIKodaliVPEvaluation of anti-cancer, anti-microbial and anti-biofilm potential of biosurfactant extracted from an Acinetobacter M6 strainJ King Saud Univ Sci Epub2018410
- HuangWLangYHakeemALeiYGanLYangXSurfactin-based nanoparticles loaded with doxorubicin to overcome multidrug resistance in cancersInt J Nanomed20181317231736
- DongJWangKSunLApplication of graphene quantum dots for simultaneous fluorescence imaging and tumor-targeted drug deliverySensors Actuators B Chem2018256616623
- SiwowskaKSchmidRCohrsSSchibliRMüllerCFolate receptor-positive gynecological cancer cells: In vitro and in vivo characterizationPharmaceuticals (Basel)2017103E7228809784
- SchroederKLGorehamRVNannTGraphene quantum dots for theranostics and bioimagingPharm Res201633102337235727207272
- Geszke-MoritzMPiotrowskaHMuriasMThioglycerol-capped Mndoped ZnS quantum dotbioconjugates as efficient two-photon fluorescent nanoprobes for bioimagingJ Mater Chem B201315698706
- LeamonCPLowPSDelivery of macromolecules into living cells: a method that exploits folate receptor endocytosisProc Natl Acad Sci19918813557255762062838
- SuiXLuoCWangCZhangFZhangJGuoSGraphene quantum dots enhance anticancer activity of cisplatin via increasing its cellular and nuclear uptakeNanomedicine20161271997200627085903
- WangXSunXLaoJMultifunctional graphene quantum dots for simultaneous targeted cellular imaging and drug deliveryColloids and Surfaces B Biointerfaces201412263864425129696
- GargMPriyankaChatterjeeMIsolation, characterization and antibacterial effect of biosurfactant from Candida parapsilosisBiotechnol Rep201818e00251
- HeYSunJFengDChenHGaoFWangLGraphene quantum dots: Highly active bifunctional nanoprobes for nonenzymatic photoluminescence detection of hydroquinoneBiosens Bioelectron20157441842226164014
- SinghGKumarMSoniUCancer cell targeting using folic acid/anti-HER2 antibody conjugated fluorescent CdSe/CdS/ZnS-MPA and CdTe-MSA quantum dotsJ Nanosci Nanotechnol201515129382939526682358
- NurunnabiMKhatunZHuhKMIn vivo biodistribution and toxicology of carboxylated graphene quantum dotsACS Nano2013786858686723829293
- JiangDChenYLiNSynthesis of luminescent graphene quantum dots with high quantum yield and their toxicity studyPLoS One20151012e014490626709828
- WuXTianFWangWChenJWuMZhaoJXFabrication of highly fluorescent graphene quantum dots using L-glutamic acid for in vitro/ in vivo imaging and sensingJ Mater Chem C Mater20131314676468423997934
- MagthalinCJVaradharajanASwarnalathaSSekaranGUtilization of chicken tallow for the production of cationic biosurfactant and thereof for decontamination of Cr(III) containing soilProcedia Environ Sci201635895913
- ZhengXTAnanthanarayananALuoKQChenPGlowing graphene quantum dots and carbon dots: properties, syntheses, and biological applicationsSmall201511141620163625521301
- JiaFLvSXuSBio-conjugation of graphene quantum dots for targeting imagingRSC Adv20177845353253536
- MaitiSKunduSRoyCNDasTKSahaASynthesis of excitation independent highly luminescent graphene quantum dots through per-chloric acid oxidationLangmuir20173351146341464229172551
- SherwoodDEmmanuel B InventorsCouncil of Scientific, Industrial Research (CSIR), assignee. Method for computing crystal shapes from X-ray diffraction data (XRD) of a substanceUnited States Patent US20107739075
- SangamSGuptaAShakeelASustainable synthesis of single crystalline sulphur-doped graphene quantum dots for bioimaging and beyondGreen Chem2018201842454259
- NwaharaNNkhahleRNgoyBPMackJNyokongTSynthesis and photophysical properties of BODIPY-decorated graphene quantum dot–phthalocyanine conjugatesNew J Chem201842860516061
- BalanSSKumarCGJayalakshmiSAneurinifactin, a new lipopeptide biosurfactant produced by a marine Aneurinibacillus aneurinilyticus SBP-11 isolated from Gulf of Mannar: purification, characterization and its biological evaluationMicrobiol Res20171941927938857
- BhattacharjeeSDLS and zeta potential – What they are and what they are not?J Control Release201623533735127297779