?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Traditional intravesical instillation treatment in bladder cancer has limited efficacy, which results in a high frequency of recurrence.
Purpose
The aim of this study was to report on an epirubicin (EPI)-loaded magnetic multi-walled carbon nanotube (mMWCNTs-EPI) system for intravesical instillation in place of the current formulation.
Methods
The mMWCNTs-EPI system was formulated with carboxylated MWCNTs, Fe3O4 magnetic nanoparticles, and EPI. Features and antitumor activity of the system were investigated.
Results
Under the effect of external magnets, the mMWCNTs-EPI system showed sustained release and prolonged retention behavior and better antitumor activity than free EPI. The mMWCNTs-EPI system had higher efficiency in enhancing cytotoxicity and inhibiting proliferation in vitro and in vivo than free EPI. Our studies also revealed the atoxic nature of mMWCNTs.
Conclusion
These findings suggested that mMWCNTs are effective intravesical instillation agents with great potential for clinical application.
Introduction
Urothelial bladder cancer (UBC) is a common malignancy with significant impact on public health. Approximately 75% of newly diagnosed UBCs are non-muscle-invasive BC (NMIBC).Citation1 After transurethral resection of bladder tumors, up to 70% of NMIBC patients experience disease recurrence.Citation2,Citation3 NMIBC has the highest recurrence rate among solid tumorsCitation4 and is the most expensive cancer over the lifetime of the patient, due to its high frequency of recurrence.Citation5,Citation6
Table S1 Serum biochemical analysis between treated group (2.5 mg/LmLmMWCNTs instilled intravesically every 3 days for 1 month) and control group
NMIBC recurs because of topical occult micrometastases in bladder.Citation7 The conventional systemic administration of drugs is ineffective in bladder diseases, due to poorly vascularized urothelium. As such, intravesical instillation has been used, whereby a drug is directly instilled into the bladder via a catheter to attain high local concentrations with minimal systemic effects.Citation8 The limitations of traditional intravesical instillation are primarily due to insufficient perfusion and limited retention of the chemotherapy drugs in bladders with periodical urination. Therefore, new strategies for improving intravesical instillation therapy are an important research topic.
Magnetic nanoparticles (NPs) with good superparamagnetic behavior have attracted much attention, due to their inherent material properties and huge potential in cancer diagnosisCitation9–Citation11 and treatment.Citation12 Our previous studies have demonstrated that thermosensitive hydrogel systems including Fe3O4 magnetic NPs prolonged the retention of chemotherapy drugs in bladders with external magnets.Citation13,Citation14 However, these systems have limited impact on disease sites. First, most drugs in hydrogel systems would separate from bladder mucosa via the loose structure of the cross-linking. Second, the hydrogel may swell and block the vesicoureteric junction. Third, temperature-dependent preparation of hydrogel systems would restrict clinical applications.
We describe a novel magnetic carboxylated multiwalled carbon nanotube (mMWCNTs)-based system with nanoscale characteristics that solves these issues. CNTs have a large surface area and are conjugated easily to chemotherapy drugs,Citation15 including single-walled CNTsCitation16 or MWCNTs.Citation17 MWCNTs have lower biological toxicity than single-walled CNTs.Citation18 Strong acid-carboxylated MWCNTs (MWCNTs-COOH) not only mitigate toxicityCitation19 but also increase the dispersibility and efficiency of loaded drugs.Citation20
For patients with NMIBC, early intravesical instillation with epirubicin (EPI) after transurethral resection of bladder tumors is an effective method. MWCNTs–COOH can efficiently load EPI via supramolecular π–π stacking because of a large surface area and hydrogen bonding.Citation20 Therefore, we propose an mMWCNTs-based delivery system for intravesical EPI instillation with external magnets. This scheme is flexible, because the magnet controls drug delivery.
Methods
Materials
MWCNTs (diameter 20–50 nm, length 10–30 µm, purity >95%) were purchased from Nanotech Port (Shenzhen, China). Reagents were acquired: sulfuric acid, nitric acid, ammonium hydroxide, FeCl2⋅4H2O, and FeCl3⋅6H2O (Analytical Reagent) from Sinopharm Chemical Reagent (Shanghai, China); antibodies to BCL2, BAX, and Ki67 from Abcam (Cambridge, UK); antibody to cleaved caspase 3 from Cell Signaling Technology (Danvers, MA, USA); EPI from Selleck Chemicals (Houston, TX, USA); a fluorescein isothiocyanate (FITC)–annexin V apoptosis-detection kit from BD Biosciences (San Jose, CA, USA); N-methyl-N-nitrosourea (MNU), DAPI, and FITC-labeled phalloidin from Sigma-Aldrich (St Louis, MO, USA); CCK8 from Dojindo Laboratories (Kumamoto, Japan); and a Cell Light ethynyl deoxyuridine (EdU) Apollo 567 in vitro imaging kit from RiboBio (Guangzhou, China). The human bladder transitional-cell carcinoma 5637 and T24 cell lines were kindly provided by the Stem Cell Bank, Chinese Academy of Sciences in 2016. Eight-week-old female Wistar rats were obtained from the Experimental Animal Center of Shandong University. Animal care and protocols were approved by the Institutional Animal Care and Use Committee of Shandong University. All animal experiments were performed in adherence with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Preparation of mMWCNTs
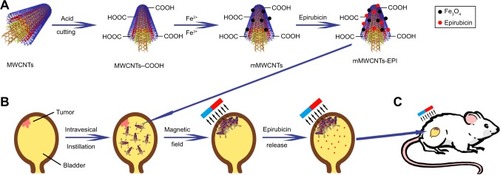
mMWCNTs preparation is illustrated in . Fe3O4 were attached to a chemically modified MWCNTs–COOH surface, producing mMWCNTs. First, 750 mg MWCNTs were dispersed in 300 mL solution of mixed acid (sulfuric acid:nitric acid 1:3) by 80 kHz sonication with a KQ-500DE digital-control ultrasonic cleaner (Kunshan Ultrasonic Instruments) for 30 minutes and then heated to 95°C for 4 hours. The MWCNTs were transformed into MWCNTs–COOH with mixed-acid treatment. The MWCNTs–COOH were subsequently dried in a drying oven. Next, 100 mg dried MWCNTs–COOH were dispersed in double-distilled water, and then 42.8 mg FeCl2⋅4H2O and 116.8 mg FeCl3⋅6H2O were added (the feed molar ratio of Fe2+:Fe3+ was 1:2). The weight ratio of MWCNTs–COOH to Fe3O4 was 2:1. After sonication for 5 minutes, the solution was purged with Ar2 for 30 minutes. The reaction system was then heated to 60°C and ammonium hydroxide added. The reaction system was kept at 60°C for 2 hours and then 90°C for 30 minutes. Finally, the resulting mMWCNTs were washed six times with 200 mL double-distilled water and dried in an oven. The dried mMWCNTs were laid out thinly on an ultraclean stage (Bolante Laboratory System Engineering, Suzhou, China) and bacteria/spore-free mMWCNTs produced by ultraviolet radiation for 6 hours.Citation21
Characterization of mMWCNTs
Transmission electron-microscopy images were obtained with a JEM-1011 (JEOL, Tokyo, Japan). Field-emission scanning electron-microscopy images were obtained with an SU-70 system (Hitachi, Tokyo, Japan). The ζ-potential measurements of the mMWCNTs were confirmed with a Delsa Nano C particle analyzer (Beckman Coulter, Brea, CA, USA). Fourier-transform infrared spectroscopy (FTIR) spectra were determined on an Alpha FTIR spectrometer (Bruker, Billerica, MA, USA). The crystalline structure of mMWCNTs was determined by powder diffractometry (X’PertCitation3; Panalytical, Almelo, Netherlands). Magnetization evaluation was carried out with a MicroMag 2900 AGM (Lake Shore Cryotronics, Westerville, OH, USA) according to the manufacturer’s instruction at room temperature.
Cell culture
T24 cells were grown in McCoy’s 5A medium supplemented with 10% FBS, and 5637 cells were grown in RPMI 1640 medium supplemented with 10% FBS. All cells were grown at 37°C in an atmosphere containing 5% CO2 and 95% air.
Cell-viability assays
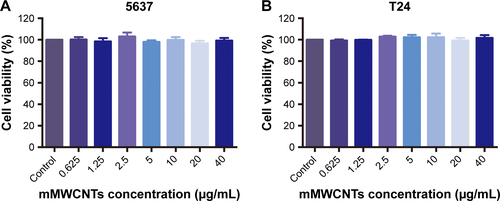
The cytotoxicity of mMWCNTs was evaluated with CCK8 assays. T24 and 5637 cells were seeded onto 96-well plates at a density of 5×103 cells/well. After attaching to the surface of a plate, cells were treated with 0, 0.625, 1.25, 2.5, 5, 10, 20, and 40 µg/mL mMWCNTs for 72 hours. Cells were subsequently treated with CCK8 solution and incubated for an additional 4 hours. Optical density was measured using an absorbance microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
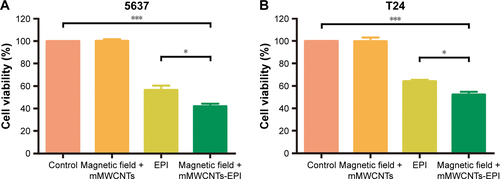
CCK8 assays were also performed to test the antitumor activity of mMWCNTs-EPI against 5637 and T24 cells. Group 1 was the control. Groups 2–4 were treated for 24 hours with a magnetic field and 40 µg/mL mMWCNTs; 2 µg/mL EPI; and a magnetic field and mMWCNTs-EPI (containing 2 µg/mL EPI), respectively. The culture medium was refreshed at 2, 4, 6, 8, 10, 12, and 24 hours to mimic urination. CCK8 solution was added in accordance with the aforementioned protocol.
Cell-morphology tests
T24 and 5637 cells were divided into control groups and experimental groups, respectively. Experimental groups were treated with 40 µg/mL mMWCNTs for 72 hours. Control groups were treated with PBS for the same time. Cells were fixed with methanol for 10 minutes and stained with FITC-labeled phalloidin for 1 hour. Nuclei were counterstained with DAPI. Images were captured using fluorescence microscopy (Olympus, Tokyo, Japan).
In vivo toxicity of mMWCNTs
The toxicity of mMWCNTs in vivo was determined in 12 female rats. Six rats were maintained in the magnetic field with 3,200 G magnets fixed near their bladders. Then, 2.5 mg/1 mL mMWCNTs were instilled intravesically every 3 days for 1 month. Another six rats were fed as controls. Blood samples were collected after 1 month for analysis of blood serum chemistry. ALT, AST, Scr, and BUN were determined with a fully automatic biochemical analyzer (Chemray 240; Rayto Life and Analytical Sciences, Shenzhen, China). Hearts, livers, spleens, lungs, kidneys, and brains were excised at the same time to detect the systemic toxicity of mMWCNTs.
Loading and release studies
EPI was dissolved in double-distilled water at a concentration of 1 mg/mL. The same mass of mMWCNTs was then mixed into EPI solution by sonication for 30 minutes. mMWCNTs-EPI were produced and then purified via external magnets. Briefly, the suspension of mMWCNTs-EPI and free EPI was placed on a magnetic rack (Solarbio Life Sciences, Beijing, China) for 10 minutes. mMWCNTs-EPI were then held tightly to the flask wall by magnetic forces. The free EPI was aspirated and measured on a Shimadzu SCL-10AVP series HPLC system coupled with ultraviolet detection set at 480 nm and C18 analytical column (150×4.6 mm, 5 µm particle size) (Beckman Coulter). The binary mobile phase was sodium dihydrogen phosphate (68%) and acetonitrile (32%) delivered at 1.0 mL/min. The loading efficiency of EPI was calculated:Citation22
In vivo evaluation of retention
The retention of mMWCNTs-EPI in bladders was determined in 15 female rats with a 3,200 G magnetic field. Rats were killed at 12, 24, 48, 72, and 96 hours after mMWCNTs-EPI instillation (three rats at each time point). Bladders excised at different time points were fixed with 4% paraformaldehyde, embedded in paraffin, and serially sectioned. The 4 µm-thick sections underwent H&E staining.
Quantitative detection of apoptosis in vitro
To measure cell apoptosis, 5637 and T24 cells were seeded onto six-well plates at a density of 105 cells/well. Group 1 was the control. Groups 2–4 were treated for 24 hours with a magnetic field and 40 µg/mL mMWCNTs; 2 µg/mL EPI; and a magnetic field and mMWCNTs-EPI (containing 2 µg/mL EPI). The culture medium was refreshed at 2, 4, 6, 8, 10, 12 and 24 hours to mimic urination. Cells were collected and subjected to annexin V–propidium iodide staining using the FITC–annexin V apoptosis-detection kit. Apoptotic cells were analyzed with a FACSDiva Flow Cytometer (BD Biosciences).
Quantitative detection of proliferation in vitro
To examine proliferation of 5637 and T24 cells, cells were seeded onto 24-well plates at a density of 5×104 cells/well. The groups were treated as described in (Cell morphology test section) and (Quantitative detection of apoptosis in vitro section). All groups were treated with EdU for 2 hours and then incubated with the Apollo 567 kit. Nuclei were counter-stained with DAPI. Images were captured using fluorescence microscopy, and digital histomorphometric analysis was performed with ImageJ software.
Chemically induced bladder cancer model in rats
An MNU-induced rat-bladder tumor model described previously was used, with some modifications.Citation23 Female Wistar rats were anesthetized with intraperitoneal injections of 3% pentobarbital (30 mg/kg). Then, MNU was instilled intravesically via shortened 3F epidural anesthesia catheters within 45 minutes of preparation after the bladders had been drained. Instillation was performed every other week for a total of four doses (8 weeks).
Antitumor activity in vivo
The 30 rats were divided randomly into five groups. Group 1 was administered as the control. The other groups were induced BC models as previously described. After 8 weeks, groups 2–5 received intravesical instillation with the 0.1 mL PBS, 0.25 mg/0.1 mL mMWCNTs, 0.1 mg/0.1 mL EPI, and 0.1 mL mMWCNTs-EPI (containing 0.1 mg EPI and 0.25 mg mMWCNTs), respectively. They received intravesical instillation on a weekly basis six times. Groups 3 and 5 were maintained in the 3,200 G magnetic field (group 5 treatment is illustrated in , B and C). All rats were killed and subjected to necropsy (one in the mMWCNTs group was killed by an unintended anesthetic overdose). A tumor was defined as a lesion >0.5 mm in diameter.Citation24 Tumor volume was calculated:
Immunohistochemistry study
The 4 µm-thick bladder-tissue sections of all groups underwent immunohistochemical staining for BCL2, BAX, cleaved caspase 3, and immunofluorescence staining of Ki67. Negative control sections were incubated without the primary antibody. Digitized images were subsequently analyzed using Image-Pro Plus 6.0, and average optical density (integrated optical density/area) positive reactions used to evaluate the expression of BCL2, BAX, and cleaved caspase 3. The ratio of Ki67+ cells was analyzed with ImageJ software.
Statistical analysis
All statistical analyses were conducted with SPSS 20.0 software. Data are expressed as mean ± SD for continuous variables. Continuous data were compared among the groups via one-way ANOVA. Tukey’s test was used to analyze multiple comparisons between groups. Two-tailed P<0.05 indicated statistical significance.
Results
Characterization of mMWCNTs
The preparation of mMWCNTs is illustrated in . show transmission electron microscopy and field-emission scanning electron microscopy of prepared mMWCNTs. The length of raw MWCNTs was 10–30 µm, and the length of MWCNTs–COOH cut by mixed acid became 200–1,000 nm. (). Close observation revealed that the MWCNTs–COOH were wrapped tightly with nano-Fe3O4 on the surface (). mMWCNTs-suspension ζ-potential was -47.31±0.16 mV, which showed good stability in aqueous solution.
Figure 1 Characterization of MWCNTs, MWCNTs-COOH, and mMWCNTs.
Notes: (A) TEM images of MWCNTs, MWCNTs-COOH, and mMWCNTs; black arrow indicates that the MWCNTs-COOH were wrapped tightly with nano-Fe3O4 on the surface. (B) FESEM images of MWCNTs-COOH and mMWCNTs; white arrow indicates the nano-Fe3O4 on the surface of MWCNTs-COOH. (C) FTIR spectra of MWCNTs-COOH (i) and mMWCNTs (ii). (D) XRD of mMWCNTs. (E) Magnetic hysteresis curve of mMWCNTs. (F) mMWCNTs dispersed in water without a magnet (i) or with a magnet for 2 minutes (ii) and 5 minutes (iii).
Abbreviations: MWCNTs, multiwalled carbon nanotubes; MWCNTs-COOH, carboxylated MWCNTs; mMWCNTs, magnetic MWCNTs-COOH; TEM, transmission electron microscopy; FESEM, field-emission scanning electron microscopy; FTIR, Fourier-transform infrared spectroscopy; XRD, X-ray diffraction.
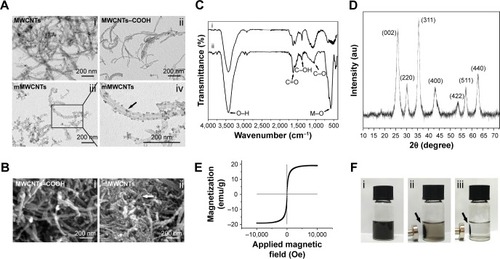
FTIR spectra of MWCNTs–COOH and mMWCNTs showed several peaks at 3,434 cm−1, 1,626 cm−1, 1,384 cm−1, and 1,045 cm−1 (). These peaks were ascribed to O–H bonds,Citation25 C=O groups,Citation26 and C–OH and C–O stretching vibrations,Citation27 respectively. The peak at 582 cm−1 of mMWCNTs () was assigned to metal oxygen.Citation26 X-ray diffraction confirmed the metal–oxygen bonds in the mMWCNTs (). According to Joint Committee on Powder Diffraction Standards file 190629 for the magnetite, six diffraction peaks of mMWCNTs at 2θ =30.16°, 35.58°, 43.22°, 53.70°, 57.22°, and 62.87° were attributed to the (220, 311, 400, 422, 511) and (440) crystal planes of cubic Fe3O4.Citation28 Furthermore, the main feature of the X-ray-diffraction pattern of CNTs was a graphite-like peak (002).Citation29 The diffraction peaks of mMWCNTs at 2θ =26.02° was attributed to the (002) peak of CNTs, which is not obvious. This suggested that mMWCNTs were wrapped by Fe3O4 NPs.
The magnetic hysteresis curve of mMWCNTs was determined by alternating-gradient magnetometry (). The mMWCNTs showed superparamagnetic behavior, and the maximum saturation magnetization value is 19.13 emu/g. Residual magnetization and coercivity were both zero. The magnetization curve showed an invertible S shape. There was no hysteresis in the sample. Macroscopic magnetic parameters of mMWCNTs were measured via the 1 mg/mL mMWCNTs suspension. When an external magnet was applied, the mMWCNTs separated from the suspension and were rapidly attracted to the magnet to clear the suspensions (). However, the mMWCNTs returned to suspensions after gentle shaking. Only a trace of mMWCNTs sediments was observed after storage for 15 days, indicating excellent aqueous stability.
Toxicity of mMWCNTs
When treated with different concentrations of mMWCNTs, mMWCNTs showed little toxicity against 5637 and T24 cells (). The ratio of EdU-labeled cells was calculated to examine the effect of mMWCNTs on cell proliferation. There was no difference between 40 µg/mL mMWCNTs groups and control groups (). F-actin staining was found predominantly in cortical structures around the cell periphery, with a few thin stress fibers located within the cell body. Alignment of F-actin fibers increased in all periods of mitosis (, red arrow). There were no obvious morphological changes or reorganization of F-actin cytoskeleton in either group ().
Figure 2 Toxicity of mMWCNTs in vitro and in vivo.
Notes: (A) Immunofluorescence-staining microscopy of F-actin cytoskeleton (phalloidin, green) and nuclei (DAPI, blue) of 5637 and T24 cells treated with 40 µg/mL mMWCNTs for 72 hours. Red arrows indicate increased alignment of F-actin fibers over all periods of mitosis. (B) Immunofluorescence-staining microscopy of EdU (red) and nuclei (blue) of 5637 and T24 cells treated with 40 µg/mL mMWCNTs for 72 hours. (C) Corresponding ratiometric analyses of ratio of EdU-labeled cells. Data presented as mean ± SD. (D) H&E-stained rat hearts, livers, spleens, lungs, kidneys, and brains after 2.5 mg/mL mMWCNTs instilled intravesically every 3 days for 1 month.
Abbreviations: mMWCNTs, magnetic multiwalled carbon nanotubes; EdU, ethynyl deoxyuridine.
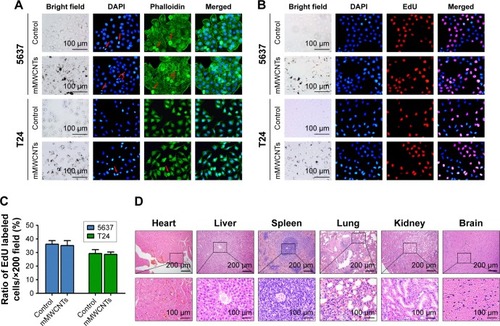
The toxicity of mMWCNTs in vivo was determined in 12 female rats. During the experiment, there was no mortality or systemic serum biochemical toxicity induced by mMWCNTs (). Neither mMWCNTs agglomerates nor any visible signs of toxicity (eg, inflammatory cells or histopathological changes) were found in major organs (). There were no abnormal behavioral changes, including diarrhea, vomiting, anorexia, or lethargy.
Sustained EPI release and prolonged retention in rat bladder
The loading procedure for mMWCNTs with EPI solutions resulted in a loading percentage of 40.4%±9.6%. shows that the release of EPI from mMWCNTs-EPI was slower, and the decrease in concentration was moderate and lasted longer than free EPI. The sustained release of EPI from mMWCNTs-EPI resulted in the area under the curve nearly tripling ().
Figure 3 The sustained release of EPI from mMWCNTs-EPI system and prolonged retention in rat bladder.
Notes: (A) The EPI release curve of mMWCNTs-EPI and EPI solution. (B) The EPI accumulative releasing ratio from mMWCNTs-EPI and EPI solution. (C) The areas under the AUC values of EPI. (D) The retention of mMWCNTs-EPI system in rat bladder. Exemplary H&E-stained tissue sections from urinary bladders of rats maintained in magnetic field of 3,200 G for 12, 24, 48, 72, and 96 hours after mMWCNTs-EPI instillation. Data presented as mean±SD. *P<0.05.
Abbreviations: EPI, epirubicin; mMWCNTs, magnetic multiwalled carbon nanotubes; AUC, area under curve (concentration–time).
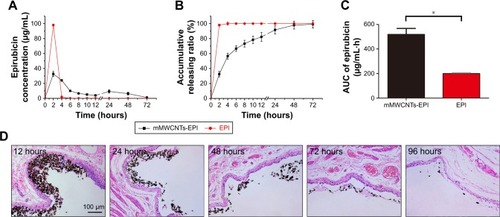
mMWCNTs-EPI were stable in rat bladder after 12 hours with external magnets (). The amount of mMW-CNTs-EPI and the mMWCNTs-EPI-covered surface areas along the urothelium decreased with time. There were some remnants until 96 hours.
In vitro antitumor activity
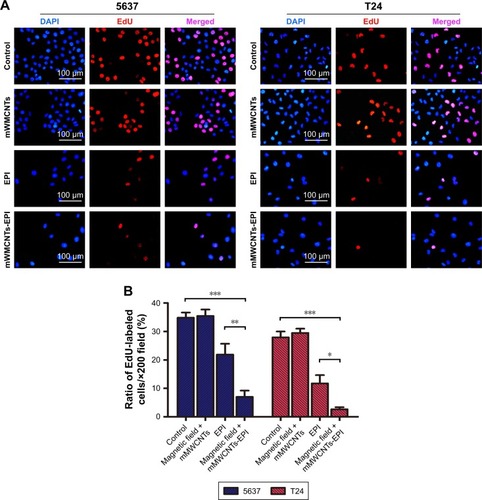
mMWCNTs-EPI showed more significant cytotoxicity on 5637 and T24 cells than free EPI when the culture medium was refreshed every 2 hours (). Flow-cytometry results demonstrated that the free EPI and mMWCNTs-EPI groups showed higher apoptotic ratios than control and mMWCNTs groups. Versus free EPI, apoptotic ratios in the mMWCNTs-EPI groups increased significantly (). Significantly lower ratios of EdU-labeled cells per high-power field (magnification 200×) were observed in mMWCNTs-EPI-treated cells relative to free EPI groups (). Statistical analysis showed that the mMWCNTs-EPI groups demonstrated significantly less proliferation than free EPI groups ().
Figure 4 In vitro apoptosis-inducing activity.
Notes: (A) Apoptotic cells in groups of 5637 and T24 cells with different treatments. Representative flow-cytometry plots. Q1 represents necrotic cells, Q2 late-apoptosis cells, Q3 living cells, and Q4 early-apoptosis cells. (B) Graphic representation of apoptosis rate of each group. Data presented as mean ± SD. *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: FITC, fluorescein isothiocyanate; PI, propidium iodide; mMWCNTs, magnetic multiwalled carbon nanotubes; EPI, epirubicin.
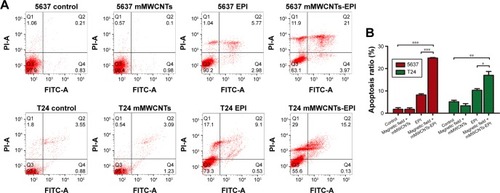
Figure 5 In vitro proliferation-inhibition activity.
Notes: (A) Immunofluorescence-staining microscopy of EdU (red) and nuclei (blue) of 5637 and T24 cells treated with 40 µg/mL mMWCNTs and control groups. (B) Graphic representation of EdU-labeled cell ratio of each group in A. *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: EdU, ethynyl deoxyuridine; mMWCNTs, magnetic multiwalled carbon nanotubes; EPI, epirubicin.
In vivo antitumor activity
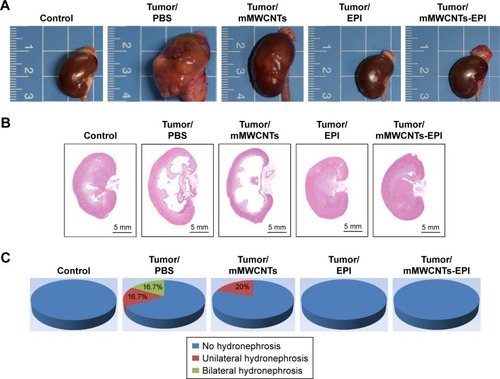
Representative bladder tumors from the five groups are shown in (black arrows). Administration of PBS and mMWCNTs caused no markedly different reduction in the growth of bladder tumors, neither total tumor volume per rat nor volume of each tumor. In terms of efficacy, the mMWCNTs-EPI group achieved better efficacy than free EPI for inhibition of tumor volume (). In addition, one unilateral hydronephrosis appeared in both the PBS and mMWCNTs groups, and one bilateral hydronephrosis appeared in the PBS group (). Representative images and H&E-stained microscopy of kidney in different groups are shown in . Corresponding ratios of hydronephrosis in different groups are displayed in . The hydronephrosis was caused by ureter or vesicoureteric junction invasion.
Figure 6 In vivo antitumor activity on bladder cancer.
Notes: (A) Representative images of bladder tumors in different groups (black arrow). (B) Summary of total tumor volume per rat. Data presented as mean ± SD. (C) Graphic representation of the tumor volume. **P<0.01; ***P<0.001.
Abbreviations: mMWCNTs, magnetic multiwalled carbon nanotubes; EPI, epirubicin.
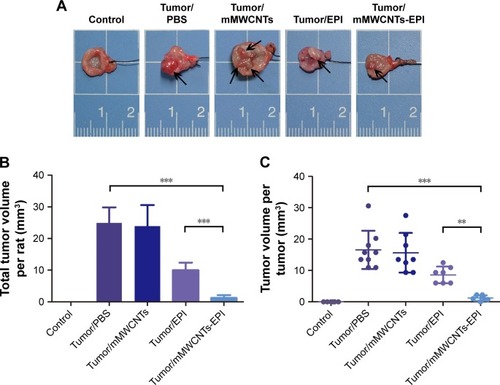
Figure 7 Representative images and H&E-stained sections of kidneys in different groups.
Notes: (A) Representative images of kidneys in different groups: normal or hydronephrosis. (B) H&E-stained exemplary tissue sections of kidneys in different groups. (C) Summary of hydronephrosis in different groups, including unilateral hydronephrosis and bilateral hydronephrosis.
Abbreviations: mMWCNTs, magnetic multiwalled carbon nanotubes; EPI, epirubicin.
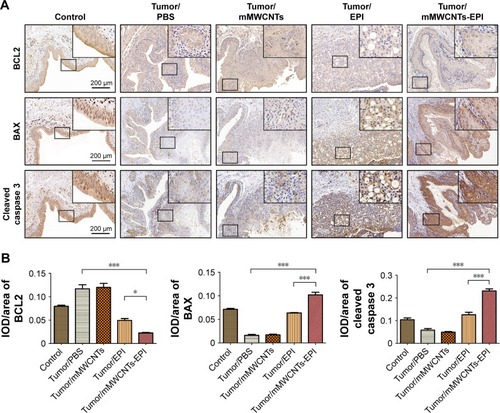
Enhanced apoptosis and inhibited proliferation by mMWCNTs-EPI
The expression of BAX and cleaved caspase 3 was weak, while BCL2 increased in the PBS and mMWCNTs groups. The EPI and mMWCNTs-EPI groups had contrasting expression patterns compared with the PBS and mMWCNTs groups (). The data also showed that the mMWCNTs-EPI group had higher expression of BAX and cleaved caspase 3 and lower expression of BCL2 than the EPI group ().
Figure 8 Immunohistochemical staining of exemplary tissue sections of bladders in different groups and statistical analysis of the IOD/area data.
Notes: (A) Representative light-microscopy sections of BCL2, BAX, and cleaved caspase 3-stained bladder tissue; (B) statistical analysis of IOD/area data of bladder-tissue sections for BCL2, BAX, and cleaved caspase 3. *P<0.05, ***P<0.001.
Abbreviations: IOD, integrated optical density; mMWCNTs, magnetic multiwalled carbon nanotubes; EPI, epirubicin.
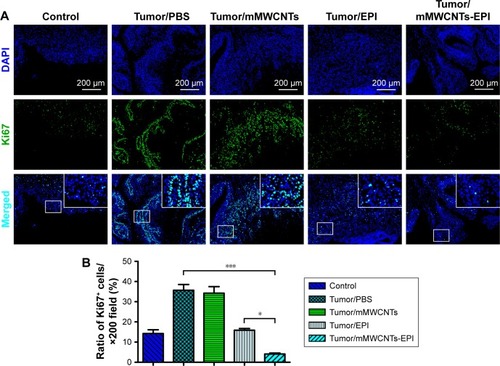
Immunofluorescence-staining results indicated that Ki67+ cells were nearly negative in the control group, but obviously increased in the PBS and mMWCNTs groups (). After intravesical instillation therapy with EPI or mMWCNTs-EPI, the ratios of Ki67+ cells decreased significantly. In addition, the mMWCNTs-EPI group showed more inhibition in Ki67 expression than the EPI group ().
Figure 9 Immunofluorescence-stained exemplary tissue sections of bladders in different groups and statistical analysis of the Ki67+ cells.
Notes: (A) Representative light-microscopy sections of Ki67 (green) and nuclei (DAPI, blue) stained bladder tissue; (B) statistical analysis of Ki67+ cells of bladder-tissue sections in different groups. *P<0.05, ***P<0.001.
Abbreviations: mMWCNTs, magnetic multiwalled carbon nanotubes; EPI, epirubicin.
Discussion
We describe a novel magnetic carboxylated mMWCNTs-based system. This is an excellent delivery system for chemotherapeutic drugs in intravesical instillation. CNTs have been investigated for possible applications in delivering chemotherapy drugs with large surface area.Citation15,Citation30 Magnetite Fe3O4 NPs were introduced to decorate MWCNTs as mMWCNTs. As such, mMWCNTs offered superparamagnetic behavior at room temperature. This superparamagnetic property is very important for the application of magnetic NPs in biomedicine, rather than bulk material. The magnetic NPs showed good magnetic response characteristics via an external magnetic field, and remained monodisperse without an external magnetic field to prevent blocking blood vessels or other lumens.Citation31
Studies have shown that chitosan,Citation13,Citation14 liposomes,Citation32 and hydrogelsCitation33 are other candidates for local drug delivery. The mMWCNTs-based delivery system has several advantages vs existing systems. First, most drugs in previous delivery systems would be separated from bladder mucosa by the encapsulation barrier or the gel system with a loose cross-linking structure. The drug effect needs to overcome steric hindrance first. However, the mMWCNTs-EPI system is tightly attached to the bladder mucosa. Aggregation of mMWCNTs-EPI under a magnetic field can increase the mucoadhesion of EPI to the tumor. Second, the gel matrix or hydrogel may swell and block the vesicoureteric junction. mMWCNTs solve this issue. mMWCNTs are more clinically convenient and do not lead to vesicoureteric junction blockage. Third, the synthetic steps underlying mMWCNTs are simple and fast. The mMWCNTs mixture was able to be stored at room temperature for a long time, and mMWCNTs-EPI can be readily prepared when needed. Moreover, the mMWCNTs system showed better retention after 96 hours ().
Toxicity, low dispersion, and poor solubility of pristine MWCNTs have limited their biomedical applications.Citation34 However, research has indicated that highly functionalized and water-dispersible MWCNTs do not accumulate in or injure any tissues.Citation35 Methods to functionalize MWCNTs include decoration with polyacrylic acid,Citation41 polysaccharide,Citation36 alginate sodium,Citation37 chitosan,Citation38 and polyethylene glycol.Citation39 However, our method utilized an acid mixture to carboxylate MWCNTs and reduce their length. This significantly decreased toxicity.Citation35 Moreover, MWCNTs–COOH were decorated with Fe3O4 via coprecipitation of Fe2+ and Fe3+, which is a facile option without any assistance from organic solvents.Citation40
Traditional NP-based drug-delivery systems will be distributed throughout most tissue after venous injection. However, the wall of the urinary bladder is an effective barrier between the urinary system and blood circulation.Citation8 In contrast to intravenous injection, intravesical instillation can further minimize the potential toxicity of the mMWCNTs. and show that the mMWCNTs had little cytotoxicity or systemic toxicity. These results suggested that mMWCNTs were safe materials for intravesical instillation.
EPI was periodically released from the mMWCNTs-EPI system with urination cycles (). In general, the uti-lization efficiency of EPI through the urothelium is governed by the degree of ionization at bladder pH, time of exposure to tissue, and urinary output frequency.Citation42 By preventing EPI from being washed away during urine voiding with magnetic fields, exposure of EPI was prolonged and the initial burst release reduced ().
Scheme 1 Schematic illustrations of preparation and use of mMWCNTs-EPI system.
Notes: (A) Synthesis of mMWCNTs; (B) release of EPI from system; (C) application of the system in rats.
Abbreviations: MWCNTs, multiwalled carbon nanotubes; MWCNTs-COOH, carboxylated MWCNTs; mMWCNTs, magnetic MWCNTs-COOH; EPI, epirubicin.
Research has indicated that the amount of EPI loaded on MWCNTs–COOH is pH-dependent.Citation20 The π–π stacking interaction, hydrophobic interaction, and hydrogen-bonding interaction between the EPI–OH and tube-surface–COOH groups lead to three possible interactions between EPI and MWCNTs–COOH.Citation20 When -OH ionizes to -O− at a high pH, the electron-donating strength is improvedCitation43 to strengthen the π–π interaction between the EPI and CNTs. This increases adsorption affinity.Citation44 On the contrary, EPI would release from the mMWCNTs-EPI system at low pH value. Urine is normally slightly acidic.Citation8 Similar to prior work,Citation14 we performed our experiments at pH 6. shows that EPI had been totally released from the mMWCNTs-EPI system in continuous PBS (pH=6) irrigation after 72 hours. EPI bioavailability via the mMWCNTs-EPI system almost tripled vs free EPI (). Therefore, EPI offered sustained-release behavior for mMWCNTs-EPI in a bladder microenvironment, as expected.
The mMWCNTs-EPI system encouraged antitumor activity both in vitro and in vivo vs traditional intravesical instillation. and confirm that the mMWCNTs-EPI group had better tumor-growth inhibition and induced more apoptosis in vitro than free EPI when given at equivalent doses as free EPI. and indicate that mMWCNTs-EPI are an effective intravesical instillation system for the treatment of BC. EPI acts by interfering with syntheses of DNA, RNA, and protein, as well as cytocidal activity.Citation45 Caspase 3 is a major executive caspase in mitochondria-dependent apoptosis.Citation46 Increased BAX and decreased BCL2 expression promote mitochondria-dependent apoptosis.Citation47 The expression of Ki67 is strongly associated with tumor-cell proliferation.Citation48,Citation49 Our findings suggested that sustained release of EPI from the mMWCNTs-EPI system promoted mitochondria-dependent apoptosis and inhibited proliferation ( and ).
mMWCNTs increased residence time of EPI in the bladder via an external magnet, but this study does have some limitations. This system could not target the tumor site accurately in rat models, even using external magnets. Some other approaches, such as decorating mMWCNTs with tumor-targeted molecules, could be used. The precise placement of magnets for postoperative care should be a good way to improve the efficacy of mMWCNTs-EPI in clinical applications. A magnetic belt for the mMWCNTs-EPI system could also be envisioned.
Conclusion
This study successfully developed a safe magnetic adhesion system for the urothelium. The results showed that the mMWCNTs-EPI system effectively extended the duration of EPI in intravesical instillation under external magnets. The mMWCNTs-EPI system enhanced EPI cytotoxicity and inhibited cell proliferation in BC, both in vitro and in vivo. This system can enhance therapeutic effects and decrease side effects of BC by utilizing the synergistic effects of magnetic retention and chemotherapy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant 81572534), Natural Science Foundation of Shandong (grant ZR2016HM32), Shandong Key Research and Development Plan (grants 2015GSF118096, 2017GSF218078, and 2018GSF118189), and the Medical and Health Technology Development Project of Shandong (grant 2016WS0444). We appreciate Professor Lijie Ci, Professor Pengchao Si, Chenglong Dong, and Xiangkun Nie for helpful discussions on the synthesis of mMWCNTs. We would like to thank Professor Jun Li and Shasha Li for HPLC measurements.
Supplementary material
Disclosure
The authors report no conflicts of interest in this work.
References
- BurgerMCattoJWDalbagniGEpidemiology and risk factors of urothelial bladder cancerEur Urol201363223424122877502
- BabjukMBöhleABurgerMEAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016Eur Urol201771344746127324428
- FrantziMvan KesselKEZwarthoffECDevelopment and validation of urine-based peptide biomarker panels for detecting bladder cancer in a multi-center StudyClin Cancer Res201622164077408627026199
- LorasATrassierraMSanjuan-HerráezDBladder cancer recurrence surveillance by urine metabolomics analysisSci Rep201881917229907864
- YeungCDinhTLeeJThe health economics of bladder cancer: an updated review of the published literaturePharmacoeconomics201432111093110425056838
- PloegMAbenKKKiemeneyLAThe present and future burden of urinary bladder cancer in the worldWorld J Urol200927328929319219610
- ZuiverloonTCTheodorescuDPharmacogenomic considerations in the treatment of muscle-invasive bladder cancerPharmacogenomics201718121167117828745580
- GuhaSarkarSBanerjeeRIntravesical drug delivery: challenges, current status, opportunities and novel strategiesJ Control Release2010148214715920831887
- TangHGuoYPengLIn vivo targeted, responsive, and synergistic cancer Nanotheranostics by magnetic resonance imaging-guided synergistic high-intensity focused ultrasound ablation and chemotherapyACS Appl Mater Interfaces20181018154281544129652130
- MariappanLShaoQJiangCMagneto acoustic tomography with short pulsed magnetic field for in-vivo imaging of magnetic iron oxide nanoparticlesNanomedicine201612368969926656627
- GambardellaABianchiMKaciulisSMagnetic hydroxyapatite coatings as a new tool in medicine: a scanning probe investigationMater Sci Eng C Mater Biol Appl20166244444926952445
- ZhaoYZhaoXChengYGuoXYuanWIron oxide Nanoparticles-Based vaccine delivery for cancer treatmentMol Pharm20181551791179929570298
- ZhangDSunPLiPA magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette-Guérin in the treatment of bladder cancerBiomaterials20133438102581026624070571
- SunXSunPLiBA new drug delivery system for mitomycin C to improve intravesical instillationMater Design2016110849857
- WongBSYoongSLJagusiakACarbon nanotubes for delivery of small molecule drugsAdv Drug Deliv Rev201365151964201523954402
- IijimaSIchihashiTSingle-shell carbon nanotubes of 1-nm diameterNature19933636430603605
- IijimaSHelical microtubules of graphitic carbonNature199135463485658
- JiaGWangHYanLCytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullereneEnviron Sci Technol20053951378138315787380
- AllegriMPerivoliotisDKBianchiMGToxicity determinants of multi-walled carbon nanotubes: the relationship between functionalization and agglomerationToxicol Rep2016323024328959543
- ChenZPierreDHeHAdsorption behavior of epirubicin hydrochloride on carboxylated carbon nanotubesInt J Pharm20114051–215316121145959
- BellucciSChiarettiMOnoratoPMicro-Raman study of the role of sterilization on carbon nanotubes for biomedical applicationsNanomedicine (Lond)20105220921520148633
- SinghRMehraNKJainVJainNKGemcitabine-loaded smart carbon nanotubes for effective targeting to cancer cellsJ Drug Target201321658159223484494
- SteinbergGDBrendlerCBIchikawaTSquireRAIsaacsJTCharacterization of an N-methyl-N-nitrosourea-induced autochthonous rat bladder cancer modelCancer Res19905020666866742208131
- ParadaBReisFFigueiredoAInhibition of bladder tumour growth by sirolimus in an experimental carcinogenesis modelBJU Int2011107113514320367636
- SuoHXuLXuCEnhancement of catalytic performance of porcine pancreatic lipase immobilized on functional ionic liquid modified Fe3O4-Chitosan nanocompositesInt J Biol Macromol201811962463230071225
- Ahmadian-Fard-FiniSSalavati-NiasariMGhanbariDHydrothermal green synthesis of magnetic Fe3O4-carbon dots by lemon and grape fruit extracts and as a photoluminescence sensor for detecting of E. coli bacteriaSpectrochim Acta A Mol Biomol Spectrosc201820348149329898431
- MohamedMMKhairyMIbrahemADispersed Ag2O/Ag on CNT-Graphene composite: an implication for magnificent photoreduction and energy storage applicationsFront Chem2018625030018950
- FanXJiaoGZhaoWJinPLiXMagnetic Fe3O4-graphene composites as targeted drug nanocarriers for pH-activated releaseNanoscale2013531143115223288110
- BelinTEpronFCharacterization methods of carbon nanotubes: a reviewMater Sci Eng B20051192105118
- BhirdeAAPatelVGavardJTargeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug deliveryACS Nano20093230731619236065
- MehtaRVSynthesis of magnetic nanoparticles and their dispersions with special reference to applications in biomedicine and biotechnologyMater Sci Eng C Mater Biol Appl20177990191628629096
- KaldybekovDBTonglairoumPOpanasopitPKhutoryanskiyVVMucoadhesive maleimide-functionalised liposomes for drug delivery to urinary bladderEur J Pharm Sci2018111839028958893
- KolawoleOMLauWMMostafidHKhutoryanskiyVVAdvances in intravesical drug delivery systems to treat bladder cancerInt J Pharm2017532110511728867449
- MamidiNLeijaHMDiabbJMCytotoxicity evaluation of unfunctionalized multiwall carbon nanotubes-ultrahigh molecular weight polyethylene nanocompositesJ Biomed Mater Res A2017105113042304928779510
- LacerdaLAli-BoucettaHHerreroMATissue histology and physiology following intravenous administration of different types of functionalized multiwalled carbon nanotubesNanomedicine (Lond)20083214916118373422
- LiuYChipotCShaoXCaiWEdge effects control helical wrapping of carbon nanotubes by polysaccharidesNanoscale2012482584258922415663
- Karkeh-AbadiFSaber-SamandariSSaber-SamandariSThe impact of functionalized CNT in the network of sodium alginate-based nano-composite beads on the removal of Co(II) ions from aqueous solutionsJ Hazard Mater201631222423327037477
- KarnatiKRWangYUnderstanding the co-loading and releasing of doxorubicin and paclitaxel using chitosan functionalized single-walled carbon nanotubes by molecular dynamics simulationsPhys Chem Chem Phys201820149389940029565091
- SobhaniZBehnamMAEmamiFDehghanianAJamhiriIPhotother-mal therapy of melanoma tumor using multiwalled carbon nanotubesInt J Nanomedicine2017124509451728684911
- LuAHSalabasELSchüthFMagnetic nanoparticles: synthesis, protection, functionalization, and applicationAngew Chem Int Ed200746812221244
- YangFJinCYangDMagnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatmentEur J Cancer201147121873188221493061
- GiannantoniADi StasiSMChancellorMBCostantiniEPorenaMNew frontiers in intravesical therapies and drug deliveryEur Urol200650611831193 discussion 119316963179
- ChenWDuanLWangLZhuDAdsorption of hydroxyl- and amino-substituted aromatics to carbon nanotubesEnviron Sci Technol200842186862686818853801
- LinDXingtBAdsorption of phenolic compounds by carbon nano-tubes: role of aromaticity and substitution of hydroxyl groupsEnviron Sci Technol200842197254725918939555
- KhasrawMBellRDangCEpirubicin: is it like doxorubicin in breast cancer? A clinical reviewBreast201221214214922260846
- PorterAGJänickeRUEmerging roles of caspase-3 in apoptosisCell Death Differ1999629910410200555
- DanialNNKorsmeyerSJCell deathCell2004116220521914744432
- YangCZhangJDingMKi67 targeted strategies for cancer therapyClin Transl Oncol201820557057529058263
- LiLTJiangGChenQZhengJNKi67 is a promising molecular target in the diagnosis of cancer (review)Mol Med Rep20151131566157225384676