Abstract
Background
Hepatic ischemia/reperfusion-induced pancreatic islet injury (HI/RIPII) was an important pathophysiological phenomenon in clinics. In the present study, we observed the effects of phycocyanin on HI/RIPII. However, the half-life of phycocyanin was extremely short and limited its use in vivo.
Materials and methods
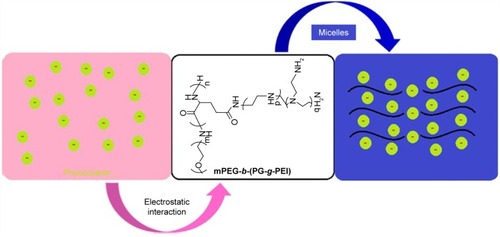
In order to overcome this shortcoming, poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine) (PEG-b-(PG-g-PEI)) was synthesized and estimated as a nanocarrier for lengthening delivery of phycocyanin through the abdominal subcutaneous injection in rats. Phycocyanin (isoelectric point=4.3) was encapsulated with PEG-b-(PG-g-PEI) via electrostatic interactions at pH 7.4.
Results
In vitro phycocyanin was fast and efficiently encapsulated and showing efficient loading and sustained release. In vivo the anti-HI/RIPII function of phycocyanin/PEG-b-(PG-g-PEI) complex was surveyed in rats using free phycocyanin as the controls, and the results showed that phycocyanin/PEG-b-(PG-g-PEI) complex reduced HI/RIPII property and enlarged islet functionality.
Conclusion
These results suggested that PEG-b-(PG-g-PEI) might be treated as a potential phycocyanin nanocarrier.
Introduction
Hepatic ischemia/reperfusion injury (HI/RI) has been widely researched during the last decades and is closely related to the pathophysiology of numerous clinical entities following liver surgery and transplantation.Citation1 HI/RI not only results in liver damage but also induces remote organ damage, especially pancreatic islet injury.Citation2 In addition, HI/RI contains two injury phases: first damage phase (<6 hours) and second damage phase (>6 hours), and many studies focus on the first damage phase rather than the second damage phase on HI/RI.Citation2 Previous literature has reported that HI/RI can aggravate the secretion of pancreatic beta cells.Citation2 Many factors are reported to get in touch with the pathogenesis of HI/RI.Citation1 Overfull reactive oxygen species (ROS) engendered in the liver after ischemia/reperfusion (I/R) is one of the main mechanisms contained in HI/RI.Citation3–Citation5 Overfull productions of ROS induces remote organ injuries via oxidative stress, and inflammatory responses.Citation6,Citation7 Therefore, clearance of ROS will be a potential therapeutic method for HI/RI.
Recently studies have shown that severe hyperglycemia occurs frequently during the period of new liver opening on HI/RI, and this affects not only the patient’s internal environmental stability and the success rate of surgery, but also the recovery of the patient after surgery.Citation2 However, whether HI/RI interacts with hyperglycemia and mediates hypoinsulinism is still unclear. Based on the abovementioned findings, we hypothesized that phycocyanin attenuated hepatic ischemia/reperfusion-induced pancreatic isle injury (HI/RIPII) and enlarged islet functionality.
Phycocyanin as deep-sea algae extract is safe and nontoxic, and its unique medicinal function has attracted widespread attention at home and abroad, especially in the field of anti-tumor research has a certain degree of breakthrough.Citation8 Recent research results suggest that phycocyanin has an antioxidant, anti-inflammatory, anticancer, anti-radiation role and improves immunity.Citation9 Moreover, phycocyanin protects brain and myocardium against I/R-induced injury.Citation10,Citation11 However, phycocyanin treatment is limited due to its short plasma half-life and poor stability. Thus, dosing is extremely difficult and frequent injections are needed, which is inconvenient for patients and leads to low compliance.Citation12 In this study, we are striving to identify appropriate delivery systems to encapsulate phycocyanin and ameliorate its poor stability. Here, we synthetize poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine) (PEG-b-(PG-g-PEI)) as a potential phycocyanin carrier. The synthesis of the PEG-b-(PG-g-PEI) polymer refers to a previous document.Citation13 The negatively charged phycocyanin (isoelectric point=4.3) combines with the positively charged PEI block via electrostatic interactions at pH 7.4 (shown in and ). The encapsulated phycocyanin guarantees sustained release, which may augment the protective effect and bioactivity of phycocyanin. The encapsulation capacity of phycocyanin is evaluated through in vitro release. In in vivo research, we keep on assessing the sustained pharmacological effect of the phycocyanin-encapsulated PEG-b-(PG-g-PEI) which significantly attenuates HI/RIPII and enlarges islet functionality in comparison with free phycocyanin as the control.
Materials and methods
Materials
PEI 600, mPEG-NH2 (molecular weight=2,000 Da) and phycocyanin were bought from Aldrich-Sigma; l-glutamic acid was purchased from Aladdin (Shanghai, China); HIT-T15 cells was bought from Shanghai Bioleaf Biotech Co., Ltd. (Shanghai, China); other reagents and kits were purchased from Sigma-Aldrich Co., St Louis, MO, USA.
Methods
Synthesis of PEG-b-(PG-g-PEI)
PEG-b-(PG-g-PEI) was synthesized via referring to previous literature.Citation13 First of all, we referred to our previous works to synthesize PEG-b-PBLG.Citation14–Citation18 PEI 600 was put in DMF and heated in a water bath at 40°C. The suitable number of PEG-b-PBLG was mixed with a quantity of PEI/DMF fluid. The corresponsive compounds were stirred for 24 hours and dialyzed. The PEG-b-(PG-g-PEI) polymeric compounds were gained through freeze-drying, and the product was characterized via 1H-nuclear magnetic resonance.
Cytotoxicity estimation of PEG-b-(PG-g-PEI)
For a detailed depiction of the estimation of cytotoxicity (HIT-T15 cells were selected) of PEG-b-(PG-g-PEI), refer to our previous works.Citation14–Citation18
Loading of phycocyanin via PEG-b-(PG-g-PEI)
To evaluate the encapsulation of phycocyanin by PEG-b-(PG-g-PEI), a certain amount of phycocyanin (5 mg/mL) in PBS (pH =7.2, 0.01 mmol/L) was mixed with PEG-b-(PG-g-PEI) in PB solutions and then the resulting solution was put in a dialysis bag to dialyze. The PEG-b-(PG-g-PEI)-encapsulated phycocyanin was observed through an ELISA kit, technique of particle size measurement and transmission electron microscopy (TEM).
Phycocyanin release in vitro
Release of phycocyanin from PEG-b-(PG-g-PEI) was observed by a dialysis method at 37°C, with 5 mL of phycocyanin-encapsulated PEG-b-(PG-g-PEI) against a PB buffer. At a certain time interval, a given volume of the release media was dislodged and supplemented with an equal volume of fresh release media. The amount of phycocyanin released was checked via ELISA methods.
Animal and surgical process of HI/RI
Sprague Dawley rats (160–240 g) were acquired from the Jiaxing University, Medical College, Jiaxing, PR China. The procedures and care of animals were approved through the Institutional Ethics Committee of Jiaxing University, Medical College, Jiaxing, PR China. The investigations conformed to the guide for the care and use of laboratory animals published by the US National Institutes of Health (NIH Publication updated in 2011). In this research, 40 male Sprague Dawley rats were assigned to four groups (each group=10 rats): (1) Sham group; (2) I/R group: male Sprague Dawley rats were anesthetized with 1% pentobarbital sodium (50 mg/kg) through intraperitoneal injection. The abdomina were opened and livers were exposed to discover the left and median liver lobes, then clamped for 1 hour in order to cause 70% hepatic ischemia, followed by 12 hours of reperfusion; (3) phycocyanin group: phycocyanin was administrated once a day (10 mg/kg) through abdominal subcutaneous injection for 16 days prior to I/R procedures; (4) phycocyanin/PEG-b-(PG-g-PEI) (phycocyanin/P) group: phycocyanin/P (10 mg/kg) was administrated once every 4 days through abdominal subcutaneous injection for 16 days prior to I/R procedures. The blood was collected from abdominal aorta and centrifuged under 3,500× g for 20 minutes to obtain the sera. Livers and pancreatic islets of male Sprague Dawley rats were collected and stored at −80°C until analysis.
Histopathologic change of liver injury
Sections (4 µm) of livers were cut and stained with HE stain. The sample was blindly analyzed to observe the degree of liver injury based on the technique outlined by Yang et al and Li et al.Citation19,Citation20 Briefly, the liver injuries were observed based on each of the following parameters: cytoplasmic discoloration, vacuolation formation, nuclear pyknosis, nuclear fragmentation, nuclear discoloration and red cell stasis. Specifically, one whole deep coronal section was observed via microscope and graded according to the degree of injury, based on the percentage of involvement of liver. Higher score represents more severe damage, with the maximum score being 4 (0, histopathological changes <10%; 1, 10%–25%; 2, 25%–50%; 3, 50%–75%; and 4, 75%–100%). The mean score for each parameter was measured and subjected to statistical analysis.
Liver function measurement
Aminotransferase (AST) and alanine aminotransferase (ALT) activities were observed by the previous methods.Citation21 The blood was collected after reperfusion for 12 hours and centrifuged at 3,500× g for 20 minutes to obtain the sera. AST and ALT activities were observed through a standard automatic biochemistry analyzer.
Histopathologic changes of pancreatic islet tissue damage
Sections (4 µm) of pancreatic islets were cut and stained with HE stain. The sample was blindly analyzed to observe the degree of pancreatic islet injury based on the technique outlined by Chuan et al.Citation22 Briefly, the pancreatic islet damages were assessed based on each of the following parameters: the islet structure disorder and atrophy, showing irregular border and volume of pancreatic islet cells increased, morphology was abnormal and nuclear distribution was eccentric. A higher score represents more severe damage, with the maximum score being 4 (0, histopathological changes <10%; 1, 10%–25%; 2, 25%–50%; 3, 50%–75%; and 4, 75%–100%). The mean score for each parameter was observed and subjected to statistical analysis.
Blood glucose, blood insulin and insulin secretion index on HI/RIPII for different groups of rats
Blood glucose was measured via micro blood glucose meter and blood insulin was evaluated by radio immunity.Citation2 Insulin secretion index was observed via the following formula: insulin secretion index=20×blood insulin/(blood glucose–3.5).Citation23
Blood insulin and metabolic rate of glucose on hyperglycemic clamp test
Blood insulin and metabolic rate of glucose on hyperglycemic clamp test were measured by previous methods.Citation2,Citation24–Citation27
The activity of superoxide dismutase (SOD) and the level of malondialdehyde (MDA) on HI/RIPII for different groups of rats
The SOD activity and MDA level of islet cells were measured by xanthine oxidase and thiobarbituric acid method via previous literature.Citation28 Absorbance was observed at 550 and 532 nm, respectively. Lipid peroxide levels were shown via “U” of SOD/mL and “nmol” of MDA/mL.
Apoptosis evaluation
To refer to previous literature,Citation29–Citation32 pancreatic islet tissue damaged was extracted, and paraffin sections preconditioning dewaxed to water, 40 g/L paraformaldehyde fixed for 15 minutes and PBS washed for 5 minutes ×2 times, 20 mg/L protease K solutions dissolved in Tris/HCL (pH 7.4–8.0) for 20 minutes at 37°C, then PBS washed for 5 minutes ×3 times and added 50 µL TUNEL reaction liquid at 37°C for 60 minutes, PBS washed for 5 minutes ×3 times and added 50 µL POD at 37°C for 30 minutes, DAB colored for 5–10 minutes and PBS washed for 5 minutes ×3 times, and hematoxylin counterstain for 1 minute. Then the slices were washed, dehydrated transparent, sealed and observed; TUNEL reaction were replaced via PBS and was acted as negative control. Through light microscope observation, the normal pancreatic islet cell nuclei were light blue and apoptosis of pancreatic islet cells were deep blue. Each slice randomly selected 5 high magnification (×400) to calculate apoptotic index: (apoptosis index, AI)=(the number of apoptotic cells/total cell number)×100% and take the mean.
ROS expression Previous methods had been referred to here.Citation33–Citation36 After 12 hours of reperfusion, Sprague Dawley rats were anesthetized with 1% pentobarbital sodium (50 mg/kg), and CM-H2DCFDA (100 µg) was injected into the pancreas circulation. After CM-H2DCFDA injection, the pancreatic islets were harvested for 45 minutes and dipped in 4% paraformaldehyde for about 24 hours. After cure with 20% sucrose for about 12 hours, the pancreatic islets were promptly frozen in liquid nitrogen. Cryostat sections were cut at −20°C and the sections were placed on Star-Frost adhesive slides and air-dried for about 3 minutes. Parts were washed in PBS and stained with DAPI for fluorescence microscopy analysis in ROS qualitative assessment. ROS quantitative assessment in isolated mitochondria was implemented by the Amplex red H2O2/peroxidase assay kit (Sigma-Aldrich Co., St Louis, MO, USA) through a spectrofluorometer and emission wavelengths of 544 and 590 nm, respectively.
Data analysis
SPSS 17.0 software was used for data administration and statistical analysis. Measurement data was expressed as ±SD. Statistical analysis was actualized via ANOVA with post-hoc testing. A P-value of less than 0.01 (P<0.01) was used for statistical significance.
Results
Synthesis and characterization of PEG-b-(PG-g-PEI)
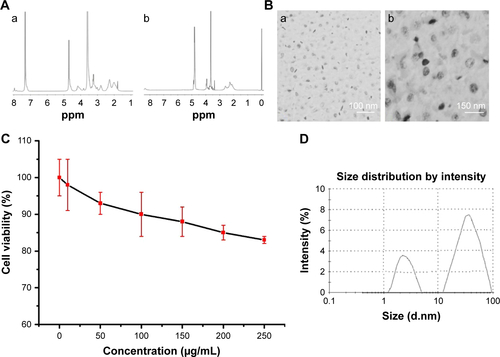
The PEG-b-(PG-g-PEI) contained a PEG block, PG and PEI ( and ); they were characterized via 1H NMR spectra, as depicted in and .
Table 1 Molecular weights, particle size, TEM and phycocyanin-loading capacity of PEG-b-(PG-g-PEI)
Cellular viability measurements
The cell toxicity from PEG-b-(PG-g-PEI) on HIT-T15 cells was measured in 24 hours culturing and the results are shown in . The PEG-b-(PG-g-PEI) kept high cell toxicity even below a level as high as 250 µg/mL.
Encapsulation capacity of phycocyanin in PEG-b-(PG-g-PEI)
Phycocyanin was efficiently encapsulated via PEG-b-(PG-g-PEI) at physiological pH via hydrophobic interaction. Phycocyanin was put in PEG-b-(PG-g-PEI) (mass ratio=1:5) and dialyzed in PB solutions. The dialysis of free phycocyanin as a control was also implemented at the same condition. To determine the encapsulation of phycocyanin in PEG-b-(PG-g-PEI), the quantity of phycocyanin was measured via ELISA. The encapsulation of phycocyanin was 8.05% through calculating, as depicted in .
Characterization measurement of PEG-b-(PG-g-PEI) and phycocyanin/PEG-b-(PG-g-PEI) compounds
The PEG-b-(PG-g-PEI) and phycocyanin/PEG-b-(PG-g-PEI) compounds were measured using a granulometer and TEM. The images of TEM were observed in and the particle diameters were measured with a granulometer in . The PEG-b-(PG-g-PEI) and phycocyanin/PEG-b-(PG-g-PEI) compounds both showed an orbicular structure and the diameters were 21 and 70 nm (TEM), respectively (); the particle sizes of PEG-b-(PG-g-PEI) and phycocyanin/PEG-b-(PG-g-PEI) compounds were 8 and 39 nm (granulometer), respectively ().
In vitro release of phycocyanin
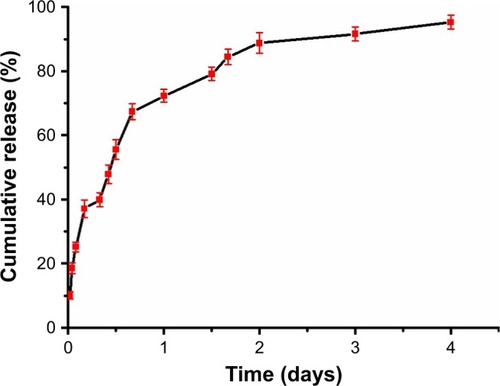
The phycocyanin release from PEG-b-(PG-g-PEI) was measured via dialysis method (MWCO=7 kDa) at 37°C, with 5 mL of phycocyanin/PEG-b-(PG-g-PEI) against a PB solution of 0.02 mol/L (physiological condition). The release curve of phycocyanin/PEG-b-(PG-g-PEI) was observed in . After 2 hours, 25.16% of the phycocyanin was released from phycocyanin/PEG-b-(PG-g-PEI), showing the beginning of a burst release of exenatide. About 72.29% of the phycocyanin was released after 1 day and 95.25% after 4 days, revealing biphase release profile from phycocyanin/PEG-b-(PG-g-PEI).
Histopathologic change of liver damage
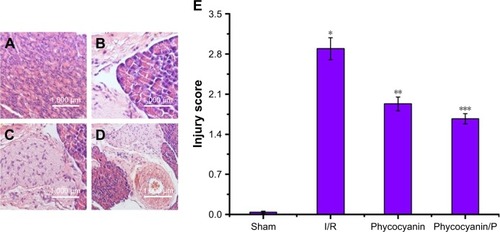
Light microscopy images of liver sections were observed in . The hepatic lobule structure disorder, hepatic sinusoid and central veins had different degrees of blood stasis; liver blood sinus narrowed or disappeared; endothelial cells and hepatocytes were widely showing edema and degeneration, neutrophil attachment and focal necrosis; cytoplasmic discoloration, vacuolation formation, nuclear pyknosis, nuclear fragmentation, nuclear discoloration and red cell stasis were measured in histological specimen from the I/R group () but were absent in the Sham group (). Histological alteration was decreased in specimens from the phycocyanin group () compared to the I/R group. Histological alteration was remarkably reduced in specimens from the phycocyanin/P group () compared to the I/R group. The corresponding quantitative analysis was observed in .
Figure 2 Histopathologic change of liver injury.
Notes: Light microscopy images (×400) in Sham group (A), I/R group (B), phycocyanin group (C) and phycocyanin/PEG-b-(PG-g-PEI) group (D), respectively. Quantitative injury scores, expressed as the mean ± SD, are shown in (E). *Significant increase relative to the Sham group (P<0.01). **Significant decrease relative to the I/R group (P<0.01). ***Significant decrease relative to the I/R group (P<0.01).
Abbreviation: PEG-b-(PG-g-PEI), poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine).
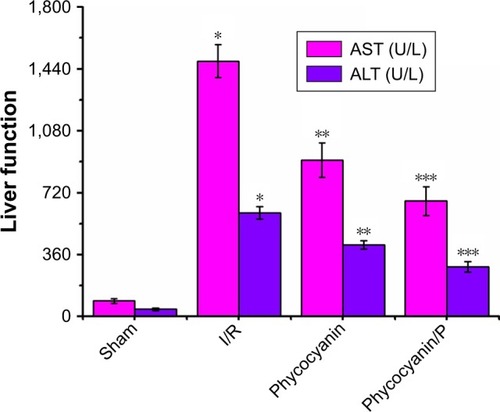
ALT and AST activities
ALT and AST activities were observed after reperfusion for 12 hours () and was higher in the I/R group than in the Sham group (P<0.01; ALT: Sham group 43±8 U/L, I/R group 604±36 U/L; AST: Sham group 92±13 U/L, I/R group 1485±96 U/L). Administration of phycocyanin reduced ALT (417±25 U/L) and AST (910±101 U/L) compared with ALT and AST in the I/R group (P<0.01). Administration of phycocyanin/P significantly reduced ALT (290±31 U/L) and AST (672±84 U/L) compared with ALT and AST in the I/R group (P<0.01).
Figure 3 The activities of ALT and AST on HI/RIPII for different groups of rats.
Notes: The blood of Sham, I/R, phycocyanin and phycocyanin/PEG-b-(PG-g-PEI) groups of rats were collected 12 hours after reperfusion and the activities of AST and ALT measured. Results are expressed as mean ± SD. *Significant increase from the Sham group (P<0.01). **Significant decrease from the I/R group (P<0.01). ***Significant decrease from the I/R group (P<0.01).
Abbreviations: I/R, ischemia/reperfusion; ALT, alanine aminotransferase; AST, amino-transferase; HI/RIPII, hepatic ischemia/reperfusion-induced pancreatic islets injury; PEG-b-(PG-g-PEI), poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine).
Histopathologic change of pancreatic islets tissue damage
Light microscopy images of pancreatic islet sections were observed in . The islet structure disorder and atrophy showing irregular border and volume of pancreatic islets cells increased, morphology was abnormal and nuclear distribution was eccentric; they were measured in histological specimens from the I/R group () but were absent in the Sham group (). Histological alteration was decreased in specimens from the phycocyanin group () compared to the I/R group. Histological alteration was remarkably reduced in specimens from the phycocyanin/P group () compared to the I/R group. The corresponding quantitative analysis was observed in .
Figure 4 Histopathologic change of pancreatic islet tissues damage.
Notes: Light microscopy images (×400) in Sham group (A), I/R group (B), phycocyanin group (C) and phycocyanin/PEG-b-(PG-g-PEI) group (D), respectively. Quantitative injury scores, expressed as the mean ± SD, are shown in (E). *Significant increase relative to the Sham group (P<0.01). **Significant decrease relative to the I/R group (P<0.01). ***Significant decrease relative to the I/R group (P<0.01).
Abbreviation: PEG-b-(PG-g-PEI), poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine).
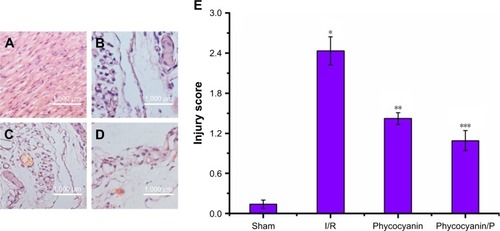
Blood glucose, blood insulin and insulin secretion index on HI/RIPII for different groups of rats
Blood glucose was measured after reperfusion for 12 hours () and was higher in the I/R group than in the Sham group (P<0.01; blood glucose: Sham group 4.05±1.07 mmol/L, I/R group 10.32±1.19 mmol/L). Administration of phycocyanin reduced blood glucose (6.14±0.88 mmol/L) compared with blood glucose in the I/R group (P<0.01). Administration of phycocyanin/P significantly reduced blood glucose (5.21±0.93 mmol/L) compared with blood glucose in the I/R group (P<0.01).
Figure 5 Blood glucose (A), blood insulin (B) and insulin secretion index (C) on HI/RIPII for different groups of rats.
Notes: The blood of Sham, I/R, phycocyanin and phycocyanin/PEG-b-(PG-g-PEI) groups of rats were collected 12 hours after reperfusion and the activities of AST and ALT measured. Results are expressed as mean ± SD. (A) *Significant increase from the Sham group (P<0.01). **Significant decrease from the I/R group (P<0.01). ***Significant decrease from the I/R group (P<0.01). (B) *Significant increase from the Sham group (P<0.01). **Significant decrease from I/R group (P<0.01). ***Significant decrease from I/R groups (P<0.01). (C) *Significant decrease from the Sham group (P<0.01). **Significant increase from the I/R group (P<0.01). ***Significant increase from the I/R group (P<0.01).
Abbreviations: I/R, ischemia/reperfusion; AST, aminotransferase; ALT, alanine aminotransferase; PEG-b-(PG-g-PEI), poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine).
Blood insulin was observed after reperfusion for 12 hours () and was higher in the I/R group than in the Sham group (P<0.01; blood insulin: Sham group 61.84±10.24 mU/L, I/R group 127.52±8.98 mU/L). Administration of phycocyanin reduced blood insulin (108.34±9.03 mU/L) compared with blood insulin in the I/R group (P<0.01). Administration of phycocyanin/P significantly reduced blood insulin (84.15±10.07 mU/L) compared with blood insulin in the I/R group (P<0.01).
Insulin secretion index was checked after reperfusion for 12 hours () and was lower in the I/R group than in the Sham group (P<0.01; insulin secretion index: Sham group 2248.73±123.45, I/R group 373.96±97.39). Administration of phycocyanin increased insulin secretion index (820.76±109.24) compared with insulin secretion index in the I/R group (P<0.01). Administration of phycocyanin/P significantly increased insulin secretion index (984.21±88.93) compared with insulin secretion index in the I/R group (P<0.01).
Blood insulin and metabolic rate of glucose on hyperglycemic clamp test
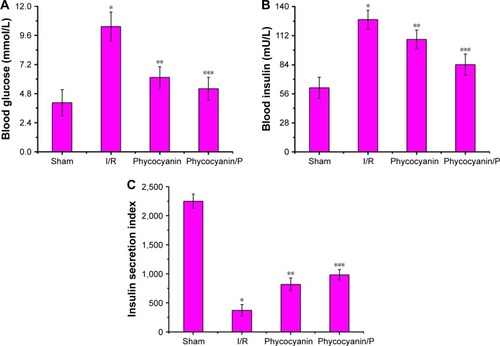
Blood insulin was observed on hyperglycemic clamp test () and was lower in the I/R group than in the Sham group (P<0.01; blood insulin: Sham group 119.95±11.27 mU/L, I/R group 70.18±9.84 mU/L). Administration of phycocyanin increased blood insulin (85.69±10.07 mU/L) compared with blood insulin in the I/R group (P<0.01). Administration of phycocyanin/P significantly increased blood insulin (99.83±8.76 mU/L) compared with blood insulin in the I/R group (P<0.01).
Figure 6 Blood insulin (A) and metabolic rate of glucose (B) on hyperglycemic clamp test.
Notes: The blood of Sham, I/R, phycocyanin and phycocyanin/PEG-b-(PG-g-PEI) groups of rats were collected after hyperglycemic clamp test and blood insulin level and metabolic rate of glucose measured. Results are expressed as mean ± SD. (A) *Significant decrease from the Sham group (P<0.01). **Significant increase from the I/R group (P<0.01). ***Significant increase from the I/R group (P<0.01). (B) *Significant decrease from the Sham group (P<0.01). **Significant increase from the I/R group (P<0.01). ***Significant increase from I/R group (P<0.01).
Abbreviation: PEG-b-(PG-g-PEI), poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine).
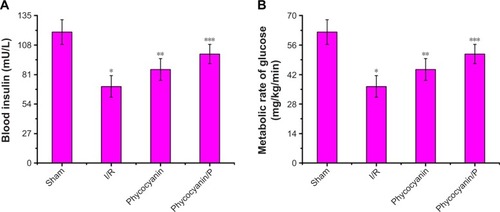
Metabolic rate of glucose was observed on hyperglycemic clamp test () and was lower in the I/R group than in the Sham group (P<0.01; metabolic rate of glucose: Sham group 64.52±5.08 mg/kg−1/min−1, I/R group 22.43±3.45 mg/kg−1/min−1). Administration of phycocyanin increased metabolic rate of glucose (30.17±3.91 mg/kg−1/min−1) compared with metabolic rate of glucose in the I/R group (P<0.01). Administration of phycocyanin/P significantly increased metabolic rate of glucose (41.24±2.77 mg/kg−1/min−1) compared with metabolic rate of glucose in the I/R group (P<0.01).
SOD activity and MDA level
Activity of SOD was measured after reperfusion for 12 hours () and was less on I/R group than in the Sham group (P<0.01; Sham group 40.17±3.08 U/mL, I/R group 10.03±1.81 U/mL). Administration of phycocyanin increased SOD (18.46±2.13 U/mL) compared with SOD in the I/R group (P<0.01). Administration of phycocyanin/P significantly increased SOD (25.68±1.94 U/mL) compared with SOD in the I/R group (P<0.01).
Figure 7 The activity of SOD and the level of MDA on HI/RIPII for different groups of rats.
Notes: The SOD activity and the MDA level of Sham, I/R, phycocyanin and phycocyanin/PEG-b-(PG-g-PEI) groups of rats were measured 12 hours after reperfusion. Results are expressed as mean ± SD. (A) *Significant decrease from the Sham group (P<0.01). **Significant increase from the I/R group (P<0.01). ***Significant increase from the I/R group (P<0.01). (B) *Significant increase from the Sham group (P<0.01). **Significant decrease from the I/R group (P<0.01). ***Significant decrease from the I/R group (P<0.01).
Abbreviations: I/R, ischemia/reperfusion; HI/RIPII, hepatic ischemia/reperfusion-induced pancreatic islets injury; MDA, malondialdehyde; PEG-b-(PG-g-PEI), poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine); SOD, superoxide dismutase.
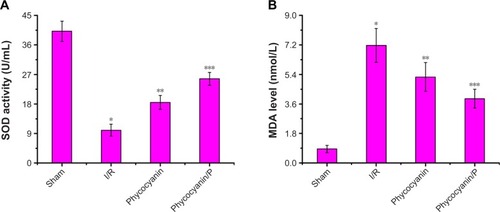
Level of MDA was checked after reperfusion for 12 hours () and was higher in the I/R group than in the Sham group (P<0.01; Sham group 0.86±0.22 nmol/mL, I/R group 7.17±1.03 nmol/mL). Administration of phycocyanin reduced MDA (5.24±0.88 nmol/mL) compared with MDA in the I/R group (P<0.01). Administration of phycocyanin/P significantly reduced MDA (3.92±0.57 nmol/mL) compared with MDA in the I/R group (P<0.01).
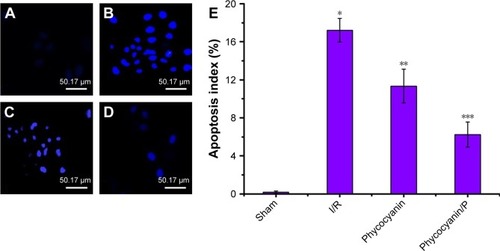
Apoptosis evaluation
Apoptosis of pancreatic islet tissue was measured after reperfusion for 12 hours () and was higher in the I/R group than in the Sham group (P<0.01). Administration of phycocyanin reduced apoptosis of pancreatic islet tissue compared with apoptosis of pancreatic islet tissue in the I/R group (P<0.01). Administration of phycocyanin/P significantly reduced apoptosis of pancreatic islet tissue compared with apoptosis of pancreatic islet tissue in the I/R group (P<0.01).
Figure 8 Apoptosis evaluation.
Notes: (A–E) The islet tissues of Sham, I/R, phycocyanin and phycocyanin/PEG-b-(PG-g-PEI) groups of rats were collected 12 hours after reperfusion and the apoptosis measured. Results are expressed as mean ± SD. *Significant increase from the Sham group (P<0.01). **Significant decrease from the I/R group (P<0.01). ***Significant decrease from the I/R group (P<0.01).
Abbreviations: I/R, ischemia/reperfusion; PEG-b-(PG-g-PEI), poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine).
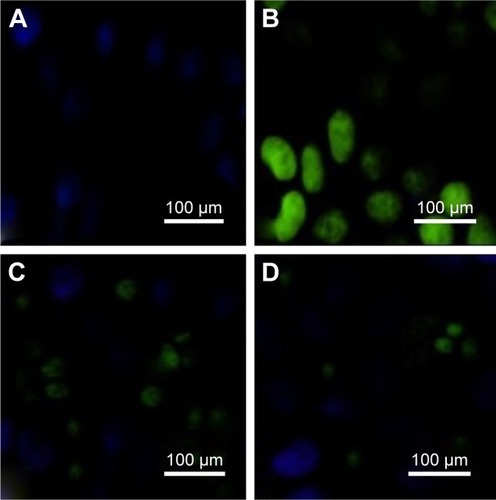
ROS expression
ROS expression of pancreatic islet tissue was measured after reperfusion for 12 hours () and was higher in the I/R group than in the Sham group (P<0.01). Administration of phycocyanin reduced ROS expression of pancreatic islet tissue compared with ROS expression of pancreatic islet tissue in the I/R group (P<0.01). Administration of phycocyanin/P significantly reduced ROS expression of pancreatic islet tissue compared with ROS expression of pancreatic islet tissue in the I/R group (P<0.01).
Figure 9 ROSexpression.
Notes: (A–D) The islet tissues of Sham, I/R, phycocyanin and phycocyanin/PEG-b- (PG-g-PEI) groups of rats were collected 12 hours after reperfusion and the ROSexpression measured. The ROS expression is significantly higher in the I/R group than in the Sham group; the ROSexpression is lower in the phycocyanin group than in the I/R group; the ROS expression is significantly lower in the phycocyanin/P group than in the I/Rgroup.
Abbreviations: PEG-b-(PG-g-PEI), poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine); ROS, reactive oxygen species.
Discussion
Hepatic ischemia/reperfusion (HI/R) had been the topic of intense research and was a complex pathophysiological phenomenon. HI/RI could be divided into two distinct phases: first damage phase (<6 hours) and second damage phase (>6 hours). During the first damage phase intracellular production of xanthine oxidase and NADPH oxidase from hepatic cells was increased, and in the second damage phase reactive oxidant species (ROS) further augmented to aggravate liver and distant organs injury.Citation1,Citation37–Citation39 In addition, the effects of HI/RI were systemic and could cause functional and organic damage to multiple organs, such as circulatory failure, pulmonary edema, acute respiratory distress syndrome, renal failure, islet damage.Citation2 Previous literatures showed that HI/RI was mainly related to free radical damage, calcium overload, lipid peroxidation, microcirculatory disturbance of organs, release of inflammatory factors and respiratory eruption.Citation2 Recently study had showed that during the first damage phase ROS activated Kupffer cells, increasing even further ROS as well as cytokine production,Citation1 and in the second damage phase neutrophil recruitment and activation were involved.Citation2 In the current study, we demonstrated that HI/RI could induce pancreatic islet damage via a remarkable augment of oxidative and apoptosis during the second damage phase. Furthermore, in this study we found that the blood glucose, blood insulin and insulin secretion index of Sprague Dawley rats were lower than those of the Sham group on HI/RIPII, and in the hyperglycemic clamp test we found that the blood insulin in the I/R group was significantly lower than that in the Sham group, and the metabolic rate of glucose was also significantly lower than that in the Sham group. These results showed that the secretory function of pancreatic islet cells was obviously impaired in HI/RIPII.
Phycocyanin as deep-sea algae extract involved abundant functions and pharmacological action, such as antioxidant, anti-inflammatory, anticancer, anti-radiation, improved immunity role and anti-I/R injury.Citation9–Citation11 In our studies, we revealed that phycocyanin attenuated pancreatic islet damage after HI/RI and enlarged islet functionality. However, phycocyanin treatment was limited due to its short plasma half-life and poor stability. Thus, dosing was extremely difficult and frequent injections were needed, which was inconvenient for patients and led to low compliance.Citation12 Consequently, in order to solve this flaw we synthesized poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine) (PEG-b-(PG-g-PEI)) (P) as potential phycocyanin carriers. We surveyed the function of phycocyanin and phycocyanin/P in the development and progression of HI/RIPII. Phycocyanin and phycocyanin/P preconditioning reduced liver and pancreatic islet injury, involving reduction of AST, ALT, MDA, apoptosis and ROS, as well as increased SOD. Moreover, this study reported a delivery system to increase both in vitro release and in vivo safety and efficacy of phycocyanin, administered as a subcutaneous abdominal injection, using encapsulation of a phycocyanin/P complex. Furthermore, PEG-b-(PG-g-PEI) could heighten blood half-life and bioactivity of phycocyanin to enhance its pharmacological treatment.
In conclusion, we authenticated that PEG-b-(PG-g-PEI) could increase the blood half-life of phycocyanin. In addition, phycocyanin/P could significantly reduce HI/RIPII and enlarged islet functionality via attenuating oxidative stress injury and apoptosis compared to free phycocyanin.
Conclusion
This study mainly synthesized PEG-b-(PG-g-PEI) to improve the bioactivity and short half-life of phycocyanin. In addition, it affirmed that phycocyanin and phycocyanin/PEG-b-(PG-g-PEI) pretreatment were both capable of attenuating HI/RIPII and enlarged islet functionality, and phycocyanin/PEG-b-(PG-g-PEI) pretreatment remarkably increased the effect.
Author contributions
All authors contributed equally to this work and share equal responsibility as the first author. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was financially supported by the Science and Technology Planning Project of Jiaxing, Zhejiang Province (2017AY33076).
Supplementary materials
Figure S1 The synthesis of PEG-b-(PG-g-PEI).
Abbreviations: BLG, γ-benzyl l-glutamate; BLG-NCA, γ-benzyl l-glutamate-N-carboxyanhydride; mPEG-NH2, methoxy poly(ethylene glycol) amine; PBLG, poly(γ-benzyl l-glutamate); PEG-b-(PG-g-PEI), poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine).
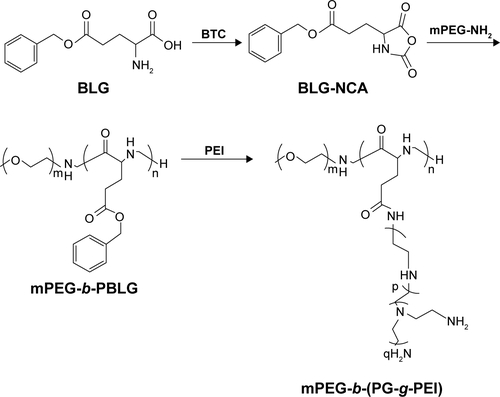
Figure S2 Characterization of PEG-b-(PG-g-PEI) and phycocyanin/PEG-b-(PG-g-PEI).
Notes: (A) 1H NMR spectra of PEG-b-PBLG (a) and PEG-b-(PG-g-PEI) (b); (B) TEM image of PEG-b-PBLG and phycocyanin/PEG-b-(PG-g-PEI); (C) cellular viability of HIT-T15 cells cultured with different concentrations of PEG-b-(PG-g-PEI); (D) diameter of PEG-b-(PG-g-PEI) (left) and phycocyanin/PEG-b-(PG-g-PEI) complexes (right) in PB.
Abbreviations: 1H NMR, 1H-nuclear magnetic resonance; PBLG, poly(γ-benzyl l-glutamate); PEG-b-(PG-g-PEI), poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethyl-enimine); TEM, transmission electron microscopy.
Disclosure
This study was supported by the Zhejiang Province Nature Fund, Zhejiang Province (LY19B040003, Fei Tong). The authors report no other conflicts of interest in this work.
References
- NastosCKalimerisKPapoutsidakisNGlobal consequences of liver ischemia/reperfusion injuryOxid Med Cell Longev201420141113
- WuYLiuCMZhuJMEffects of the second phase injury of hepatic ischemia-reperfusion on the secretion of pancreatic beta cellsJ Clin Anesthesiol200925252254
- ChanKCLinCJLeePHPropofol attenuates the decrease of dynamic compliance and water content in the lung by decreasing oxida-tive radicals released from the reperfused liverAnesth Analg200810741284128918806041
- CastroAPCastro JuniorMALauzSFacinESimõesMJFagundesDJThe role of N-acetyl-cysteine in the lung remote injury after hepatic ischemia and reperfusion in rabbitsActa Cir Bras2012271495522159439
- JaeschkeHRamachandranAReactive oxygen species in the normal and acutely injured liverJ Hepatol201155122722821238521
- GeMChenCYaoWOverexpression of Brg1 alleviates hepatic ischemia/reperfusion-induced acute lung injury through antioxidative stress effectsOxid Med Cell Longev201720174319
- HanSJJangHSSeuSYHepatic ischemia/reperfusion injury disrupts the homeostasis of kidney primary cilia via oxidative stressBiochim Biophys Acta1863201718171828
- ShenWMaXLiuLThe effect of phycocyanin on the expression of iNOS in myocardial cells of type 2 diabetes mellitus in ratsActa Academiae Medicinae Qingdao Universitatis (Chin)201046283285
- Bani-SadrFTeissiereFCurieIAnti-infection prophylaxis after sexual assault. Experience of the Raymond Poincaré-Garches HospitalPresse Med200130625325811252969
- Pentón-RolGMarín-PridaJPardo-AndreuGC-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbilsBrain Res Bull2011861–2425221669260
- CaoMLqXLiuQZProtective effects of phycocyanin on myocardium in rats with acute myocardial ischemia reperfusion injuriesChina Medical Herald2017142528
- CyLLiBYangPOptimized preparation of novel c-phycocyanin-carboxymethyl chitosan nanoparticles and its inhibitory effects on human cervical carcinoma HeLa cells proliferationChin J Cancer Biother2015223440
- HeNSunHXuHDongXShaoZSynthesis and characterization of PEG-b-(PG-g-PEI) for gene deliveryNan Fang Yi Ke Da Xue Xue Bao2013331116431647 Chinese24273269
- TongFTangXLuoLSustained delivery of insulin-loaded block copolymers: Potential implications on renal ischemia/reperfusion injury in diabetes mellitusBiomed Pharmacother20179153454528482291
- TongFDongBChaiRSimvastatin nanoparticles attenuated intestinal ischemia/reperfusion injury by downregulating BMP4/COX-2 pathway in ratsInt J Nanomedicine2017122477248828408819
- TongFTangXLiXXiaWLiuDThe effect of insulin-loaded linear poly(ethylene glycol)-brush-like poly(l-lysine) block copolymer on renal ischemia/reperfusion-induced lung injury through downregulating hypoxia-inducible factorInt J Nanomedicine2016111717173027175073
- TongFZhangHPoly (ethylene glycol)-block-brush poly (l-lysine) copolymer as an efficient nanocarrier for human hepatocyte growth factor with enhanced bioavailability and anti-ischemia reperfusion injury efficacyKidney Blood Press Res201742349550828854424
- TongFPreparation of exenatide-loaded linear poly(ethylene glycol)-brush poly(l-lysine) block copolymer: potential implications on diabetic nephropathyInt J Nanomedicine2017124663467828721043
- YangJWuRQiangXHuman adrenomedullin and its binding protein attenuate organ injury and reduce mortality after hepatic ischemia-reperfusionAnn Surg2009249231031719212187
- LiYLiTQiHYuanFMinocycline protects against hepatic ischemia/reperfusion injury in a rat modelBiomed Rep201531192425469240
- YuYLiSWangZInterferon regulatory factor-1 activates autophagy to aggravate hepatic ischemia-reperfusion injury via the P38/P62 pathway in miceSci Rep2017714368428266555
- HeCMyersMAForbesBEGrütznerFImmunohistochemical analysis of pancreatic islets of platypus (Ornithorhynchus anatinus) and echidna (Tachyglossus aculeatus ssp.)J Anat2015226437338025682842
- HaffnerSMKennedyEGonzalezCSternMPMiettinenHA prospective analysis of the HOMA model. The Mexico City Diabetes StudyDiabetes Care19961910113811418886564
- YhHPanZHMethods for catheterization of carotid and jugular veins in ratsChin J Lab Anim Sci200010250252
- XiongHBShengJNingYTechnical improvement of jugular vein catheterization in ratsXinjiang Med J200427415
- DefronzoRATobinJDAndresRGlucose clamp technique: a method for quantifying insulin secretion and resistanceAm J Physiol19792373E214E223382871
- ZhuMJiaWPBaoYQEstablishment of hyperglycemic clamp techniqueChin J Diabetes2004122327
- YangMWangYMMengQNAn experimental study of the impacts of magnetic fields on superoxide dismutase and malondialdehyde in rat pancreatic islet cellsChin J Phys Med Rehab201234733736
- ChuvinNVincentDFPommierRMAcinar-to-ductal metaplasia induced by transforming growth factor beta facilitates KRASG12D-driven pancreatic tumorigenesisCell Mol Gastroenterol Hepatol20174226328228752115
- RankinMMWilburCJRakKShieldsEJGrangerAKushnerJAβ-cells are not generated in pancreatic duct ligation-induced injury in adult miceDiabetes20136251634164523349489
- GohSKBerteraSOlsenPPerfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineeringBiomaterials201334286760677223787110
- WestmorelandJJWangQBouzaffourMBakerSJSosa-PinedaBPdk1 activity controls proliferation, survival, and growth of developing pancreatic cellsDev Biol2009334128529819635472
- KristiansenKAJensenPEMøllerIMSchulzAMonitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H(2)DCFDA and confocal laser microscopyPhysiol Plant2009136436938319493304
- OparkaMWalczakJMalinskaDQuantifying ROS levels using CM-H2DCFDA and HyPerMethods201610931127302663
- TanXZhangLJiangYPostconditioning ameliorates mitochondrial DNA damage and deletion after renal ischemic injuryNephrol Dial Transplant201328112754276524021677
- DoiKSuzukiYNakaoAFujitaTNoiriERadical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidneyKidney Int20046551714172315086910
- JaeschkeHMolecular mechanisms of hepatic ischemia-reperfusion injury and preconditioningAm J Physiol Gastrointest Liver Physiol20032841G15G2612488232
- SmyrniotisVFarantosCKostopanagiotouGArkadopoulosNVascular control during hepatectomy: review of methods and resultsWorld J Surg200529111384139616222453
- GarceaGGescherAStewardWDennisonABerryDOxidative stress in humans following the Pringle manoeuvreHepatobiliary Pancreat Dis Int20065221021416698577