?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Doxorubicin (DOX) is one of the most effective treatments for hepatocellular carcinoma (HCC), but is restricted by its poor pharmacokinetics. Herein, we exploited efficient targeted drug delivery systems and they have been found to be a worthy strategy for liver cancer therapy.
Materials and methods
We investigated polymeric nanoparticles which were synthesized based on host–guest interaction between β-cyclodextrin and benzimidazole. The properties of nanoparticles with regard to size/shape, encapsulation efficiency, and drug release were investigated using conventional experiments. Cell proliferation assay in vitro, cell uptake assay, and cell apoptosis analysis were used to investigate cytotoxicity, uptake, and mechanism of targeted supramolecular prodrug complexes (TSPCs)-based self-assemblies and supramolecular prodrug complexes (SPCs)-based self-assemblies.
Results
The pH-sensitive lactobionic acid (LA)-modified pH-sensitive self-assemblies were synthesized successfully. The results of in vitro released assay showed that the accelerated released of DOX from TSPCs-based self-assemblies with the decrease of pH value. When TSPCs-based self-assemblies were taken up by HepG2 cells, they demonstrated a faster release rate under acidic conditions and proved to have higher cytotoxicity than in the presence of LA. A mechanistic study revealed that TSPCs-based self-assemblies inhibited liver cell proliferation by inducing cell apoptosis.
Conclusion
The pH-sensitive nanocomplex, as liver-targeted nanoparticles, facilitated the efficacy of DOX in HepG2 cells, offering an appealing strategy for the treatment of HCC.
Introduction
Cancer is a major public health problem worldwide and is the second leading cause of death.Citation1 Hepatocellular carcinoma (HCC) is a prevalent liver malignancy with poor prognosis and accounts for 85%–90% of all primary liver cancers.Citation2 Chemotherapy, as one of the most effective treatment methods for HCC, is still used as the frontline approach in clinics,Citation3 for instance doxorubicin (DOX), which can inhibit the synthesis of DNA and RNA.Citation4 However, one major handicap of DOX is the systemic distribution in normal tissue of patients because of its poor pharmacokinetics.Citation5 Based on this situation, nanotechnologies are appealing because they can facilitate a combined effect, which is commonly used in HCC therapy.Citation6 Many efficient drug delivery systems with all kinds of biomaterials have been designed to reduce severe side effects including micelles,Citation7 liposomes,Citation8 macromolecules,Citation9 supramolecular polymers, etc.Citation10 Among these classes of carriers, supramolecular polymer-based carriers have attracted considerable attention because of their excellent biocompatibility and structural versatility.Citation11 β-cyclodextrin (β-CD) is regarded as an important kind of supramolecular system, which is the commonest host compound, exhibiting good complexation capacities with all kinds of hydrophobic drugs.Citation12 The property of β-CD indicates that it can be used as functional delivery system by means of molecular recognition and self-assembly. As early as 1994, the first report of β-CD-based supramolecular hydrogel proved its potential application in the field of nanomedicine.Citation13 Benzimidazole and its derivatives are important materials and intermediate products, which can self-assemble with β-CD by host–guest interaction.
In the meantime, because of the intense demands for intelligent drug delivery systems, more and more multifunctional drug carriers have been exploited, such as those possessing features like temperature-, pH-, ultrasound-, light-sensitivity etc.Citation14–Citation18 And, worth mentioning, is that tumor cells have a lower pH than normal cells because of anaerobic glucose metabolism in tumor cells,Citation19 so the pH-triggered approach is one of the most efficient tactics for anti-tumor drug delivery systems, which is expected to stabilize the drug in the natural environment. For instance, the chitosan-coated mixed micellar nanocarriers loaded with DOX and siRNA were used to provide pH responsiveness, and the nanocomplexes showed better DOX release in acidic tumor pH environments.Citation20 In addition, many tumor cells have specific expression of numerous receptors which are very important for malignant tumor proliferation, such as folate receptorCitation21 and biotin receptor.Citation22 Particularly in HCC, the galactose and N-acetyl-galactosamine-terminated glycoproteins are recognized by asialoglycoprotein receptor (ASGPR) located on the surface of liver cancer cells,Citation23 hence ASGPR-mediated targeted delivery system has been regarded as one of the most efficient methods for treating HCC. Nowadays, several galactose-containing ligands including lactobionic acid (LA) have been used for hepatocyte-targeted drug delivery.Citation24 As an endogenous molecule possessing wonderful biocompatibility,Citation25 LA has great advantages in modifying nanoparticles for liver cancer therapy.
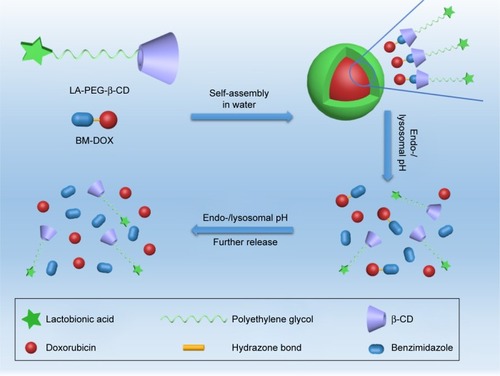
In this research, we developed a targeted prodrug based on host–guest interaction containing benzimidazole for pH-sensitivity. The host molecule β-CD and targeted molecule LA were coupled with polyethylene glycol (PEG) successively by alkynation and click reaction. We had a hypothesis that targeted supramolecular prodrug complexes (TSPCs)-based self-assemblies possess good biocompatibility and PEG could prolong the half-life of the prodrug in vivo. Meanwhile, the object–chain segment, benzimidazole modified DOX (BM-DOX), was synthesized from benzimidazole via a three-step reaction including esterification, hydrazination, and hydrazone formation. The hydrazone bond was expected to be cleaved in the intracellular acidic environment with rapid release of DOX from the prodrug,Citation26,Citation27 resulting in enhanced inhibition of cellular proliferation. Finally, β-CD-modified PEGylated LA (LA-PEG-β-CD) and BM-DOX were assembled with each other to form a self-assembled polymer by host–guest interaction,Citation28 and the supramolecular force inside these complexes resulted in nanoscale self-assemblies (). The TSPCs- and supramolecular prodrug complexes (SPCs)-based self-assemblies were synthesized and characterized by regular characterization methods. Besides, the pH-sensitive release of DOX from prodrug was investigated under different pH conditions. Finally, the tumor cell inhibition properties of TSPCs- and SPCs-based self-assemblies were tested through cell culture of human hepatoma HepG2 cells in vitro.
Materials and methods
Materials
For details of the main chemical reagents, please refer to the Supplementary materials. DMEM, RPMI-1640 medium, and PBS were obtained from HyClone (Logan, UT, USA). FBS was purchased from ExCell Bio (Shanghai, China). Trypsin-EDTA solution and Penicillin-Streptomycin solution, 100X were acquired from Solarbio Science & Technology Co., Ltd (Beijing, China). MTT, DMSO, propidium iodide (PI), and Hoechst 33258 were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). LA was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Annexin V-fluorescein isothiocyanate FITC reagent kit was purchased from KeyGen Biotech Inc (Nanjing, China). Human normal liver cells L02 (GNHu6), human non-small-cell lung cancer cells A549 (TCHu150), and human liver cancer cells HepG2 (TCHu72) were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). All other reagents used were of analytical grade. A549 cells were cultured in RPMI-1640 medium with 10% FBS (v/v), L02 and HepG2 cells were cultured in DMEM with 10% FBS (v/v). All cell lines were maintained in an incubator with a humidified atmosphere of 5% CO2 at 37°C.
Preparation of TSPCs- and SPCs-based self-assemblies
The subject-chain segment, LA-PEG-β-CD and PEGylated β-CD (mPEG-β-CD) were obtained by substitution reaction and click reaction. And object-chain segment (BM-DOX) was synthesized by successive esterification, hydrazination, and hydrazone formation. The specific routes, which can be referred to, were shown in Scheme S1–S5 in the Supplementary materials. In order to obtain TSPCs- and SPCs-based self-assemblies, 5 mg of LA-PEG-β-CD was dissolved in 2 mL deionized water, then 1 mg of BM-β-CD was added into the solution. The mixture was constantly stirred for 6 hours at room temperature after being exposed to ultrasound for 10 minutes to accelerate molecular diffusion, which was critical for uniformity during host–guest interaction. TSPCs- and SPCs-based self-assemblies were collected via dialysis (molecular weight cut off: 1,000 D) in water for 6 hours and lyophilization. The dialysate containing the BM-DOX was evaluated by means of UV-visible spectrophotometry after acidification. Subtraction method was implemented as follows: the amount of drug in dialysate was subtracted from the total amount and fitted into the calibration equation of DOX solution (Figure S1) to calculate drug loading capacity (DLC) and drug loading efficiency (DLE).
All polymer precursors were well-defined and characterized by 1H spectroscopy, 13C nuclear magnetic resonance (NMR) spectroscopy, 1H-1H nuclear Overhauser effect spectroscopy (NOESY), and Fourier transform infrared spectroscopy.
Particle size and zeta potential analysis
The particle size and zeta potential analysis were conducted by means of Malvern Zetasizer Nano ZS at 25°C. TSPCs- and SPCs-based self-assemblies were suitably dissolved in deionized water or PBS, and diluted to a final concentration of 1 mg/mL. HCl and NaOH were used in treatment of acidization and alkalization, respectively. The average size and zeta potential of samples were evaluated by dynamic light scattering (DLS) and laser Doppler microelectrophoresis at room temperature, respectively.
Transmission electron microscopy (TEM)
The TEM images used to assess size and morphology were obtained with Hitachi H-7650 transmission electron microscope at an acceleration voltage of 80 kV. Samples were diluted to 1 mg/mL, and were prepared by dropping 10 µL of polymer solution on the copper grids. The polymer settled on the copper grids under static condition for a few minutes before aqueous solution was removed with filter paper. The samples were then counter-stained with phosphotungstic acid and dried at room temperature for testing.
In vitro release study of TSPCs-based self-assemblies
Dialysis method was the main way to realize the release study in vitro. The study was performed in PBS of different pH values. An amount of 15 mg of freeze-dried TSPCs-based self-assemblies was dissolved in 7.5 mL deionized water, and 2.5 mL solution was added into a dialysis bag (MWCO 500). Then, three dialysis bags were soaked in a glass bottle containing 30 mL of release medium with different pH values at 37°C under continuous shaking (110 rpm). At each predetermined time point, 3 mL of the release medium was withdrawn from the bottle for characterization. Meanwhile, the same volume of fresh buffer solution was added to the bottle to maintain the total volume. The amount of DOX in each sample was evaluated by UV-2550 (Shimadzu, Japan) spectroscopy (the excitation wavelength was 485 nm [Figure S2]), and could be calculated with the aid of calibration curve of DOX. The total amount of released DOX was calculated using the following formula, and averaged from three repeated measurements.
Cn and Cn-1 are the concentration of DOX determined by UV spectrometry for the nth time and the (n-1)th time to take 3 mL dialyzate as sample from glass bottle respectively, W0 is the total amount of DOX, at t=0, present in the dialysis bag.
Cell proliferation assay
The biocompatibility of materials and the effect of self-assemblies were detected by MTT assay. L02, HepG2, and A549 cells were seeded in 96-well plates in complete medium and cultivated overnight. Then, the cells were treated with different treatments at final DOX concentrations of 1, 3, 5, 7, and 10 µg/mL for 48 hours. To test the liver target of TSPCs-based self-assemblies, HepG2 cells were incubated with LA at a concentration of 100 µg/mL for 1 hour. Meanwhile, to investigate the pH-sensitivity of self-assemblies, SPCs- and TSPCs-based self-assemblies were pretreated with PBS (pH =5.0) for 1 hour. After treatment, cells were cultured with serum-free medium and MTT (5 mg/mL) for 4 hours, followed by the addition of 150 µL DMSO for 15 minutes. The absorbance was measured using a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA) at a wavelength of 490 nm. Three independent experiments were performed.
Cell uptake assay
Exponentially growing cells were seeded in 6-well plates at a density of 2×105 cells per well. After incubating for 24 hours, the cells were treated with different treatments at a final DOX concentration of 5 µg/mL for 12 hours, Then, the cells were harvested and the cell suspension was collected in a 1.5 mL Eppendorf tube, and washed with PBS three times followed by centrifugation at 1,000 rpm for 5 minutes at 37°C. Then, the cells in each tube were suspended by the addition of 400 µL PBS. The DOX content in the cells was detected and analyzed by FACS (ACEA Novocyte, San Diego, CA, USA).
Exponentially growing cells were seeded in 96-well plates at a density of 2×104 cells per well. After incubating for 24 hours, the cells were washed with PBS three times after removing the medium. Then, the cells were fixed in 4% paraformaldehyde for 15 minutes at room temperature. Then, cells were washed with PBS and stained with DAPI in the dark at room temperature for 10 minutes. Images were obtained using an inverted fluorescence microscope (DM505, Nikon Corporation, Tokyo, Japan).
Flow cytometric analysis of cell apoptosis
A549 and HepG2 cells were plated in 6-well plates in complete medium and cultivated overnight. Then, cells were treated with indicated treatments at a final DOX concentration of 5 µg/mL for 24 hours. Then cells were harvested, washed with PBS, and subjected to sequential staining with 5 µL Annexin V-FITC. After 5 minutes, 10 µL PI (20 µg/mL) was added and cells were incubated in the dark, at room temperature for 10 minutes. All stained cells were analyzed by FACS (ACEA Novocyte).
Hoechst staining assay
Tumor cells were seeded in 12-well plates at a density of 4×105 cells per well and then treated with different treatments for 24 hours. Cells were washed with PBS and fixed with 4% paraformaldehyde for 10 minutes. Then, cells were washed with PBS and stained with Hoechst 33258 in the dark, at room temperature for 20 minutes. Images were obtained using an inverted fluorescence microscope (DM505).
Results and discussion
Synthesis of SPCs and TSPCs
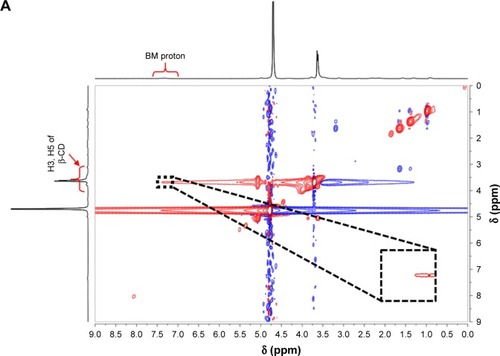
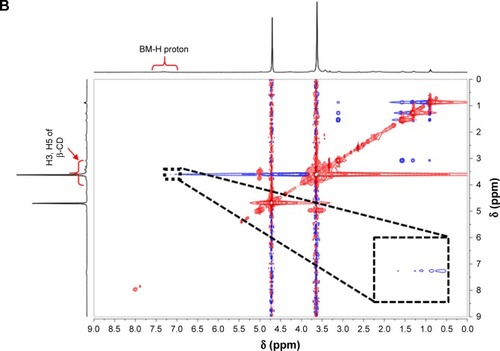
To obtain TSPCs and SPCs, building blocks including LA-PEG-β-CD and BM-DOX were first synthesized by click reaction and Schiff-base reaction, according to the routes shown in Schemes S1–S5 in the Supplementary materials. All polymer precursors were well-defined and characterized, and the data were provided in Figures S3–S12. SPCs and TSPCs were then constructed through host–guest interaction between mPEG-β-CD/LA-PEG-β-CD and BM-DOX in aqueous solutions, respectively. NMR spectra of SPCs and TSPCs ( and S13) were firstly utilized to confirm the formation of host–guest complexes. shows the 2D NMR NOESY spectra of an equimolar solution of LA-PEG-β-CD (4 mmol/L) and BM-DOX (4 mmol/L) in D2O at 25°C, which indicated that the signals of BM protons were correlated with those of inner protons in β-CD. The signals at δ 6.9–7.4 ppm attributed to the BM moieties of BM-DOX show cross-peaks, resulting from dipolar interactions with the signals at 3.5–4.0 ppm ascribed to the H-3 and H-5 protons located within the cavity of β-CD moieties in LA-PEG-β-CD. This phenomenon proved that BM moieties were deeply embedded within the β-CD cavities. Similarly, the 2D NMR NOESY spectra of SPC solutions () revealed the appearance of the correlation peaks between the protons of BM and the inner protons of β-CD, indicating the formation of host–guest complexes.
Figure 1 2D NOESY NMR spectra of TSPCs-based self-assemblies and SPCs-based self-assemblies.
Note: 2D NOESY NMR spectra of TSPCs- (A) and SPCs-based self-assemblies (B) in 0.5 mL D2O (inset: partial enlargement).
Abbreviations: β-CD, β-cyclodextrin; BM, benzimidazole modified; NMR, nuclear magnetic resonance; NOESY, nuclear Overhauser effect spectroscopy; SPCs, supramolecular prodrug complexes; TSPCs, targeted supramolecular prodrug complexes.
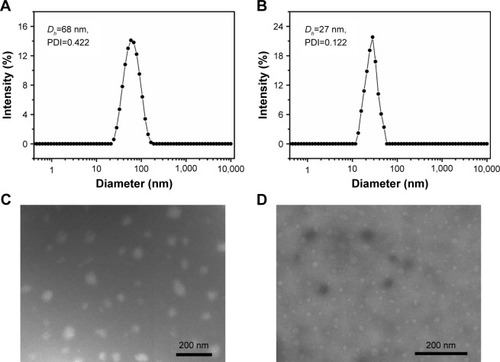
Size and morphology of TSPCs- and SPCs-based self-assemblies
The self-assembly behaviors of SPCs and TSPCs in aqueous solutions were studied by DLS and TEM. The average size of SPCs-based self-assemblies was 27±1 nm while the size of TSPCs-based self-assemblies obviously increased to 68±3 nm due to the introduction of LA (). Furthermore, TEM images revealed clear spherical morphology in both SPCs- and TSPCs-based self-assemblies, and the sizes observed in TEM images were consistent with the results determined by DLS (). It should be noted that, the morphology of TSPCs-based self-assemblies was not uniformly distributed and the size was much larger than those of SPCs-based self-assemblies. A reasonable explanation is that charged groups like LA and amino acid may have had some effects on the properties of self-assemblies or complexes such as size and zeta potential.Citation29
Figure 2 Physicochemical characterization of TSPCs- and SPCs-based self-assemblies.
Notes: Particle size distribution of TSPCs-based self-assemblies (A) and SPCs-based self-assemblies (B). TEM images of TSPCs-based self-assemblies (C) and SPCs-based self-assemblies (D).
Abbreviations: PDI, polydispersity index; SPCs, supramolecular prodrug complexes; TEM, transmission electron microscopy; TSPCs, targeted supramolecular prodrug complexes.
pH-responsive reversibility of SPCs- and TSPCs-based self-assemblies
As we know, host–guest interactions between β-CD and BM can be cleaved under acidic conditions, leading to disassembly and subsequent drug release.Citation30,Citation31 The pH-triggered reversibility of TSPCs- and SPCs-based self-assemblies upon acid stimulus was further investigated by DLS and UV-visible spectrophotometry. In , the hydrodynamic diameter (Dh) value of TSPCs-based self-assemblies changed from 68±3 nm to 18±2 nm under acidic environment due to the protonation of BM, because the exclusion of the water-soluble BM from the hydrophobic inner cavity of β-CD would lead to the dissociation of microsphere.Citation31 The subsequent alkali additive led to an increase in size to hundreds of nanometers, indicating the pH-induced reversibility of TSPCs-based self-assemblies. Similarly, the same phenomenon observed as the reversible process was confirmed in SPCs-based self-assemblies solution ().
Figure 3 Redox-triggered reversibility confirmation of TSPCs- and SPCs-based self-assemblies in deionized water.
Notes: Particle size distribution of TSPCs-based self-assemblies (A) and SPCs-based self-assemblies (B); initial state (black line), after acidification for 12 hours (red line), and after acidification for 12 hours and alkalization for 12 hours (blue line). UV-visible absorption spectra of TSPCs-based self-assemblies (C) and SPCs-based self-assemblies (D); initial state (black line), after acidification for 12 hours (red line), and after acidification for 12 hours and alkalization for 12 hours (blue line).
Abbreviations: SPCs, supramolecular prodrug complexes; TSPCs, targeted supramolecular prodrug complexes.
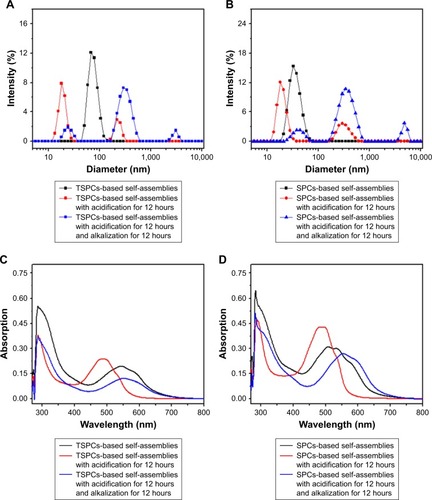
UV-visible absorption spectra also confirmed the pH-triggered reversible feature of TSPCs- and SPCs-based self-assemblies. In , compared with λmax=544 nm of initial absorbance, the corresponding λmax absorbance of DOX decreased to 485 nm upon acidification for 12 hours because DOX was released from self-assemblies and exposed to the solution owing to the destruction of self-assemblies under acidic condition. With subsequent alkalization for 12 hours, the λmax absorbance of DOX increased from 485 nm to 553 nm. According to the data shown in Figure S2 (λmax=541 nm of DOX’s absorbance in alkaline solution), the red shift of λmax of absorption of TSPCs-based self-assemblies in alkaline condition indicated the formation of reversible dynamic covalent bonds (hydrazone bond) which could affect the absorbance of DOX, which has been reported by Gao et al.Citation32 There was a similar occurrence in SPCs-based self-assemblies, as shown in .
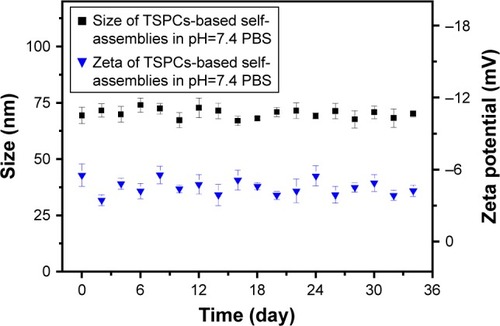
Stability of TSPCs-based self-assemblies
As a promising prodrug, the favorable ability to maintain size fluctuation within a controlled range is a critical property.Citation33 Thus, the size and zeta stability of TSPCs-based self-assemblies were further evaluated via real-time DLS testing in pH 7.4 PBS. As shown in , TSPCs-based self-assemblies were fairly stable and maintained a constant size of 70 nm in PBS, and only a slight fluctuation was observed within 1 month. Additionally, the zeta potential of TSPCs-based self-assemblies presented similar stability and maintained a potential of −4.46 mV. Nano-prodrugs with diameter larger than 200 nm are known to induce non-specific scavenging by monocytes and the reticuloendothelial system.Citation34 Hence, these results indicated that TSPCs-based self-assemblies may possess prolonged circulation time and enhance tumor accumulation in vivo.
Controlled drug release of TSPCs-based self-assemblies in vitro
The DLC and DLE of TSPCs-based self-assemblies were 11.74% and 12.96% determined by aforesaid subtraction method, respectively. The drug release study was performed in pH 6.0, pH 7.4 (corresponding to the normal blood environment), and pH 5.0 (simulating the pH in mature endosomes of tumor cells) PBS, as shown in .Citation35 It could be observed that the accelerated release of DOX was obvious with the decrease in pH value. What is more, the sustained release of the loaded DOX from TSPCs-based self-assemblies indicated that the relatively hydrophobic dendrimer interior could prevent the drug’s burst release.Citation36 Specifically, TSPCs-based self-assemblies displayed a fairly slow DOX release rate in pH 7.4 PBS, and 21.41% of DOX was released within 24 hours. In contrast, 83.38% of DOX was released from TSPCs-based self-assemblies in pH 5.0 PBS at the same time. The amount of DOX released in pH 6.0 PBS was between that of pH 5.0 and pH 7.0, reaching 53.45% within 24 hours. These results indicated that an acidic environment could accelerate the breakdown of self-assemblies, further giving rise to faster release of the drug. As stated previously, benzimidazole could be protonated in an acidic environment, resulting in the dissociation of host–guest interaction. Furthermore, an acidic environment caused the cleavage of hydrazone bond and decomposition of the hydrophobic block to release DOX molecules from TSPCs-based self-assemblies.Citation37 The release mechanism of DOX from TSPCs-based self-assemblies was thus proposed to elucidate the release behavior by studying the release kinetics with a simple semi-empirical equation (EquationEquation 3(3) ) and a modified equation (EquationEquation 4
(4) ) to describe the release behavior.Citation38
Figure 5 Time-dependent release of DOX from TSPCs-based self-assemblies.
Notes: (A) Cumulative release curves of DOX from TSPCs-based self-assemblies as a function of time over a period of 30 hours in PBS with different pH values at 37°C. (B) Corresponding fitted curves for release kinetics. All quantitative data represent mean ± SD (n=3).
Abbreviations: DOX, doxorubicin; SPCs, supramolecular prodrug complexes; TSPCs, targeted supramolecular prodrug complexes.
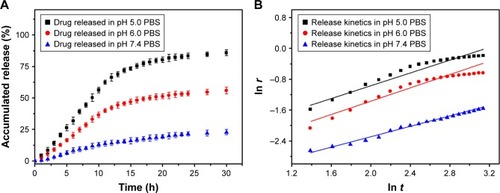
Mt and M∞ are the cumulative amounts of guest molecule released at time t and infinity, respectively; k is the release constant; and n describes the kinetic and release mechanism. For spherical particles, n is usually between 0.43 and 0.85. When n is close to 0.43, diffusion, referred to as “Fickian diffusion”, is the major driving force. When n is close to 0.85, the release is mainly controlled by degradation.Citation39 As was shown in and , the cumulative release amount and release time of DOX from TSPCs-based self-assemblies exhibited a linear correlation during the test time at 37°C. As a result of n value being close to 0.85, the release mechanism of DOX in an acidic condition was mainly dominated by degradation. In particular, n values decreased from 8.2–6.5 with the increase of pH value from 5.0–7.4. The phenomenon indicated that the dominance of the degradation mechanism increased to a greater extent for TSCPs-based self-assemblies when exposed to an acidic environment due to the effect of hydrazination and β-CD/BM binding sites.
Table 1 Release kinetics parameters of DOX from TSPCs-based self-assemblies in PBS with different pH values at 37°C fitted with Peppas’Citation36 formulaTable Footnotea
Biocompatibility and cytotoxicity of SPCs- and TSPCs-based self-assemblies
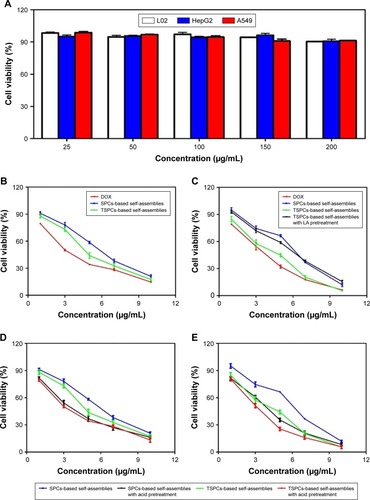
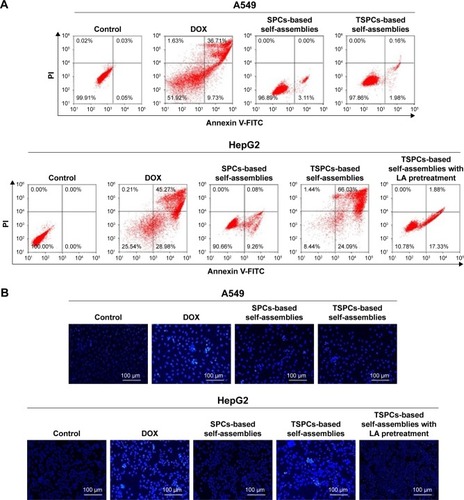
The biocompatibility of drug-free self-assemblies is of crucial importance for the further use of materials as drug carriers. Herein, the biocompatibility of self-assemblies was investigated using normal liver cells L02, human non-small-cell lung cancer cells A549, and human liver cancer cells HepG2, by MTT assay. As shown in , the drug-free self-assemblies did not show cytotoxicity against L02, A549, and HepG2 cells even if the concentration of polymers was 200 µg/mL, indicating that the biocompatibility of the materials used for drug carriers is decent. Additionally, MTT assay was conducted to detect the targeting and pH-responsiveness of SPCs- and TSPCs-based self-assemblies in vitro, the results indicated that TSPCs-based self-assemblies showed more toxicity than SPCs-based self-assemblies on HepG2 cells instead of A549 cells (). Furthermore, to verify the TSPCs-based self-assemblies’ uptake through LA receptors, a competitive inhibition experiment was conducted in the presence of free LA (100 µg/mL). The cytotoxicity was decreased and there was no distinct difference between the two self-assemblies (), which suggested that the free LA competed with TSPCs-based self-assemblies for LA receptor binding sites. Moreover, when the polymers were pretreated in PBS solution (pH =5.0), which was used to mimic tumor microenvironment conditions, for 1 hour, as shown in , SPCs- and TSPCs-based self-assemblies could further inhibit tumor cell proliferation, which could be due to the acidic environment which facilitated the release of DOX. All these results revealed that TSPCs-based self-assemblies have liver cancer-targeting and pH-responsiveness features, hence, it can be used as drug carrier and targeting agent for liver cancer therapy.
Figure 6 Biocompatibility and cytotoxicity of TSPCs- and SPCs-based self-assemblies.
Notes: (A) Effect of drug-free self-assemblies in L02, A549, and HepG2 cells. (B, C) Effect of free DOX, TSPCs-based self-assemblies in the absence or presence of free LA for 1 hour and SPCs-based self-assemblies at final DOX concentrations of 1, 3, 5, 7, 10 µg/mL in A549 (B) and HepG2 (C) cells. (D, E) Effect of SPCs- and TSPCs-based self-assemblies pretreated in PBS solution (pH =5.0) for 1 hour in A549 (D) and HepG2 (E) cells. Data were expressed as mean ± SD (n=5).
Abbreviations: DOX, doxorubicin; LA, lactobionic acid; SPCs, supramolecular prodrug complexes; TSPCs, targeted supramolecular prodrug complexes.
Cellular uptake of SPCs- and TSPCs-based self-assemblies
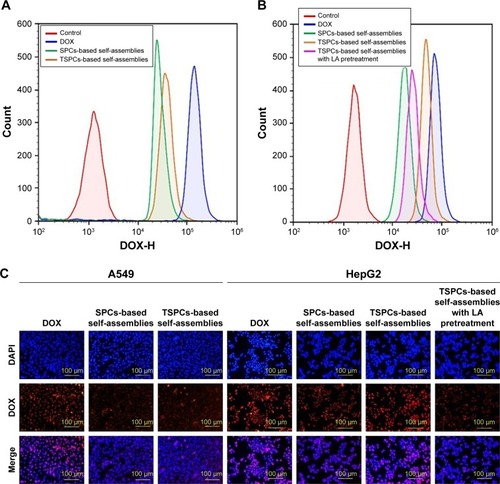
A major requirement for the therapeutic efficacy of drugs is cellular uptake efficiency.Citation40 To further confirm the host–guest interaction-enhanced adjustable release of DOX from TSPCs-based self-assemblies, we evaluated the cellular uptake behavior with flow cytometry and by using an inverted fluorescence microscope. Flow cytometry analysis indicated that only the HepG2 cells treated with TSPCs-based self-assemblies had the same high fluorescence signal compared with the cells treated with free DOX after 12 hours’ co-culture (). In addition, the competitive inhibition was investigated using flow cytometry in the presence and absence of free LA by measuring the fluorescence intensity of DOX. As shown in , the fluorescence intensity of DOX was decreased in the presence of free LA after 1 hour of incubation, which confirmed that TSPCs-based self-assemblies were taken up via LA receptors. To comparatively investigate the intracellular internalization of free DOX, SPCs-, and TSPCs-based self-assemblies by A549 and HepG2 cells, we further carried out a fluorescence-based localization study using an inverted fluorescence microscope – the stained cell nuclei and DOX showed a blue and red fluorescence. As shown in , the DOX fluorescence intensity could clearly be observed in both the cytoplasm and nuclei of HepG2 cells after 12 hours’ incubation with TSPCs-based self-assemblies, while free DOX mainly appeared in the nucleus, which may be correlated to the slow release of DOX in self-assemblies compared with free DOX. Moreover, the DOX fluorescence signal in the HepG2 cells was significantly reduced in the presence of free LA, which further confirmed that the TSPCs-based self-assemblies were taken up through LA receptors in HepG2 cells. In summary, the results were consistent with the results obtained by flow cytometry, which demonstrated that TSPCs-based self-assemblies could be internalized by liver cancer cells.
Figure 7 Cellular uptake and intracellular distribution of TSPCs- and SPCs-based self-assemblies.
Notes: (A, B) The DOX fluorescence signal of A549 (A) and HepG2 (B) cells after being treated with TSPCs- or SPCs-based self-assemblies for 12 hours at a final DOX concentration of 5 µg/mL. (C) Photographs of A549 and HepG2 cells stained with DAPI (blue) and incubated with DOX (red); cells were incubated with TSPCs- or SPCs-based self-assemblies at a final DOX concentration of 5 µg/mL for 12 hours.
Abbreviations: DOX, doxorubicin; LA, lactobionic acid; TSPCs, targeted supramolecular prodrug complexes.
Cell apoptosis analysis of SPCs- and TSPCs-based self-assemblies
Because DOX could induce tumor cells’ apoptosis by intercalating with DNA,Citation41 flow cytometry and Hoechst 33258 staining were carried out. The apoptotic cells were stained with Annexin V. The results () indicated that after being treated with TSPCs-based self-assemblies for 24 hours, the HepG2 cells’ apoptosis rate (right quadrant) was 90.12%, which was a significant increase compared with those treated with SPCs-based self-assemblies (9.45%) or TSPCs-based self-assemblies (19.21%) in the presence of LA. To further confirm these results, Hoechst 33258 staining was carried out to study the nuclear morphology. As depicted in , HepG2 cells treated with free DOX or TSPCs-based self-assemblies for 24 hours, and A549 cells treated with free DOX showed intense blue fluorescence, archetypal of apoptotic cells. At the same time, the condensation of chromatin, shrinkage of nuclei, and condensed bright blue apoptotic nuclei in HepG2 cells were observed only after being treated with free DOX or TSPCs-based self-assemblies for 24 hours. Hence, these data suggest that TSPCs-based self-assemblies were able to induce apoptosis in HepG2 cells.
Figure 8 Cell apoptosis analysis of TSPCs- and SPCs-based self-assemblies.
Notes: (A) Cell apoptosis ratio measured by flow cytometry analysis in A549 and HepG2 cells treated with TSPCs- or SPCs-based self-assemblies for 24 hours. (B) Photographs of the morphology of apoptotic nuclei using Hoechst 33258 staining in A549 and HepG2 cells after treatment with TSPCs- or SPCs-based self-assemblies.
Abbreviations: DOX, doxorubicin; LA, lactobionic acid; SPCs, supramolecular prodrug complexes; TSPCs, targeted supramolecular prodrug complexes.
Scheme 1 Schematic illustration of preparation, proposed degradation mechanism of TSPCs-based self-assemblies, and consequent release of DOX.
Abbreviations: BM-DOX, benzimidazole modified DOX; β-CD, β-cyclodextrin; DOX, doxorubicin; LA-PEG-β-CD, β-CD-modified PEGylated LA; PEG, polyethylene glycol; TSPCs, targeted supramolecular prodrug complexes.
Conclusion
In summary, we developed biocompatible, pH-sensitive self-assemblies based on host–guest interaction between BM and β-CD for liver cancer therapy. We demonstrated that TSPCs-based self-assemblies have excellent size stability and a well-defined spherical structure. In vitro drug release assay revealed that TSPCs-based self-assemblies, as a pH-sensitive drug carrier, released more drug at pH 5.0 than at the physiological pH value. In vitro cytotoxicity of TSPCs-based self-assemblies was greater than SPCs-based self-assemblies toward HepG2 liver cancer cells. Meanwhile, cell uptake assay proved that the uptake of TSPCs-based self-assemblies was decreased in the presence of LA, which further verified the cell uptake behavior via LA receptors. In addition, the cell apoptosis analysis indicated that TSPCs-based self-assemblies could induce HepG2 cells apoptosis, which may be one of the mechanisms in inhibiting cell proliferation. In conclusion, the DOX-loaded polymers exhibited excellent effects on HCC tumor cell growth, and could potentially be a good candidate to deliver DOX. More importantly, the experimental data we acquired provide a foundation for further in vivo application of DOX-loaded polymers, which offers a strategy for liver cancer-targeting therapy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21674086), the Natural Science Basic Research Plan in Shaanxi Province of China (2018JZ2003), the National Natural Science Foundation of China (grant number 81773772), and the Fundamental Research Funds for the Central Universities. Tianfeng Yang and Guowen Du should be regarded as co-first authors. Wei Tian and Yanmin Zhang should be regarded as co-corresponding authors.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin201868173029313949
- MoukhadderHMHalawiRCappelliniMDTaherATHepatocellular carcinoma as an emerging morbidity in the thalassemia syndromes: a comprehensive reviewCancer2017123575175827911488
- LiYZhuDHouLTRB3 reverses chemotherapy resistance and mediates crosstalk between endoplasmic reticulum stress and AKT signaling pathways in MHCC97H human hepatocellular carcinoma cellsOncol Lett20181511343134929391905
- WangWPengHLiJControllable inhibition of hepatitis B virus replication by a DR1-targeting short hairpin RNA (shRNA) expressed from a DOX-inducible lentiviral vectorVirus Genes201346339340323397077
- KebsaWLahouelMRouibahHReversing multidrug resistance in chemo-resistant human lung adenocarcima (A549/DOX) cells by Algerian propolis through direct inhibiting the Pgp efflux-pump, G0/G1 cell cycle arrest and apoptosis inductionAnticancer Agents Med Chem20181891330133730088453
- BertrandNWuJXuXKamalyNFarokhzadOCCancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biologyAdv Drug Deliv Rev20146622524270007
- CongZShiYWangYA novel controlled drug delivery system based on alginate hydrogel/chitosan micelle compositesInt J Biol Macromol2018107Pt A85586428935541
- LiSGoinsBZhangLBaoANovel multifunctional theranostic liposome drug delivery system: construction, characterization, and multimodality MR, near-infrared fluorescent, and nuclear imagingBioconjug Chem20122361322133222577859
- YeJShinMCLiangQHeHYangVC15 years of ATTEMPTS: a macromolecular drug delivery system based on the CPP-mediated intracellular drug delivery and antibody targetingJ Control Release2015205586925483423
- DaveVYadavRBKushwahaKLipid-polymer hybrid nanoparticles: Development & statistical optimization of norfloxacin for topical drug delivery systemBioact Mater20172426928029744436
- RossiFFerrariRCastiglioneFPolymer hydrogel functionalized with biodegradable nanoparticles as composite system for controlled drug deliveryNanotechnology201526101560225490351
- SoltaniYGoodarziNMahjubRPreparation and characterization of self nano-emulsifying drug delivery system (SNEDDS) for oral delivery of heparin using hydrophobic complexation by cationic polymer of β-cyclodextrinDrug Dev Ind Pharm201743111899190728685625
- LiuGYuanQHollettGCyclodextrin-based host–guest supra-molecular hydrogel and its application in biomedical fieldsPolym Chem201892534363449
- WangXGDongZYChengHA multifunctional metal-organic framework based tumor targeting drug delivery system for cancer therapyNanoscale2015738160611607026372069
- YuFWuHTangYTemperature-sensitive copolymer-coated fluorescent mesoporous silica nanoparticles as a reactive oxygen species activated drug delivery systemInt J Pharm20185361112029146540
- DabiriSMHLagazzoABarberisFNew in-situ synthetized hydrogel composite based on alginate and brushite as a potential pH sensitive drug delivery systemCarbohydr Polym201717732433328962775
- PapaALKorinNKanapathipillaiMUltrasound-sensitive nanoparticle aggregates for targeted drug deliveryBiomaterials201713918719428618348
- ZuoJTuLLiQNear Infrared Light Sensitive Ultraviolet-Blue Nanophotoswitch for Imaging-Guided “Off-On” TherapyACS Nano20181243217322529489327
- ChenBDaiWHeBCurrent multistage drug delivery systems based on the tumor microenvironmentTheranostics20177353855828255348
- ButtAMAminMCKatasHDoxorubicin and siRNA Codelivery via chitosan-coated pH-responsive mixed micellar polyplexes for enhanced cancer therapy in multidrug-resistant tumorsMol Pharm201613124179419027934479
- AkGYilmazHGüneşAHamarat SanlierSIn vitro and in vivo evaluation of folate receptor-targeted a novel magnetic drug delivery system for ovarian cancer therapyArtif Cells Nanomed Biotechnol201846sup1926937
- JungKHParkJWPaikJYEGF receptor targeted tumor imaging with biotin-PEG-EGF linked to (99m)Tc-HYNIC labeled avidin and streptavidinNucl Med Biol20123981122112722819251
- RoggenbuckDMytilinaiouMGLapinSVReinholdDConradKAsialoglycoprotein receptor (ASGPR): a peculiar target of liver-specific autoimmunityAuto Immun Highlights20123311912526000135
- WangXHeLWeiBBromelain-immobilized and lactobionic acid-modified chitosan nanoparticles for enhanced drug penetration in tumor tissuesInt J Biol Macromol201811512914229665385
- BansalDYadavKPandeyVLactobionic acid coupled liposomes: an innovative strategy for targeting hepatocellular carcinomaDrug Deliv201623114014624786484
- SonawaneSJKalhapureRSGovenderTHydrazone linkages in pH responsive drug delivery systemsEur J Pharm Sci201799456527979586
- XuZGZhangKLHouCLA novel nanoassembled doxorubicin prodrug with a high drug loading for anticancer drug deliveryJ Mater Chem B201422234333437
- ZhangZDingJChenXIntracellular pH-sensitive supramolecular amphiphiles based on host–guest recognition between benzimidazole and β-cyclodextrin as potential drug delivery vehiclesPolym Chem20134113265
- ZhaoYFanZShenMShiXCapturing hepatocellular carcinoma cells using lactobionic acid-functionalized electrospun polyvinyl alcohol/polyethyleneimine nanofibersRSC Adv20155867043970447
- ChipemFABeheraSKKrishnamoorthyGEnhancing excited state intramolecular proton transfer in 2-(2′-hydroxyphenyl)benzimidazole and its nitrogen-substituted analogues by β-cyclodextrin: the effect of nitrogen substitutionJ Phys Chem A2013117204084409523621742
- MaHWangYWuDA novel controlled release immunosensor based on benzimidazole functionalized SiO2 and cyclodextrin functionalized goldSci Rep201661979726791418
- GaoLZhengBChenWSchalleyCAEnzyme-responsive pillar[5] arene-based polymer-substituted amphiphiles: synthesis, self-assembly in water, and application in controlled drug releaseChem Commun201551801490114904
- StephensonAWuZYuanXThe preformulation stability studies of a proline prodrug of methotrexateMed Chem20128462262822571189
- Di-WenSPanGZHaoLImproved antitumor activity of epirubicin-loaded CXCR4-targeted polymeric nanoparticles in liver cancersInt J Pharm20165001–2546126748365
- GaoGHLiYLeeDSEnvironmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapyJ Control Release2013169318018423195533
- ZhanFChenWWangZAcid-activatable prodrug nanogels for efficient intracellular doxorubicin releaseBiomacromolecules201112103612362021905663
- WangLRenKFWangHBWangYJiJpH-sensitive controlled release of doxorubicin from polyelectrolyte multilayersColloids Surf B Biointerfaces201512512713325461920
- SiepmannJPeppasNAModeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC)Adv Drug Delivery Rev2001482–3139157
- YangXGrailerJJPillaSSteeberDAGongSTumor-targeting, pH-responsive, and stable unimolecular micelles as drug nanocarriers for targeted cancer therapyBioconjug Chem201021349650420163170
- Zakeri-MilaniPMussa FarkhaniSShiraniACellular uptake and anti-tumor activity of gemcitabine conjugated with new amphiphilic cell penetrating peptidesEXCLI J20171665066228694765
- HajraSPatraARBasuABhattacharyaSPrevention of doxorubicin (DOX)-induced genotoxicity and cardiotoxicity: Effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammationBiomed Pharmacother201810122824329494960