Abstract
Purpose
The purpose of this study was to overcome the clinical defects of 7-ethyl-10-hydroxycamptothecin (SN-38) and explore its characteristics and antitumor effects.
Materials and methods
An amorphous solid dispersion of SN-38 with disodium glycyrrhizin (Na2GA) was prepared by mechanical ball milling (Na2GA/SN-38-BM). Moreover, an untreated mixture of Na2GA and SN-38 (Na2GA/SN-38-UM), a pure drug SN-38, was prepared for comparison with Na2GA/SN-38-BM. The samples were characterized by powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), dynamic light scattering, and transmission electron microscopy. Then, further in vitro and in vivo studies were performed including cell uptake, cytotoxicity, antitumor efficacy, tissue distribution, and histopathological evaluation (H&E staining).
Results
SN-38 loaded in Na2GA was self-formed as nano-micelles in water. The particle size of nano-micelle was 69.41 nm and ζ-potential was −42.01 mV. XRD and SEM analyses showed that the ball milling transformed SN-38 crystals into amorphous form and that solubility increased by 189 times. Compared with SN-38 and Na2GA/SN-38-UM, Na2GA/SN-38-BM has a stronger cytotoxicity to tumor cells and exhibited a significant inhibition of tumor growth. Then, pharmacokinetic studies showed that the bioavailability of Na2GA/SN-38-BM was about four times that of SN-38 suspension.
Conclusion
Na2GA/SN-38-BM (69 nm, −42 mV) nanoparticles which had excellent phar-macokinetic and distribution properties can dramatically enhance the anticancer efficacy of SN-38 in vitro and in vivo, suggesting a promising formulation for efficient anticancer therapy.
Introduction
Irinotecan (CPT-11) and its active metabolite 7-ethyl-10-hydroxyl-camptothecin (SN-38) are both alkaloid-derived topoisomerase I interactive compounds, which exhibit a broad spectrum of antitumor activity.Citation1,Citation2 CPT-11 can be converted to SN-38 by carboxylesterase-catalyzed hydrolysis that are predominantly present in liver, and SN-38 generated from irinotecan is eventually glycuro-conjugated for metabolic degradation by UGT1A1.Citation3–Citation5 Owing to its slow hydrolysis rate, irinotecan has an in vitro activity 100- to 1,000-fold lower than SN-38 and also very limited therapeutic efficacy in humans. On the other hand, because of the instability of the active lactone ring, lack of pharmaceutically acceptable excipients, and the extreme insolubility of SN-38,Citation6–Citation8 its clinical application is markedly restricted.
Several approaches to overcome the problem of SN-38 solubility and bioavailability have been proposed, including incorporation into nanoparticles (NPs),Citation9,Citation10 liposomes,Citation11 polymeric micelles,Citation12,Citation13 polymer conjugates,Citation14,Citation15 peptides,Citation16 carbohydrates,Citation17 and so on.
In the abovementioned methods, expensive excipients (such as lipids, cholesterol, etc), long time for preparation, complicated process, and various organic solvents (ethanol, dichloromethane, dimethyl sulfoxide, etc) are usually required. Most of these procedures are not environment-friendly and may increase the risk and cost of production. In recent years, mechanochemistry has become an important topic in pharmaceutical research because of its role in green synthesis.Citation18,Citation19 When solid molecular compounds are subjected to high-energy grinding, their structural and microstructural characteristics as well as their physicochemical stability will vary greatly. The proposed mechanochemical method has significant advantages over traditional “liquid-phase way,” and therefore, the bioavailability and solubility will be enhanced and the required dose and side effects will be reduced.
Disodium glycyrrhizinate (Na2GA) is the salt of glycyrrhizic acid (GA) which can undergo hydrolysis in aqueous solutions and form a free GA. Like GAs, it has anti-inflammatory and anti-cancer properties,Citation20 and the resulting solution is less viscous and can help improve the solubility and permeability of hydrophobic drugs.Citation21 Considering the environment-friendly and low-cost mechanochemical techniques and potential dissolution enhancement ability of Na2GA, an amorphous solid dispersion of SN-38 was prepared by mechanical milling, which was combined with Na2GA.Citation22 Moreover, we evaluated the physical characteristics, dissolution law, in vitro and in vivo properties, pharmacokinetics, tissue distribution, and antitumor effects. The main purposes of this study are twofold: 1) to prepare a new dosage form of SN-38 with higher antitumor efficiency by mechanical milling and 2) to study the characterization and behavior of Na2GA/SN-38-BM in vitro and in vivo.
Materials and methods
Materials
SN-38 was purchased from Shanxi Pioneer Biotech Co. Ltd. (Shanxi, China; purity >99%). Disodium glycyrrhizin (Na2GA) was purchased from Shanxi Pioneer Biotech Co. Ltd. (Xian, China, purity >98%). Methanol (HPLC grade) was obtained from Tedia Company, Inc (Fairfield, OH, USA). The human lung cancer cell line A549, the human hepatocellular carcinoma cell line HepG2, and the human breast cancer cell line Bcap-37 were purchased from China Center for Type Culture Collection (Wuhan, China).
Male and female Sprague Dawley rats (CD® (SD) IGS) weighing 330–350 g and BALB/c nude mice (6–8 weeks) weighing 18–20 g were obtained from the Shanghai Slac Laboratory Animal Co. Ltd. All the animal experiments in this study were conducted with the approval of the animal experiment center of Zhejiang University of Technology. Animal ethics review number was No. 25/2018. The animal experiments in this study were performed under the guidance of the care and use of laboratory animals in Zhejiang University of Technology (Hangzhou, China) and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23).
Preparation of SN-38 solid dispersion
Roll mill ML007 was used to prepare samples. Briefly, 1 g SN-38 and 100 g Na2GA were added to 500 mL ball mill pot with three specifications of steel balls (diameter 4, 8, and 12 mm, respectively). The grinding time was 24 hours, and the rotation speed was 20 rpm. Ball milling product was described as Na2GA/SN-38-BM. Moreover, an untreated mixture of Na2GA and SN-38 (Na2GA/SN-38-UM), a pure drug SN-38, was prepared for comparison with Na2GA/SN-38-BM. Finally, a mixture of SN-38 and GA was prepared by ball milling in the above ratio (GA/SN-38-BM).
Solubility determination
Solubility test was conducted on pure SN-38 and Na2GA/ SN-38-BM. Samples were made into a saturated solution and filtered through a filter paper (0.45 µm). Then, 1 mL filtrate solution was taken out. The filtrate solution was analyzed by an HPLC (Shimadzu LC-20D, Kyoto, Japan) with column Shimadzu ODS-3, 4.6×250 mm at +30°C and ultraviolet-array detector. The eluent was methanol-formate water (80:20, pH 3.0), with flow rate of 1.0 mL/min, and detection wavelength is 380 nm.
Dissolution determination
The dissolution behaviors of SN-38, Na2GA/SN-38-UM, Na2GA/SN-38-BM, and GA/SN-38-BM samples were tested in a dissolution tester (RC-6ST; Tianjin, China). The dissolution medium was 900 mL of phosphate buffer (pH 1.2), and the paddle speed was 200 rpm and maintained at 37°C±0.5°C. The doses of four groups were equivalent to 100 mg SN-38. At the set time point, 2 mL of the sample was extracted, and the phosphoric acid medium was added to the container in time. The samples collected in 1 mL vial were treated with methanol and then filtered into liquid phase vials using a syringe with a filter head. Finally, samples were detected using HPLC detector.
Differential scanning calorimetry (DSC)
The prepared samples (3 mg) in hermetically closed aluminum pans using a DSC-250 cell (Thermal Analysis Co. Ltd., New Castle, DE, USA) were heated from 50°C to the temperature of 30°C higher than melting points at the rate of 10°C/min, and the final temperature was maintained for 10 minutes; rapid temperature drop to 0°C was operated at the rate of −15°C/min. A second heating run was repeated. N2 was used as protective gas for 50 mL/min. All the data were analyzed using Trios software.
Powder X-ray diffraction (PXRD)
PXRD was performed using Bruker D2 Phaser diffractometer (Bruker, Germany) by using CuKα radiation to determine SN-38, Na2GA, Na2GA/SN-38-BM, and Na2GA/SN-38-UM. The sampling parameter is the step range 5°–40° at a speed of 2°/min.
Scanning electron microscopy (SEM)
SEM (Nano nova 450, FEI, USA) was used to acquire microphotographs on the surface and cross-section of the samples SN-38, Na2GA, Na2GA/SN-38-BM, and Na2GA/SN-38-UM. Dual adhesive was used to place the four samples onto the metal stubs and then the samples were gold-coated before imaging by electron microscopy.
Particle characterization, zeta potential, and stability
The physicochemical characteristics of the sample that dissolved in water were detected by Zetasizer NanoZS (Malvern Instruments, Malvern, UK). Before being measured, all samples were diluted with physiological saline. The particle size of the nano-micelle, polydispersity index (PDI), and the charge on its surface (ζ-potential) were detected by dynamic light scattering (DLS) and laser Doppler anemometry. The sample was dissolved in PBS. Then the change in particle size and PDI of nano-micelles was detected by DLS for 60 hours.
Determination of the critical micelle concentration (CMC)
Nile red (1 µg) in CH2Cl2 (30 µL) was added to a series of vials. After CH2Cl2 was evaporated, Na2GA/SN-38-BM aqueous solutions (3 mL) with various concentrations were added into the vials, stirred for 12 hours. The fluorescence intensity of these solutions was measured by a microplate reader (Flexstation 3; Molecular Devices LLC, Sunnyvale, CA, USA) at the wavelength of 620 nm (excited at 579 nm). The junction of the tangents of the two linear parts of the graph of the fluorescence intensity as a function of Na2GA/SN-38-BM concentration gave the CMC value.
Transmission electron microscopy (TEM)
Na2GA/SN-38-BM was configured into 1 mg/mL solution. One drop of solution was dropped on the surface of the copper sheet and the sample was observed by Hitachi H7600 transmission electron microscope (TEM; Hitachi Ltd., Tokyo, Japan).
Multicellular tumor spheroids
Multicellular tumor spheroid models consisting of A549 cancer cell line were prepared by modifying a previously reported protocol.Citation23 Briefly, 50 µL DMEM containing 1.5% agarose (wt/vol) was plated onto each well of a 96-well microtiter plate under sterile conditions. After the agarose solidifies, the plates were stored at room temperature until use.
The multicellular spheroids of A549 cells were prepared by a hanging drop technique. Generally, cell suspensions were diluted in medium containing 0.24% (w/v) methylcel-lulose at a density around 105 cells per milliliter. Then, 25 µL diluted cells were dropped on the lids of 10 cm cell culture plates. After 24 hours, the spheroids were formed and transferred to agarose-coated 96-well plates with one spheroid in each well. The spheroids were incubated for another 72 hours and obtained multicellular tumor spheroids (400–500 µm in diameter). Subsequently, SN-38, Na2GA/SN-38-UM, and Na2GA/SN-38-BM at 10 µg/mL SN-38 concentration were added to the 96-well plate, and the diameter change of each well was determined for a week.
Cellular uptake studies
Fluorescent labeling
Fluorescent labeling dyes, 7 mg curcumin (Frontier Scientific, Inc., Logan, UT, USA), were mixed with 7 mg Na2GA/SN-38-BM powders and then dissolved in 100 µL tetrahydrofuran completely. About 1 mL distilled water was then added dropwise with continuously stirring for extra 2 hours. Subsequently, 20 kDa MWCO cartridge Spectrum Lab was used for dialysis of the solution. The labeled NPs were stored at −20°C before use.
Na2GA/SN-38-BM/curcumin localization
Cells were plated on glass bottom dishes (MatTek, Shanghai, China) at a density of 6×103 cells per cm2 and incubated for 24 hours before use. The cells were incubated with 3.5 µg/ mL Na2GA/SN-38-BM/curcumin for 4 hours. Subsequently, after 3× washing with cold PBS, the cells were fixed with 4% formalin for 15 minutes, followed by 1.5 µg/mL DAPI staining at room temperature. The images of cells were acquired with a Zeiss LSM 510 Meta confocal microscope (confocal laser scanning microscopy [CLSM]; Carl Zeiss Meditech AG, Jena, Germany).
In vitro cytotoxicity studies
The cytotoxicity of SN-38, Na2GA/SN-38-UM, and Na2GA/ SN-38-BM on human breast cancer cell line Bcap-37, lung cancer cell line A549, and hepatocellular carcinoma cell line HepG2 was determined by MTT cell proliferation assay.Citation24–Citation26 All the cells were cultivated in the incubator which had a humidified atmosphere containing 5% CO2 at 37°C. The medium was supplemented with 10% (v/v) FBS and 1% (w/v) penicillin-streptomycin. Bcap-37 cells were cultured in 1,640 medium while A549 and HepG2 cells were cultured in DMEM. In short, ~5,000–10,000 cells per well were evenly plated into 96-well plates and grown overnight.
Meanwhile, the cell-free wells were used as the background, and the wells which implanted cells but with no drug treatment were used as the control group. The media was replaced with different concentrations of SN-38, Na2GA/SN-38-UM, and Na2GA/SN-38-BM for 24 hours. Subsequently, 20 µL MTT (5 mg/mL) in PBS was added to each well, and the cells were further incubated at 37°C for 4 hours. Next, the medium containing MTT was removed and 150 µL DMSO was added to each well, and the mixture was shaken at room temperature for 10 minutes to dissolve the formazan crystals produced by living cells. Finally, the optical density of each well was measured at 570 nm using a microplate reader (Flexstation 3; Molecular Devices LLC).
In vivo antitumor efficacy
The antitumor effects of SN-38 suspensions, Na2GA/SN-38-UM, and Na2GA/SN-38-BM on Nu/Nu nude mice were further studied. About 0.2 mL of Bcap-37 cells (1×106 cells) was injected subcutaneously on mice right flank, and when the tumor volume reached to 50–70 mm3 (about 7 days after inoculation), they were randomly divided into four groups (n=6/ group). Each nude mouse was intragastrically administered with a daily dose of 1 mg/kg equivalent SN-38 concentration, whereas the control group was given a 0.9% saline solution. The width and length of the tumors were measured with a caliper and the mice body weight was weighed every day. Tumor volume was estimated as [(length) × (width)2/2].Citation27 The daily weight of each nude mouse was used as an indicator of systemic toxicity.Citation28 On the 16th day, the tumors of four groups were dissected, washed with PBS solution, and then weighed. Moreover, tumor paraffin sections of four groups were stained with H&E staining to observe pathological changes.
Pharmacokinetic evaluation
Nine SD rats were randomly divided into three groups including SN-38 suspensions, Na2GA/SN-38-UM, and Na2GA/SN-38-BM groups. The dose for each intragastric administration was 1 mg/kg (equivalent to SN-38 concentration). At the set time point, 0.5 mL of blood was taken from the eyelids and placed in the prepared heparinized tube and then centrifuged at 5,000 rpm for 5 minutes. About 100 µL of the upper serum was taken, and 200 µL of methanol and 100 µL of CPT-11 solution (as the internal standard, concentration: 200 ng/mL) were added thereto. The mixture was vortexed for 1 minute, centrifuged at 10,000 rpm for 10 minutes, and then filtered through a syringe with a filter into a liquid vial. Finally, the samples were detected by HPLC.
Tissue biodistribution studies
To investigate the tissue biodistribution of Na2GA/SN-38-UM, Na2GA/SN-38-BM, and SN-38 suspensions, three groups of Bcap-37-bearing mice were intragastrically administered with three drugs at dose about 1 mg/kg equivalent to SN-38 before 24 hours. Major organs including heart, kidney, spleen, lung, stomach, liver, and tumors were excised and washed with 0.9% saline before weighed. The organs or tissues were cut into small pieces and homogenized. Tissue homogenate (100 µL) was added with 1 µL of 20% trifluoroacetic acid. SN-38 was extracted with 200 µL ice-cold acetonitrile containing 0.5% acetic acid. The mixture was centrifuged at 12,000 rpm for 10 minutes and 200 µL of the supernatant was transferred to a HPLC sample vial. The drug concentrations in the solutions were determined using HPLC as described above, and the corresponding SN-38 tissue concentrations were calculated accordingly.
Statistical analysis
Data were reported as mean ± SD. Student’s t-test was used for statistical comparison/analysis. A value of P<0.05 was considered statistically significant.
Results and discussion
Solubility and dissolution determination
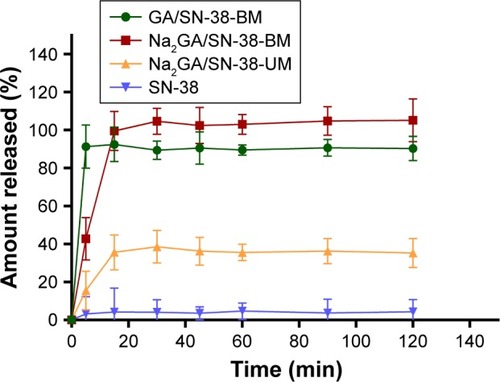
Through HPLC detection and analysis, the solubility of the pure drug SN-38 was 1.12 µg/mL. Compared with SN-38, the solubility of ball-milling products was increased 189 times. Dissolution profiles of SN-38, Na2GA/SN-38-UM, Na2GA/SN-38-BM, and GA/SN-38-BM are shown in . Compared with SN-38 and Na2GA/SN-38-UM, GA/SN-38-BM and Na2GA/SN-38-BM exhibited better dissolution properties. In PBS solution, GA/SN-38-BM dissolved a little bit faster than Na2GA/SN-38-BM which had the better solubility. The cumulative amount of SN-38 dissolved after 5 minutes was 4.2%, 35.6%, 92.5%, and 99.6% for SN-38, Na2GA/SN-38-UM, GA/SN-38-BM, and Na2GA/SN-38-BM, respectively. We speculated that the drug was encapsulated in a hydrophilic carrier and formed as the amorphous sample which had better wettability and dispersibility leading to more excellent properties. Na2GA/SN-38-BM and GA/SN-38-BM form micelles in water, resulting in excellent solubility. While Na2GA/SN-38-UM was not uniformly dispersed in Na2GA, therefore only part of SN-38 dissolves rapidly.
Figure 1 In vitro release profiles of SN-38, Na2GA/SN-38-UM, Na2GA/SN-38-BM and GA/SN-38-BM (n=3).
Abbreviations: Na2GA, disodium glycyrrhizin; Na2GA/SN-38-UM, untreated mixture of Na2GA and SN-38; Na2GA/SN-38-BM, amorphous solid dispersion of SN-38 with Na2GA)was prepared by mechanical ball milling; SN-38, 7-ethyl-10-hydroxycamptothecin.
Particle size, morphological analysis, and stability
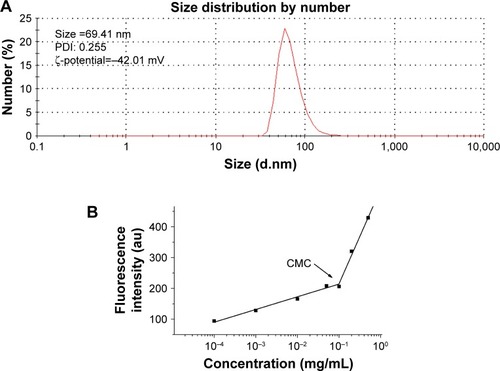
When Na2GA/SN-38-BM dissolved in water, Na2GA encapsulated SN-38 to form micelles. The size distribution of the nano-micelles was measured by DLS as shown in . At 25°C, the average particle size has a narrow distribution, which is about 69.41 nm. The PDI value of Na2GA/SN-38-BM is about 0.255. In addition, the ζ-potential of the particle was −42.01 mV. The CMC was 99.26 µg/mL (). The average particle sizes <200 nm were considered to be at a range suitable to evade filtration in reticuloendothelial system (RES) organs and were more easily absorbed by the tumor.Citation29 In general, the abovementioned ζ-potential ±30 mV can form a stable dispersion.Citation30 The negative charge was also proved more easily to evade the RES system’s capture so that the tumor tissue can accumulate more drugs. The uniform particle size was further identified by TEM as shown in . The nano-micelles are spherical and have a medium uniform particle size which is consistent with the results measured by DLS. In order to test the stability of the obtained Na2GA/SN-38-BM NPs, they were suspended in 1× PBS at a concentration of 1 mg/mL, and then the particle size and PDI were monitored by DLS (). The particle size increased from 68 to 81 nm over a span of 60 hours, and the PDI remained relatively the same at 0.26. The change of the particle size indicated that the nano-micelle was relatively stable.
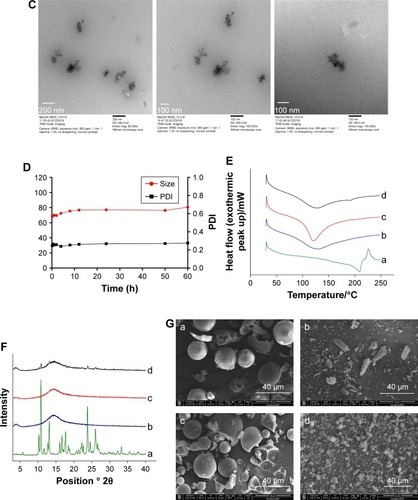
Figure 2 (A) Dynamic light scattering size measurement of Na2GA/SN-38 nanoparticles. (B) The CMC value of Na2GA/SN-38-BM. (C) Transmission electron micrograph (TEM) of Na2GA/SN-38 nanoparticles, the magnification from left to right was 80,000×, 100,000×, 150,000×. (D) The stability of Na2GA/SN-38-BM. (E) DSC thermograms of (a) SN-38, (b) Na2GA, (c) Na2GA/SN-38-BM, (d) Na2GA/SN-38-UM. (F) X-ray powder diffraction spectra of (a) SN-38, (b) Na2GA, (c) Na2GA/SN-38-BM, (d) Na2GA/SN-38-UM. (G) The electron micrographs of (a) Na2GA, (b) SN-38, (c) Na2GA/SN-38-UM, (d) Na2GA/SN-38-BM.
Abbreviations: CMC, critical micelle concentration; PDI, polydispersity index.
Crystalline state analysis
The DSC thermograms of Na2GA, SN-38, Na2GA/SN-38-UM, and Na2GA/SN-38-BM are shown in . The DSC curves of SN-38 exhibited endothermic peaks around 215°C and a characteristic peak was at 200°C–250°C. There was also a characteristic peak observed in Na2GA/SN-38-UM, but the peak intensity of Na2GA/SN-38-BM disappeared. It was speculated that SN-38 had been transformed into an amorphous state during the mechanical grinding process, and therefore, SN-38 had been uniformly distributed in Na2GA.
The PXRD thermograms of Na2GA, SN-38, Na2GA/ SN-38-UM, and Na2GA/SN-38-BM are shown in . The characteristic peak 2θ values of SN-38 were detected to be 10.40, 10.96, 13.24, 17.75, 23.90, and 26.10, indicating that it was a crystal. In the Na2GA/SN-38-UM diffraction spectrum of the physical mixture, the characteristic crystallization peak of SN-38 was significantly decreased, and no characteristic peaks were found in the Na2GA/SN-38-BM after grinding for 24 hours. From the diffraction pattern of Na2GA/SN-38-BM and the DSC thermograms, we can further identify that SN-38 was dispersed in Na2GA to form an amorphous sample by physical ball milling.
Electron micrographs of SN-38, Na2GA, Na2GA/SN-38-UM, and Na2GA/SN-38-BM are shown in . It could be visually seen that SN-38 was elongated and the Na2GA was in a spherical state. However, after 24 hours of mechanochemical milling, the intact morphology of the SN-38 and Na2GA particles was destroyed, forming fine and irregularly shaped particles. Grinding made the particles more uniform, which increased the surface area of the particles, thereby increasing the rate of dissolution.
In vitro cellular studies and cytotoxicity
The colocalization and internalization by A549 cells of Na2GA/SN-38-BM/curcumin was confirmed by CLSM. As shown in , the fluorescence from Na2GA/SN-38/ curcumin (green) was observed in the cytoplasm after 4 hours of incubation at 37°C, indicating that Na2GA/SN-38/ curcumin was quickly taken up by A549 cells and located in cytoplasm of the tumor cells.
Figure 3 (A) Cell uptake of Na2GA/SN-38-BM/Curcumin. Nucleus was stained with DAPI. Images were taken from the DAPI channel (blue), Na2GA/SN-38-BM/Curcumin channel (green), and the overlapped image. (B) In vitro cytotoxicity of SN-38, Na2GA/SN-38-UM and Na2GA/SN-38-BM. (a) suppression ratio on A549 cells, (b) suppression ratio on Bcap-37 cells, (c) suppression ratio on HepG2 cells (n=3). (C) Image-based quantification of the relative average diameter of untreated and treated cells spheroids, The image of multicellular tumor spheroids. (D) Data represent the average of n≥20 cell spheroid (±SEM). *P<0.05, **P<0.01, and ***P<0.001, compared with untreated group.
Abbreviations: Na2GA, disodium glycyrrhizin; Na2GA/SN-38-UM, untreated mixture of Na2GA and SN-38; Na2GA/SN-38-BM, amorphous solid dispersion of SN-38 with Na2GA) was prepared by mechanical ball milling; SN-38, 7-ethyl- 10-hydroxycamptothecin.
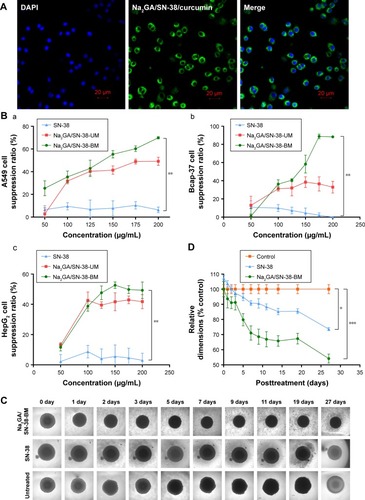
The cytotoxicity of SN-38 suspensions, Na2GA/SN-38-UM, and Na2GA/SN-38-BM was evaluated in cancerous A549, Bcap-37, and HepG2 cell lines by MTT assay. As shown in , compared with SN-38 suspensions, Na2GA/SN-38-UM and Na2GA/SN-38-BM have the significant inhibition ability in all three cancer cell lines. The half maximal inhibitory concentrations (IC50) of Na2GA/SN-38-BM and Na2GA/SN-38-UM were 1.25 and 4.71 µg/mL on Bcap-37 cells, 1.30 and 1.77 µg/mL on A549 cells, and 1.62 and 2.18 µg/mL on HepG2 cells, respectively. Compared with SN-38 suspension, the comparison was statistically significant (P<0.05). Furthermore, Na2GA/SN-38-BM and Na2GA/SN-38-UM were two or three times stronger than SN-38 suspension (P<0.05). Therefore, Na2GA/SN-38-BM enhanced the cytotoxic ability of SN-38. In addition, all obtained cytotoxic action of Na2GA/SN-38-BM was due to the SN-38 effect. As shown in , the survival rate of all three cell lines was above 90%, which confirmed that Na2GA itself possessed no cytotoxicity in all tested cell lines in the concentration range of 50–200 µg/mL. There are some possible explanations for these results. First, the lactone ring of SN-38 is prone to hydrolysis at pH 7.4,Citation31 and the stability of the lactone ring in Na2GA/SN-38-BM NPs might be enhanced significantly compared to the solution.Citation26,Citation32 Second, SN-38 is a P-gp substrate.Citation33 Part of SN-38 molecules in tumor cells will be effluxed outside cells by P-gp transporters, while Na2GA/SN-38-BM NPs is not affected by P-gp transporters due to its pinocytosis pathway to enter the cells.Citation34 Finally, mechanical ball milling and Na2GA significantly increase the solubility of the drug. Excess Na2GA/SN-38-BM and pure drug SN-38 dissolved the same dose of water, and Na2GA/SN-38-BM gave a modified concentration of SN-38. All these reasons might explain the enhancement of cytotoxicity of Na2GA/SN-38-BM NPs.
Further multicellular tumor spheroids were used to investigate NP cytotoxicity in 3D models. As shown in , apart from untreated group, SN-38 suspensions and Na2GA/SN-38-BM treatment groups both decreased the spheroid diameter, and there was a similar downward trend. The relative diameter of SN-38 group dropped to about 25%, and the Na2GA/SN-38-BM group dropped to about 46% (). It maybe is due to their cytotoxic effects on A549 cells. Moreover, compared with SN-38 group, a large number of dead cells were seen in the MCTSs of Na2GA/SN-38-BM groups.
Table 1 Cytotoxicity of Na2GA in A549, Bcap-37, and HepG2 cell lines
In vivo antitumor efficacy
Next, we investigated the antitumor effects of SN-38 suspensions, Na2GA/SN-38-UM, and Na2GA/SN-38-BM on nude mice bearing Bcap-37 tumors. Each nude mouse was intragastrically administered with a daily dose of about 1 mg/kg equivalent to SN-38, and the control group was given a 0.9% saline solution. Animal body weight and tumor size were monitored every day. As shown in , compared with the control group, Na2GA/SN-38-BM showed better tumor inhibition ability throughout the treatment, nearly 53.70% tumor inhibitory rate. Meanwhile, Na2GA/SN-38-UM and SN-38 suspensions showed no significant differences compared with the control group (P<0.001 for Na2GA/SN-38-BM).
Figure 4 In vivo antitumor effect tested in Bcap-37-bearing mice (n=6). SN-38 suspensions, Na2GA/SN-38-UM and Na2GA/SN-38-BM were intragastric administration to mice at the dose of 100 mg/kg, and 0.9% saline was used as control. (A) The profile change of tumor volume. **P<0.01, Na2GA/SN-38-BM group compared with SN-38 group. (B) The body weight curve of mice. **P<0.01, Na2GA/SN-38-BM group compared with SN-38 group. (C) Mean weight of tumors in each group upon animal termination. (D) H&E staining of tumor tissue sections. Tumor paraffin sections dyed with H&E reagent were well prepared for observation of the pathological change separately under the microscope (×200). The mice were administered (a) 0.9% saline, (b) SN-38 suspensions, (c) Na2GA/SN-38-UM, and (d) Na2GA/SN-38-BM.
Abbreviations: Na2GA, disodium glycyrrhizin; Na2GA/SN-38-UM, untreated mixture of Na2GA and SN-38; Na2GA/SN-38-BM, amorphous solid dispersion of SN-38 with Na2GA) was prepared by mechanical ball milling; SN-38, 7-ethyl- 10-hydroxycamptothecin.
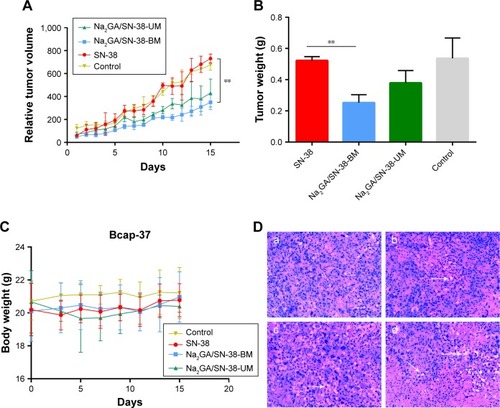
The potential systemic toxicities of different SN-38 formulations to nude mice were assessed based on changes in body weight (). Two days after the start of intragastric administration, mild diarrhea occurred in the nude mice treated with the SN-38 suspensions. Compared with the control group, the treatment group had mild weight loss (<10%) during intragastric administration and recovered after ~5 days. The mice treated with Na2GA/SN-38-UM exhibited slightly more body weight loss after 15 days (about 1 g). Meanwhile, the mice in the Na2GA/SN-38-BM treatment groups did not lose weight, but increased slightly. This result indicates that the severe side effects caused by the formulation can be negligible at the test dose.
On the 16th day, the tumors of the four groups of nude mice were dissected and washed with 0.9% saline and then weighed to calculate the tumor inhibition rate (IR) (). The average tumor weight of the group administered with saline, SN-38 suspensions, Na2GA/SN-38-UM, or Na2GA/SN-38-BM was 0.54, 0.52, 0.38, or 0.25 g, respectively. The IR of SN-38 suspensions, Na2GA/SN-38-UM, or Na2GA/SN-38-BM groups was 3.70%, 29.63%, or 53.70%, respectively, relative to the control group.
The tumor tissue sections were subjected to H&E staining, and the pharmacological and toxicological effects of the three groups on the tumor cells were observed. From , we could find that the cells of control group were dense and had abundant vascular tissue. However, different degrees of apoptosis were seen in tumors treated with SN-38 suspension, Na2GA/SN-38-UM, and Na2GA/SN-38-BM. Especially in the Na2GA/SN-38-BM group, a large amount of excessive vacuolization was observed in the tissue sections. This indicated that Na2GA/SN-38-BM was the most cytotoxic. The result was consistent with the above tumor inhibition data. In summary, the antitumor effect of Na2GA/SN-38-BM is the most prominent of the three formulations.
Pharmacokinetics study
The SN-38 blood concentration–time curves are depicted as . It could be clearly seen from the figure that Na2GA/SN-38-BM had the better bioavailability compared with Na2GA/SN-38-UM and SN-38. After intragastric administration, SN-38 distributed rapidly into the whole body and reached the highest blood concentration at 0.5 hour, after that SN-38 distributed to the whole body and cleared slowly and finally expelled from the body at about 2 hours. As shown in , the bioavailability of the SD rats treated with SN-38 suspensions, Na2GA/SN-38-UM, and Na2GA/SN-38-BM were 212, 351, and 818 µg/L*h, respectively. Compared with the SN-38 suspension, the area under the curve of Na2GA/SN-38-BM increased about four times larger, and the maximum blood concentration increased about five times higher.
Table 2 Pharmacokinetic parameters after intragastric administration of SN-38 suspensions, Na2GA/SN-38-UM, and Na2GA/SN-38-BM at a dose of 100 mg/kg in Sprague Dawley rats (n=3)
Figure 5 (A) Concentration of SN-38 in rat plasma after intragastric administration of SN-38 suspensions, Na2GA/SN-38-UM and Na2GA/SN-38-BM to rats at the dose of 1 mg/kg (n=3), *P<0.05, statistical signifcance compared to SN-38 suspensions. (B) Biodistribution of SN-38 in Bcap-37-bearing mice after 24 h treatment with SN-38, Na2GA/SN-38-UM and Na2GA/SN-38-BM (n=3). *P<0.05, Na2GA/SN-38-BM vs SN-38, #P<0.05, Na2GA/SN-38-UM vs SN-38.
Abbreviations: Na2GA, disodium glycyrrhizin; Na2GA/SN-38-UM, untreated mixture of Na2GA and SN-38; Na2GA/SN-38-BM, amorphous solid dispersion of SN-38 with Na2GA) was prepared by mechanical ball milling; SN-38, 7-ethyl- 10-hydroxycamptothecin.
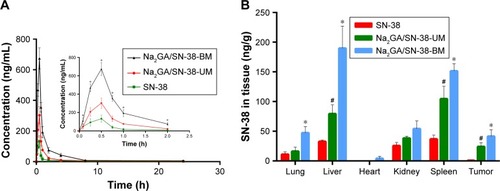
There were several reasons that could explain the increase in bioavailability of Na2GA/SN-38-BM. First, it is well known that the bioavailability of the solution was the best, followed by suspension, and powder was worst.Citation35 In water, Na2GA/SN-38-BM formed a homogeneous solution by forming nano-micelles. Some of SN-38 and Na2GA/ SN-38-UM samples could not dissolve completely. So the bioavailability was worse than Na2GA/SN-38-BM. Second, the crystalline vs amorphous properties (SN-38 and Na2GA/SN-38-BM) might also play a role. The drug encapsulated in a hydrophilic carrier formed as the amorphous sample which had better wettability and dispersibility, and thus had more excellent pharmacokinetics properties. Third, it was reported that GA increases drug permeability not only due to increased solubility but also due to increased drug permeability through cell membranes.Citation20,Citation36 Na2GA has similar properties to GA and can penetrate more into the cells than SN-38 suspension, therefore leading to a better bioavailability.
SN-38 biodistribution in tumor-bearing mice
The concentration of SN-38 in tumors and major organs of the heart, liver, spleen, lungs, and kidneys were studied after three groups of different formulations administered intragastrically at a dose of about 1 mg/kg equivalent to SN-38. As shown in , SN-38 in the formulation of Na2GA/SN-38-BM can be detected in the abovementioned organ tissues after 24 hours. The amount of SN-38 in the tumors administered with SN-38 suspensions was almost undetectable, and the drug accumulated in the tumors treated with Na2GA/SN-38-UM and Na2GA/SN-38-BM was 32- and 56-fold higher than that treated with SN-38 suspensions, respectively. The amount of SN-38 accumulated in liver and spleen of the mice treated with Na2GA/SN-38-UM and Na2GA/SN-38-BM was remarkably higher than that treated with SN-38 suspensions. In addition, the average particle sizes of <200 nm were considered to be at a range suitable to evade filtration in RES organs. The nano-micelles size of Na2GA/SN-38-BM formed in water was about 69 nm, which can prolong the residence time in the blood. Na2GA/SN-38-UM could also form part of nano-micelles. Therefore, the formulations of Na2GA/SN-38-UM and Na2GA/SN-38-BM have better tumor inhibition than SN-38 suspensions.
Conclusion
In this study, Na2GA/SN-38-BM was successfully developed by mechanical ball milling and overcame the clinical defects of SN-38. Crystalline state analysis showed that SN-38 was uniformly dispersed in the hydrophilic carrier and became amorphous in physical ball milling. Compared with conventional physical mixture, the dissolution of SN-38 was increased markedly after grinding. SN-38-loaded nano-micelles were self-formed by Na2GA when Na2GA/SN-38-BM dissolved in water. Compared with SN-38 and Na2GA/SN-38-UM, Na2GA/SN-38-BM showed good properties in vitro and in vivo, significantly improving the antitumor effect of SN-38. In Bcap-37 tumor-bearing mice, the antitumor effect of Na2GA/SN-38-BM was better than SN-38 suspensions and Na2GA/SN-38-UM because Na2GA/SN-38-BM can be better absorbed by tumors and had longer blood circulation time, which can kill tumor cells more effectively. In summary, the new formulation Na2GA/SN-38-BM using mechanical ball milling is feasible, portable, and environment-friendly and may be a promising formulation of SN-38 in antitumor therapy.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (no. 21506192) and the Zhejiang Provincial Natural Science Foundation of China (no. LY16E030010).
Disclosure
The authors report no conflicts of interest in this work.
References
- JaxelCKohnKWWaniMCWallMEPommierYStructure-activity study of the actions of camptothecin derivatives on mammalian topoisomerase I: evidence for a specific receptor site and a relation to antitumor activityCancer Res1989496146514692538227
- BisseryMCVrignaudPLavelleFChabotGGExperimental antitumor activity and pharmacokinetics of the camptothecin analog irinotecan (CPT-11) in miceAnticancer Drugs1996744374608826613
- SlatterJGSuPSamsJPSchaafLJWienkersLCBioactivation of the anticancer agent CPT-11 to SN-38 by human hepatic microsomal carboxylesterases and the in vitro assessment of potential drug interactionsDrug Metab Dispos19972510115711649321519
- IyerLHallDDasSPhenotype-genotype correlation of in vitro SN-38 (active metabolite of irinotecan) and bilirubin glucuronidation in human liver tissue with UGT1A1 promoter polymorphismClin Pharmacol Ther199965557658210340924
- IyerLKingCDWhitingtonPFGenetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomesJ Clin Invest199810148478549466980
- ZhangJAXuanTParmarMDevelopment and characterization of a novel liposome-based formulation of SN-38Int J Pharm20042701–29310714726126
- RogerELagarceFBenoitJPDevelopment and characterization of a novel lipid nanocapsule formulation of SN38 for oral administrationEur J Pharm Biopharm201179118118821303693
- SapraPZhaoHMehligMNovel delivery of SN38 markedly inhibits tumor growth in xenografts, including a camptothecin-11-refractory modelClin Cancer Res20081461888189618347192
- YellepeddiVKVangaraKKPalakurthiSIn vivo efficacy of PAMAM-Dendrimer-Cisplatin complexes in SKOV-3 xenografted BALB/c nude miceJ Biotechnol Biomater213003
- VangaraKKLiuJLPalakurthiSHyaluronic acid-decorated PLGA-PEG nanoparticles for targeted delivery of SN-38 to ovarian cancerAnticancer Res20133362425243423749891
- SadzukaYTakabeHSonobeTLiposomalization of SN-38 as active metabolite of CPT-11J Control Release20051082–345345916182400
- GuQXingJZHuangMHeCChenJSN-38 loaded polymeric micelles to enhance cancer therapyNanotechnology2012232020510122543761
- CarieARios-DoriaJCostichTIT-141, a polymer micelle encapsulating SN-38, induces tumor regression in multiple colorectal cancer modelsJ Drug Deliv201120111–219
- ZhaoHRubioBSapraPNovel prodrugs of SN38 using multiarm poly(ethylene glycol) linkersBioconjug Chem200819484985918370417
- KurzrockRGoelSWhelerJSafety, pharmacokinetics, and activity of EZN-2208, a novel conjugate of polyethylene glycol and SN38, in patients with advanced malignanciesCancer2012118246144615122674635
- Meyer-LosicFNicolazziCQuinoneroJDTS-108, a novel peptidic prodrug of SN38: in vivo efficacy and toxicokinetic studiesClin Cancer Res20081472145215318381956
- SerafinoAZonfrilloMAndreolaFCD44-targeting for antitumor drug delivery: a new SN-38-hyaluronan bioconjugate for locoregional treatment of peritoneal carcinomatosisCurr Cancer Drug Targets201111557258521486216
- SuXWuLHuMGlycyrrhizic acid: a promising carrier material for anticancer therapyBiomed Pharmacother20179567067828886526
- TsaiYMChienCFLinLCTsaiTHCurcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetrationInt J Pharm2011416133133821729743
- PolyakovNELeshinaTVGlycyrrhizic acid as a novel drug delivery vector: synergy of drug transport and efficacyOpen Conf Proc J2011216472
- PiperJTSinghalSSSalamehMSMechanisms of anticarcinogenic properties of curcumin: the effect of curcumin on glutathione linked detoxification enzymes in rat liverInt J Biochem Cell Biol19983044454569675878
- VangaraKKAliHILuDSN-38-cyclodextrin complexation and its influence on the solubility, stability, and in vitro anticancer activity against ovarian cancerAAPS PharmSciTech201415247248224477982
- LiuHWangYWangMXiaoJChengYFluorinated poly (propylenimine) dendrimers as gene vectorsBiomaterials201435205407541324709522
- YellepeddiVKVangaraKKKumarAPalakurthiSComparative evaluation of small-molecule chemosensitizers in reversal of cisplatin resistance in ovarian cancer cellsAnticancer Res20123293651365822993302
- KumarAYellepeddiVKVangaraKKStrycharKBPalakurthiSMechanism of gene transfection by polyamidoamine (PAMAM) dendrimers modified with ornithine residuesJ Drug Target201119977078021457075
- JwalaJBodduSHSShahSOcular sustained release nanoparticles containing stereoisomeric dipeptide prodrugs of acyclovirJ Ocul Pharmacol Ther201127216317221500985
- MamotCDrummondDCNobleCOEpidermal growth factor receptor–targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivoCancer Res20056524116311163816357174
- ZhangHHollisCPZhangQLiTPreparation and antitumor study of camptothecin nanocrystalsInt J Pharm20114151–229330021679755
- Landesman-MiloDRamishettiSPeerDNanomedicine as an emerging platform for metastatic lung cancer therapyCancer Metastasis Rev201534229130125948376
- YoshiokaKSakaiEDaimonMKitaharaARole of steric hindrance in the performance of Superplasticizers for ConcreteJ Am Ceram Soc1997801026672671
- BalaVRaoSBoydBJPrestidgeCAProdrug and nanomedicine approaches for the delivery of the camptothecin analogue SN38J Control Release20131721486123928356
- TangXJHanMYangBNanocarrier improves the bioavailability, stability and antitumor activity of camptothecinInt J Pharm20144771–253654525445532
- ChuCAbbaraCTandiaMCetuximab increases concentrations of irinotecan and of its active metabolite SN-38 in plasma and tumour of human colorectal carcinoma-bearing miceFundam Clin Pharmacol201428665266024588516
- des RieuxAFievezVGarinotMSchneiderYJPréatVNanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approachJ Control Release2006116112717050027
- VeberDFJohnsonSRChengHYMolecular properties that influence the oral bioavailability of drug candidatesJ Med Chem200245122615262312036371
- PolyakovNEKispertLDWater soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid deliveryCarbohydr Polym201512820721926005157
