?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
The purpose of this study was to investigate the acute toxic potential of cerium oxide nanoparticles (CNPs) synthesized by pullulan in adult male Wistar rats.
Patients and methods
Thirty male Wistar rats randomly were divided into five experimental groups of six animals each. The animals were received 50, 100, 200, and 400 mg/kg CNPs for 14 consecutive days. At the end of the experiment, the rats were euthanized and histopathological evaluation of the liver and renal tissues, as well ass, the markers of serum oxidative stress including thiobarbituric acid reactive substances, total sulfhydryl content, and antioxidant capacity (using ferric reducing/antioxidant power assay) were assessed. Hematological parameters and the activity of liver function enzymes were also measured.
Results
The results of this study showed that CNPs caused no significant changes in the activity of liver enzymes, hepatic and renal histopathology and hematological parameters, while significantly improved serum redox status.
Conclusion
Acute administration of pullulan-mediated CNPs is safe and possess antioxidant activity.
Introduction
In the last few decades, nanotechnology has been employed throughout biomedical research studies.Citation1–Citation3 Cerium oxide nanoparticles (CNPs), consisting of cerium atoms linked by oxygen atoms, have been used in commercial, industrial, and biological applications.Citation4–Citation6 CNPs are considered as one of the most interesting nanomaterials due to their catalytic attributes and seem to be practical in therapy applications.Citation7,Citation8 Furthermore, these nanoparticles are extensively employed for producing oxygen sensors,Citation9 automotive catalytic converters,Citation10 and so on. Pullulan, a unique natural material, is a water-soluble polysaccharide that could be used in various biomedical/bioengineering applications due to its non-toxic, non-immunogenic, non-mutagenic, and non-carcinogenic nature.Citation11,Citation12 In our pervious study, we have prepared ceria nanoparticles by using pullulan as a capping agent that could affect the morphology of CNPs. In fact, the presence of hydroxyl groups in the backbone would provide a medium that would interact effectively with cationic cerium ions.Citation13 It was illustrated from the cytotoxicity results that CNPs have no toxicity against Neuro2A cells of <125 μg/mL.Citation14 The disease progression of many neurodegenerative conditions is strongly related to the oxidative damage that is caused by increasing the generation of reactive oxygen species (ROS).Citation15 Nowadays, the antioxidant properties of CNPs have been discovered to be throughout the Ce3+/Ce4+ redox reaction on the surface of nanoparticles,Citation4,Citation16 which could be beneficial in biomedical applications and protect cells from damage due to radiation, oxidative stress, or inflammation.Citation17 Since CNPs are capable of stimulating the catalytic potency of superoxide dismutase (SOD),Citation18 it could be applied as a potent antioxidant. Redox properties of CNPs could also detoxify the existing free radicals for prolonged time intervals by maintaining its bioactivity within the tissues.Citation19 The main objective of this work was to evaluate the effects of biosynthesized CNPs on oxidative stress status and biochemical and hematological parameters, along with the histopathological alterations in adult male Wistar rats.
Materials and methods
CNP synthesis and its cytotoxicity
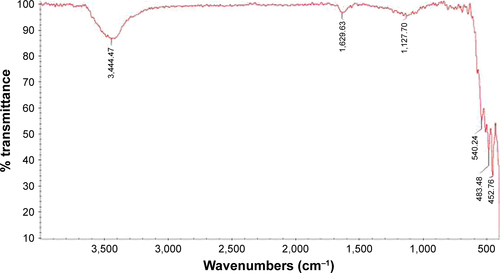
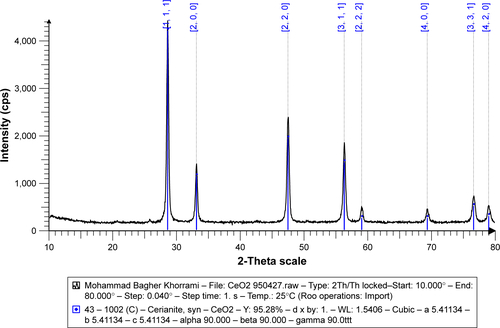
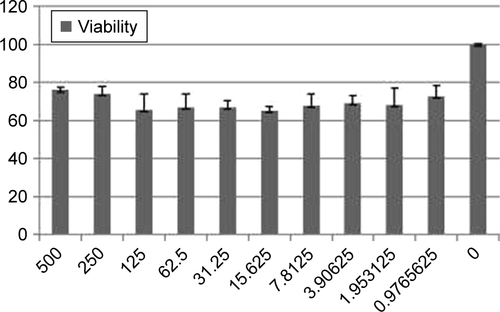
CNPs were prepared from a previously reported method.Citation14 Briefly, to prepare CNPs, Ce(NO3)3⋅6H2O (10.1 g) was dissolved in double distilled water (ddH2O, 25 mL) and stirred for 30 minutes. Then, dissolved pullulan (1.5 g in 50 mL ddH2O) was added at 60°C with string for 8 hours. Finally, the obtained resin was calcined (rate: 3°C/min) at 500°C for 2 hours. The CNPs were characterized by FTIR (), FESEM (), and X-ray diffraction (XRD) (). Cell viability of CNPs was determined by MTT assay against Neuro2A cells as described in the literature.Citation14 The detailed procedure is provided in the Supplementary materials. The absorbance of the amount of formazan formation was measured by a plate reader (statfax303) at λmax =570 and 620 nm ().
Animals and experimental procedure
We obtained male Wistar rats (weighing 200–230 g) from the Animal Center of School of Medicine, Mashhad University of Medical Sciences (MUMS), Mashhad, Iran. Then, they were provided with accommodations, which included housing and constant temperature (22°C±2°C), as well as the standard conditions of 12 hour light/dark cycle and free access to food pellets and tap water. The rats had been allowed to acclimatize for 1 week prior to the testing process. All the designated rats were handled in conformity with the National Institutes of Health Guidance for Care and Use of Laboratory Animals, while the Animal Ethics Committee of MUMS had authorized their usage for this particular project. The procedure was performed in five separate groups, each comprising six rats divided randomly. Animals in the experimental groups have been treated intraperitoneally with CNPs in order to obtain 50, 100, 200, and 400 mg/kg in 14 consecutive days. The negative control group has been injected with normal saline for 14 days.
Laboratory analyses
The anesthetizing process has been carried out by using ketamine and xylazine on the rats subsequent to the passage of the 14-day experiment in animal laboratory of MUMS, Faculty of Medicine. The blood samples were collected from the abdominal aorta and were accumulated and kept within sterile tubes with an anticoagulant (K3-EDTA) in order to investigate the hematological parameters. Non-anticoagulant was used in biochemical testing procedures such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), glucose, urea, creatinine (Cr), and direct and total bilirubin (Bill-D/T) by using the related commercial kits and a Biotecnica BT-3500 chemistry analyzer (Diamond Diagnostics, Holliston, MA, USA).Citation20 After blood serums were separated by centrifuge, they were analyzed by Biotechnica BT-3500 chemistry analyzer (Diamond Diagnostics; USA) using glucose, Cr, Bill-D/T, urea, AST, ALT, ALP and LDH reagents. The experimental assays were performed at a commercial laboratory (Laboratory of Modern Sciences and Technology Department at Mashhad University of Medical Sciences) using Man company kits in Iran under license of ELItech group, France. The detailed protocols could be obtained from the Man Company website (http://mancompany.com/en/; in biochemistry products). Routine hematological parameters including white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (Hb) concentration, hematocrits (HTC), mean corpuscular volume (MCV), platelet (PLT) count, and RBC distribution width (RDW) have been determined by the application of a blood cell counter (Sysmex KX-21N™; Sysmex, Hyogo, Japan). Serum was used for evaluating malondialdehyde (MDA), SOD activity, total sulfhydryl (SH) groups, catalase activity, and ferric reducing/antioxidant power (FRAP). The results were analyzed by one-way ANOVA using GraphPad Prism version 6.01 (GraphPad Software, Inc., La Jolla, CA, USA). Data were expressed as mean ± SEM, and values were considered statistically significant at P<0.05. The protocol was confirmed by the Animal Use and Care Advisory Committee of MUMS.
Histopathological examination
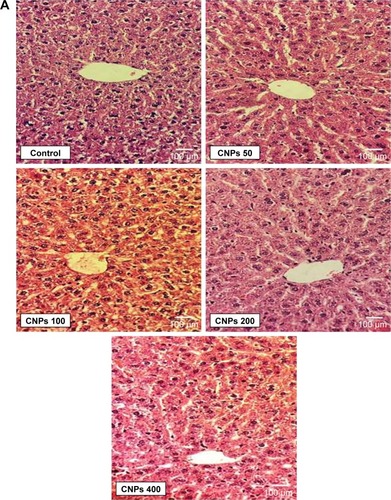
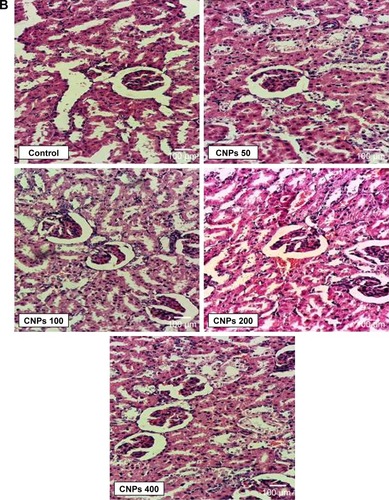
After collecting the blood samples, the rats were euthanized and the liver and kidney tissues were removed and fixed in 10% formalin. After mounting, a 5 μm section of each tissue was prepared and then stained with H&E. Histopathological changes were then investigated and blindly scored by a pathologist.Citation21,Citation22
Measurement of MDA
The levels of MDA, as the final product of lipid peroxidation process, were determined using thiobarbituric acid (TBA), as the reagent.Citation23,Citation24 Briefly, 100 μL of serum was mixed with 2 mL of TCA-TBA-HCl reagent (15% trichloroacetic acid, 0.67% TBA, and 0.25 N HCl) and heated for 45 minutes in a boiling water bath. After cooling, the mixture was centrifuged at 3,000 rpm for 10 minutes. The supernatant was collected, and the absorbance was read against the blank reagent at 532 nm. The amount of MDA produced was calculated in μmol/L.Citation23
Determination of SOD activity
SOD activity in serum was evaluated using a spectrophotometric method.Citation25 Briefly, the sample was incubated in a solution containing Tris-HCl buffer (pH 8.2, 50 mM) and EDTA (1 mM). Reaction was initiated by the addition of 0.2 mM pyrogallol. Oxidation of pyrogallol was measured at 420 nm for 10 minutes, at intervals of 1 minute. The percentage inhibition of pyrogallol autoxidation was determined using the following equation:
Assay of total SH groups
Total SH groups were measured using 5,5′-dithiobis-2-nitrobenzoic acid (DTNB).Citation26 Briefly, 1 mL of Tris-EDTA buffer (pH 8.6) was added to 50 μL of serum and 50 μL of the sample. The sample absorbance was observed at 412 nm against Tris-EDTA buffer (A1). Afterward, 20 μL of DTNB reagent (10 mM in methanol) was added to the mixture and, after 15 minutes, the sample absorbance was read again (A2). Moreover, the absorbance of DTNB reagent was read as a blank (B). Total thiol concentration (mM) was determined using the following equation:
Assay of catalase activity
The decomposition of hydrogen peroxide (H2O2) by catalase can be measured directly by the decrease in H2O2absorbance at 240 nm. The difference in absorbance per unit time is a measure of catalase activity. The results obtained for the sample containing 500 μL of serum and 500 μL of the substrate solution (10 mM H2O2 prepared in 50 mM phosphate buffer, pH 7.0) were compared with those of a blank containing 500 μL of phosphate buffer, instead of substrate solution, and 500 μL of sample. The reaction was initiated by adding the substrate solution and the solution was incubated at 20°C for 1 minute. Catalase activity was manifested as mmol H2O2/min/mg protein (U/L). An enzyme unit has been defined as the quantity of enzymes that can catalyze the release of 1 μmol of H2O2 per minute at the temperature of 20°C. Specific activity has been measured with reference to units per milligram of protein.Citation27
Assay of FRAP
The serum antioxidant (reducing) power was measured using the FRAP assay.Citation26 Briefly, 50 μL of serum was added to 1.5 mL of freshly prepared and pre-warmed (37°C) FRAP reagent (300 mM acetate buffer, 10 mM TPTZ in 40 mM HCl, and 20 mM FeCl3 in the ratio of 10:1:1) in a test tube and incubated at 37°C for 8 minutes. The optical density of the blue colored complex was read against the blank reagent at 593 nm. The data were expressed as μmol of ferric ions reduced to ferrous form per liter (FRAP-value).Citation26
Results
Effect of CNPs on hematological parameters
As it is illustrated in , there has been no significant changes in the WBC, RBC, HCT, Hb, PLT, MCV, and RDW indices in all of the experimental groups in comparison with the control group (P>0.05 for all).
Table 1 Effect of CNPs on hematological parameters in male Wistar rats
Effect of CNPs on biochemical markers
According to the obtained data (), significant differences in serum BS, urea, Cr, Bill-D, and Bill-T levels, as well as the ALP, LDH, AST, and ALT activities were seen throughout the experimental groups compared to the control (P>0.05 for all).
Table 2 Biochemical serum profile of male Wistar rats after 14 days exposure to CNPs
Histopathological examinations
Liver
The liver tissue was investigated for the existence of any pathological changes such as inflammatory reactions, necrosis, apoptosis, and fibrosis in different liver zones, ports, and regional centrilobular space. Light microscopic evaluation of liver tissue in all the doses of CNPs did not display any notable variation in comparison with the control (, P>0.05).
Kidney
Structural changes in the renal glomerular tubules and vessels were also investigated. The analyses of the kidneys revealed no significant pathological changes (P>0.05) between the CNP-treated group and the control group ().
Effect of CNPs on MDA level
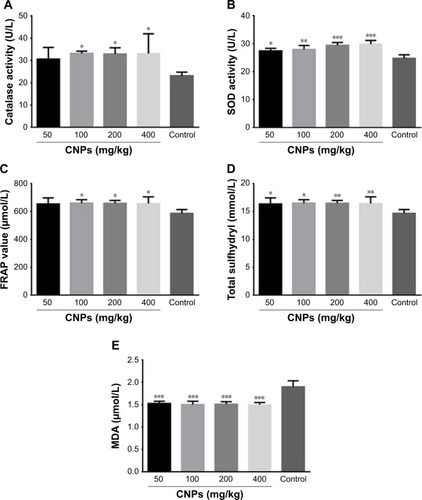
shows that that CNP administration at doses of 50, 100, 200, and 400 mg/kg significantly (P<0.001 for all) decreased the MDA level to 1.5±0.02, 1.5±0.03, 1.5±0.03, and 1.5±0.02 μmol/L, as compared to control (1.9±0.02 μmol/L), respectively.
Figure 2 Effects of CNPs on serum oxidant/antioxidant status of male Wistar rats after treatment with CNPs for 14 days. (A) Effect of CNPs on MDA level. (B) Effect of CNPs on SOD activity. (C) Effect of CNPs on total SH level. (D) Effect of CNPs on CAT activity. (E) Effects of CNPs on FRAP values are expressed as mean ± SEM (n=7). *P<0.05, **P<0.01, ***P<0.0001 as compared with the control group.
Abbreviations: CNPs, cerium oxide nanoparticles; MDA, malondialdehyde; SOD, superoxide dismutase; SH, sulfhydryl; CAT, catalase; FRAP, ferric reducing/antioxidant power.
Effect of CNPs on SOD level
As illustrated in , administration of CNPs resulted in significant increase in SOD activity in CNP-treated rats (27.4±0.4 U/L at 50 mg/kg, P<0.05; 28.0±0.6 U/L at 100 mg/kg, P<0.01; 29.4±0.4 U/L at 200 mg/kg, P<0.001; and 29.8±0.6 U/L at 400 mg/kg, P<0.001), as compared to the control (24.7±0.5 U/L).Citation28
Effect of CNPs on SH level
As shown in , administration of CNPs at doses of 50, 100, 200, and 400 mg/kg increased the total SH content to 15.6±0.3 mM (P<0.05), 15.6±0.8 mM (P<0.05), 16.0±0.9 mM (P<0.01) and 16.3±0.5 mM (P<0.01) respectively, in comparison with control (12.3±0.5 mM).
Effect of CNPs on CAT level
As shown in , the administration of CNPs at doses of 50, 100, 200, and 400 mg/kg has caused an increase in the CAT level toward 30.6±2.4, 33.1±0.5, 32.9±1.2, and 33.0±4.0 U/L, as compared to the control (23.0±0.7 U/L), respectively (P<0.05 for all).
Effect of CNPs on FRAP level
We have perceived a notable increase within the FRAP level following the administration of CNPs with doses of 50 (653.3±22.12), 100 (659.8±12.55), 200 (659.0±9), and 400 mg/kg (657.8±21.48) in comparison with the control (586.6±12), respectively (P<0.05 for all) ().
Discussion
Investigation of the effects of nanomaterials on cellular function is necessary to confirm the safety of utilization of these materials in medical applications.Citation10 Biochemical parameters have a significant role in determining toxicity because changes in their levels are considered as one of the best general indicator of the toxicity.Citation29–Citation31 Moreover, these particular biochemical tests can be easily carried out in most of the diagnostic laboratories.Citation32 On the other hand, CNPs were known as antioxidants because of their dual redox states on the surface.Citation33–Citation35 Antioxidants could be harmful per se, because of pro-oxidant activity.Citation36–Citation38 For example, Srinivas et al suggested that acute exposure of CNPs through inhalation route may induce cytotoxicity via oxidative stress and may lead to a chronic inflammatory response.Citation39 Adebayo et al also showed that administration of 100–300 mg/kg CNPs thrice in a week for 5 consecutive weeks to mice induces testicular dysfunction via disruption of antioxidant/oxidant balance and endocrine suppression.Citation40
In compliance with our previous studies, CNPs were characterized by FTIR, FESEM, and XRD. The results of characterization were in good agreement with our previous report.Citation14 Nanoparticles can be potentially hazardous to the central nervous system because they can readily bypass or cross the blood–brain barrier.Citation41–Citation43 One of the limitations of our study is the lack of brain pathology data following CNP administration to rat. Therefore, Neuro2A cells (as neuroblastoma) was used for the evaluation of possible toxicity of CNPs, which showed low dose-toxicity under 500 μg/mL. Intraperitoneal administration is a commonly used technique to administer large quantity of medicine in small animals. Although it is parenteral route, but unlike intravenous injection, the drug delivery is much slower.Citation44 Therefore, intravenous injection usually uses lower doses of nanoparticles that is usually under 5 mg/kg for CNPs,Citation45–Citation47 but in intraperitoneal injection, it is safer if higher doses were used.Citation48 Herein, we want to show that CNPs are safe following acute administration. To evaluate the toxicity of the substances in vivo, CNPs were administered to rat and then some toxicological parameters such as histopathological parameters were evaluated and hematotoxicity analyses were performed. CNPs did not cause any vital alteration throughout the hematological and biochemical parameters of experimental groups relative to negative controls. In addition, histopathological examination of liver and kidney tissues, which were performed by using light microscopy technique, has not pointed out any notable pathological changes. This seems to be in parallel with the obtained normal results from biochemical parameters and partly confirms the safety of CNPs. In support of our results, Srinivas et al also reported that CNPs did not cause any harmful effects on liver and kidney functions or hematology or blood biochemistry of rats exposed to CNPs at 14-day post exposure period.Citation39 Furthermore, the measures of serum oxidative/anti-oxidative indices including MDA, SOD activity, SH groups, and the catalase (CAT) indices among all experimental doses in treated rats have a significant change in comparison with the control group, which have indicated that they can considerably effect on the potency of antioxidant. Antioxidant effects of CNPs may not be very obvious in healthy non-injured animals (ie, physiological conditions), but it has been reported that protective antioxidant effects are found under pathological conditions.Citation49 On the other hand, according to the FRAP test on serums obtained from rats that had received CNPs, a stronger antioxidant activities in contrast to the control group was observed. These data are in agreement with other studies indicating the ability of CNPs to bind to oxygen under reducing and oxidizing circumstances, which plays a key role in neutralizing a variety of ROS.Citation50,Citation51 CNPs have been so far applied for ameliorating the signs of several oxidative stress-based animal model’s diseases, including Alzheimer’s, cardiomyopathy, and cancers.Citation10,Citation52,Citation53 Indeed, the balance between the oxidant production and antioxidant content is pivotal for a correct cell function.Citation54 Therefore, anti-oxidant agents might have toxic or nontoxic effects. In general, CNPs exhibited protective effects against oxidative stress, in vitro and in vivo.Citation55,Citation56 The current research was carried out to exactly evaluate the safety/toxicity of CNP (synthesized with pullulan) administration, as an anti-oxidant agent. The scientific databases revealed that there are several studies about the effects of CNPs on different types of cells. For instance, a study performed on the rat retina cells showed that nanoceria was found to be non-toxic.Citation57 Similarly, the results of another study stated the non-toxicity of 2,000 mg/kg body weight per day of CNPs that was administrated orally.Citation58 In contrast, cerium oxide was toxic to bronchial epithelial lung fibroblasts in culture,Citation59 but non-toxic to mammary epithelial cells,Citation60 macrophages,Citation61 immortalized keratinocytes,Citation62 or immortalized pancreatic epithelial cells.Citation56 They are not toxic in normal cells, while the physiological pH is suitable for enabling canonical radical scavenging by CNPs.Citation63,Citation64
Conclusion
Based on the findings demonstrated here, it was found that pullulan-mediated CNPs is safe following acute administration. In this work the safety and antioxidant ability of CNPs have been studied by evaluating several markers of serum oxidative stress, hematological parameters, and some liver enzymes. It could be concluded that CNPs have no damage to liver enzymes, liver, and renal tissues. CNPs seem safe to be used for medical applications and have a protective behavior against the oxidative stress. Of course, further studies, including more doses, are required to prove its safety in subacute/chronic situations. At the same time, further research studies should also be performed on pullulan-mediated CNPs to further develop its potential as an effective antioxidant for the management of illnesses caused by uncontrolled oxidative stress.
Acknowledgments
We would like to thank the Vice Chancellery for Research and Technology, MUMS for financial support (grant no 930954) and facilities.
Supplementary materials
Disclosure
The authors report no conflicts of interest in this work.
References
- SrivastavaVGusainDSharmaYCCritical review on the toxicity of some widely used engineered nanoparticlesInd Eng Chem Res201554246209623310.1021/acs.iecr.5b01610
- HaganCTMedikYBWangAZNanotechnology Approaches to Improving Cancer ImmunotherapyAdv Cancer Res2018139353629941106
- SridharanKEmerging Trends of Nanotechnology in Environment and Sustainability: A Review-Based ApproachSpringer2018
- ReedKCormackAKulkarniAExploring the properties and applications of nanoceria: is there still plenty of room at the bottom?Environ Sci Nano201415390405
- HasanzadehLOskueeRKSadriKGreen synthesis of labeled CeO2 nanoparticles with 99mTc and its biodistribution evaluation in miceLife Sci201821223324010.1016/j.lfs.2018.10.01030304691
- MaRZhangSWenTA critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutantsCatal Today201810.1016/j.cattod.2018.11.016
- WasonMSZhaoJCerium oxide nanoparticles: potential applications for cancer and other diseasesAm J Transl Res20135212613123573358
- NourmohammadiEOskueeRKHasanzadehLCytotoxic activity of greener synthesis of cerium oxide nanoparticles using carrageenan towards a WEHI 164 cancer cell lineCeram Int20184416195701957510.1016/j.ceramint.2018.07.201
- IzuNShinWMatsubaraIMurayamaNDevelopment of resistive oxygen sensors based on cerium oxide thick filmJ Electroceramics2004131–370370610.1007/s10832-004-5179-7
- NiuJAzferARogersLMWangXKolattukudyPECardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathyCardiovasc Res200773354955910.1016/j.cardiores.2006.11.03117207782
- RekhaMSharmaCPPullulan as a promising biomaterial for biomedical applications: a perspectiveTrends Biomater Artif Organs2007202116121
- AbediniFEbrahimiMRoozbehaniAHDombAJHosseinkhaniHOverview on natural hydrophilic polysaccharide polymers in drug deliveryPolym Adv Technol201829102564257310.1002/pat.v29.10
- NonsuwanPPuthongSPalagaTMuangsinNNovel organic/inorganic hybrid flower-like structure of selenium nanoparticles stabilized by pullulan derivativesCarbohydr Polym201818491910.1016/j.carbpol.2017.12.02929352947
- KhorramiMBSadeghniaHRPasdarAGhayour-MobarhanMRiahi-ZanjaniBDarroudiMRole of Pullulan in preparation of ceria nanoparticles and investigation of their biological activitiesJ Mol Struct2018115712713110.1016/j.molstruc.2017.12.053
- ShahVShahSShahHAntibacterial activity of polymer coated cerium oxide nanoparticlesPLoS One2012710e4782710.1371/journal.pone.004782723110109
- NelsonBJohnsonMWalkerMRileyKSimsCAntioxidant cerium oxide nanoparticles in biology and medicineAntioxidants2016521510.3390/antiox5020015
- PelletierDASureshAKHoltonGAEffects of engineered cerium oxide nanoparticles on bacterial growth and viabilityAppl Environ Microbiol201076247981798910.1128/AEM.00650-1020952651
- PaganoGRare Earth Elements in Human and Environmental Health: At the Crossroads Between Toxicity and SafetyPan Stanford2016
- PourkhaliliNHosseiniANili-AhmadabadiABiochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic ratsWorld J Diabetes201121120421010.4239/wjd.v2.i11.20422087357
- AbbasalipourkabirRMoradiHZareiSToxicity of zinc oxide nanoparticles on adult male Wistar ratsFood Chem Toxicol20158415416010.1016/j.fct.2015.08.01926316185
- ForouzanfarFAminBGhorbaniANew approach for the treatment of neuropathic pain: Fibroblast growth factor 1 gene-transfected adipose-derived mesenchymal stem cellsEur J Pain201722229531028949091
- GhorbaniAMohebbatiRJafarianAHToxicity evaluation of hydroalcoholic extract of Ferula gummosa rootRegul Toxicol Pharmacol201677354110.1016/j.yrtph.2016.02.00826893240
- ForouzanfarFHosseinzadehHEbrahimzadeh BideskanASadeghniaHRAqueous and ethanolic extracts of Boswellia serrata protect against focal cerebral ischemia and reperfusion injury in ratsPhytother Res201630121954196710.1002/ptr.570127515127
- BotsoglouNAFletourisDJPapageorgiouGEVassilopoulosVNMantisAJTrakatellisAGRapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samplesJ Agric Food Chem19944291931193710.1021/jf00045a019
- MarklundSMarklundGInvolvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutaseFEBS J1974473469474
- SadeghniaHRShaterzadehHForouzanfarFHosseinzadehHNeuroprotective effect of safranal, an active ingredient of Crocus sativus, in a rat model of transient cerebral ischemiaFolia Neuropathol201755320621310.5114/fn.2017.7048528984113
- QujeqDRezvaniTCatalase (antioxidant enzyme) activity in streptozotocin-induced diabetic ratsInt J Diabetes Metab2007152224
- RajabianABoroushakiMTHayatdavoudiPSadeghniaHRBoswellia serrata protects against glutamate-induced oxidative stress and apoptosis in PC12 and N2a cellsDNA Cell Biol2016351166667910.1089/dna.2016.333227494534
- KalyanaramanVNaveenSVMohanaNBiocompatibility studies on cerium oxide nanoparticles–combined study for local effects, systemic toxicity and genotoxicity via implantation routeToxicol Res2019812537
- SinglaRSharmaCShuklaAKAcharyaAToxicity concerns of therapeutic nanomaterialsJ Nanosci Nanotechnol20191941889190710.1166/jnn.2019.1650230486930
- YangTWuTLvLCeria oxide nanoparticles an ideal carrier given little stress to cells and ratsJ Nanosci Nanotechnol20181863865386910.1166/jnn.2018.1501829442720
- WangBFengWYWangTCAcute toxicity of nano- and microscale zinc powder in healthy adult miceToxicol Lett2006161211512316165331
- PopovAPopovaNTarakinaNVIntracellular delivery of antioxidant CeO2 nanoparticles via polyelectrolyte microcapsulesACS Biomater Sci Eng201842453246210.1021/acsbiomaterials.8b00489
- SerebrovskaZSwansonRPortnichenkoVAnti-inflammatory and antioxidant effect of cerium dioxide nanoparticles immobilized on the surface of silica nanoparticles in rat experimental pneumoniaBiomed Pharmacother201792697710.1016/j.biopha.2017.05.06428531802
- ShabrandiAAziziSHobbenaghiROwnaghAKeshipourSThe healing effect of chitosan supported nano-CeO2 on experimental excisional wound infected with pseudomonas aeruginosa in ratIran J Vet Surg2017122920
- ErikssonPTalAASkallbergACerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancementSci Rep201881699910.1038/s41598-018-25390-z29725117
- DograYArkillKPElgyCCerium oxide nanoparticles induce oxidative stress in the sediment-dwelling amphipod Corophium volutatorNanotoxicology201610448048710.3109/17435390.2015.108858726554927
- PešićMPodolski-RenićAStojkovićSAnti-cancer effects of cerium oxide nanoparticles and its intracellular redox activityChem Biol Interact2015232859310.1016/j.cbi.2015.03.01325813935
- SrinivasARaoPJSelvamGMurthyPBReddyPNAcute inhalation toxicity of cerium oxide nanoparticles in ratsToxicol Lett2011205210511510.1016/j.toxlet.2011.05.102721624445
- AdebayoOAkinloyeOAdaramoyeOCerium oxide nanoparticle elicits oxidative stress, endocrine imbalance and lowers sperm characteristics in testes of balb/c miceAndrologia2018503e1292010.1111/and.2018.50.issue-3
- Biddlestone-ThorpeLMarchiNGuoKNanomaterial-mediated CNS delivery of diagnostic and therapeutic agentsAdv Drug Deliv Rev201264760561310.1016/j.addr.2011.11.01422178615
- CzajkaMSawickiKSikorskaKPopekSKruszewskiMKapka-SkrzypczakLToxicity of titanium dioxide nanoparticles in central nervous systemToxicol In Vitro20152951042105210.1016/j.tiv.2015.04.00425900359
- FengXChenAZhangYWangJShaoLWeiLCentral nervous system toxicity of metallic nanoparticlesInt J Nanomedicine201510432126170667
- TurnerPVBrabbTPekowCVasbinderMAAdministration of substances to laboratory animals: routes of administration and factors to considerJ Am Assoc Lab Anim Sci201150560061322330705
- KarakotiAMonteiro-RiviereNAggarwalRNanoceria as antioxidant: synthesis and biomedical applicationsJOM2008603333710.1007/s11837-008-0029-820617106
- DasSReed McDonaghPSelvan SakthivelTTissue deposition and toxicological effects of commercially significant rare earth oxide nanomaterials: material and physical propertiesEnviron Toxicol201732390491710.1002/tox.2229027255187
- DasSChigurupatiSDowdingJTherapeutic potential of nanoceria in regenerative medicineMRS Bull2014391197698310.1557/mrs.2014.221
- NajafiRHosseiniAGhaznaviHMehrzadiSSharifiAMNeuroprotective effect of cerium oxide nanoparticles in a rat model of experimental diabetic neuropathyBrain Res Bull201713111712210.1016/j.brainresbull.2017.03.01328373151
- MitraRNMerwinMJHanZConleySMAl-UbaidiMRNaashMIYttrium oxide nanoparticles prevent photoreceptor death in a light-damage model of retinal degenerationFree Radic Biol Med20147514014810.1016/j.freeradbiomed.2014.07.01325066531
- RobinsonRDSpanierJEZhangFChanS-WHermanIPVisible thermal emission from sub-band-gap laser excited cerium dioxide particlesJ Appl Phys20029241936194110.1063/1.1494130
- EschFFabrisSZhouLElectron localization determines defect formation on ceria substratesScience2005309573575275510.1126/science.111156816051791
- D’AngeloBSantucciSBenedettiECerium oxide nanoparticles trigger neuronal survival in a human Alzheimer disease model by modulating BDNF pathwayCurr Nanosci20095216717610.2174/157341309788185523
- GiriSKarakotiAGrahamRPNanoceria: a rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancerPLoS One201381e5457810.1371/journal.pone.005457823382918
- RiahiBRafatpanahHMahmoudiMEvaluation of suppressive effects of paraquat on innate immunity in Balb/c miceJ Immunotoxicol201181394510.3109/1547691X.2010.54309521299353
- ZhouXWongLLKarakotiASSealSMcGinnisJFNanoceria inhibit the development and promote the regression of pathologic retinal neovascularization in the Vldlr knockout mousePLoS One201162e1673310.1371/journal.pone.001673321364932
- WasonMSColonJDasSSensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS productionNanomedicine20139455856910.1016/j.nano.2012.10.01023178284
- WongLLHirstSMPyeQNReillyCMSealSMcGinnisJFCatalytic nanoceria are preferentially retained in the rat retina and are not cytotoxic after intravitreal injectionPLoS One201383e5843123536794
- RameshARatlaNNIndukurRAcute and sub-acute oral toxicity assessment of the cerium oxide nanoparticles in wistar ratsInt J Pharmacol2014514650
- ParkE-JChoiJParkY-KParkKOxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cellsToxicology200824519010010.1016/j.tox.2007.12.02218243471
- TarnuzzerRWColonJPatilSSealSVacancy engineered ceria nanostructures for protection from radiation-induced cellular damageNano Lett20055122573257710.1021/nl052024f16351218
- HirstSMKarakotiASTylerRDSriranganathanNSealSReillyCMAnti-inflammatory properties of cerium oxide nanoparticlesSmall20095242848285610.1002/smll.20090104819802857
- SinghSKumarAKarakotiASealSSelfWTUnveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticlesMol Biosyst20106101813182010.1039/c0mb00014k20697616
- XiaTKovochichMLiongMComparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress propertiesACS Nano20082102121213410.1021/nn800511k19206459
- ChenJPatilSSealSMcGinnisJFRare earth nanoparticles prevent retinal degeneration induced by intracellular peroxidesNat Nanotechnol20061214215010.1038/nnano.2006.9118654167