Abstract
Aims
Despite daily increase in diabetic patients in the world, currently approved medications for this disease, at best, only reduce its progression speed. Using novel technologies is a solution for synthetizing more efficient medicines. In the present study, we evaluated anti-diabetic effects of DIBc, a nano metal–organic framework, which is synthetized based on nanochelating technology.
Methods
High-fat diet and streptozotocin-induced diabetic rats were treated by DIBc or metformin for 6 weeks.
Results
DIBc decreased plasma glucose, triglyceride, cholesterol, high-density lipoprotein, and low-density lipoprotein compared with diabetic and metformin groups. In DIBc-treated rats, significant homeostasis model assessment of insulin resistance index, malondialdehyde, and tumor necrosis factor-α decrease was observed. H&E staining showed increased islet number and area in DIBc-treated rats compared with diabetic controls.
Conclusion
The results showed anti-diabetic effects of nanochelating-based framework. So DIBc, as a nano structure, has the capacity to be evaluated in future studies as a novel anti-diabetic agent.
Introduction
“Despite the significant scientific advances of the last century, diabetes remains a major health challenge across the world, is responsible for millions of deaths annually and limits many lives with life-threatening complications”.Citation1 Unfortunately, the available drugs, including metformin, which is the first-line drug in diabetes, can only delay the disease progression.Citation2
Other than that, hypoglycemic adverse effects, lactic acidosis, overweight, and liver dysfunction are the side effects in patients using these drugs.Citation2,Citation3 In many patients, after the treatments start, tolerance is seen, so it is necessary to combine multiple drug regimens to control the disease.Citation4
Oxidative stress, inflammatory conditions (increase in inflammatory cytokines, eg, tumor necrosis factor [TNF]-α) and impaired metabolism of metal ions, such as iron, zinc, selenium, and magnesium are the factors involved in the initiation, progression, and development of diabetes and also its complications, which have already been investigated and proven by numerous studies.Citation5–Citation8
In recent years, nanotechnology and smart nano structures have shown considerable potential for a large number of biomedical applications like monitoring, diagnosis, repair, and treatment of human biological systems.Citation9 Nanoscale metal–organic frameworks (NMOFs) are categorized as one group of hybrid materials, which are created by self-assembly of polydentate bridging ligands and metal-connecting points having a number of benefits compared with conventional nanomedicines, including their intrinsic biodegradability and structural and chemical variety.Citation10,Citation11
Nanochelating technology is a modern field capable of designing and synthetizing efficient nano structures by self-assembly method. In the previous studies, therapeutic aspects of nanochelating-based structures were evaluated in vitro and in vivo.Citation12–Citation15 In the current study, we assessed the effects of DIBc, a nanochelating-based NMOF on high-fat diet and streptozotocin (STZ)-induced diabetic rats.Citation16,Citation17
According to the beneficial effects of selenium, zinc, and chromium supplementation on diabetic patients and also diabetic animal models, the NMOF structure contained these elements.Citation18–Citation23 We compared anti-diabetic effects of DIBc NMOF with metformin as a first-line medication of diabetic patients and evaluated their effects on key blood parameters and pancreatic islets’ tissue as well as hepatic iron content.
Materials and methods
DIBc NMOF synthesis
Sodour Ahrar Shargh company designed and synthesized DIBc by using nanochelating technology, registered at USPTO (US20120100372A1). DIBc is a structure from the family of MOFs in which carboxylic ligands are used. The clusters used in it include chrome, zinc, and selenium. Due to the chelate form of these elements and their nanoscale and porous structure, they have taken a new spatial structure with new and specific properties. First, 3 g of Zn (NO3) and 1 g of CrO should be completely dissolved in distilled water. Then, the solution should be heated a little and kept, and stirred at 40°C for 15 minutes. After that, 20 g of carboxylic acid should be slowly added to the solution while it is still being stirred. After stirring is finished, 0.5 g of Na2SeO3 is added to let the reaction start. The heater should be turned off exactly when the exothermic reaction is finished and then the solution should be allowed to get mixed gently and lose its temperature during 8 hours so that it slowly reaches the temperature of the surrounding environment. The resulting solution contains nano MOFs of DIBc, which will be separated from the solution through dialysis and then dried slowly at 40–45°C.
Brunauer, Emmett and Teller (BET) test was done on this drug to specify its porosity; the test showed that the porosity of the drug is 4,460 m2/g, which is the best amount of porosity for carboxylic MOFs ().
Table 1 BET test results on DIBc NMOF
High-resolution transmission electron microscopy (HRTEM)
DIBc HRTEM images were captured by a transmission electron microscope (Philips CM30-250KV model) in the University of Tehran Science and Technology Park.
Evaluation of DIBc toxicity
To assess the toxicity of DIBc, standard tests were carried out to evaluate the median lethal dose (LD50) according to the guidelines of the Organization for Economic Co-operation and Development (guideline 420), in the School of Pharmacy at Tehran University of Medical Sciences.
Animal model and treatment protocols
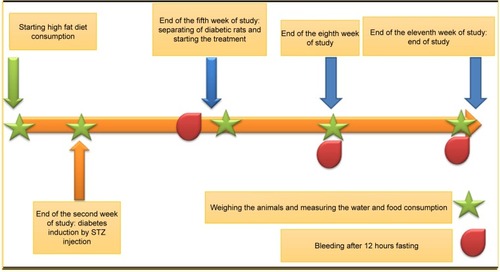
Male Wistar rats (150–180 g) were used in this study. The study started 1 week after animals’ environmental adaptation. All animals were maintained under 12-hour dark/light cycles with proper access to food and water, 3 animals in every polycarbonate conventional cages. The grouping pattern is shown in and the time line of the study is shown in . The high-fat diet consisted of 65% normal chow and 35% butter. The composition of normal chow and high-fat diet is shown in . Regular diet was given to normal control groups. Following 2 weeks of dietary intervention and 12 hours of fasting, the rats in groups C to G were injected intraperitoneally (i.p) with low dose of STZ (35 mg/kg, dissolved in 0.1 M sodium citrate buffer, pH 4.4), while the normal control group received injection of citrate buffer vehicle in a dose volume of 1 mL/kg. Fasting blood glucose was measured 3 weeks after the injection. The rats with fasting blood glucose levels >250 mg/dL were considered diabetic. The diabetic rats were divided into five groups: low (n=8), medium (n=8) and high dose (n=8) DIBc groups, Metformin (n=8), and control groups (n=8). All diabetic groups were fed on the high-fat diet for another 6 weeks. DIBc was dissolved in distilled water and injected i.p once a day during 6 weeks (). Body weight and food and water intake were measured at weeks 5, 8, and 11 () by measuring the difference between food or water amount in the cage and the remaining amount after 24 hours. At the end of the study, rats were euthanized using an overdose of isoflurane anesthesia. All the animal studies were conducted according to the relevant national and international guidelines of Shahid Beheshti University of Medical Sciences for the care and use of laboratory animals and Shahid Beheshti University of Medical Sciences also approved the experiments (SBMU.REC.1392.73).
Table 2 Groups of rats and their regimen
Table 3 Composition of normal and high-fat diet
Blood sampling and biochemical analysis
The sample collection was performed at weeks 5, 8, and 11 (). The samples were put in micro tubes, which contained 0.5% heparin, centrifuged at 3,000 rpm for 5 minutes and then stored at −20°C for the analysis of glucose, insulin, total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) (commercial ELISA kits, Pars Azmoon, Tehran, Iran). On the basis of fasting plasma insulin and glucose levels, homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated according to the formula: HOMA-IR = fasting insulin (µg/L)× fasting blood glucose (mg/dL)/405.Citation24,Citation25 The contents of plasma malondialdehyde (MDA) and TNF-α (ELISA kits, Zellbio, Ulm, Germany) were determined by commercially available kits according to the manufacturer’s directions at week 11.
Hepatic iron level and the hematologic parameters
The blood samples collected at week 11 were used for hema-tologic parameters assay. The hepatic iron level and hemato-logic parameters were assayed based on the previous study.Citation26
Histopathological examination of the pancreas
Optical microscopy was applied to conduct histopathological analyses on paraffin material. The fixation of pancreas tissue sections was carried out in 10% buffered formalin. After fixation, water was used to clean the sample, then it was soaked in 70% ethyl alcohol for 3–4 days and submerged in paraffin. Paraffin sections of 5 µm were obtained from rotational microtome and stained with H&E to evaluate the size and number of Langerhans islets.
Statistics
One-way ANOVA was performed followed by post hoc tests (Bonferroni’s test) for multiple comparisons. P-value of <0.05 was considered statistically significant.
Results
DIBc characteristics
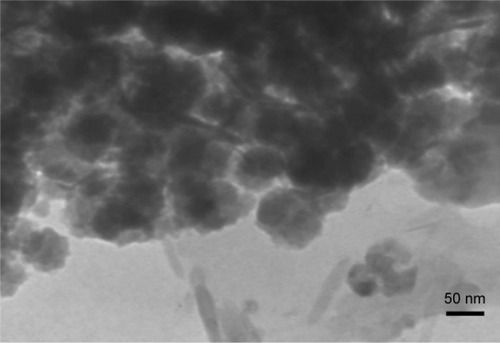
Imaging and analytical characterization of nanoparticles by HRTEM is carried out to determine the nanoparticle size. The image of DIBc indicates that the size of the NMOF is almost 25–30 nm ().
DIBc toxicity
DIBc toxicity report showed that intraperitoneal and oral LD50 of this NMOF was 37 and 130.5 mg/kg in mice, respectively.
DIBc effects on food and water intake and weight gain
The high-fat diet induced significant weight gain and decreased water intake in group B (High-fat diet group without STZ injection) compared with normal controls (P<0.05). The consumption of this diet decreased food intake, but it was not significant (). In group C (diabetic control group), STZ injection induced significant water intake and decreased weight gain (P<0.05).
Table 4 DIBc effects on food and water intake and weight gain
DIBc in group D (low dose) significantly decreased water intake compared with group C (diabetic controls) (P<0.05). It had no significant effects on food intake and weight gain. DIBc in group E (medium dose) had no significant effects on weight gain, and water and food intake. The DIBc NMOF in group F (high dose) significantly increased weight gain and decreased water intake compared with group C (diabetic controls) (P<0.05). It had no significant effects on food intake ().
Metformin had no significant effects on water and food intake and weight gain compared with group C (diabetic controls).
DIBc effects on food and water intake and weight gain
The high-fat diet induced significant increase in level of plasma glucose, insulin, TG, TC, LDL, and HDL (high fat diet group without STZ injection) compared with normal controls (P<0.05). In group C (diabetic control group), STZ injection induced significant increase in level of plasma glucose, TG, TC, LDL, and HDL (P<0.05). At the end of week 5 and 8 of the study, significant increase in plasma insulin level was seen in this group, but at the end of the study (P<0.05), there was no significant difference between group C and A ().
Table 5 DIBc effects on plasma glucose, insulin, TG, cholesterol, LDL, and HDL level
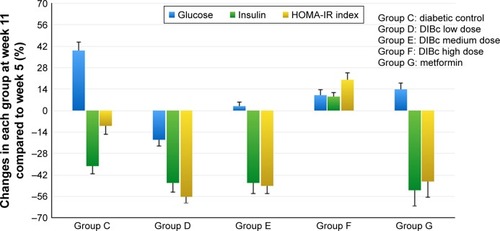
DIBc at all three doses significantly reduced glucose levels compared with diabetic control group (P<0.05). The high dose of DIBc increased insulin level compared with diabetic controls (P<0.05). This NMOF decreased HOMA-IR index at low and medium dose significantly (P<0.05), while it increased HOMA-IR index at high dose. The greatest hypoglycemic effect was observed in the low-dose group ( and ).
Figure 3 The percentage of HOMA-IR index and plasma glucose and insulin changes in each group at week 11 compared with the fifth week of the study. Each column shows mean ± SEM for eight rats.
Abbreviation: HOMA-IR, homeostasis model assessment of insulin resistance.
DIBc in group D (low dose) significantly decreased plasma level of TG, TC, HDL, and LDL () compared with group C (diabetic controls) (P<0.05). DIBc in group E (medium dose) significantly decreased plasma level of TC, HDL, and LDL compared with group C (diabetic controls) (P<0.05). It had no significant effects on plasma level of TG. The DIBc NMOF in group F (high dose) signifi-cantly decreased plasma level of TC, HDL, and LDL and increased TG level compared with group C (diabetic controls) (P<0.05).
At the end of the third week of the treatment (eighth week of the study, ), metformin significantly reduced glucose level (P<0.05). But at the end of the treatment (eleventh week of the study), glucose level increased significantly in this group ( and ), in a way that glucose level was significantly higher in metformin-treated group compared with group C (P<0.05). At the end of the study, insulin level and HOMA-IR index in the metformin group were lower than the diabetic control group, but not significant. Metformin had no significant effects on plasma level of TG, TC, HDL and LDL compared to group C (diabetic controls).
DIBc effects on plasma TNF-α and MDA level
In high-fat diet group (B), no significant change of TNF-α was seen compared with normal controls (). But combination of high-fat diet and STZ injection increased TNF-α level significantly (P<0.05). DIBc significantly decreased this cytokine in three doses compared with diabetic controls (P<0.05)
Figure 4 The plasma level of TNF-α (A) and MDA (B). Each column shows mean ± SEM for eight rats.
Notes: Significant differences vs the normal control group have been specified by square (#) meaning P-value <0.05. Significant differences vs the diabetic group have been specified by asterisk (*) meaning P-value <0.05.
Abbreviation: MDA, malondialdehyde.
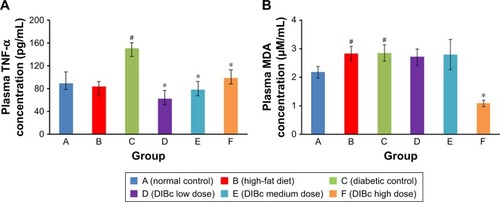
In high-fat diet (B) and diabetic groups, plasma MDA level increased () compared with normal control (P<0.05). DIBc high-dose treated rats had lower MDA concentration compared with diabetic controls (P<0.05).
DIBc effects on liver iron level and hematological indices
Liver iron level significantly increased in the high-fat diet group () compared with the normal control group (P<0.05). However, no significant difference in the liver iron level of all diabetic groups was observed (P<0.05).
Figure 5 Hepatic (A) and plasma (B) iron level. Each column shows mean ± SEM for eight rats.
Note: Significant differences vs the normal control group have been specified by square (#) meaning P-value <0.05.
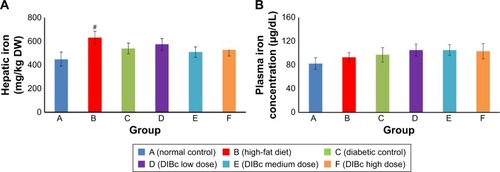
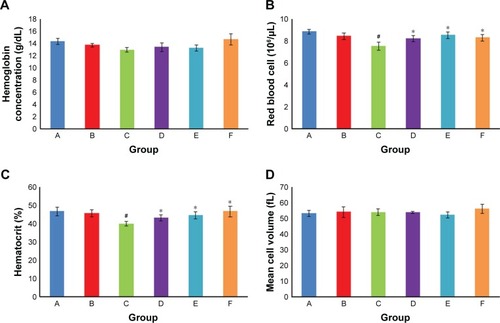
Hemoglobin concentration and mean corpuscular volume in different groups were not significantly different from each other (). But the number of red blood cells and hematocrit percentage in the diabetic control group signifi-cantly decreased compared with the normal control group (P<0.05). DIBc significantly increased (P<0.05) the red blood cells count and hematocrit percentage compared with diabetic control group.
Figure 6 Hemoglobin concentration (A), RBC count (B), hematocrit (C) and mean cell volume (D). Each column shows mean ± SEM for eight rats.
Notes: Significant differences vs the normal control group have been specified by square (#) meaning P-value <0.05. Significant differences vs the diabetic group have been specified by asterisk (*) meaning P-value <0.05.
DIBc effects on islet count and area
The results showed that the consumption of high-fat diet significantly increased the area of the islets of Langerhans in group B compared with the normal control group (P<0.05). The number of islets in the two groups was not significantly different from each other ( and ).
Figure 7 The area of islets (A) and their numbers (B).
Notes: Each column shows mean ± SEM for eight rats. Significant differences vs the normal control group have been specified by square (#) meaning P-value <0.05. Significant differences vs the diabetic group have been specified by asterisk (*) meaning P-value <0.
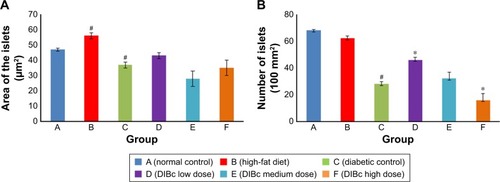
Figure 8 H&E images from pancreatic islet of rats in different groups: normal control group (A), high-fat diet group (B), diabetic control group (C), DIBc low-dose group (D), DIBc medium-dose group (E), and DIBc high-dose group (F).
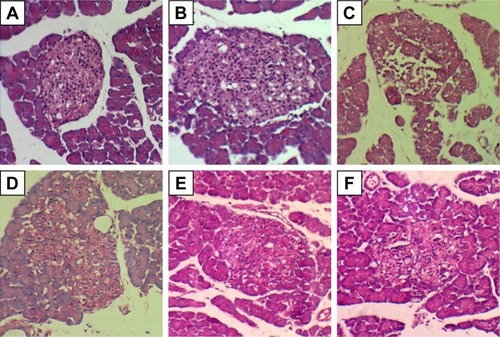
The number and area of Langerhans islets in the diabetic control group decreased significantly (P<0.05). H&E stained samples of pancreatic islet in diabetic control group showed cells with degenerated hydropic cytoplasm in unclear boundaries.
Treatment with DIBc in low-dose group significantly increased the number and area of islets compared with diabetic control group (P<0.05). In the group treated with the intermediate dose of NMOF, no significant difference in these two parameters was observed compared with diabetic control group. In the group treated with high doses, the number of islets decreased compared with diabetic control group.
Discussion
The global spread of diabetes and disorders of glucose metabolism have strongly motivated the international communities to solve this problem. Also, the International Diabetes Federation has announced research on new medications as priorities.
Access to innovative technologies has opened new doors to designing efficient methods for the treatment of this disease. For example, by using nanocarriers, nanotechnology has introduced non-invasive methods for insulin therapy.Citation27 Recently, in dozens of studies in animal models induced by STZ, with or without high-fat diet, multiple herbal or synthetic compounds have been evaluated compared with metformin. In many of these articles, like the study done by Ugochukwu and Babady, the tested compounds had similar anti-diabetic studies compared to metforminCitation15,Citation28 and a very small handful of studies have shown better effects than metformin.Citation29 In the present study, the anti-diabetic effect of DIBc metal framework, synthesized based on the novel technology of nanochelating by self-assembly method, was evaluated in a diabetic animal model, and its higher therapeutic potency in controlling plasma glucose and lipids level was proved compared with metformin.
In the present animal model of diabetes, we used a combination of high-fat diet and low dose of STZ.Citation16,Citation30,Citation31 In the pilot study, hyperinsulinemia appeared 2 weeks after high-fat diet consumption similar to the study by Skovsø.Citation17 As shown in , the combination of high-fat diet and STZ induced hyperglycemia, hyperinsulinemia, and HOMA-IR index increase (as a parameter, which shows insulin resistance) at weeks 5, 8, and 11. So it can simulate diabetes disease in animals.
The NMOF reduced glucose level and insulin resistance at low and medium dose and suppressed hyperglycemic process at high dose, while increased HOMA-IR index at high dose. According to histopathological examination in the group treated with low dose of DIBc, the number and area of Langerhans islets were improved. It should be mentioned that studies have shown that nano structures evoke nonlinear responses in living systems and sometimes low vs high doses of a nanoparticle can exert effects in opposite directions.Citation32 So in the present study, low, medium, and high doses showed different effects, and as mentioned previously, low dose of DIBc decreased HOMA-IR index and high dose increased it. But all three doses decreased plasma glucose compared with diabetic control group. Various studies have proved the link between oxidative stress and diabetes.Citation33,Citation34 Kamalakanthan et al in a diabetic model with STZ showed that the decrease in MDA is one of the mechanisms through which the complications of diabetes can be avoided.Citation35 Also, studies show that, in people with diabetes, the level of inflammatory cytokines increasesCitation36 and a direct link has been found between chronic inflammation and diabetes.Citation37 TNF-α was the first cytokine whose direct impact on the development of diabetes was detected.Citation38 In the present study, DIBc in all doses decreased TNF-α and in high dose, reduced MDA level. So it seems that one mechanism of anti-diabetic effects of this NMOF can be its anti-oxidative and immunomodulatory effects (). In the previous studies, it was revealed that nanochelating-based structures could protect cell viability against oxidative stress.Citation13,Citation39,Citation40
Numerous studies have reported pivotal role of iron in diabetes and also its complications. Free iron can make a vicious cycle, which progresses more oxidative stress and more inflammation.Citation41 So modification of iron metabolism and using structures with chelating property can be a candidate to affect this cycle.Citation42 But it should be noted that due to the importance and vital physiological role of iron in the body, chelating the iron should not lead to the removal of this element. In diseases, such as diabetes, real iron overload does not exist and true purpose of chelating the iron should be redistribution of iron and not its disposal.Citation43 In the present study, DIBc improved glucose and lipid metabolism in diabetic animals but did not have any negative effects on liver iron level and also hematological indices. So another benefit of DIBc metal framework is its chelating property. However, in the study by Kalanaky et al, in vitro and in vivo studies showed that another nanochelating-based structure, named TLc-A nano chelator, had greater performance in hepatic iron decrease than the commercially available and extensively used deferoxamine for iron removal in thalassemia models. The difference between structures comes from the technology, which is used for synthesis of the noted nano structures because self-assembly is defined as engineering the interactions among particles by functionalizing their surfaces chemically in a way that the desired structure is formed by this method. Self-assembly, as a strategy, is full of merits. This method can be used to perform the most difficult steps in nanofabrication such as atomic-level modification of structures by fully developed techniques of synthetic chemistry. Due to the need for target structures to be thermodynamically stable and open to the system, this method mostly produces defect-free and self-healing structures.Citation44 So DIBc benefits from the advantages of this method of synthesis.
Treatment by DIBc in three doses decreased TC, HDL, and LDL, and in the group treated with low-dose plasma, TG level was also reduced. Studies show that high-fat diet, by increasing acetyl coenzyme A in the liver, increases the production of cholesterol in the body leading to increase in the plasma density of cholesterol, LDL, and HDL. Any disorders in insulin secretion or function and an increase in the density of free fatty acids cause the liver to pick the fatty acids in plasma and make more cholesterol. In this case, the density of plasma lipoproteins increases.Citation45 In a survey conducted by Hayek et al, it was found that the consumption of high-fat diet increases HDL serum concentrations due to an increase in transferring speed and slows down the catabolism of HDL A-esters and apolipoprotein A-1.Citation46 Nicholls et al found that dietary intake of saturated fatty acids results in disruption of anti-inflammatory properties of HDL.Citation47 Thus, based on the findings of the present study, DIBc could improve fat metabolism in diabetic animals by reducing LDL, HDL, and TC levels. Metformin failed to decrease lipid profile in the present study. In the study by Erejuwa et al and also Salemi et al on diabetic rats, metformin could not decrease cholesterol and even increased it.Citation48,Citation49 Furthermore, in several clinical trials, metformin failure in decreasing TC level is reported.Citation50 It is recommended that initiating metformin consumption soon after diabetes diagnosis is important to prevent failure of metformin monotherapy.Citation50 As we started treatment 3 weeks after STZ injection in all groups, met-formin failure may have been due to the starting time of treatment and also type of animal model. On the other hand, the role of supplementation of micronutrients like selenium, zinc, and chromium in lowering plasma lipids is mentioned in several studies.Citation51–Citation54 Moreover, Matthews et al showed that iron chelator deferiprone (L1) significantly decreased total plasma cholesterol and LDL cholesterol.Citation55 Since DIBc has iron chelating property and contains selenium, zinc, and chromium, maybe its lowering effects on lipid parameters could be related to the previously mentioned items, but the exact mechanisms should be evaluated in future studies.
In numerous studies, beneficial effects of selenium, zinc, and chromium supplementation in diabetic patients and also diabetic animals were evaluated. In the study by Dkhil et al, they demonstrated the effects of selenium nanoparticles in attenuating diabetes-induced oxidative damage.Citation19 Shahin et al, evaluated anti-diabetic effects of chromium picolinate and showed anti-diabetic activities of this supplement in high-fat diet and STZ animal model.Citation56 Pattar et al reported that “chromium picolinate positively influences the glucose transporter system”.Citation57 Also, Barman et al demonstrated that zinc supplementation can ameliorate the severity of diabetic hyperglycemia and insulin resistance in STZ-induced diabetic rats.Citation21 So it seems that DIBc anti-glycemic effects and insulin sensitivity increase can be partially because of having these minerals.
Toxicity evaluation survey showed that DIBc i.p LD50 is equivalent to 37 mg/kg. The highest dose used in this study was 40 mg/kg. The highest dose used for this NMOF in the present study was >900 times less than LD50. DIBc low dose of the active ingredient proves it as a biocompatible NMOF.
In conclusion, the DIBc showed anti-diabetic effects, probably through its antioxidant, iron chelating properties and also delivery of three beneficial elements: zinc, selenium, and chromium. Even, its anti-diabetic effects were more potent than metformin and could be considered a novel anti-diabetic agent, which should be further evaluated in future studies. Because this is the first study on DIBc NMOF, we could only evaluate anti-diabetic effects of this nanostructure. In further studies, it is needed to study the pharmacokinetic and pharmacodynamics of DIBc, the effects of DIBc on complications (nephropathy, neuropathy, etc.) of diabetes and also other detailed mechanisms of this nano structure.
Data sharing statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank the Department of Research and Development at Sodour Ahrar Shargh and the Department of Physiology at School of Medicine, Shahid Beheshti University of Medical Sciences, for their kind support in this project.
Disclosure
The authors report no conflicts of interest in this work.
References
- https://www.idf.org [homepage on the Internet]Strategic Implementation PlanInternational Diabetes Federation2016 Available from: https://www.idf.org/component/attachments/?task=download&id=394Accessed March 20, 2016
- RojasLBAGomesMBMetformin: an old but still the best treatment for type 2 diabetesDiabetol Metab Syndr201351623415113
- RazIEldorRRational therapy for diabetes: early recognition of adverse effects and avoidance of disruptive false alarmsDiabetes Metab Res Rev201228432132422173845
- ChenZCZhangSLYanLWuMCChenLHJiLNAssociation between side effects of oral anti-diabetic drugs and self-reported mental health and quality of life among patients with type 2 diabetesZhonghua Yi Xue Za Zhi201191422923321418865
- TahraniAAPiyaMKKennedyABarnettAHGlycaemic control in type 2 diabetes: targets and new therapiesPharmacol Ther2010125232836119931305
- FosterMSammanSZinc and regulation of inflammatory cytokines: implications for cardiometabolic diseaseNutrients20124767669422852057
- ZhengYLiXKWangYCaiLThe role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: therapeutic effects by chelatorsHemoglobin2008321–213514518274991
- DominguetiCPDusseLMCarvalhoMde SousaLPGomesKBFernandesAPDiabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complicationsJ Diabetes Complications201630473874526781070
- HeJQiXMiaoYWuHLHeNZhuJJApplication of smart nanostructures in medicineNanomedicine2010571129113820874025
- DincăMLongJRHydrogen storage in microporous metal-organic frameworks with exposed metal sitesAngew Chem Int Ed2008473667666779
- RowsellJLCYaghiOMStrategies for hydrogen storage in metal-organic frameworksAngew Chem Int Ed2005443046704679
- KalanakySHafiziMSafariSTLc-A, the leading nanochelating-based nanochelator, reduces iron overload in vitro and in vivoInt J Hematol2016103327428226830968
- FakharzadehSSahraianMAHafiziMThe therapeutic effects of Msc1 nanocomplex, synthesized by nanochelating technology, on experimental autoimmune encephalomyelitic C57/BL6 miceInt J Nanomedicine201493841385325143732
- ReisCPDamgéCNanotechnology as a promising strategy for alternative routes of insulin deliveryMethods Enzymol201250827129422449931
- UgochukwuNHBabadyNEAntihyperglycemic effect of aqueous and ethanolic extracts of Gongronema latifolium leaves on glucose and glycogen metabolism in livers of normal and streptozotocin-induced diabetic ratsLife Sci200373151925193812899918
- SrinivasanKViswanadBAsratLKaulCLRamaraoPCombination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screeningPharmacol Res200552431332015979893
- SkovsøSModeling type 2 diabetes in rats using high fat diet and streptozotocinJ Diabetes Investig201454349358
- FebiyantoNYamazakiCKameoSEffects of selenium supplementation on the diabetic condition depend on the baseline selenium status in KKAy miceBiol Trace Elem Res20181811718128429286
- DkhilMZrieqRAl-QuraishySAbdel MoneimASelenium nanopar-ticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic ratsMolecules201621111517
- JayawardenaRRanasinghePGalappatthyPMalkanthiRConstantineGKatulandaPEffects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysisDiabetol Metab Syndr2012411322515411
- BarmanSSrinivasanKZinc supplementation alleviates hyperglycemia and associated metabolic abnormalities in streptozotocin-induced diabetic ratsCan J Physiol Pharmacol201694121356136527782759
- San Mauro-MartinIRuiz-LeónAMCamina-MartínMAChromium supplementation in patients with type 2 diabetes and high risk of type 2 diabetes: a meta-analysis of randomized controlled trialsNutr Hosp20163312727019254
- SundaramBAggarwalASandhirRChromium picolinate attenuates hyperglycemia-induced oxidative stress in streptozotocin-induced diabetic ratsJ Trace Elem Med Biol201327211712123122718
- AntunesLCElkfuryJLJornadaMNFolettoKCBertoluciMCValidation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar ratsArch Endocrinol Metab201660213814227191048
- Al-TradBAlkhateebHAlsmadiWAl-ZoubiMEugenol ameliorates insulin resistance, oxidative stress and inflammation in high fat-diet/ streptozotocin-induced diabetic ratLife Sci201921618318830448265
- KarunakaranUParkKGA systematic review of oxidative stress and safety of antioxidants in diabetes: focus on islets and their defenseDiabetes Metab J201337210611223641350
- SharmaGSharmaARNamJSDossGPCLeeSSChakrabortyCNanoparticle based insulin delivery system: the next generation efficient therapy for type 1 diabetesJ Nanobiotechnol201513174
- ViannaRBraultAMartineauLCCoutureRArnasonJTHaddadPSIn vivo anti-diabetic activity of the ethanolic crude extract of Sorbus decora C.K.Schneid. (Rosacea): a medicinal plant used by Canadian James Bay Cree Nations to treat symptoms related to diabetesEvid Based Complement Alternat Med2011201123794119887507
- XieWZhaoYGuDDuLCaiGZhangYScorpion in combination with gypsum: novel antidiabetic activities in streptozotocin-induced diabetic mice by up-regulating pancreatic PPAR-β and PDX-1 expressionsEvid Based Complement Alternat Med20112011319
- AliSMKhalifaHMostafaDKEl SharkawyASuppression of connective tissue growth factor mediates the renoprotective effect of sitagliptin rather than pioglitazone in type 2 diabetes mellitusLife Sci201615318018727049870
- SrimaroengCOntawongASaowakonNAntidiabetic and renoprotective effects of Cladophora glomerata Kutzing extract in experimental type 2 diabetic rats: a potential nutraceutical product for diabetic nephropathyJ Diabetes Res2015201532016725883984
- BellIRIvesJAWayneBJJonasWBNonlinear effects of nanoparti-cles: biological variability from hormetic doses, small particle sizes, and dynamic adaptive interactionsDose Response201412220223224910581
- MaritimACSandersRAWatkinsJBDiabetes, oxidative stress, and antioxidants: a reviewJ Biochem Mol Toxicol2003171243812616644
- Nourooz-ZadehJRahimiATajaddini-SarmadiJRelationships between plasma measures of oxidative stress and metabolic control in NIDDMDiabetologia19974066476539222643
- KamalakkannanNPrincePSMAntihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic Wistar ratsBasic Clin Pharmacol Toxicol20069819710316433898
- MaiorinoMIBellastellaGGiuglianoDEspositoKFrom inflammation to sexual dysfunctions: a journey through diabetes, obesity, and metabolic syndromeJ Endocrinol Invest201841111249125829549630
- El-RefaeiMFAbduljawadSHAlghamdiAHAlternative medicine in diabetes – role of angiogenesis, oxidative stress, and chronic inflammationRev Diabet Stud2014113–423124426177484
- HotamisligilGSShargillNSSpiegelmanBMAdipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistanceScience1993259509187917678183
- HafiziMHajarizadehAAtashiANanochelating based nano-complex, GFc7, improves quality and quantity of human mesenchymal stem cells during in vitro expansionStem Cell Res Ther20156122626597909
- MaghsoudiAFakharzadehSHafiziMNeuroprotective effects of three different sizes nanochelating based nano complexes in MPP(+) induced neurotoxicityApoptosis201520329830925451011
- HublerMJPetersonKRHastyAHIron homeostasis: a new job for macrophages in adipose tissue?Trends Endocrinol Metab201526210110925600948
- LehmannCIslamSJaroschSThe utility of iron chelators in the management of inflammatory disordersMediators Inflamm201520153112
- KakhlonOBreuerWMunnichACabantchikZIIron redistribution as a therapeutic strategy for treating diseases of localized iron accumulationCan J Physiol Pharmacol201088318719620393584
- FreddyCAdamsCBNanoscience, nanotechnology and spectrometrySpectrochim Acta Part B Atomic Spectrosc2013863313
- GuytonACHallJEGuyton and Hall Textbook of Medical Physiology (Guyton Physiology)11th edPhiladelphiaSaunders2006
- HayekTItoYAzrolanNDietary fat increases high density lipoprotein (HDL) levels both by increasing the transport rates and decreasing the fractional catabolic rates of HDL cholesterol ester and apolipoprotein (Apo) A-I. Presentation of a new animal model and mechanistic studies in human Apo A-I transgenic and control miceJ Clin Invest1993914166516718473509
- NichollsSJLundmanPHarmerJAConsumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial functionJ Am Coll Cardiol200648471572016904539
- ErejuwaOOSulaimanSAWahabMSSirajudeenKNSallehMSGurtuSGlibenclamide or metformin combined with honey improves glycemic control in streptozotocin-induced diabetic ratsInt J Biol Sci20117224425221448302
- SalemiZRafieEGoodarziMTGhaffariMAEffect of metformin, acar-bose and their combination on the serum visfatin level in nicotinamide/ streptozocin-induced type 2 diabetic ratsIran Red Crescent Med J2016183e2381427247792
- BrownJBConnerCNicholsGASecondary failure of metformin monotherapy in clinical practiceDiabetes Care201033350150620040656
- WangNTanHYLiSXuYGuoWFengYSupplementation of micronutrient selenium in metabolic diseases: its role as an antioxidantOxid Med Cell Longev20172017747852329441149
- GhaffariTNouriMIrannejadERashidiMREffect of vitamin E and selenium supplement on paraoxonase-1 activity, oxidized low density lipoprotein and antioxidant defense in diabetic ratsBioimpacts20111212112823678416
- RanasinghePWathurapathaWSIsharaMHEffects of zinc supplementation on serum lipids: a systematic review and meta-analysisNutr Metab201512126
- SundaramBSinghalKSandhirRAnti-atherogenic effect of chromium picolinate in streptozotocin-induced experimental diabetesJ Diabetes20135435022650796
- MatthewsAJVercellottiGMMenchacaHJIron and atherosclerosis: inhibition by the iron chelator deferiprone (L1)J Surg Res199773135409441790
- SahinKTuzcuMOrhanCAnti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocinBr J Nutr2013110219720523211098
- PattarGRTackettLLiuPElmendorfJSChromium picolinate positively influences the glucose transporter system via affecting cholesterol homeostasis in adipocytes cultured under hyperglycemic diabetic conditionsMutat Res20066101–29310016870493