?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The inflammatory basis of diabetes mellitus directed the researchers’ attention to the immune system for better management and prevention of complications. Metoclopramide (MCA; the only US Food and Drug Administration-approved for gastroparesis) has the ability to restore immune function through increasing prolactin secretion. This study aimed to test the effect of BSA/MCA nanoparticles (NPs) on modulating immune response.
Methods
BSA/MCA NPs were fabricated by desolvation and evaluated in vitro via measuring loading efficiency, particle size, and surface charge. The selected formula was further evaluated via differential scanning calorimetry and release behavior. Then, NPs were injected into rats (25 mg MCA/kg/week) for 3 weeks to be evaluated histopathologically and immunologically via measuring proinflammatory cytokines, such as IL1β, IL6, and TNFα, in addition to measuring regulatory T-cell frequency.
Results
MCA was successfully loaded on BSA, achieving high encapsulation efficiency reaching 63±2%, particles size of 120–130 nm with good polydispersity, and a negative surface charge indicating that entire positively charged drug was encapsulated inside NPs. Differential scanning calorimetry thermography of selected NPs showed an obvious interaction between components and cross-linking of BSA molecules using glutaraldehyde, resulting in sustained release of MCA (around 50% within 3 days). MCA NPs significantly restored the immune response via decreasing proinflammatory cytokines and increasing regulatory T-cell frequency when compared to control and free MCA (drug not loaded in NPs)-treated groups. Histopathological examination of this MCA NPs-treated group did not show the characteristic lesions of diabetes, and apoptosis nearly disappeared.
Conclusion
BSA/MCA NPs could be considered a new modality for treatment of gastro-paresis, in addition to management of diabetes itself and preventing its complications via an MCA-immunomodulatory effect.
Introduction
Diabetes mellitus (DM) is a chronic disease, divided mainly into type 1 diabetes (T1D) and type 2 diabetes (T2D). Autoimmunity is recognized as the main cause of T1D, where the immune system attacks insulin-producing (β) cells, resulting in insufficient insulin. However, T2D is associated with inability of the body to use the produced insulin effectively. Autoimmunity has been reported as an overlapping issue between T1D and T2D, due to the presence of circulating islet autoantibodies, self-reactive T cells, and defects in regulatory T (Treg) cells of T2D patients.Citation1,Citation2
For a long time, it has been known that inflammation is involved in T1D pathogenesis, and currently there is strong evidence that inflammation also has a critical role in the pathogenesis of T2D.Citation3–Citation5 T2D has been suggested to compose the final result of an acute-phase reaction during which cytokines, including IL1β, IL6, TNFα, and IL17, are released in large amounts, sustaining inflammation.Citation6–Citation11 The uncontrolled inflammation results in pathogenesis of T2D through tissue destruction and activation of autoimmune response toward β-cells.Citation1,Citation12,Citation13
Accordingly, extensive attention has been paid to the role of the immune system and inflammation in progression and complications of DM. In general, reduction in inflammation and restoring normal functions of the immune system should be kept in mind while controlling DM to decrease the risk of any further complications.Citation14,Citation15 Metoclopramide (MCA; dopamine D2-receptor antagonist), which is the only US Food and Drug Administration (FDA)-approved drug for diabetes-induced gastroparesis, has been used extensively as an immunomodulating agent, due to its ability to restore depressed cellular immune function through increasing prolactin secretion, which consequently affects cytokine release.Citation16–Citation19
In this study, we hypothesized that MCA use in DM could be beneficial in modulating immune response. To test this hypothesis, diabetic male rats were injected intraperitoneally with MCA. Serum levels of both proinflammatory cytokines (IL1β, IL6, and TNFα) and peripheral Treg-cell frequency were measured. Additionally, liver, lymph nodes, and spleen tissue were histopathologically examined for further support for this hypothesis.
An additional challenge in this study was combining the advantage of using MCA as an immunoenhancing agent and nanotechnology. MCA nanoparticles (NPs) were encapsulated into BSA, and outcomes of BSA/MCA NPs were compared with those of free MCA (MCA not loaded in NPs). The successful implementation of NPs in drug delivery depends on their ability to penetrate several anatomical barriers and sustain release of their contents, especially with drugs of short biological half-life (such as MCA). Dependently, using MCA NPs, it was possible to decrease frequency of administration to once a week (25 mg/kg/week). The use of albumin NPs has several advantages, including stability in blood, biocompatibility, biodegradability, and nonimmunogenicity.Citation20–Citation22
In this work, BSA/MCA NPs were fabricated and evaluated in vitro via measurement of drug-loading efficiency, particle size, surface charge, and transmission electron microscopy (TEM). Differential scanning calorimetry (DSC) and in vitro release also were studied to detect any interactions between NP components, achieving retarded drug release from these NPs. Then, the optimized NPs were injected into rats and evaluated histopathologically and immunologically.
Materials and methods
Materials
MCA hydrochloride and BSA were purchased from Sigma-Aldrich (St Louis, MO, USA). Glutaraldehyde was purchased from El-Gomhoria (Cairo, Egypt). Ethanol, sodium hydroxide, and saline (0.9%) were obtained from El-Nasr Chemical (Cairo, Egypt). An ELISA kit was purchased from RayBiotech (Peachtree Corners, GA, USA). Fluorescein isothiocyanate-conjugated antirat CD4 (Isotype rat IgG2b), PE-Cy5-conjugated antirat CD25 (Isotype rat IgG1) and PE-conjugated antirat FOXP3 (Isotype rat IgG2a) were purchased from eBioscience (San Diego, CA, USA).
Animals
Adult male Wistar rats weighing 200–250 g were obtained from the animal house at Assiut University. All animal experiments were done according to guidelines of the Council for International Organizations of Medical Sciences, which was founded under the auspices of the World Health Organization (WHO) and United Nations Educational, Scientific, and Cultural Organization. The study was approved by the ethical committee of the Faculty of Medicine, Assiut University, Assiut, Egypt. Rats were housed in clean capacious Macrolane cages under standard laboratory conditions, including a well-aerated room with suitable temperature (25°C±5°C) and maintained in good light.
Fabrication of metoclopramide nanoparticles
BSA powder was dissolved in distilled water. The pH of the solution was adjusted to be in the range of 8–9 using 0.1 M NaOH. An ethanol solution of MCA (10 mg/mL) was added dropwise under magnetic stirring into BSA solution. Excess desolvating agent (ethanol) was added dropwise under continuous stirring until the appearance of turbidity. After 10 minutes of continuous stirring, 100 μL (8% v:v) glutaraldehyde was added as a cross-linker. Overnight stirring at room temperature was done. MCA NPs were collected via centrifugation (14,000 rpm, 4°C for 30 minutes). The collected MCA particles were dried by solvent evaporation. The composition of different MCA NPs is shown in .
Table 1 Composition of metoclopramide (MCA) nanoparticles
Drug entrapment efficiency and loading capacity
The amount of MCA entrapped inside the BSA NPs was determined indirectly. NPs were centrifuged (14,000 rpm, 4°C for 30 minutes) and the supernatant diluted with distilled water and analyzed spectrophotometrically at 310 nm against blank prepared similarly (ultraviolet spectrophotometry; Jenway, Stone, UK). Drug entrapment efficiency (EE) was calculated according to EquationEquation 1(1) . Drug loading (DL) capacity was determined via measuring the MCA content of 1 mL formulation using the supernatant divided by the total weight of the 1 mL formulation using the dried pellet via solvent evaporation. DL% was calculated according to EquationEquation 2
(2) .
Particle-size and Zeta-potential measurements
Particle size, polydispersity index and Zeta-potential of different MCA NPs were measured using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Pelleted NPs were diluted with distilled water for size and polydispersity measurements at 25°C and diluted with 0.05 M phosphate buffer pH 7.4 for Zeta-potential measurements.
Transmission electron microscopy (TEM)
BSA/MCA NP morphology was revealed by TEM (JEM-100CX II; Jeol, Tokyo, Japan). Selected NPs were negatively stained using 2% w:v uranyl acetate solution and deposited on 200-mesh carbon-coated copper grids. A clear image of deposited NPs was captured using digital microscopy and the image analyzed using imaging-viewer software.
Differential scanning calorimetry (DSC)
DSC analysis was performed for selected NPs and components to investigate the presence of any possible interactions (DSC50; Shimadzu, Kyoto, Japan). MCA, BSA, physical mixture, and selected BSA/MCA NPs were weighed in an aluminum pan (3–5 mg), then the temperature of all samples was elevated to 300°C at a constant rate (10°C/min).
In vitro release studies
In vitro MCA release from BSA NPs was carried out in a release tube capped with a standard semipermeable cellophane membrane at one side.Citation23–Citation25 A selected NP suspension (1 mL) was placed on the surface of the membrane. The tube was moved into a container containing 25 mL PBS pH 7.4, which was placed in a shaking water bath (50 strokes/minute and 37°C±0.2°C). Released MCA was calculated by measuring the absorbance of aliquots at 310 nm (ultraviolet spectrophotometry). Kinetic analysis of release data using the kinetic models zero- and first-order kinetics and Higuchi and Korsmeyer–Peppas models was done to determine the exact mechanism of MCA release from BSA NPs.
In vivo experimental protocol
Animals were divided into four groups (ten each group). The first group was control nondiabetic rats, the second was control diabetic rats, the third was MCA-treated diabetic rats, and the fourth was MCA NP-treated diabetic rats. Induction of diabetes was done in 1 week, then the third and forth groups were injected intraperitoneally with MCA (25 mg/kg/day)Citation24 and MCA NPs (25 mg/kg/week), respectively, for 3 weeks. On the last day of the experiment, rats were sacrificed by decapitation. Blood samples were collected in sterile heparinized tubes and examined. The liver, lymph nodes, and spleen were dissected and fixed in buffered 10% formalin for tissue histopathology.
Diabetic induction
Diabetes was induced in the rats using streptozotocin (60 mg/kg intraperitoneally).Citation26 Streptozotocin induces diabetes within 3 days through damaging β-cells. Diabetic animals were separated from nondiabetic animals and maintained under feeding and metabolism control. Blood samples were obtained from the tail vein to detect glucose levels in rats that exceeded 135 mg/dL.
Evaluation of proinflammatory cytokines in plasma
Proinflammatory cytokines (IL1β, IL6, and TNFα) were measured in all groups with the ELISA kit according to manufacturer protocols. Kit levels of sensitivity were 80 pg/mL for IL1, 30 pg/mL for IL6, and 25 pg/mL for TNFα.
Flow cytometric analysis of rat Treg cells
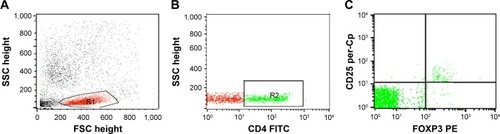
Treg cells were assessed in blood by staining blood samples for 20 minutes at 4°C in the dark with fluorescein isothiocyanate–conjugated antirat CD4 (Isotype rat IgG2b and PE-Cy5-conjugated antirat CD25 (Isotype rat IgG1). Following incubation for 15 minutes at 4°C, red blood cells lysis and washing with PBS were done. Then, fixation, permeabilization, and intracellular staining with PE-conjugated antirat FOXP3 (Isotype rat IgG2a) for 15 minutes were done. After washing with PBS, flow cytometric analysis was performed with a FACSCalibur with CellQuest software (BD Biosciences, San Jose, CA, USA). An isotype-matched negative control was used for each sample. A forward and side scatter histogram was used to define the lymphocyte population (R1). CD4+ cells were gated, and then CD25 and FOXP3 expression was assessed on CD4+ lymphocytes to detect Treg cells (CD4+CD25+Foxp3+Treg cells), as shown in .
Figure 1 Flow cytometry detection of regulatory T (Treg) cells.
Notes: (A) Forward and side scatter histogram depicting lymphocyte population (R1). (B) The expression of CD4 was assessed in the lymphocyte population (R1) and CD4+ T cells were gated. (C) Then, the expression of CD25 and FOXP3+ cells on CD4+ T cells was assessed to detect CD4+CD25+FOXP3+ Treg cells.
Abbreviations: SSC, side scatter; FSC, forward scatter; FITC, fluorescein isothiocyanate.
Histopathological examination
Fresh specimens from spleen, liver, and lymph nodes of rats of all experimental groups were collected and fixed in 10% neutral buffered formalin. Tissue samples were processed in routine fashion, sectioned at 4 μm thickness, and stained with hematoxylin and eosin (H&E) for histopathological examination by light microscopy (CX31; Olympus, Tokyo Japan) and photographed using a digital camera (Camedia C-506; Olympus).Citation27
Statistical analysis
All experiments were done at least in triplicate, and results are reported as mean ± SD. Statistical differences among various treatment groups were assessed. P<0.05 was considered statistically significant (Prism 6.0; GraphPad Software, San Diego, CA, USA).
Results
Drug entrapment efficiency (EE%) and loading capacity (DL%)
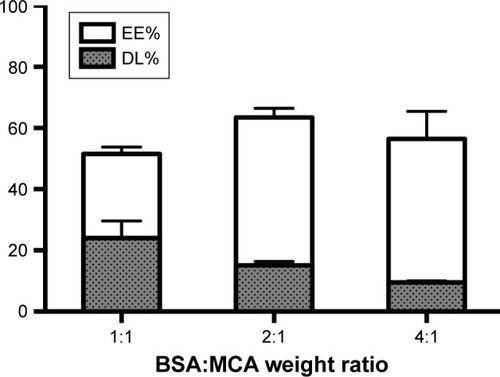
MCA EE was increased with increasing BSA content, reaching 63%±2%. EE was significantly higher at BSA:MCA ratios of 2:1 and 4:1. DL was decreased with increasing BSA:MCA ratio. Both EE% and DL% are shown in .
Particle-size and Zeta-potential measurements
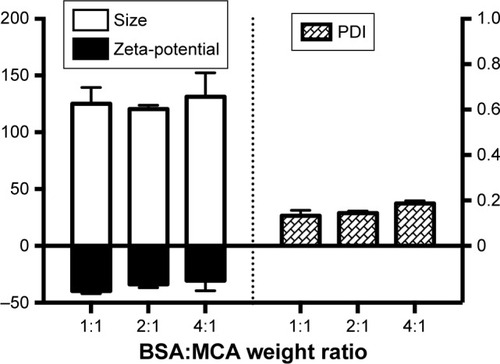
shows that particle sizes with the different BSA:MCA ratios were not significantly different. However, these different BSA:MCA ratios resulted in NPs with good polydispersity index, ranging from 0.13±0.02 to 0.19±0.01 (). The NPs formed were negatively charged, and surface charge ranged from −31±9 to −40±2 mV.
Figure 3 Particle size, polydispersity index (PDI), and Zeta-potential of nanoparticles at different BSA:metoclopramide (MCA) weight ratios.
Based on the aforementioned in vitro evaluations for the different formulated NPs, a BSA:MCA ratio of 2:1 was selected for further evaluation, due to its relatively higher EE and DL values.
Transmission electron microscopy (TEM)
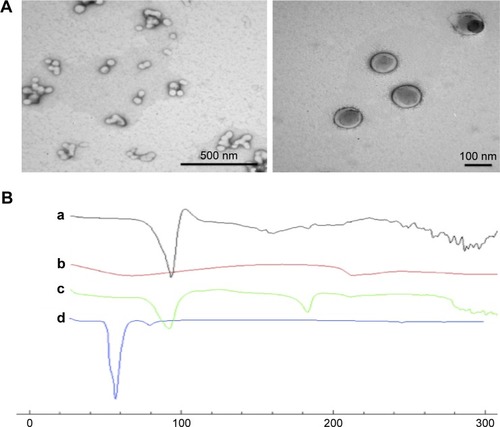
TEM of selected BSA/MCA NPs () showed spherical NPs and the particle size of these NPs using TEM was ≤100 nm.
Figure 4 (A) Transmission electron microscopy of selected BSA/metoclopramide nanoparticles (NPs). (B) Differential scanning calorimetry thermography of individual components of NPs, physical mixture, and selected NPs: metoclopramide HCl (a), BSA (b), physical mixture of components (c), and selected NPs (d; BSA:metoclopramide at weight ratio of 2:1).
Differential scanning calorimetry (DSC)
Thermal analysis of MCA HCl, BSA, physical mixture, and selected NPs was performed to detect any possible interactions (). MCA HCl thermography showed characteristic endothermic peaks at 95°C, 158°C and 185°C. BSA thermography showed characteristic broad endothermic peaks at 69°C and 210°C. The thermal behavior of the physical mixture of BSA and MCA (1:1) in the absence of glutaraldehyde exhibited the characteristic peaks of the two components. However, thermography of developed NPs did not show endothermic BSA peaks. Furthermore, shifted endothermic peaks at 58°C and 80°C were observed.
In vitro release studies
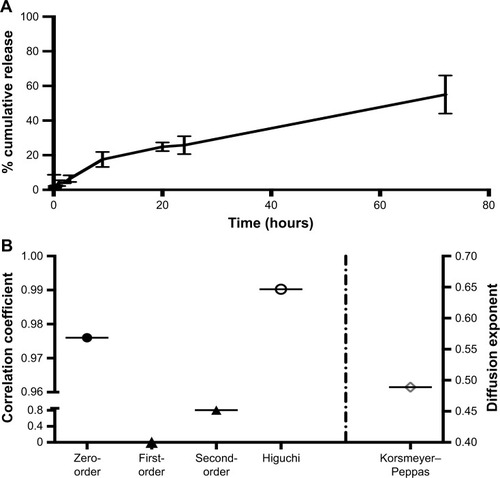
In vitro MCA release from desolvated BSA NPs was studied and showed controlled and sustained release behavior. MCA was released from the NPs at 26%±5% after 24 hours, and only around 55% had released after 3 days (). However, the release pattern of free MCA revealed that around 100% of the drug had been released after 24 hours. Kinetic analysis of these data showed that the highest correlation was observed with the Higuchi model, indicating that MCA was released by diffusion (). The diffusion exponent n of the Korsmeyer–Peppas model was 0.489, which revealed that MCA release from BSA NPs followed non-Fickian diffusion.
Effect of MCA and MCA NPs on serum levels of proinflammatory cytokines
Serum levels of IL1β, IL6, and TNFα were significantly higher among control diabetic rats than control nondiabetic rats (P<0.001 for all). Serum levels of IL1β, IL6, and TNFα were significantly decreased in diabetic rats treated with MCA when compared to untreated diabetic rats (P=0.01, P=0.04, and P=0.001, respectively). Moreover, serum levels of IL1β, IL6, and TNFα were significantly decreased in diabetic rats treated with MCA NPs when compared to untreated diabetic rats (P<0.001, P=0.01, and P=0.03, respectively). Also, there were significant decreases in serum levels of IL6 and TNFα in diabetic rats treated with MCA NPs when compared to free MCA-treated rats (P=0.04 and P=0.01, respectively), as shown in .
Table 2 Proinflammatory cytokines, lymphocytes, and regulatory T (Treg) cells in the study subjects
Effect of MCA and MCA NPs on frequency of Treg cells
The frequency of lymphocytes was significantly decreased in diabetic rats compared to nondiabetic rats (P=0.01). Specifically, the frequency of CD4+ T cells was significantly decreased in diabetic rats compared to nondiabetic rats (P=0.012). Additionally, the frequency of Treg cells was significantly lower in diabetic rats than nondiabetic rats (P=0.04).
shows that the frequency of CD4+ T cells and Treg cells was significantly increased in diabetic rats treated with MCA when compared to the control diabetic rats (P=0.03 and P=0.0002), respectively. Moreover, the frequency of CD4+ T cells and frequency of Treg cells were significantly increased in diabetic rats treated with MCA NPs when compared to untreated diabetic rats (P=0.01 and P=0.02, respectively). However, MCA NPs showed significant increases in only Treg cells when compared to MCA-treated rats (P=0.001).
Histopathological examination
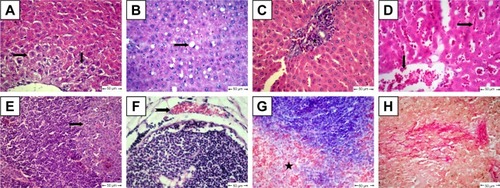
The diabetic rat group showed different lesions in different organs. Livers showed centrilobular hepatic necrosis and clear nonstaining vacuoles in hepatic cells, which may have contained intracytoplasmic material, such as lipids, Kupffer-cell proliferation in the portal area, and vascular changes characterized by dilatation and congestion of central veins and hepatic sinusoids (). Apoptosis of some lymphocytes characterized by cell shrinkage and fragmentation of cell cytoplasm appeared in cortical lymphoid follicles in lymph nodes, accompanied by subcapsular vascular congestion (). Histopathological examination of spleens showed collagen deposition in different areas of red pulp ().
Figure 6 Histopathological changes in diabetic rats.
Notes: (A) Liver shows centrilobular hepatic necrosis (arrows); (B) liver shows clear nonstaining vacuoles in hepatic cells (notched arrows); (C) liver shows focal areas of Kupffer-cell proliferation in portal area (star); (D) liver shows dilatation and congestion of central veins and in hepatic sinusoids (arrows); (E) lymph nodes show apoptotic lymphocytes (arrow); (F) lymph nodes show subcapsular vascular congestion (notched arrow); (G) spleen shows accumulation of collagen deposition in red pulp (star); H&E. (H) Collagen fibers in spleen confirmed by van Gieson stain.
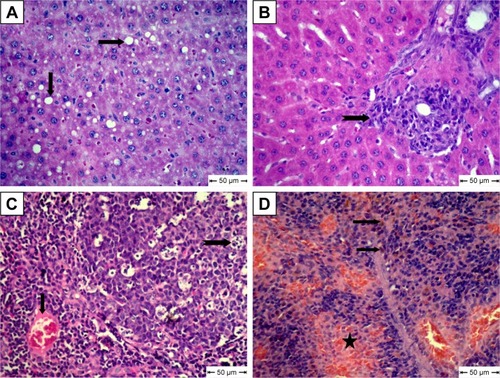
For the MCA-treated diabetic group, vacuolar degeneration in hepatocytes, which appeared as clear vacuoles, were detected in moderate distribution in liver tissue (). Also, Kupffer-cell proliferation was seen in the portal area (). Congestion of blood vessels associated with apoptosis in lymphocytes of lymphoid follicles was seen in most examined lymph nodes in this group (). Focal areas of hemorrhage accompanied by mild hemosiderosis were observed in red pulp of spleens ().
Figure 7 Histopathological changes in free metoclopramide-treated group.
Notes: (A) Liver shows clear vacuoles in hepatic cells (arrow); (B) liver shows Kupffer-cell proliferation (notched arrow); (C) lymph nodes show congestion (arrow) and apoptosis in some lymphocytes in lymphoid follicles (notched arrow); (D) Spleen shows focal areas of hemorrhage (star) and hemosiderosis (arrow); H&E.
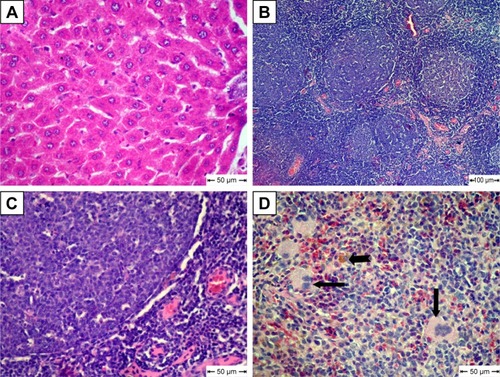
The MCA NPs-treated group showed normal radiated hepatic cords (). Examination of lymph nodes showed follicular hyperplasia with variably sized reactive germinal centers (). Lymphoid follicles were heavily populated with small lymphocytes (). Extramedullary formation of hematopoietic cells accompanied with hemosiderosis was very obvious in red pulp of spleen ().
Figure 8 Histopathological changes in metoclopramide nanoparticle–treated group.
Notes: (A) Liver shows normal hepatic cords; (B) lymph nodes show follicular hyperplasia; (C) lymphoid follicles heavily populated with small lymphocytes; (D) spleen shows extramedullary formation of hematopoietic cells (arrow) and hemosiderosis (notched arrow); H&E.
Discussion
In 2016, DM was classified by the WHO as the seventh-leading cause of death. Diabetes and its consequences can be managed with proper medical and physical intervention. Current antidiabetic medications might be able to ameliorate disease-induced inflammation via its hypoglycemic effect; however, this antidiabetic effect is inconsistent.Citation5 Therefore, targeting the uncontrolled inflammation and improving the immune response of patients open new venues for better control of this disease and preventing its progression. Due to the reported immunomodulatory effect of MCA,Citation16–Citation19 this study aimed to test the effects of free MCA and BSA/MCA NPs on the immune system of diabetic rats via measuring the levels of IL1β, IL6, TNFα, and Treg cells frequency and additionally, via detection of histopathological changes on immunity-related organs (such as the liver, spleen, and lymph nodes).
BSA, which is homologous to human serum albumin, is a common carrier for many drugs in the form of microparticles and NPs, due to in vivo stability, low toxicity, nonimmunogenicity, and biodegradability.Citation28 Despite the water-solubility of MCA, we successfully loaded it on BSA by desolvation followed by cross-linking with glutaraldehyde.Citation29 Increasing BSA content showed a positive effect on EE and negative effect on DL (). The positive effect on EE may be attributed to the well-defined structure of BSA. With fabrication pH of 8–9, BSA has negatively charged amino groups, which could electrostatically bind the positively charged aliphatic amines on MCA. Negative charges (−31±9 to −40±2 mV) on NPs revealed complete drug entrapment (). Additionally, entrapping MCA inside the BSA matrix cross-linked using glutaraldehyde prevented MCA escape. This interaction between NP components was also revealed on DSC thermography (). The thermal behavior of the physical mixture of BSA and MCA (1:1) in the absence of glutaraldehyde exhibited characteristic endothermic peaks of both MCA and BSA, confirming absence of any interaction (). These characteristic endothermic peaks of MCA (at 95°C, 158°C, and 185°C) correspond to melting of the hydrated form, recrystallization of an anhydrous form, and final melting, respectively,Citation30–Citation32 and the characteristic endothermic peaks of BSA (69°C and 210°C) correspond to its melting ().Citation25,Citation33 However, thermography of BSA/MCA NPs did not show characteristic endothermic peaks of BSA or MCA, but new peaks appeared, indicating the interaction between NP components and cross-linking of BSA molecules using glutaraldehyde (cross-linker), where it is known that glutaraldehyde changes the thermal properties and crystallization temperatures of compounds.Citation34 As a consequence of the aforementioned interactions between NP components, controlled and sustained release of MCA was observed () over the faster release rate of the free MCA (100% release within 24 hours). The selected ratio (BSA:MCA 2:1) released only half the MCA content within 3 days, due to slow diffusion from the NP matrix (non-Fickian diffusion). MCA is considered a suitable candidate for sustained drug delivery due to its short half-life and has been incorporated in solid-lipid NPs, which controlled its release to around 15%–50% over 12 hours.Citation24 Despite high EE of these solid-lipid NPs, our BSA NPs were characterized with fabrication simplicity, slower release pattern, and higher DL efficiency (10%–24% versus 5%–8%).
Sustained MCA release from NPs in small concentrations resulted in avoidance of most MCA side effects, such as extrapyramidal symptoms.Citation35 The varied particle content of BSA had a slight effect on particle size (120–130 nm, ), similarly to previously reported.Citation36,Citation37 TEM shed light on particle morphology (), showing spherical particles smaller than obtained using light scattering in solution (120±3 nm), which has been attributed to determination of particle size in the dry form.Citation38 The negative surface charge resulted in electrostatic repulsion among the formed NPs, preventing coagulation (polydispersity index <0.2, ).Citation39,Citation40 Protein–water interactions, which occur at any pH away from the isoelectric point of BSA (around 4.9), also decreased the coagulation of formed particles, resulting in more stabilized NPs in our preparation vehicles (pH 8–9).Citation39,Citation41 Small particles (120–130 nm) are beneficial in decreasing elimination from the body and increasing circulation time.Citation32 Additionally, this size enables good penetration of biological barriers and interaction with biomolecules, which present in/on the cells, affecting the cellular responses.Citation42 These promising in vitro findings of high EE, small particles and sustained release encourage us to use BSA/MCA NPs for further in vivo evaluation.
Currently, diabetes management is focusing on either optimizing available treatment or developing new anti-diabetic drugs. MCA, which is the FDA-approved drug for diabetes-induced gastroparesis, is suggested in this study to be an adjunct for improving diabetes and decreasing its complications via working on the immune system. The principle behind this suggestion is the reported ability of MCA to improve inflammation and immune system dysfunction.Citation16–Citation19 This suggestion was supported with our findings that diabetic rats had significantly higher levels of TNFα, IL6, and IL1β but decreased percentage of peripheral CD4+CD25+Foxp3+ Treg when compared to nondiabetic rats (). T2D is currently regarded as a chronic inflammatory disease due to the implication of the inflammatory system in the pathogenesis of diabetes.Citation43 Cytokines, a group of proteins that are expressed by several cell types, act as immune mediators and regulators. Treg cells and cytokines play important roles in the development of T2D. Moreover, research has demonstrated that the changes in the number and functions of Treg in the body are closely associated with the pathogenesis and development of various diabetic complications.Citation44 Studies have reported that insulin resistance was associated with abnormal secretion of proinflammatory cytokines, such as TNFα, IL6 and IL1β, and decreased production of anti-inflammatory mediators, such as IL4 and IL10.Citation45–Citation47
When the diabetic rats were injected with free MCA (25 mg/kg/day) for 3 weeks, serum levels of proinflammatory cytokines (IL1β, IL6, and TNFα) were significantly decreased and the frequency of CD4+ T cell and Treg cells was significantly increased when compared to untreated diabetic rats (). The obvious MCA-lowering effect on proinflammatory cytokines is indirect via increased secretion of prolactin (immunoenhancing hormone).Citation19,Citation48–Citation51 MCA (dopamine antagonist) increases prolactin secretion through blocking D2-receptors, preventing the inhibitory effect of dopamine (neurotransmitter) on prolactin release.Citation19,Citation52 Prolactin, which is responsible for many physiological functions, plays an important role in regulation of the immune system, where it can downregulate the expression of inflammatory cytokines.Citation19,Citation53
Treg cells, a small subset of T lymphocytes constituting only 5%–20% of the CD4+ compartment, are thought to be important to prevent excessive inflammatory responses and limit tissue impairment.Citation54–Citation58 In T2D, Treg cells can inhibit the inflammatory response by various pathways, such as surpassing cytokine secretion, modulating the microenvironment, and changing the expression of surface receptors.Citation59
Nanomedicine, which is the integration of nanotechnology with medicine, has been extensively used to give an entirely new outlook to current medications. Advantages of NPs, such as sustained drug release, decreasing frequency of administration, decreasing side effects, and enabling cellular/molecular interactions due to smallness, make NPs an eye-catching tool for better management of diabetes. When diabetic rats were injected with BSA/MCA NPs (25 mg/kg/week) for 3 weeks, significant results were obtained. It was found that serum levels of IL1β, IL6, and TNFα were significantly decreased when compared to untreated diabetic rats, and when compared to free MCA-treated rats, both IL6 and TNFα showed a significant decrease (). Moreover, the frequency of CD4+ T cells and Treg cells was significantly increased when compared to untreated diabetic rats, and these NPs showed significant increases in Treg cells when compared to free MCA-treated rats. This significant difference produced with NPs, administered weekly, as opposed to the free drug, which was administered daily, could be attributed to the different benefits of nanotechnology. It was expected that albumin, which has a long half-life in circulation and has the ability to form NPs with sustained release (), would increase the circulation time of MCA.Citation60 Long-circulating BSA NPs and release of small amounts of MCA guarantee that MCA in small concentrations will be available to work both peripherally and centrally through crossing the blood–brain barrier and exerting its effect through blocking the D2-receptors.Citation61,Citation62 Despite MCA readily crossing the blood–brain barrier, NPs are considered an efficient delivery carrier to the brain, where MCA releases gradually from NPs.Citation60 Body exposure to small concentrations of MCA and consequently decreasing side effects is another advantage of using NPs over free drug.
Parallel to these serum-positive findings using MCA NPs, histopathological examination of the different animal groups confirmed the effect of MCA (–). Histopathological examination of diabetic rats showed hepatic lesions characterized by centrilobular hepatic necrosis, clear vacuoles in hepatic cells and cytoplasm that may have contained fat, Kupffer-cell proliferation in the portal area, and vascular changes, similar to typical hepatic lesions found in some studies.Citation63,Citation64 The liver plays a vital role in regulating glucose levels in pathological states of diabetes. Inflammation, and proliferation of Kupffer cells related to the role of liver in controlling glucose homeostasis by modifying the expression of hepatokines. Fetuin is one of the hepatokines that is responsible for increased inflammation and insulin resistance via inhibiting the insulin-receptor tyrosine kinase in hepatocytes.Citation65 Also, accumulation of fat in hepatic cell cytoplasm is a characteristic lesion in diabetic rats. In diabetes, the liver usually stops the process of oxidizing fatty acids and uses them instead to synthesize triglycerides, which then accumulate abnormally in the liver. Additionally, in DM, insulin deficiency upregulates hormone-sensitive lipase in adipose tissue, subsequently leading to increased lipolysis and the circulation of free fatty acids, which then accumulate in the liver. The hepatic uptake of very-low-density lipoproteins increases due to these processes.Citation66,Citation67
The effect of diabetes on the immune system of rats in our experiment was demonstrated pathologically by the appearance of apoptotic lymphocytes in large number in lymphoid follicles accompanied by subcapsular vascular congestion in lymph nodes. Collagen deposition was observed in different areas in spleen red pulp, which is similar to previous findings.Citation68 Diabetes decreases immune response, affecting immune cell function and the structure of immune organs, causing atrophy.Citation69 In diabetes, increasing free-radical generation stimulates the release of proinflammatory cytokines, causing stressed lymphocytes and programmed cell death. TNF causes stimulation of death domains, such as Fas, which leads to activation of a signal transduction pathway inducing apoptosis.Citation69,Citation70
In this study, no significant improvement was observed on histopathological examination of livers or lymphatic tissue in free MCA-treated diabetic rats, while livers of MCA NPs-treated rats showed normally radiated hepatic cords without characteristic lesions of diabetes. There was hyperplasia of heavily populated lymphoid follicles with variably sized reactive germinal centers associated with extramedullary formation of hematopoietic cells. Apoptosis nearly disappeared in this group. These results are in line with previous studies, which showed that MCA treatment enhanced the depressed peritoneal and splenic macrophage function. The treatment of immunosuppressive effects of diabetes depends upon controlling apoptosis of lymphocytes in lymphatic tissue.Citation71 MCA does not encourage apoptosis or influence the activation of caspases that complete apoptosis.Citation72 MCA modifies splenocyte apoptosis and cytokine release in a murine model of sepsis.Citation18 These encouraging histopathology results could be attributed to the aforementioned ability of MCA NPs to improve the immune function via decreasing proinflammatory cytokines and increasing the level of Treg cells ().
Conclusion
NPs have been considered promising drug delivery systems for the treatment of many diseases. As DM prevalence has been increasing more rapidly, nanotechnology may provide better modalities for management of diabetes and prevention of its complications through reducing inflammation and restoring normal functions of the immune system. BSA/MCA NPs were designed and developed to have high DL efficiency, be small, and sustain the release of MCA from NPs (only 50% MCA content within 3 days).
MCA (dopamine D2-receptor antagonist) stimulates the release of prolactin, which in turns downregulates inflammatory cytokines and additionally increases the frequency of Treg cells. BSA/MCA NPs (25 mg/kg/week) injected into diabetic rats for 3 weeks showed significant reductions in serum levels of proinflammatory cytokines when compared to both control diabetic rats and free MCA-treated rats. Furthermore, these albumin NPs showed significant increases in frequency of Treg cells when compared to control diabetic rats and free MCA-treated rats. Based on the inflammatory basis of diabetes, immunomodulatory effects of MCA NPs on diabetic rats could help better control of the disease and prevent progression. Depending on these findings, implementation of immunotherapy should be considered in the management of diabetes.
Disclosure
The authors report no conflicts of interest in this work.
References
- ItariuBKStulnigTMAutoimmune aspects of type 2 diabetes mellitus – a mini-reviewGerontology201460318919624457898
- WentworthJMFourlanosSHarrisonLCReappraising the stereotypes of diabetes in the modern diabetogenic environmentNat Rev Endocrinol20095948348919636326
- GeerlingsSEHoepelmanAIImmune dysfunction in patients with diabetes mellitus (DM)FEMS Immunol Med Microbiol1999263–425926510575137
- PelegAYWeerarathnaTMcCarthyJSDavisTMCommon infections in diabetes: pathogenesis, management and relationship to glycaemic controlDiabetes Metab Res Rev200723131316960917
- PollackRMDonathMYLeroithDLeibowitzGAnti-inflammatory agents in the treatment of diabetes and its vascular complicationsDiabetes Care201639Suppl 2S244S25227440839
- KohKKHanSHQuonMJInflammatory markers and the metabolic syndrome: insights from therapeutic interventionsJ Am Coll Cardiol200546111978198516325028
- DandonaPAljadaAChaudhuriAMohantyPGargRMetabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammationCirculation2005111111448145415781756
- RuanHLodishHFInsulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alphaCytokine Growth Factor Rev200314544745512948526
- El-DeebTSBakkarSMEltoonyLThe adipokine chemerin and fetuin-A serum levels in type 2 diabetes mellitus: relation to obesity and inflammatory markersEgypt J Immunol201825119120230243011
- HettaHFElkadyAMorsyKHMohamedISIbrahimMASerum level of IL17A among cirrhotic hepatitis C virus infected patients with incidence of diabetes mellitusEgypt J Immunol2017241798829120580
- HettaHFEz-EldeenMEMohamedGAVisfatin serum levels in obese type 2 diabetic patients: relation to proinflammatory cytokines and insulin resistanceEgypt J Immunol201825214115130600957
- HettaHFKhairyHMIsmailSMCirculating IL17A and IFN-gamma serum levels in cirrhotic hepatitis C virus infected patients with autoimmune thyroiditisInt J Curr Microbiol Appl Sci20176319721983
- HettaHFElkadyAMeshaalAKTH17/TH1 role in endocrine disorders among chronic HCV infected patientsInt J Curr Microbiol App Sci20176825422551
- HotamisligilGSShargillNSSpiegelmanBMAdipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistanceScience1993259509187917678183
- MaedlerKSergeevPRisFGlucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic isletsJ Clin Invest2002110685186012235117
- KnöferlMWAngeleMKAyalaACioffiWGBlandKIChaudryIHInsight into the mechanism by which metoclopramide improves immune functions after trauma-hemorrhageAm J Physiol Cell Physiol20002791C72C8010898718
- ZellwegerRWichmannMWAyalaAChaudryIHMetoclopramide: a novel and safe immunomodulating agent for restoring the depressed macrophage immune function after hemorrhageJ Trauma Acute Care Surg19984417077
- OberbeckRSchmitzDWilsenackKMetoclopramide and cellular immune functions during polymicrobial sepsisEur Surg Res200436211612215007265
- JarrarDWangPSongGYMetoclopramide: a novel adjunct for improving cardiac and hepatocellular functions after trauma-hemorrhageAm J Physiol Endocrinol Metab20002781E90E9510644541
- SebakSMirzaeiMMalhotraMKulamarvaAPrakashSHuman serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: preparation and in vitro analysisInt J Nanomedicine2010552553220957217
- LomisNWestfallSFarahdelLMalhotraMShum-TimDPrakashSHuman serum albumin nanoparticles for use in cancer drug delivery: process optimization and in vitro characterizationNanomaterials201666E11628335244
- ElzoghbyAOSamyWMElgindyNAAlbumin-based nanoparticles as potential controlled release drug delivery systemsJ Control Release2012157216818221839127
- IbrahimE-SAIsmailSFetihGShaabanOHassaneinKAbdellahNHDevelopment and characterization of thermosensitive pluronic-based metronidazole in situ gelling formulations for vaginal applicationActa Pharm2012621597022472449
- MohamedRAAbassHAAttiaMAHeikalOAFormulation and evaluation of metoclopramide solid lipid nanoparticles for rectal suppositoryJ Pharm Pharmacol201365111607162124102470
- JenitaJLChocalingamVWilsonBAlbumin nanoparticles coated with polysorbate 80 as a novel drug carrier for the delivery of antiretroviral drug-EfavirenzInt J Pharm Investig201443142
- AkbarzadehAMehrabiMRMehrabiMRInduction of diabetes by streptozotocin in ratsIndian J Clin Biochem2007222606423105684
- BancroftJDTheory and Practice of Histological TechniquesNYChurchill Livingstone1982
- LinWCoombesAGGarnettMCPreparation of sterically stabilized human serum albumin nanospheres using a novel Dextranox-MPEG crosslinking agentPharm Res19941111158815927532861
- XuRFisherMJulianoRLTargeted albumin-based nanoparticles for delivery of amphipathic drugsBioconjug Chem201122587087821452893
- GattaniSGKhabiyaSSAmrutkarJRKushareSSFormulation and evaluation of bilayer tablets of metoclopramide hydrochloride and diclofenac sodiumPDA J Pharm Sci Technol201266215116022492600
- HemalathaKFormulation and evaluation of metoclopramide hydrochloride microbeads by ionotropic gelation methodInt J Pharm Biol Arch201123
- MitchellAGPolymorphism in metoclopramide hydrochloride and metoclopramideJ Pharm Pharmacol19853796016042867177
- MichnikAThermal stability of bovine serum albumin DSC studyJ Therm Anal Calorim2003712509519
- SathiyanarayananaPKarunakaranaRJGomathibTSudhabPSynthesis and characterization of carboxymethyl cellulose/polyethylene glycol/montmorillonite clay blendsInt J Novel Trends Pharm Sci2015523641
- Yadav DeepakRAyyappanTShanmugamSSundaramoorthyKVetrichelvanTDevelopment and in-vitro evaluation of buccoadhesive metoclopramide hydrochloride tablet formulationsDevelopment201131516525
- SebakSMirzaeiMMalhotraMKulamarvaAPrakashSHuman serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: preparation and in vitro analysisInt J Nanomed20105525
- LangerKBalthasarSVogelVDinauerNvon BriesenHSchubertDOptimization of the preparation process for human serum albumin (HSA) nanoparticlesInt J Pharm20032571–216918012711172
- Radovic-MorenoAFLuTKPuscasuVAYoonCJLangerRFarokhzadOCSurface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibioticsACS Nano2012654279428722471841
- JunJYNguyenHHChunHSKangBCKoSPreparation of size-controlled bovine serum albumin (BSA) nanoparticles by a modified desolvation methodFood Chem2011127418921898
- RahimnejadMJahanshahiMNajafpourGProduction of biological nanoparticles from bovine serum albumin for drug deliveryAfr J Biotechnol2006520
- HoffmannMAvan MilPJHeat-induced aggregation of beta-lactoglobulin as a function of pHJ Agric Food Chem19994751898190510552468
- YanSZhangHPiaoJStudies on the preparation, characterization and intracellular kinetics of JD27-loaded human serum albumin nanoparticlesProcedia Eng2015102590601
- XiaoJLiJCaiLChakrabartiSLiXCytokines and diabetes researchJ Diabetes Res2014201492061324551859
- YangTTSongSJXueHBShiDFLiuCMLiuHRegulatory T cells in the pathogenesis of type 2 diabetes mellitus retinopathy by miR-155Eur Rev Med Pharmacol Sci201519112010201526125263
- Fernández-RealJMBrochMRicartWPlasma levels of the soluble fraction of tumor necrosis factor receptor 2 and insulin resistanceDiabetes19984711175717629792545
- de LucaCOlefskyJMInflammation and insulin resistanceFEBS Lett200858219710518053812
- JagerJGrémeauxTCormontMLe Marchand-BrustelYTantiJFInterleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expressionEndocrinology2007148124125117038556
- MccallumRWSowersJRHershmanJMSturdevantRAMetoclopramide stimulates prolactin secretion in manJ Clin Endocrinol Metab197642611481152777023
- McNeillyAThornerMVolansGBesserGMetaclopramide and prolactinBr Med J197425921729
- HealyDLBurgerHGIncreased prolactin and thyrotrophin secretion following oral metoclopramide: dose-response relationshipsClin Endocrinol197773195201
- FalaschiPFrajeseGSciarraFRoccoAContiCInfluence of hyperprolactinaemia due to metoclopramide on gonadal function in menClin Endocrinol197885427433
- Van den BergheGde ZegherFAnterior pituitary function during critical illness and dopamine treatmentCrit Care Med1996249158015908797634
- ZhuXHZellwegerRAyalaAChaudryIHProlactin inhibits the increased cytokine gene expression in Kupffer cells following haemorrhageCytokine1996821341408777271
- ZhengYRudenskyAYFOXP3 in control of the regulatory T cell lineageNat Immunol20078545746217440451
- HettaHFMekkyMAKhalilNKAssociation of colonic regulatory T cells with hepatitis C virus pathogenesis and liver pathologyJ Gastroenterol Hepatol201530101543155125708446
- MehtaMHettaHFAbdel-HameedEAAssociation between IL28b rs12979860 single nucleotide polymorphism and the frequency of colonic Treg in chronically HCV-infected patientsArch Virol2016161113161316927544760
- ZahranAMMohammed SalehMFSayedMMRayanAAliAMHettaHFUp-regulation of regulatory T cells, CD200 and Tim3 expression in cytogenetically normal acute myeloid leukemiaCancer Biomark201822358759529843224
- ZahranAMZharanKMHettaHFSignificant correlation between regulatory T cells and vitamin D status in term and preterm laborJ Reprod Immunol2018129152230029057
- Guzmán-FloresJMPortales-PérezDPMecanismos de supresión de las células T reguladoras (Treg) [Mechanisms of suppression of regulatory T-cells (Treg)]Gac Med Mex20131496630638 Spanish24276186
- BergonziMCGuccioneCGrossiCAlbumin nanoparticles for brain delivery: a comparison of chemical versus thermal methods and in vivo behaviorChemMedChem201611161840184926947767
- ShiloMSharonABaranesKMotieiMLelloucheJPMPopovtzerRThe effect of nanoparticle size on the probability to cross the blood-brain barrier: an in-vitro endothelial cell modelJ Nanobiotechnol201513119
- MariamJSivakamiSDongrePMAlbumin corona on nanoparticles-a strategic approach in drug deliveryDrug Deliv20162382668267626056719
- MohamedJNazratun NafizahAHZariyanteyAHBudinSBMechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammationSultan Qaboos Univ Med J2016162e132e14127226903
- RegnellSELernmarkÅHepatic steatosis in type 1 diabetesRev Diabet Stud20118445422580727
- StefanNSunQFritscheAImpact of the adipokine adiponectin and the hepatokine fetuin-A on the development of type 2 diabetes: prospective cohort- and cross-sectional phenotyping studiesPLoS One201493e9223824643166
- LongoDKasperDKauserSLoscalzoJFauciAJamesonJHarrison’s Principle of Internal MedicineEdisi ke-18New YorkMcGraw-Hill2011
- Al-HussainiAASulaimanNMAlzahraniMDAleniziASKhanMPrevalence of hepatopathy in type 1 diabetic childrenBMC Pediatr201212116023039762
- EbaidHAl-TamimiJMetwalliAEffect of STZ-induced diabetes on spleen of rats: improvement by camel whey proteinsPakistan J Zool201547411091116
- EbaidHPromotion of immune and glycaemic functions in streptozotocin-induced diabetic rats treated with un-denatured camel milk whey proteinsNutr Metab201411131
- ParkGBKimYSLeeHKChoDHKimDHurDYCD80 (B7.1) and CD86 (B7.2) induce EBV-transformed B cell apoptosis through the Fas/FasL pathwayInt J Oncol20134351531154024008628
- WangNSunHShenYAugmenter of liver regeneration inhibits apoptosis of activated human peripheral blood lymphocytes in vitroImmuno Immunotoxicol2013352257263
- JaberBMPetroianuGARizviSAProtective effect of metoclopramide against organophosphate-induced apoptosis in the murine skin fibroblast L929J Appl Toxicol201838332934029027213