Abstract
Background
EGFP-EGF1-conjugated poly(lactic-co-glycolic acid) (PLGA) nanoparticle (ENP) has a specific affinity to tissue factor (TF). The aim of this study was to investigate the target delivery of ENP to plaques and its uptake in a mouse model of atherosclerosis in vivo and in vitro.
Materials and methods
Coumarin-6- and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbo cyanine iodide (DiR)-loaded ENPs were synthesized using a double-emulsion method. Mouse vascular smooth muscle cells (VSMCs) were induced with MCP-1 to obtain an increased TF expression. Fluorescence microscopy and flow cytometry assay were performed to examine the uptake of coumarin-6-loaded ENPs in cellular models. An animal model of atherosclerosis was established with an ApoE (−/−) mouse fed with continuous high-fat diets for 14 weeks. DiR-loaded ENPs (DiR-ENPs) were injected via the caudal vein. The distribution of DiR-ENPs was examined through organ imaging and confocal laser scanning microscopy.
Results
Results indicated TFs were highly expressed in the cellular model. The uptake of coumarin-6-loaded ENPs was significantly higher than that of common PLGA nanoparticles. Thickening of intima and lipid deposition in the aorta could be observed in atherosclerosis mouse models. Confocal laser scanning microscopy organ imaging showed ENPs accumulated in vessels with atherosclerotic plaques, which coincided with high expressions of TF.
Conclusion
This study showed that EGFP-EGF1-conjugated PLGA nanoparticles could be effectively delivered to atherosclerotic plaques in vivo and taken up by VSMCs with high TF expressions in vitro. Thus, it could be a promising carrier for targeted therapy of atherosclerosis.
Introduction
Atherosclerosis is an inflammatory disease that leads to the hardening and thickening of the arterial walls and subsequently results in the formation of plaques, which are comprised of immune cells, mesenchymal cells, lipids, and extracellular matrix.Citation1–Citation3 It is a predominant cause of a variety of cardiovascular disorders including myocardial infarction, ischemic stroke, and peripheral vascular disease and accounts for significant morbidity and mortality rates in the aged population.Citation4,Citation5 Atherosclerosis is initiated by the dysfunction of endothelial cells.Citation6,Citation7 The vicious cycle promotes the proliferation of vascular smooth muscle cells (VSMCs) and reconstruction of extracellular matrix, ultimately leading to the formation of atherosclerotic plaques.Citation8,Citation9
Tissue factor (TF), a key molecular component in initiating the external coagulation pathway, has been reported to increase in the plasma of patients with myocardial infarction and hyperlipidemia.Citation10,Citation11 TF participates in nearly all pathological processes of atherosclerosis.Citation12 Inflammatory mediators such as monocyte chemoattractant protein-1 (MCP-1) are secreted by activated monocytes/macrophages inducing more TF expression in VSMCsCitation13,Citation14 and endotheliums.Citation15 As the disease progresses, multiple TFs can be detected in the lipid core and extracellular matrix of plaques. Increased expression of TF mediates the migration and proliferation of smooth muscle cells into the intima of arteries, promoting angiogenesis, vascular reconstruction, and weakening of the stability of plaques.Citation16 As unstable plaques rupture, foam cells and micro-particles with high TF contents are released from the plaque, resulting in a hypercoagulable state and thrombosis-related complications such as acute myocardial infarction.Citation17,Citation18
There are generally no symptoms in the early stages of atherosclerosis. Traditional detection techniques cannot easily, safely, and effectively detect the lesions in the early stages. In recent years, there has been a rapid development in using nanoparticle (NP) technique as a tool for molecular imaging of atherosclerotic lesion.Citation19–Citation21 By incorporating peptides, antibodies, or other ligands on its surface, a NP can target adhesion molecules in lesion components.Citation22 Engineered supramolecular micelles targeting cellular components in atherosclerotic plaques, such as monocytes, exhibit promising characteristics for the diagnosis of the disease.Citation23,Citation24 In our previous research, we developed a novel fusion protein EGFP-EGF1, which is synthesized based on the TF gene in the EGF1 region and has a specific affinity to TF.Citation25 It has been proved that EGFP-EGF1-poly(lactic-co-glycolic acid) (PLGA) NPs can mediate target delivery of siRNA to injured brain microvascular endothelial cells (BMECs) in rats.Citation26 As TF is significantly expressed in macrophages and VSMCs in atherosclerotic lesions, it is hypothesized that EGFP-EGF1-PLGA NPs (ENPs) could also be delivered to atherosclerotic plaques by virtue of its specific binding ability to TF, and thus could be utilized as a tool for imaging and target treatment of the disease. The purpose of this study was to evaluate the distribution of ENPs in a mouse atherosclerosis model in vivo and their uptake in cultured VSMCs in vitro.
Methods
Materials and animals
The Escherichia coli strain BL21 (DE3) and plasmid pET-28a-EGFP-EGF1 were maintained in our laboratory as previously described.Citation25 PLGA (50:50, inherent viscosity of 0.89, MW 100 kDa) was purchased from Absorbable Polymers (Pelham, AL, USA). Methoxy-poly-(ethylene glycol) (M-PEG, MW 3,000 Da) was purchased from the NOF Co. (lot no 14530; Tokyo, Japan) and maleimide-PEG (Mal-PEG, MW 3,400 Da) was purchased from Nektar Co. (lot no PT-08D-16; Santa Ana, CA, USA). Rabbit polyclonal antibody against rat TF was purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). DMEM (high glucose), RPMI 1640 medium, and FBS were purchased from Thermo Fisher Scientific (Waltham, MA, USA). MCP-1 was purchased from R&D Biological Co. (Minneapolis, MN, USA). Real-time PCR primers were synthesized by Thermo Fisher Scientific. All other chemicals were analytical reagent grade, and were purchased from the Sino-pharm Chemical Reagent Co. (Hangzhou, China). C57/B background mice with ApoE gene knockout were provided by the Center of Experimental Animals at Peking University (Beijing, China). The animal procedures were approved by the Animal Care Committee of the Tongji Medical College of Huazhong University of Science and Technology and followed the Regulations of the Administration of Affairs Concerning Experimental Animals.
Cell culture and preparation
In vitro cellular model preparation
VSMCs were isolated from C57/B mouse (20–25 g) as previously describedCitation27,Citation28 and cultured in DMEM supplemented with 20% FBS at 37°C in a humidified atmosphere containing 5% CO2. TF expression was induced by incubating VSMCs with MCP-1 (10 ng/mL)Citation29 for 0, 1, 2, 4, 8, 16, and 24 hours. Then the real-time PCR was performed and the optimal induction time was selected. The primers used for PCR were as follows: TF, sense: 5′-GCACCGAGCAATGGAAGAG-3′, antisense: 5′-CAGAGATATGGACAGGAGGATGAT-3′; GAPDH: sense: 5′-ATGGTGGTGAAGACGCCAGTA-3′, antisense: 5′-GGCACAGTCAAGGCTGAGAATG-3′.
Preparation of nanoparticles
The PLGA NPs were prepared using water-in-oil-in-water (W/O/W) double-emulsion solvent evaporation method and conjugated with EGFP-EGF1 fusion protein similar to a previously described protocol.Citation26,Citation30 Coumarin-6- or DiR (DiIC18(7); 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide)-loaded NPs/ENPs were prepared with the same procedure except that 30 mg of coumarin-6 or 200 mg of DiR was additionally added to dichloromethane containing copolymers before primary emulsification.
Characterization of nanoparticles
The mean diameter and zeta potential of the NPs were determined by dynamic light scattering (DLS) using the zeta-potential/particle sizer Nicomp 380 ZLS (Particle Sizing Systems, Santa Barbara, CA, USA). The NPs were morphologically examined by transmission electron microscopy (H-600; Hitachi, Tokyo, Japan).
Establishment of atherosclerosis model in vivo
ApoE gene knockout mice were fed with high-fat (0.2% cholesterol, 21% fat) diet (HFD) for 14 weeks to establish the classical atherosclerosis model. The control group mice were fed with normal diet (CHD). Oil red O staining of aorta was performed to confirm the development of atherosclerosis.Citation31 The mRNA was extracted from the aorta of ApoE (−/−) mice after they were fed with HFD for 0, 8, 9, 10, 12, and 14 weeks. Five mice were included in each group.
Cellular uptake study
VSMCs were seeded at a density of 1×106 per well in six-well plates and incubated for 20 hours. Then the medium was exchanged with a serum-free medium. VSMCs were treated with MCP-1 (10 ng/mL) for 2 hours. Coumarin-6-labeled ENPs and NPs were mixed with cultured cells and incubated for 30 minutes at 37°C. The cells were washed two times with PBS (0.01 M, pH =7.4) and immobilized with 4% paraformaldehyde for 20 minutes at room temperature. Then, the fluorescence intensity of the cells was detected by flow cytology and fluorescence microscopy. Untreated cells were incubated with NPs to serve as a control.
Distribution of nanoparticles in vivo
Mice in the HFD group were randomly divided into three groups with eight mice in each group. They were separately injected with DiR-loaded ENPs (DiR-ENPs, 0.05 mg/g), DiR-loaded NPs (DiR-NPs, 0.05 mg/g), and PBS (0.01 M, pH =7.4) via the caudal vein. Mice in the CHD group were injected with DiR-ENP (0.05 mg/g) to serve as a control. One hour later, the mice were sacrificed. Then, the fluorescence images of the organs were obtained by placing the tissue in a living-body imager, and the relative light intensity of each organ was obtained.
Distribution of nanoparticles in aorta
Organ imaging and immune fluorescence microscopy examination of aorta were performed. Then frozen sections from the specimen were incubated with TF antibody (1:100) for 4°C after 5% BSA closure. Then CY3-labeled secondary antibody (1:100) was added to the sample at room temperature for 30 minutes after washing. The nuclei were dyed with DAPI. Finally, the aortal specimens were observed under a confocal laser microscope.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± standard error of mean (SEM). Statistical comparisons between the two groups were evaluated by Student’s t-test and one-way ANOVA was used for more than two groups. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of nanoparticles
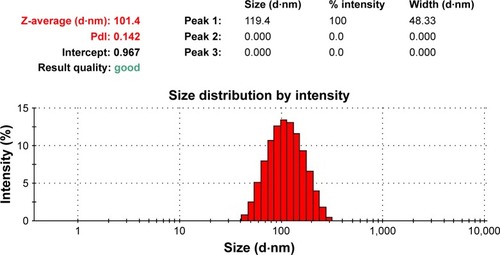
The PLGA NPs were prepared using double-emulsion solvent evaporation method and conjugated with the EGFP-EGF1 fusion protein. After coumarin-6 or DiR was loaded into the NPs, DLS showed the mean size of coumarin-6 and DiR-loaded NPs/ENPs to be about 100 nm (, ). Their zeta potential was around −11 mV ().
Table 1 Particle size and zeta potential of nanoparticles
Establishment of cellular model
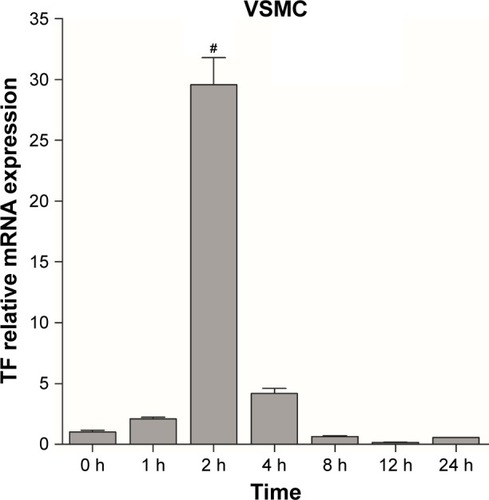
To establish cellular models of atherosclerosis, VSMCs were induced with MCP-1 (10 ng/mL). TF molecules were expressed in the cultured cellular model as in an athero-sclerotic plaque. Real-time PCR was used to investigate the relationship between time and TF expression levels. TF mRNA reached its peak after a 2-hour exposure to MCP-1 in VSMCs (). Thus, VSMCs were induced with MCP-1 for 2 hours to establish a cellular model of atherosclerosis for in vitro NPs research.
Figure 2 TF expression in VSMCs at different timepoints. The experiments were performed in triplicate to calculate the average value of each group. Data are presented as mean ± SEM. The TF mRNA levels from the different treated cells were normalized to the control one; #P<0.05.
Abbreviations: TF, tissue factor; VSMCs, vascular smooth muscle cells; SEM, standard error of mean; h, hours.
Fluorescence microscopy examination to determine nanoparticle uptake
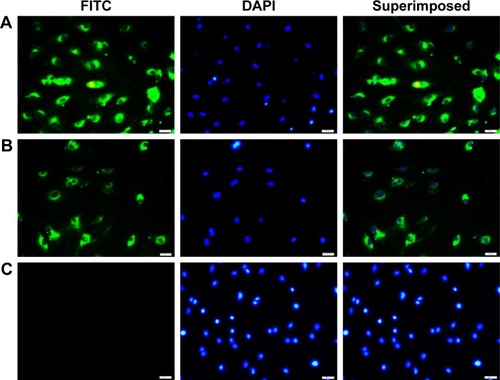
VSMCs were exposed to MCP-1 (10 ng/mL) for 2 hours. Then the cellular model was incubated with coumarin-6-loaded ENPs and NPs. Under a fluorescence microscope, a much stronger fluorescence could be observed in the ENP group than in the NP group ().
Figure 3 Uptake of coumarin-6 nanoparticles by stimulated VSMCs.
Notes: (A) Shows the ENP group of cells. (B) Shows the NP group of cells. (C) Shows cells incubated with PBS only (0.01 M, pH 7.4). Nuclei are stained with DAPI (blue). Scale bar: 100 nm.
Abbreviations: VSMCs, vascular smooth muscle cells; ENP, EGFP-EGF1-conjugated poly(lactic-co-glycolic acid) nanoparticle; NP, nanoparticle; FITC, fluorescein isothiocyanate.
Flow cytometry to determine nanoparticle uptake
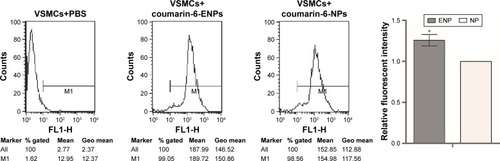
VSMCs were exposed to MCP-1 (10 ng/mL) for 2 hours. Then they were incubated with coumarin-6-loaded ENPs and NPs. Flow cytometry was performed for the quantitative analysis of the fluorescence intensity. It revealed that fluorescence intensity in the ENPs group was significantly higher than in the NPs group (), suggesting that ENPs were more easily taken up by the cells than NPs.
Figure 4 Cell fluorescence intensity of VSMCs.
Notes: The experiment was repeated three times to calculate the average fluorescence intensity of each group, and the histogram was obtained based on NP group. Data are presented as mean ± SEM; *P=0.001.
Abbreviations: VSMCs, vascular smooth muscle cells; NP, nanoparticle; SEM, standard error of mean; ENP, EGFP-EGF1-conjugated poly(lactic-co-glycolic acid) nanoparticle; Geo, geometric.
Identification of mouse atherosclerosis model
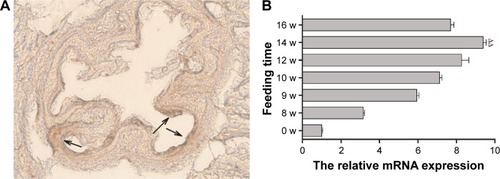
Male ApoE gene knockout mice, aged 3–4 weeks with a body weight of 20–25 g, were fed with HFD continuously for 14 weeks. Oil red O staining of the aorta was done to confirm the development of atherosclerosis. Microscopy showed that the intima of the aorta was thickened and reddish brown lipid was deposited on the arterial wall (). These findings proved the successful establishment of the mouse atherosclerosis model. Mice were sacrificed at different week intervals after they were fed with HFD. The tissue of aorta was harvested, and real-time PCR revealed that TF expression reached its peak after feeding HFD for 14 weeks ().
Figure 5 (A) Oil red O staining of frozen sections of the aorta, arrowhead showing lipid deposition on the arterial wall. (B) TF mRNA expressions in aorta of mouse atherosclerosis models fed with high-fat diets for various lengths of time. Data are presented as mean ± SEM; ∆∆P<0.05.
Abbreviations: TF, tissue factor; SEM, standard error of mean; w, weeks.
Distribution of nanoparticles in mouse atherosclerosis model
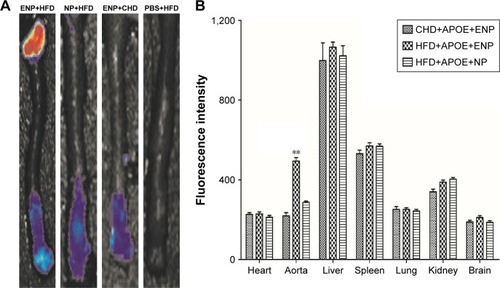
The fluorescence signal of DiR-ENP group was significantly stronger than that of DiR-NP group, especially at the aortic arch (). The fluorescence signals were collected from other major organs of the animal. It could be clearly observed that the NPs were highly aggregated in the liver, spleen, and lung of mice after exposure for a brief period. There was no significant difference between the two groups, but the uptake of Dir-ENPs in the aortic region of atherosclerotic plaques was significantly higher than that of Dir-NPs, and the fluorescence signal was found to be enhanced by about 1.73 times ().
Figure 6 Organ imaging of DiR-ENPs in atherosclerosis mice.
Notes: (A) Shows the fluorescence imaging of the mouse aorta. (B) Shows the fluorescence intensity of different organs, and the histogram was analyzed by statistical analysis. Data are presented as mean ± SEM (n=6 per group); **P<0.05.
Abbreviations: DiR, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide; ENPs, EGFP-EGF1-conjugated poly(lactic-co-glycolic acid) nanoparticle; SEM, standard error of mean; NP, nanoparticle; HFD, high-fat diet; CHD, normal diet.
Distribution of DiR-ENPs in aorta of mouse atherosclerosis model
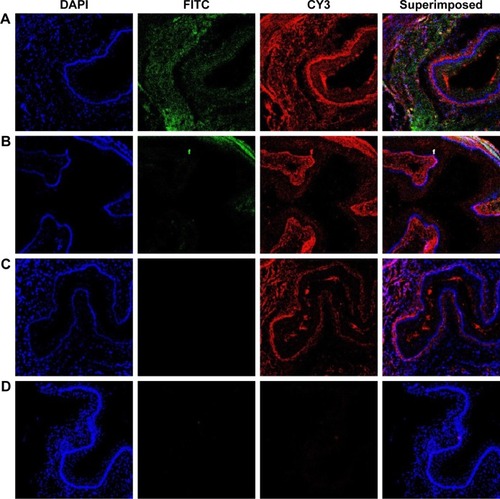
After the injection of NPs, the mouse model was sacrificed and a slice of aorta tissue was examined under a fluorescence microscope (). Tissues with DiR-ENP deposition exhibited green fluorescence and tissues with high TF expression exhibited red fluorescence. It could be observed that more TFs were expressed in the aorta of mice fed with HFD () than in mice fed with normal diet (). ENPs preferentially accumulated in the tissues exhibiting TF expression (). Only a small amount of ENPs was taken up by the mice fed with a normal diet (CHD) (). No NP deposition could be observed in the aorta of the mouse atherosclerosis model following DiR-NP injection ().
Figure 7 Laser confocal observation of the distribution of nanoparticles in the aorta of atherosclerosis model mice.
Notes: (A) Shows the aorta after injection of DiR-ENPs into the atherosclerosis mouse model of HFD group. (B) Shows the aorta after injection of DiR-ENPs in CHD group. (C) Shows the aorta after injection of DiR-NPs in HFD group. (D) is the aorta after PBS injection in HFD group, no incubation with TF antibody. FITC-labeled EGFP-EGF1 site is shown in green and CY3-labeled TF expression site in red. Nuclei were stained with DAPI (blue).
Abbreviations: DiR, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide; ENPs, EGFP-EGF1-conjugated poly(lactic-co-glycolic acid) nanoparticle; TF, tissue factor; HFD, high-fat diet; CHD, normal diet; FITC, fluorescein isothiocyanate.
Discussion
Current preventive and therapeutic modalities of atherosclerosis focus on improving blood lipid profile, inhibiting thrombus formation, and decreasing blood pressure, but the treatment cannot directly target specific molecules, cells, and processes involved in the formation of atherosclerotic lesions.Citation32 PEG-PLGA NPs have been used as drug carriers due to their physical stability and property of sustained drug release, which is believed to be suitable for the treatment of chronic diseases, and hence is approved by US FDA.Citation33,Citation34 In our previous research, we established a stable EGFP-EGF1-PLGA NP with a specific affinity to TF. The NPs could be delivered to injured BMECs, neuroglial cells, and glioma cells and can be used as a carrier for target therapy.Citation26,Citation35
Numerous studies have shown that TF expression increased significantly in atherosclerosis patients. TF plays a key role in the pathogenesis of the disease.Citation10,Citation36,Citation37 If ENP could be target-delivered to atherosclerotic lesions by binding with TF, it would serve as an ideal carrier for therapeutic agents, thereby achieving a controlled and target delivery of the drug with better efficacy and fewer side effects.
VSMCs are one of the main TF resources and contributing cell types for the formation of atherosclerotic plaque.Citation38 MCP-1 is the main inducer of TF expression in VSMCs. Through the FVIIa receptor pathway, TF promotes migration and proliferation of VSMCs into the intima of arteries, leading to vessel injury and vascular reconstruction.Citation10,Citation39 Based on their involvement in the formation of atherosclerotic plaque, tests to determine the uptake of ENPs were performed in VSMCs.
MCP-1 was used to induce TF expression in VSMCs. TF mRNA in cultured VSMCs reached its peak after being stimulated with MCP-1 for 2 hours. Coumarin-6, a fluorescent marker, was used for tracing the uptake of ENPs in cultured cells. Both fluorescence microscopy and flow cytometry results proved that more ENPs were taken up than NPs in the cellular model. The results strongly suggested that EGFP-EGF1-NPs could specifically bind to the EGF1 receptors, ie, TF, on the surface of cultured VSMCs.
The structure of atherosclerotic plaques includes a fibrin cap, a large lipid (primarily cholesterol) core, proliferated smooth muscle cells, and remodeled extracellular matrix.Citation16 The dense fibrin cap may obstruct the entry of ENP into the plaques. To further confirm the in vitro findings of this study with regard to specificity and sensitivity of ENPs in atherosclerosis, DiR, a near-infrared dye, was employed as a tag for ENPs. ApoE (−/−) mice were the rodents of choice for establishing the animal atherosclerosis model. After continuous feeding of HFD, the animal models exhibited symptoms similar to human atherosclerosis disease. Oil red staining revealed plaque formation in the aorta, and the serum cholesterol and lipid levels were found to be markedly elevated. All these observations proved the successful establishment of atherosclerosis models.
The fusion protein, EGFP-EGF1, was synthesized based on the TF gene in rats. Based on the fact that the similarity of EGF1 region between rats and mice is 95%, we assume that the affinity of EGFP-EGF1 to TF molecule in mice is similar to that of rat. To study the distribution of NPs in vivo, DiR-ENPs were administered via the caudal vein of the atherosclerotic animal. By organ imaging technique, it could be observed that there was no statistical difference with regard to fluorescence intensity in the liver, spleen, kidney, brain, and lungs among the three groups. It showed that the distribution of NPs in these organs was nonspecific, which was determined by blood flow to the organs. However, in the aorta, there was a significant statistical difference between DiR-ENPs group and DiR-NPs group (P<0.05). It indicated that ENPs have a specific affinity to the plaque in a mouse atherosclerosis model.
To further confirm the relationship between the distribution of ENPs and expression of TF in plaques, fluorescence-labeled TF antibody CY3 was cocultured with the aortic tissue. Confocal laser scanning microscopy was employed, and the results indicated that TF was significantly expressed at the sites in the aorta where ENPs were highly accumulated. It suggested that ENPs entered into the plaque through dysfunctional endothelial layer or penetrated through the unstable fibrin cap of plaques, via binding with TF molecules on the cellular surface. VSMC is one of the targeting cell types as it is one of the main resources for TF in atherosclerosis. By a mechanism of ligand-receptor-mediated endocytosis, the ENPs were taken up into the cytoplasm of the cells constituting the plaques.
Diagnostic dyes or contrast can be incorporated into NPs, which can be detected using modalities including magnetic resonance imaging, positron emission tomography/single photon emission computed tomography, computed tomography, and optical near-infrared fluoroscopy.Citation40 Our study proved that ENPs specifically and effectively bind to plaques. If ENPs are incorporated with the imaging agents, they will be useful for the diagnosis of atherosclerosis. Imaging of specific cells or components in lesions can disclose lesion biology and features, especially vulnerability, which can help prevent and treat major cardiovascular events. Thus, ENPs can also work as a tool for target therapy of atherosclerosis by providing specific medication, siRNA, etc, to decrease the inflammation, inhibit progression, and stabilize the plaques. Studies showed that TF is expressed by dysfunctional endothelial cells, VSMCs, and monocyte/macrophages at the early stage of atherosclerosis. Compared with other molecular NPs, which target single cellular type of atherosclerosis, ENPs could not only be taken up by VSMCs, a major cellular component of atherosclerotic plaques, but there is also a high possibility that ENPs could be taken up by other cell types such as dysfunctional endothelial cells and monocytes in atheromatous plaque, as TFs are also expressed in these cells. Based on the high affinity and sensitivity of the NP to TF protein, ENPs could also be beneficial for early imaging and prevention of atherosclerosis.
However, there are some limitations in our research. In vivo distribution of NPs was studied for just 1 hour after the administration. This short time period may be adequate for diagnostic purposes. However, to work as an effective drug carrier for use in targeting therapy, the information we obtained from the research is obviously not enough. Clotting and other conditions associated with abnormally high TF expression may affect the distribution and drug delivery of the NP. As macrophages/foam cells in atherosclerotic plaques are TF-rich cellular types, they might also contribute to the imaging results after ENP administration. Hence, the uptake of ENPs by these cells should also be studied. With respect to these findings, some questions need to be answered: Can these particles remain in the localized lesions for longer time? Will these NPs support targeted drug delivery in vivo? Is there any systemic effect on a healthy body after ENP administration? Further research needs be done to obtain more information about ENP distribution and their systemic effect in atherosclerosis, especially their value as a promising carrier for targeting prevention strategies and therapy for atherosclerosis needs to be studied.
In summary, this research proved that the EGFP-EGF1-conjugated NP could be effectively taken up by VSMCs in the atherosclerosis cellular model and be delivered into the sites of atherosclerotic plaque in vitro and in vivo. This NP presents a promising advantage for target drug delivery in diagnosis and therapy of atherosclerosis.
Author contributions
CC contributed to the conception and design of this study. ZW contributed to data acquisition. WS, BZ, and DL carried out animal experiments. GD and HW contributed to the analysis and interpretation of the data. JRJD and LT drafted the manuscript and critically revised it for important intellectual content. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos 81500342 and 81600175). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- WeberCNoelsHAtherosclerosis: current pathogenesis and therapeutic optionsNat Med201117111410142210.1038/nm.253822064431
- FeigJEParathathSRongJXReversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaquesCirculation2011123998999810.1161/CIRCULATIONAHA.110.98414621339485
- WilliamsKJFeigJEFisherEARapid regression of atherosclerosis: insights from the clinical and experimental literatureNat Clin Pract Cardiovasc Med2008529110210.1038/ncpcardio108618223541
- WeberCZerneckeALibbyPThe multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse modelsNat Rev Immunol200881080281510.1038/nri241518825131
- LibbyPInflammation in atherosclerosisNature2002420691786887410.1038/nature0132312490960
- MinKChoKKwonTKThe effect of oxidized low density lipoprotein (oxLDL)-induced heme oxygenase-1 on LPS-induced inflammation in RAW 264.7 macrophage cellsCell Signal20122461215122110.1016/j.cellsig.2012.02.00122349240
- TsimikasSMillerYIOxidative modification of lipoproteins: mechanisms, role in inflammation and potential clinical applications in cardiovascular diseaseCurr Pharm Des2011171273721226665
- TabasIMacrophage death and defective inflammation resolution in atherosclerosisNat Rev Immunol2010101364610.1038/nri267519960040
- MooreKJSheedyFJFisherEAMacrophages in atherosclerosis: a dynamic balanceNat Rev Immunol2013131070972110.1038/nri352023995626
- TatsumiKMackmanNTissue factor and atherothrombosisJ Atheroscler Thromb201522654354910.5551/jat.3094026016513
- SteffelJTissue factor in cardiovascular diseases: molecular mechanisms and clinical implicationsCirculation2006113572273110.1161/CIRCULATIONAHA.105.56729716461845
- MackmanNRole of tissue factor in hemostasis, thrombosis, and vascular developmentArterioscler Thromb Vasc Biol20042461015102210.1161/01.ATV.0000130465.23430.7415117736
- SchecterADRollinsBJZhangYJTissue factor is induced by monocyte chemoattractant protein-1 in human aortic smooth muscle and THP-1 cellsJ Biol Chem19972724528568285739353321
- SchepersAEeftingDBontaPIAnti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivoArterioscler Thromb Vasc Biol20062692063206910.1161/01.ATV.0000235694.69719.e216825596
- NiuJKolattukudyPERole of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implicationsClin Sci (Lond)200911739510910.1042/CS2008058119566488
- LibbyPInflammation in AtherosclerosisArterioscler Thromb Vasc Biol20123292045205110.1161/ATVBAHA.108.17970522895665
- LiSChenHRenJMicroRNA-223 inhibits tissue factor expression in vascular endothelial cellsAtherosclerosis2014237251452010.1016/j.atherosclerosis.2014.09.03325463083
- ten CateHTissue factor-driven thrombin generation and inflammation in atherosclerosisThromb Res2012129Suppl 2S38S4010.1016/j.thromres.2012.02.028
- ScharlachCKratzHWiekhorstFSynthesis of acid-stabilized iron oxide nanoparticles and comparison for targeting atherosclerotic plaques: evaluation by MRI, quantitative MPS, and TEM alternative to ambiguous Prussian blue iron stainingNanomedicine-Uk20151151085109510.1016/j.nano.2015.01.002
- YeKQinJPengZPolyethylene glycol-modified dendrimer-entrapped gold nanoparticles enhance CT imaging of blood pool in atherosclerotic miceNanoscale Res Lett20149152910.1186/1556-276X-9-52925288918
- NoukeuLCWolfJYuanBBanerjeeSNguyenKTNanoparticles for detection and treatment of peripheral arterial diseaseSmall2018e180064410.1002/smll.20180064429952061
- NieSZhangJMartinez-ZaguilanRDetection of atherosclerotic lesions and intimal macrophages using CD36-targeted nanovesiclesJ Control Release2015220Pt A617010.1016/j.jconrel.2015.10.00426450668
- MlinarLBChungEJWonderEATirrellMActive targeting of early and mid-stage atherosclerotic plaques using self-assembled peptide amphiphile micellesBiomaterials201435308678868610.1016/j.biomaterials.2014.06.05425043572
- ChungEJMlinarLBNordKMonocyte-targeting supramolecular micellar assemblies: a molecular diagnostic tool for atherosclerosisAdv Healthc Mater20154336737610.1002/adhm.20140033625156590
- MeiHShiWPangZEGFP-EGF1 protein-conjugated PEG–PLA nanoparticles for tissue factor targeted drug deliveryBiomaterials201031215619562610.1016/j.biomaterials.2010.03.05520413154
- ChenCMeiHShiWEGFP-EGF1-conjugated PLGA nanoparticles for targeted delivery of siRNA into injured brain microvascular endothelial cells for efficient RNA interferencePLoS One201384e6086010.1371/journal.pone.006086023593330
- EllmarkSHDustingGJFuiMNGuzzo-PernellNDrummondGRThe contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscleCardiovasc Res200565249550410.1016/j.cardiores.2004.10.02615639489
- SakataYXiangFChenZTranscription factor CHF1/Hey2 regulates neointimal formation in vivo and vascular smooth muscle proliferation and migration in vitroArterioscler Thromb Vasc Biol200424112069207410.1161/01.ATV.0000143936.77094.a415345511
- SalcedoRPonceMLYoungHAHuman endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progressionBlood2000961344010891427
- ShiWMeiHDengJA tissue factor targeted nanomedical system for thrombi-specific drug deliveryBiomaterials201233307643765410.1016/j.biomaterials.2012.06.09422819496
- GetzGSReardonCAAnimal models of atherosclerosisArterioscler Thromb Vasc Biol20123251104111510.1161/ATVBAHA.111.23769322383700
- TaylorAShawLJFayadZTracking atherosclerosis regression: a clinical tool in preventive cardiologyAtherosclerosis2005180111010.1016/j.atherosclerosis.2004.12.02415823269
- DanhierFAnsorenaESilvaJMCocoRLe BretonAPréatVPLGA-based nanoparticles: an overview of biomedical applicationsJ Control Release2012161250552210.1016/j.jconrel.2012.01.04322353619
- VandervoortJLudwigABiocompatible stabilizers in the preparation of PLGA nanoparticles: a factorial design studyInt J Pharm20022381–2779211996812
- ZhangBWangHLiaoZEGFP-EGF1-conjugated nanoparticles for targeting both neovascular and glioma cells in therapy of brain gliomaBiomaterials201435134133414510.1016/j.biomaterials.2014.01.07124529623
- BreitensteinATannerFCLuscherTFTissue factor and cardiovascular disease: quo vadis?Circ J201074131219996531
- CameraMToschiVBrambillaMThe role of tissue factor in atherothrombosis and coronary artery disease: insights into platelet tissue factorSemin Thromb Hemost201541773774610.1055/s-0035-156404126408918
- LibbyPRidkerPMHanssonGKProgress and challenges in translating the biology of atherosclerosisNature2011473734731732510.1038/nature1014621593864
- ChenDXiaMHayfordCExpression of human tissue factor pathway inhibitor on vascular smooth muscle cells inhibits secretion of macrophage migration inhibitory factor and attenuates atherosclerosis in ApoE −/− miceCirculation2015131151350136010.1161/CIRCULATIONAHA.114.01342325677604
- ZhangJZuYDhanasekaraCSDetection and treatment of atherosclerosis using nanoparticlesWiley Interdiscip Rev Nanomed Nanobiotechnol201791e141210.1002/wnan.1412