?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nanomedicine, known as the application of nanotechnology in medicine, has been applied to overcome the problems of poor bioavailability, in vitro and in vivo stability, and targeted delivery in the preparation of pharmaceutical products. Sirolimus, a water-insoluble immunosuppressant, has been formulated into an oral solid dosage form by using NanoCrystal® technology to increase the water solubility and thereby the bioavailability. The efficacy, safety, and pharmacokinetic properties are not significantly different between liquid and solid formulations except that less fluctuation of sirolimus blood concentration was observed in solid dosage form. The tablet formulation offers the advantages of better palatability and more convenience for long-term use. Sirolimus tablets are not only a successful example of nanomedicine, but also a more cost-effective treatment in renal transplantation than cyclosporine and tacrolimus.
Introduction to nanomedicine and issues of transplant rejection
It has been more than 51 years since the first successful human kidney transplantation at the Peter Ben Brigham Hospital in Boston (CitationMerrill et al 1956). Graft rejection is one of the greatest challenges to transplantation. It is the outcome of the natural response of the immune system to a foreign substance, or antigen. This complex process is mainly T-lymphocyte mediated, although it involves serial interactions between foreign antigens, T lymphocytes, macrophages, cytokines (also known as lymphokines or interleukins), adhesion molecules (ie, co-stimulatory molecules), and membrane proteins that enhance binding of T lymphocytes and B lymphocytes (CitationHalloran and Miller 1996; CitationValente and Alexander 1998). The goal of immunosuppressive therapy is to prevent and treat organ rejection as well as prolong graft and patient survival. With the development of immunosuppressive agents (), kidney transplantation has become one of the most important renal replacement therapies nowadays (CitationMorris 2004).
Table 1 Immunosuppressive agents commonly used in transplantation
During the “azathioprine era” from the 1960s to the early 1980s, 1-year cadaveric graft survival rate was around 60% (CitationMorris 2004; CitationSayegh and Carpenter 2004). With the introduction in 1978 of cyclosporine, a calcineurin inhibitor (CNI), the 1-year graft survival rate increased to more than 80% (CitationSayegh and Carpenter 2004). In the 1990s, the availability of several other immunosuppressive agents with different mechanisms of action (tacrolimus, mycophenolate mofetil, and sirolimus) made combination immunosuppressive therapy a general modality in organ transplantation. However, due to the potency of these agents and inter- and intra-individual variability in pharmacokinetics, dose individualization is required to maintain adequate immunosuppression while minimizing adverse reactions.
Poor water solubility and bioavailability contribute to the complexity of dosing cyclosporine and sirolimus. Pharmaceutical companies have been formulating insoluble drugs into commercially available products and minimizing the problem of large intra- and inter-individual variability in pharmacokinetic profiles. A limited number of methods such as generation of ionized drugs, co-solvents, surfactants, soft-gel technology, micronization, and nanotechnology have been utilized in formulating water-insoluble drugs. In addition, other particular drug carriers, such as emulsion, liposome, and polymeric micelle, are considered as alternative methods to deliver water insoluble drugs (CitationYokoyama 2005). For example, cyclosporine has been emulsified by bile salts to form micelles and further modified into microemulsion from the olive oil-based solution to enhance bioavailability (CitationThomas et al 2005). The liposomal formulation of tacrolimus also has been demonstrated as an effective strategy to increase efficacy and decrease toxicity (CitationMcAlister et al 1999; CitationAlemdar et al 2004). The formulation of amphiphilic block co-polymer micelles has been used to prepare injectable formulations of the highly lipophilic sirolimus (CitationAshok et al 2004; CitationForrest et al 2006).
After the introduction of tacrolimus (a CNI with a potency 32–100 times that of cyclosporine), less variation in bioavailability and not requiring bile salts for oral absorption made tacrolimus a better choice than cyclosporine (Sandimmune®, Novartis) in liver transplantation (CitationScott et al 2003). This forced a reformulation of cyclosporine to retain its market share. The new formulation of cyclosporine (Neoral®, Novartis) is a microemulsion utilizing 9.5% w/v alcohol, corn oil-mono-di-triglyceride, polyoxyl 40 hydrogenated castor oil, and polyethylene glycol as vehicle (CitationPDR 2006). This is an application of microtechnology to drug delivery, a breakthrough in the 1990s.
In the 2000s, a more advanced technology called nanotechnology was applied to medicine, which led to the emergence of nanomedicine (CitationWeber 1999). Nanomedicine is the application of nanotechnology to medicine (CitationFreitas 2005). More broadly, it is the process of applying molecular tools and molecular knowledge of the human body in diagnosis, treatment, and prophylaxis of disease, as well as preserving and improving human health (CitationFreitas 2005). Many nanomedicine technologies have been developed and are close to fruition. Among them, nanostructured materials such as fullerenes have been used in pharmaceuticals (CitationFreitas 2005). Sirolimus (Rapamune®; Wyeth-Ayerst, Philadelphia, PA, USA) tablet is another example of the successful application of nanotechnology in pharmaceuticals to overcome the problems of formulation, poor bioavailability, and erratic absorption.
Chemistry, nanocrystal technology used, and formulation of sirolimus
Sirolimus is a triene marcolide antibiotic isolated from Streptomyces hygroscopicus (CitationVezina et al 1975; CitationSehgal 1998). It is challenging to formulate sirolimus into either an intravenous or oral dosage form due to its water insolubility and a logP (log of the octanol-water partition coefficient, a measure of a drug’s lipophilicity) of greater than 5. The solubility of sirolimus is 2.6 μg/mL, which is far below the target solution concentration of 1 mg/mL (CitationSimamora et al 2001). It is impossible to enhance the solubility of sirolimus by the generation of a salt form because of the lack of an ionizable group of sirolimus in the pH range of 1–10 (CitationSimamora et al 2001). The solubility of sirolimus in a single organic solvent, such as ethanol, γ-butyrolactone, dimethyl isosobide, and glycerol formal (a mixture of 5-hydroxy-1,3-dioxane and 4-hydroxymethyl-1,3-dioxolane in a ratio of 60:40), increases thousands-fold to greater than 90 mg/mL. Although a thousand-fold decrease in the solubility of sirolimus was observed in single cosolvent–water mixtures, the solubility could be increased to more than 10 mg/mL when it was prepared in multiple cosolvent and hydrotrope mixtures, such as 10% ethanol, 40% propylene glycol, 5% benzyl alcohol, and 3%–5% benzoate buffer (CitationSimamora et al 2001). In 1999, the first commercially available product of sirolimus was thus an oral solution with a concentration of 1 mg/mL in Phosal 50 PG (a dispersion of 50% phosphatidylcholine in a propylene glycol/ethanol carrier) and polysorbate 80 (CitationPDR 2003). The taste, and the requirement for refrigerator storage, protection from light, and disposal of the oral syringe after a single use make the oily solution an inconvenient dosage form (CitationVasquez 2000).
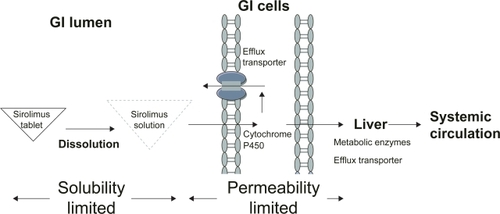
To deliver sirolimus orally in a solid dosage form, the major problem of the dissolution of sirolimus from the dosage form had to be overcome, as well as its permeability across the biological barrier, such as first-pass effect via intestinal and hepatic metabolism and efflux transporter P-glycoprotein (). It took one more year to explore the oral tablet formulation of sirolimus using NanoCrystal® technology acquired by Elan Corporation (CitationRosen and Abribat 2005). This product, launched in 2002, offers greater palatability and convenience of administration and storage (at 20–25°C).
Figure 1 Factors influencing the bioavailability of oral tablet sirolimus. Generally, the bioavailability of oral tablet sirolimus is determined by the solubility and permeability of sirolimus. The nanocrystalline sirolimus improves sirolimus dissolution, saturation solubility, and stability in gastrointestinal (GI) lumen and thereby improves the aborption. However, sirolimus is the substrate for the metabolic enzyme (cytochrome P450 3A) and efflux transporter (P-glycoprtoein) in intestinal and hepatic cells. Therefore, less than 20% of sirolimus can reach systemic circulation.
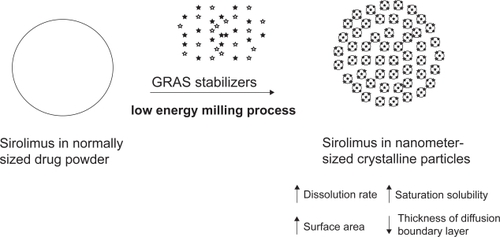
Sirolimus is formulated as nanometer-sized drug crystals by NanoCrystal technology using high-shear media mills. According to the Noyes-Whitney equation of dissolution (CitationNoyes and Whitney 1897), the rate of dissolution is proportional to the effective surface area (A) and the difference (Cs–C)/h:
Where D is the diffusion coefficient of the drug, h is the thickness of diffusion boundary layer, Cs is the saturation solubility of the drug, and C is the concentration of drug in the medium. Nanocrystalline sirolimus not only increases the effective surface area (A), but also increases the saturation solubility (Cs) and decreases the thickness of the diffusion boundary layer (h) (CitationMuller et al 2001; CitationMerisko-Liversidge et al 2003; CitationMuller and Keck 2004). The saturation solubility can be increased only when the particle size is reduced in a submicron range (CitationMuller and Keck 2004). In addition, it possesses long-term physical stability without Ostwald ripening (the tendency for larger particles to grow in diameter over time, while the smaller particles dissolve in a highly dispersed systems) due to the uniform nanometer-sized particles (CitationMuller and Keck 2004). High adhesiveness on biological surfaces and high endocytosis of nanocrystalline drugs were also reported (CitationKayser 2000; CitationMuller and Keck 2004). Therefore, nanocrystalline sirolimus may provide a range of improvements in bioavailability, dose proportionality, absorption variability, and absorption rate.
In the process of sirolimus nanocystallization, generally recognized as safe (GRAS) stabilizers were added to avoid the agglomeration/aggregation of the drug crystals due to surface energy of nanocrystalline particles () (CitationMerisko-Liversidge et al 2003). A mixture of non-ionic and ionic stabilizers, such as cellulosics, poloxamer, polysorbates, lecithin, sodium glycocholate and polyvinylpyrrolidones, is required to create a steric barrier and an electrostatic repulsion among particles, respectively (CitationPatravale et al 2004). The inactive ingredients of sirolimus tablet include microcrystalline cellulose, polyethylene glycol 8000, polyethylene glycol 20000, poloxamer 188, sucrose, lactose, calcium sulfate, pharmaceutical glaze, talc, titanium dioxide, magnesium stearate, povidone, glyceryl monooleate, carnauba wax, and other ingredients (CitationPDR 2003). Some of the disclosed and concealed inactive GRAS ingredients were the essential components in the process of sirolimus nanocrystallization.
Immunopharmacology and pharmcokinetics of sirolimus
Sirolimus, with a chemical structure related to tacrolimus, is one of the most potent immunosuppressive agents for prevention of rejection (CitationSehgal 1998; CitationVasquez 2000). When combined with cyclosporine- or tacrolimus-based regimen in kidney transplantation, sirolimus increases immunosuppressive activity through a sequential synergistic mechanism.
Like cyclosporine and tacrolimus, sirolimus binds to cytosolic receptors, an intracellular protein, known as immunophilins (). Cyclosporine binds to cyclophilin of the immunophilin family, forming a complex that then engages calcinueurin (CitationClipstone and Crabtree 1992). Both sirolimus and tacrolimus engage another immunophilin called FK-binding protein-12 (FKBP-12). Tacrolimus-FKBP complex, like cyclosporine-cyclophilin complex, inhibits the catalytic activity of calcineurin-calcium-calmodulin complex. The inhibition of calcineurin activity decreases the production of IL-2 and other cytokines, thereby blocking their activation of T-lymphocytes in the G0 and G1 phases of cell cycle.
Figure 3 Mechanism of action of cyclosporine, tacrolimus, and sirolimus. Adapted with permission from CitationWu FL, Tsai MK, et al. 2005. Effects of conversion from sirolimus oral solution to tablets in stable Taiwanese renal transplant recipients. J Formos Med Assoc, 104:22–8. © The Formosan Medical Association.
Abbreviations: CsA, cyclosporine; Ca, calcium; FK506, tacrolimus; KBP, FK binding protein; INF-γ, γ-interferon; IL, interleukin; mTOR; mammalian target of rapamycin; RAD; everolimus; SRL, sirolimus.
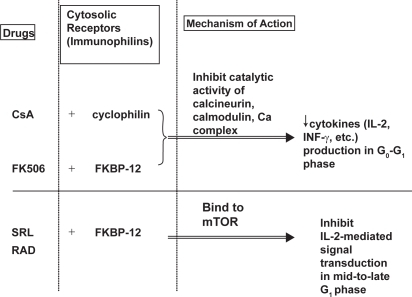
In contrast, sirolimus-FKBP complex binds to the mammalian target of rapamycin (mTOR), thereby inhibiting T-lymphocyte proliferation through inhibition of interleukin-2-mediated (IL-2-mediated) signal transduction in mid to late G1 phase and preventing cell-cycle progression from the G1 phase to the S phase (CitationVathsala et al 1990; CitationSehgal 1998). It also inhibits IL-2 dependent and independent B-lymphocyte proliferation and antibody production (CitationAagaard-Tillery and Jelinek 1994).
Sirolimus oral solution has an oral bioavailability of about 15% and distributes widely in tissue (volume of distribution 19 L/kg; CitationYatscoff 1996; CitationMahalati and Kahan 2001). The mean oral bioavailability of sirolimus tablets is not significantly different from that of oral solution (CitationKelly et al 1999; CitationHariharan and Zimmerman 2000; CitationKelly et al 2000; CitationPDR 2003). The tablets are not bioequivalent to the oral solution. However, clinical equivalence has been demonstrated at the 2-mg dose level (CitationVanBuren 2000; CitationPDR 2003). At doses between 3 and 12 mg/m2, there is a linear correlation between the whole-blood concentration and dosage of sirolimus solution within the same transplant individuals (CitationPDR 2003). The mean time-to-peak concentration (tmax) is about 1 hour after a single dose in healthy volunteers and 2 hours after multiple oral doses in renal transplant recipients (CitationPDR 2003). A study by CitationKelly et al (1999) indicated that there were no differences between the liquid and tablet in trough concentrations (C0) and area under the concentration–time curve (AUC) during dosage form conversion in stable kidney transplant recipients. However, tablets had a lower whole-blood peak concentration (Cmax) and dose corrected Cmax. The tmax of tablets was longer, but was significant only at 4 weeks post-conversion. The inter-individual variability and percentage fluctuation of sirolimus serum concentration were less in tablets than in liquids (CitationKelly et al 2000). These results indicate that sirolimus tablets provide more consistent immunosuppressive exposure over the dosing interval in each individual than the liquid formulation.
Sirolimus is metabolized extensively by liver enzymes, with only 2.2% excreted unchanged in urine (CitationPDR 2003). A study by CitationWu et al (2005) found that the dose-adjusted trough concentration of sirolimus in patients with persistent liver enzyme elevation was significantly higher than in those with normal liver function. Like cyclosporine and tacrolimus, sirolimus is a substrate for both cytochrome P450 (CYP) 3A and P-glycoprotein (P-gp) (CitationSattler et al 1992; CitationTurgeon et al 1994; CitationLampen et al 1995; CitationFricker et al 1996; CitationYatscoff 1996; CitationLown et al 1997; CitationMasuda et al 2000), and thus has potential drug interactions similar to those of CNIs. In addition, the potential drug interactions between CNIs and sirolimus have been a concern. Sirolimus did not significantly affect the pharmacokinetics of either an olive oil-based or the microemulsion-formulated cyclosporine in human studies (CitationZimmerman and Kahan 1997; CitationKahan et al 1998; CitationMacDonald et al 2000). A limited number of available studies did not reveal an effect of tacrolimus on the pharmacokinetics of sirolimus (CitationMacDonald et al 2000; CitationMcAlister et al 2002; CitationKuypers et al 2003; CitationUndre 2003). However, both an olive oil-based or the microemulsion-formulated cyclosporine may increase the bioavailability of sirolimus (CitationPDR 2003). In addition, the bioavailability and trough concentrations of sirolimus are significantly higher when microemulsion-formulated cyclosporine and sirolimus are administered concomitantly than when they are staggered by 4 hours (CitationKaplan et al 1998; CitationMacDonald et al 2000; CitationPDR 2003). Thus, the manufacturer of sirolimus, Wyeth Ayerst (Philadelphia, PA), has suggested that the two drugs be given 4 hours apart. However, a randomized, parallel-group study has shown that during multiple-dose administration, the bioavailability and trough concentration of sirolimus were, respectively, 1.46 and 1.42 times higher in the cyclosporine group than in the tacrolimus group, even though sirolimus was administered 6 hours after CNIs (CitationWu et al 2005). Staggered administration cannot completely prevent a drug interaction between cyclosporine and sirolimus solution. Whether sirolimus tablets exert the same drug interaction with cyclosporine remains to be studied.
Efficacy, comparative studies, and prolonged therapy
In September 1999, sirolimus was approved by the US Food and Drug Administration (FDA) for use in combination with cyclosporine and steroids. In November 2000, the drug was registered by the European Agency as an alternative to CNI for maintenance therapy. In April 2003, the FDA approved a new regimen to reduce the use of cyclosporine by substituting higher doses of sirolimus (CitationKahan 2004).
The safety and efficacy of sirolimus oral solution and tablets for the prevention of graft rejection following kidney transplantation has been compared in a 477-patient, randomized, multicenter, controlled trial. There was no significant difference between the oral solution and tablet formulation for rejection, graft, and patient survival at 3, 6, and 12 months after transplantation (CitationVanBuren 2000; CitationPDR 2003).
A CNI-free sirolimus-based protocol may avoid CNI nephrotoxicity. The incidences of acute rejection, chronic graft failure, and graft loss associated with this sirolimus-based therapy were 19%, 14%, and 10%, respectively (CitationKahan 2004). The long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal have been demonstrated by CitationKreis et al (2004). Lipid profiles were similar between groups, while blood pressures and incidence of abnormal kidney function, edema, hyperuricemia, hyperkalemia, gingival hyperplasia, and Herpes zoster were significantly lower in the sirolimus-based therapy. Recipients with low to moderate rejection risk may consider stopping cyclosporine 2–4 months after transplantation (CitationHalloran 2004; CitationKreis et al 2004). Compared with other immunosuppressive agents, regimens containing sirolimus have a lower incidence of cytomegalovirus (CMV) and BK polyoma virus infection (CitationHalloran 2004; CitationKahan 2004).
Different from other immunosuppressive agents, m-TOR inhibitors may have antineoplastic and arterial protective effects. Sirolimus slows the growth of established experimental tumors (CitationGuba et al 2002). According to Kahan’s study group (CitationKahan 2004), sirolimus therapy oughtweighs other immunosuppressive agents in the low incidence of malignancy. Compared with the general US population, the sirolimus/cyclosporine therapy showed equal incidence of skin cancers, and a 4-fold increase in post-transplant lymphoproliferative disorder (PTLD) and renal cell carcinoma. It was also reported to inhibit the progression of dermal Kaposi’s sarcoma in kidney-transplant recipients while providing effective immunosuppression (CitationStallone et al 2005). The incidence of Kaposi’s sarcoma among solid organ recipients is about 500 times that in the general population (CitationHayward 2003). Three months after switching from cyclosporine to sirolimus in 15 kidney-transplant recipients who had biopsy-proven Kaposi’s sarcoma, all cutaneous Kaposi’s sarcoma lesions disappeared in all patients (CitationGuba et al 2002).
Incorporation of sirolimus into coronary stents inhibits restenosis (CitationMorice et al 2002; CitationDibra et al 2005). Combination of everolimus with CNIs reduced the incidence of graft coronary artery disease associated with heart transplantation (CitationEisen et al 2003). However, the potential arterial protective effects of m-TOR inhibitors must be weighed against their hyperlipidemia side-effect (CitationBlum 2002).
Tolerability
The major adverse reactions of sirolimus include hyper-lipedemia, thrombocytopenia, and impaired wound healing (CitationPDR 2003). Delayed recovery from acute tubular necrosis in kidney transplants, aggravation of proteinuria, mouth ulcers, pneumonitis, and skin lesions were also reported (CitationHalloran 2004). Theoretically, sirolimus should not cause nephrotoxicity due to lack of CNI activity (CitationSehgal 1998). Its combination with CNIs was expected to decrease nephrotoxicity of CNIs due to dose reduction of CNIs (CitationMcAlister et al 2000; CitationKahan and Camardo 2001). However, the combinations have been reported to increase nephrotoxicity, the hemolytic-uremic syndrome, and hypertension (CitationGonwa et al 2003; CitationHalloran 2004). It should be noted that the dose and whole-blood concentrations of CNIs and sirolimus should be taken into consideration in interpreting these data. For example, in the study by CitationGonwa et al (2003), there was no difference between the two groups in median tacrolimus whole-blood trough concentration (8.5 ng/mL and 8.7 ng/mL in the sirolimus- and mycophenolate-treatment groups, respectively). That is, there was no dose reduction of tacrolimus in the sirolimus-treatment group. The median sirolimus dose was 3 mg/day with a corresponding median whole-blood trough concentration 7.3 ng/mL at 6 months, which was higher than 2 mg/day (the dosage usually recommended). Dose reduction of CNIs as well as minimizing the dose of sirolimus is crucial to minimize toxicity of this combination. Withdrawing one of the drugs in the combination of a m-TOR inhibitor and cyclosporine can also reduce renal dysfunction and hypertension, with a small increase in rejection episodes (CitationJohnson et al 2001).
Pharmacoeconomic data and patient acceptability
With the help of the nanotechnology, tablet formulation of sirolimus was successfully introduced onto the market one year after the launch of the oil-based liquid formulation. Patient satisfaction is greater with sirolimus tablet than oil-based liquid form because of improvement in palatability, less restricted storage conditions, and greater convenience of administration. Tablet sirolimus is now the preferred dosage form except in those who require a lower dose or cannot take tablets.
Sirolimus has been recognized as a potent immunosuppressive agent with less nephrotoxicity than CNIs, such as tacrolimus and cyclosporine. The cost-effectiveness and cost-utility studies of sirolimus versus tacrolimus and cyclosporine in kidney transplant patients have been reported by CitationMcEwan et al (2005, Citation2006). A stochastic simulation model was developed using clinical trial and observational data to forecast the incidence of graft failures, hemodialysis, peritoneal dialysis, retransplants, and acute rejections. Using historical data on 937 renal transplant recipients, the costs of the different events from the perspective of the UK National Health Service, valued at 2003 prices with discount, were then used to evaluate both cost-effectiveness and cost-utility over 10 and 20 years after transplantation (CitationMcEwan et al 2005, Citation2006). Treatment with sirolimus was projected to gain 0.72 and 1.8 discounted years of functioning graft over tacrolimus over 10-year and 20-year time horizons, respectively (CitationMcEwan et al 2005). Similarly, when compared with cyclosporine, sirolimus gained 0.6 and 1.59 discounted years of functioning graft and a cost saving of US$486 and US$13033 per patient over 10-year and 20-year time horizons, respectively (CitationMcEwan et al 2006).
Conclusions
Sirolimus is one of the most potent immunosuppressive agents in transplantation which is useful in combination with CNI or as an alternative to CNI for maintenance therapy. Although sirolimus is correlated with higher incidence of hyperlipedemia, thrombocytopenia, impaired wound healing, delayed graft function, proteinuria, mouth ulcers, pneumonitis, and skin lesions, it has antineoplastic and arterial protective effects, and is associated with lower incidence of CMV infection in transplant recipients. With the application of nanotechnology, NanoCrystal formulation overcomes the problems of formulation, poor bioavailability, and erratic absorption of sirolimus. The tablet formulation has a better palatability, and is more convenient for long-term use. In addition, cost-effectiveness and cost-utility analysis also demonstrated the benefits of long-term use of sirolimus in kidney transplantation. Using nanocrytalline sirolimus as a successful prototype, nanotechnology is a prominent approach in the formulation of water-insoluble drugs for clinically applicable dosage forms in the future.
Disclosures
Neither of the authors has any conflict of interest.
References
- Aagaard-TilleryKMJelinekDF1994Inhibition of human B lymphocyte cell cycle progression and differentiation by rapamycinCell Immunol1564935077517796
- AlemdarAYSadiD2004Liposomal formulations of tacrolimus and rapamycin increase graft survival and fiber outgrowth of dopaminergic graftsCell Transplant132637115191164
- AshokBArlethL2004In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: effects of PEG chain length and PC incorporationJ Pharm Sci9324768715349957
- BlumCB2002Effects of sirolimus on lipids in renal allograft recipients: an analysis using the Framingham risk modelAm J Transplant2551912118900
- ClipstoneNACrabtreeGR1992Identification of calcineurin as a key signalling enzyme in T-lymphocyte activationNature35769571377362
- DibraAKastratiA2005Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patientsN Engl J Med3536637016105990
- EisenHJTuzcuEM2003Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipientsN Engl J Med3498475812944570
- ForrestMLWonCY2006In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(epsilon-caprolactone) micellesJ Control Release110370716298448
- FreitasRA2005What is namomedicineNanomedicine: Nanotechnology, Biology, and Medicine139
- FrickerGDreweJ1996Relevance of p-glycoprotein for the enteral absorption of cyclosporin A: in vitro-in vivo correlationBr J Pharmacol118184178842452
- GonwaTMendezR2003Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 monthsTransplantation7512132012717205
- GubaMvon BreitenbuchP2002Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factorNat Med81283511821896
- HalloranPF2004Immunosuppressive drugs for kidney transplantationN Engl J Med35127152915616206
- HalloranPFMillerLW1996In vivo immunosuppressive mechanismsJ Heart Lung Transplant15959718913912
- HariharanSZimmermanJJ2000A comparative study of the pharmacokinetic profiles of sirolimus oral solution and tablets in renal allograft patients [abstract]Transplantation69SupplS154no. 159
- HaywardGS2003Initiation of angiogenic Kaposi’s sarcoma lesionsCancer Cell31312559168
- JohnsonRWKreisH2001Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressureTransplantation727778611571437
- KahanBD2004Sirolimus: a ten-year perspectiveTransplant Proc3671515013304
- KahanBDCamardoJS2001Rapamycin: clinical results and future opportunitiesTransplantation7211819311602840
- KahanBDPodbielskiJ1998Immunosuppressive effects and safety of a sirolimus/cyclosporine combination regimen for renal transplantationTransplantation66104069808489
- KaplanBMeier-KriescheHU1998The effects of relative timing of sirolimus and cyclosporine microemulsion formulation coadministration on the pharmacokinetics of each agentClin Pharmacol Ther6348539465841
- KayserO2000Nanosuspensions for the formulation of aphidicolin to improve drug targeting effects against leishmania infected macrophagesInt J Pharm196253610699730
- KellyPNapoliKL2000Comparison of the pharmacokinetics of sirolimus (Rapamune) in renal transplant recipients following administration of the liquid or solid tablet formulations [abstract]Transplantation69SupplS154no. 158.
- KellyPANapoliK1999Conversion from liquid to solid rapamycin formulations in stable renal allograft transplant recipientsBiopharm Drug Dispos202495310594869
- KreisHOberbauerR2004Long–term benefits with sirolimus-based therapy after early cyclosporine withdrawalJ Am Soc Nephrol158091714978184
- KuypersDRClaesK2003Long-term pharmacokinetic study of the novel combination of tacrolimus and sirolimus in de novo renal allograft recipientsTher Drug Monit254475112883227
- LampenAChristiansU1995Metabolism of the immunosuppressant tacrolimus in the small intestine: cytochrome P450, drug interactions, and interindividual variabilityDrug Metab Dispos231315248689938
- LownKSMayoRR1997Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporineClin Pharmacol Ther62248609333100
- MacDonaldAScarolaJ2000Clinical pharmacokinetics and therapeutic drug monitoring of sirolimusClin Ther22Suppl BB10112110823378
- McAlisterVCGaoZ2000Sirolimus-tacrolimus combination immunosuppressionLancet355376710665560
- McAlisterVCKeshavamurthyM1999Oral delivery of liposomal tacrolimus: increased efficacy and reduced toxicityTransplant Proc31111010083495
- McAlisterVCMahalatiK2002A clinical pharmacokinetic study of tacrolimus and sirolimus combination immunosuppression comparing simultaneous to separated administrationTher Drug Monit243465012021624
- McEwanPBaboolalK2005Evaluation of the cost-effectiveness of sirolimus versus cyclosporin for immunosuppression after renal transplantation in the United KingdomClin Ther2718344616368455
- McEwanPDixonS2006Evaluation of the cost effectiveness of sirolimus versus tacrolimus for immunosuppression following renal transplantation in the UKPharmacoeconomics24677916445304
- MahalatiKKahanBD2001Clinical pharmacokinetics of sirolimusClin Pharmacokinet405738511523724
- MasudaSUemotoS2000Effect of intestinal P-glycoprotein on daily tacrolimus trough level in a living-donor small bowel recipientClin Pharmacol Ther689810310945321
- Merisko-LiversidgeELiversidgeGG2003Nanosizing: a formulation approach for poorly-water-soluble compoundsEur J Pharm Sci181132012594003
- MerrillJPMurrayJE1956Successful homortransplantation of the human kidney between identical twinsJAMA16027782
- MoriceMCSerruysPW2002A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularizationN Engl J Med34617738012050336
- MorrisPJ2004Transplantation--a medical miracle of the 20th centuryN Engl J Med35126788015616201
- MullerRHJacobsC2001Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the futureAdv Drug Deliv Rev4731911251242
- MullerRHKeckCM2004Challenges and solutions for the delivery of biotech drugs – a review of drug nanocrystal technology and lipid nanoparticlesJ Biotechnol1131517015380654
- NoyesAAWhitneyWR1897The rate of solution of solid substances in their own solutionsJ Am Chem Soc199304
- PatravaleVBDateAA2004Nanosuspensions: a promising drug delivery strategyJ Pharm Pharmacol568274015233860
- PDR2003Rapamune oral solution and tablets Physicians’ Desk ReferenceMontvaleMedical Economics Company
- PDR2006Neoral soft gelatin capsules (Novartis) Physicians’ Desk ReferenceMontvaleThomson PDR
- RosenHAbribatT2005The rise and rise of drug deliveryNat Rev Drug Discov4381515864267
- SattlerMGuengerichFP1992Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and ratDrug Metab Dispos20753611385058
- SayeghMHCarpenterCB2004Transplantation 50 years later--progress, challenges, and promisesN Engl J Med3512761615616214
- ScottLJMcKeageK2003Tacrolimus: a further update of its use in the management of organ transplantationDrugs6312479712790696
- SehgalSN1998Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progressionClin Biochem31335409721431
- SimamoraPAlvarezJM2001Solubilization of rapamycinInt J Pharm21325911165091
- StalloneGSchenaA2005Sirolimus for Kaposi’s sarcoma in renal–transplant recipientsN Engl J Med35213172315800227
- ThomasKKoelwelC2005Three generations of cyclosporine a formulations: an in vitro comparisonDrug Dev Ind Pharm313576616093201
- TurgeonDKLeichtmanAB1994P450 3A activity and cyclosporine dosing in kidney and heart transplant recipientsClin Pharmacol Ther56253607924120
- UndreNA2003Pharmacokinetics of tacrolimus-based combination therapiesNephrol Dial Transplant18Suppl 1i12512738758
- ValenteJFAlexanderJW1998Immunobiology of renal transplantationSurg Clin North Am781269531932
- VanBurenCT2000Sirolimus oral solution and tablets demonstrate equivalent safety and efficacy in real allografts [abstract]Transplantation69SupplS153no 157.
- VasquezEM2000Sirolimus: a new agent for prevention of renal allograft rejectionAm J Health Syst Pharm5743748quiz 449–51. 10711524
- VathsalaAChouTC1990Analysis of the interactions of immunosuppressive drugs with cyclosporine in inhibiting DNA proliferationTransplantation49463721689520
- VezinaCKudelskiA1975Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principleJ Antibiot (Tokyo)2872161102508
- WeberDO1999NanomedicineHealth Forum J4232367
- WuFLTsaiMK2005Effects of calcineurin inhibitors on sirolimus pharmacokinetics during staggered administration in renal transplant recipientsPharmacotherapy256465315899725
- WuFLTsaiMK2005Effects of conversion from sirolimus oral solution to tablets in stable Taiwanese renal transplant recipientsJ Formos Med Assoc10422815660173
- YatscoffRW1996Pharmacokinetics of rapamycinTransplant Proc2897038623483
- YokoyamaM2005Drug targeting with nano-sized carrier systemsJ Artif Organs8778416094510
- ZimmermanJJKahanBD1997Pharmacokinetics of sirolimus in stable renal transplant patients after multiple oral dose administrationJ Clin Pharmacol37405159156373