Abstract
The polyene antibiotic Amphotericin B (AMB) is one of the first therapeutic agents to be marketed commercially as nanosized formulations in which the drug is associated with lipids as liposomes or complexes. In this way, its renal toxicity is reduced and its therapeutic index improved. This review summarizes the particular properties of AMB which justify this type of formulation and the early work leading up to their development. The clinical results obtained in the treatment of fungal infections are reviewed and their activity against leishmaniasis is also evoked. Some newer formulations of AMB, based on both lipids and polymers are described. In particular, their potential by the oral and pulmonary routes are discussed. Finally, the development of targeted systems to deliver the drug to specific cells and tissues is considered.
Introduction
The polyene antibiotic Amphotericin B (AMB) is one of the first therapeutic agents to be marketed commercially as nanosized formulations. This review will summarize the particular properties of AMB which justify this type of formulation and the early work leading up to their development. The clinical results obtained in the treatment of fungal infections will be reviewed. Finally, some newer formulations, in which the drug has been associated with polymers as well as lipids, and new directions in the use of AMB will be considered.
Properties of AMB and early work with AMB in liposomes
Properties of AMB
The antimicrobial properties of AMB, a macrolide extracted from Streptomyces nodosus, were first noted in the 1950s (CitationVandeputte et al 1955–1956) and the antibiotic arrived on the market in 1958 (CitationUtz et al 1958–1959). The drug possesses a wide spectrum of activity, encompassing a large number of fungal species as well as protozoan parasites (Leishmania species) and amoebae (Naegleria species) (for a recent review, see CitationKleinberg 2006). More recently, it was found to have some activity against prion diseases (CitationHartsel and Weiland 2003; CitationMangé et al 2000). Despite its therapeutic importance, the physicochemical properties of AMB lead to some difficulties in its formulation and utilization, and solutions based on “nanotechnology” have been developed in response to these.
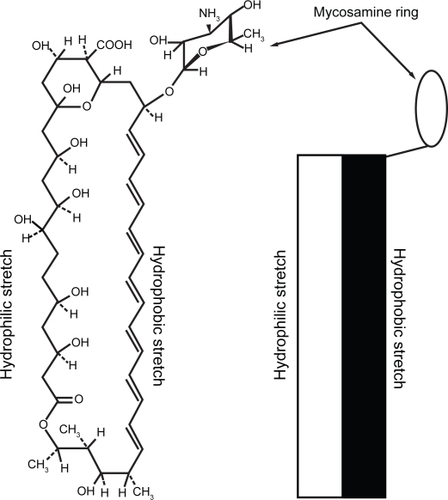
AMB () is an asymmetrical, cyclic molecule with one hydrophobic and one hydrophilic face, and an aminosugar (mycosamine) group. It has a very limited solubility profile, being almost completely insoluble in water, sparingly soluble in alcohols and soluble in organic solvents such as DMSO and DMF (CitationBrittain 1994). In water, AMB aggregates, forming first dimers by apposition of two hydrophobic faces followed by larger aggregates (CitationMazerski et al 1982). This insolubility in aqueous media also leads to low bioavailability of AMB by the oral route. Its use is therefore limited to intravenous infusion and local application. The conventional formulation of AMB (Fungizone®) is mixed micelles with the detergent sodium deoxycholate, and in this form it is the drug of choice for systemic infections with sensitive fungal species (CitationGeorgopapadakou and Walsh 1996). It is usually administered by slow perfusion diluted in 5% glucose. However, dose-limiting side-effects are frequent, the most severe being renal toxicity (CitationBrezis et al 1984).
Both the therapeutic and toxic effects of AMB derive from its interaction with lipids, and in particular, membrane sterols. The antibiotic can form complexes with both ergosterol, the principal sterol in fungal cell membranes, and cholesterol in mammalian cell membranes. The result of this is the formation of pores leading to the leakage of electrolytes and other cell components (CitationDe Kruijff and Demel 1974). The selectivity for fungal cell membranes is the result of greater affinity for ergosterol than cholesterol, due to the presence of a double bond on carbon 22 in the former (CitationCybulska et al 1986).
Given the affinity of the antibiotic for biological membranes, incorporation of AMB into lipid-based nanosystems in order to improve its therapeutic index has been studied since the 1980s. Three of these systems are now commercially available.
Mechanisms of action
In 1996, Hartsel and Bolard reviewed the mechanisms by which the selectivity of AMB towards fungal cells can be improved by association with lipid systems. One mechanism is related to the molecular state of the AMB when it is released from the formulation. It has been observed that while both monomeric and self-aggregated AMB associate with ergosterol, only self-aggregated AMB forms pores in cholesterol-containing membranes. It follows that a formulation that can assure that AMB is released only as monomers will have an improved therapeutic index. On dilution in the plasma, AMB is rapidly released from Fungizone® in the aggregated form and toxicity to mammalian cells ensues. However, AMB can also bind to membrane phospholipids, so the relative affinity for cell and drug delivery system lipids may contribute to determining the reduction of toxicity. When AMB binds to cell membranes it has a pro-oxidative effect, and this may be as important as pore formation in generating cell damage (CitationBratjburg et al 1985).
Furthermore, the affinity of AMB for lipids means that it is readily incorporated into plasma lipoproteins, particularly low density lipoprotein (CitationBratjburg et al 1984). Receptor-mediated uptake of low density lipoprotein carrying AMB by renal epithelial cells is one mechanism of toxicity in this organ. Therefore, the rate of transfer between the drug delivery system and circulating lipoproteins will be another factor which determines the efficacy of the system (CitationLegrand et al 1996).
AMB also has effects on the immune system, and in particular can modulate the functions of macrophages. For example, it stimulates production of cytokines such as interleukin 1 (CitationChia and McManus 1990) and tumor necrosis factor alpha (TNF-α) (CitationTokuda et al 1993), reactive oxygen intermediates (CitationWilson et al 1991) and nitric oxide (NO) (CitationHerrmann et al 1994; CitationMozaffarian et al 1997), as well as chemotaxis and phagocytosis. These properties could contribute to the antimicrobial activity of AMB, but could also increase its toxicity, for example by causing fever and chills. CitationLarabi et al (2001) compared the production of NO and TNF-α induced in non infected mouse peritoneal macrophages by different lipid formulations of AMB compared with free AMB, in association with co-stimulants. At equivalent AMB concentration, mediator production was always less with the lipid formulations than with the free drug, and the liposomal forms (eg, AmBisome®) reduced this more than lipid complexes (eg, Abelcet®). In macrophages infected with Leishmania donovani, AMB also contributed to stimulating NO and TNF-α production, but the concentrations at which this occurred were much higher than those causing parasite killing (Larabi et al unpublished results), suggesting that in this case at least, the immunostimulating effects contribute more to the side-effects of AMB than to its antiparasitic activity.
Early liposomal formulations
The first study incorporating AMB into liposomes was performed by CitationNew et al in 1981. Their interest was in the antileishmanial properties of the antibiotic, and followed on from studies of encapsulated antimonial drugs. The fact that Leishmania parasites are located within phagocytic cells, and that liposomes are also preferentially accumulated by these cells made this approach particularly attractive (CitationHeath et al 1984). However, the main effect of the liposomal formulation was to reduce the toxicity of AMB, allowing higher doses to be administered and thus increasing “efficacy”. Soon afterwards, similar results were obtained in infections with Cryptococcus (CitationGraybill et al 1982) and in histoplasmosis (CitationTaylor et al 1982). However, the antimicrobial activity of AMB per se was not increased by encapsulation. During this period, the influence of the liposome composition, and size, on the activity of AMB was studied. In one study (CitationLopez-Berestein et al 1983), it was found that liposomes containing phospholipids alone were more efficient than liposomes containing sterols (cholesterol or ergosterol). One explanation for this could be that the strong binding of AMB to sterol prevents its release from the liposomes and its interaction with fungal cell membranes. A study by CitationSzoka et al (1987) of a range of liposome sizes and compositions found that there was no correlation between the extent of reduction of toxicity against mouse macrophages in vitro and the reduction of lethality in vivo. In vivo, small sterol-containing liposomes were less toxic than larger ones, and liposomes without sterol but containing phospholipids which were in a “solid” (liquid crystal) state at physiological temperatures were less toxic than ones in which the phospholipids were in a fluid state. A large number of different AMB formulations were tested, leading to the commercialization of three of them. These have quite different physico-chemical structures (see below), but all reduce the toxicity of AMB compared with Fungizone®.
Commercial formulations of AMB
Three lipid-based formulations of AMB are at present licensed for clinical use. Their physico-chemical properties are listed in .
Table 1 Commercial formulations of AMB
Physico-chemical properties
AmBisome® is the only true liposomal formulation of the three. It is composed of small, unilamellar vesicles composed of lipids which yield a very stable bilayer, in the gel state at physiological temperature. AMB is incorporated into this bilayer at 10 moles %. The size of the liposomes (about 80 nm) means that they have a long circulating half-life and a good penetration into tissues. The stable bilayer composition should reduce exchanges with lipoproteins and contribute to the very low toxicity of this formulation (CitationAdler-Moore and Proffitt 2002).
Amphotec® (Amphocil™ in Europe and Amphotec® in the US) is composed of complexes between cholesteryl sulfate and AMB in equimolar proportions. These have the form of thin discs of about 120 nm in diameter. However, despite the small size, their circulation time is much less than that of AmBisome® and they deliver AMB rapidly to phagocytic cells (CitationGuo 2001).
Abelcet® is composed of two synthetic phospholipids – dimyristoyl phosphatidylcholine and dimyristoyl phosphatidylglycerol in a 7 to 3 molar ratio with an equimolar amount of AMB. These components assemble in ribbons of 1 to 10 micrometers in length. These larger objects are rapidly accumulated in the mononuclear phagocyte system (CitationJanoff et al 1993).
Clinical studies in fungal infections
Systemic fungal infections, dominated by Candida and Aspergillus infections, remain the leading cause of infection-related mortality and morbidity in many populations of immunocompromised patients. Azoles are often recommended for Candida infection but the epidemiology of Candida infection has changed over the last few years. C albicans now comprises less than half of the isolates of candidemia worldwide (CitationEggimann et al 2003; CitationPappas et al 2003). The other half is represented by a variety of non-albicans species, for some of which the susceptibility to azoles, particularly fluconazole, is decreased. For Aspergillus and other less common moulds, the mortality rates are greater than 60% and even higher in patients with disseminated infection, although the extended-spectrum azoles represent a major advance as a first-line treatment (CitationHerbrecht et al 2002). Therefore, there is a need for more effective antifungal drugs with a wide spectrum.
In this respect, AMB has the advantage of covering most of the fungal pathogens involved in human disease. However, the use of AMB formulated with deoxycholate (Fungizone®) has been limited by infusion-related side effects and cumulative nephrotoxicity which, in fine, actually increase overall healthcare expenses, despite its primarily low cost (CitationMaertens et al 2001). For these reasons, and because other alternatives are now available, primary therapy with Fungizone® is more and more challenged by new antifungal therapies for use in many systemic mycoses, including moulds, such as Aspergillus. These new antifungal therapies include extended-spectrum triazoles, the echinocandins, and also lipid formulations of AMB, as described above (CitationHerbrecht et al 2003). Among the lipophilic formulations of AMB commercially available, the majority of studies have been carried out with liposomal AMB (AmBisome®).
Clinical efficacy of liposomal AMB
Due to the paucity of diagnostic means for fungal infections and the poor prognosis of full-blown invasive fungal infections, clinicians use several strategies when faced with fungal infections. Antifungal drugs are given for demonstrated infections but in high-risk patients, they may be administered empirically, in the case of persistent fever despite appropriate antibacterials, or as prophylaxis, in every patient at risk of fungal infection whatever the clinical signs. The efficacy of liposomal AMB has been studied in these different settings, both in open and randomized studies. Liposomal AMB has also proved effective in the treatment of visceral leishmaniasis (see below).
Demonstrated infections
In full-blown fungal infections, initial open studies involving patients refractory to, or intolerant of, Fungizone® showed improvement or cure in 66% and 81% of patients with invasive aspergillosis and invasive candidiasis respectively, with the liposomal form (CitationRingden et al 1991). Two studies have suggested a superior efficacy of liposomal AMB compared to Fungizone® in probable or proven fungal invasive infections but were insufficient to give a definitive answer (CitationLeenders et al 1997; CitationLeenders et al 1998).
Another important issue is the dose to be administered. It was expected that the good tolerance profile would allow high liposomal AMB doses to be given and achieve better efficacy without increasing toxicity. In a randomized trial comparing two doses of liposomal AMB (1 versus 4mg/kg/day) for the primary treatment of invasive aspergillosis, an overall response rate of 55% was observed, regardless of the dose, with no difference in either arm (CitationEllis et al 1998). This substantial response rate demonstrates evidence of the efficacy of liposomal AMB in first-line therapy of invasive aspergillosis. To determine the appropriate daily dose for the initial treatment of invasive aspergillosis and other filamentous fungal infections in immunocompromised patients, a phase 3, multi-center, randomized, double-blind study of the safety and efficacy of an liposomal AMB loading dose regimen versus a standard liposomal AMB regimen was performed (CitationCornely 2005). The study compared a loading regimen of 10 mg/kg/day × 14d versus the standard regimen of 3 mg/kg/day for 14 days. The standard regimen had a favorable overall response rate of 50% and a 12-week survival rate of 72% comparable to those previously reported for voriconazole in a similarly designed trial (CitationHerbrecht et al 2002). However, the high-dose regimen did not demonstrate any improvement in overall response or survival.
In an attempt to sum up the efficacy of lipid formulations of AMB, not all in the liposomal form, a meta-analysis of seven randomized studies was performed (CitationBarrett et al 2003). This analysis did not show any difference in the response rate between the lipid formulations of AMB and Fungizone® but showed a decrease in mortality (OR = 0.72; 95% CI = 0.54 – 0.97).
The poor outcome of mould infections and the availability of several antifungal drugs of different classes have stimulated the evaluation of alternatives based on combinations of different antifungal drugs (CitationJohnson et al 2004). Apart from the association of Fungizone® and 5-fluorocytosine which has been the recommended treatment for cryptococcal meningitis for a long time, the other associations, mainly between Fungizone® and azoles, raised several concerns about possible toxicities (CitationPolak 1999). In vivo studies have shown encouraging results, for the associations of liposomal AMB with both voriconazole and echinocandins in models as different as rat models of invasive aspergillosis (CitationKirkpatrick et al 2006), a murine model of cerebral aspergillosis (CitationClemons et al 2005), or a murine model of C. glabrata systemic infection (CitationOlson et al 2005). In humans, a few case reports and small series of benefic results of associations have been reported but none are randomized studies (CitationAliff et al 2003; CitationKontoyiannis et al 2003; CitationMarr et al 2004).
Empirical therapy
In the setting of empirical therapy for persistent febrile neutropenia, comparative studies concluded that liposomal AMB is as effective as Fungizone® (CitationPrentice et al 1997; CitationWalsh et al 1999). A double-blind study compared the safety of liposomal AMB (3 or 5 mg/kg/day) and AMB lipid complex (5 mg/kg/day) (CitationWingard et al 2000). Neither of the two liposomal AMB dosages yielded a better outcome than AMB lipid complex.
Primary prophylaxis
Three randomized trials have assessed the efficacy of low doses of liposomal AMB as prophylaxis in bone marrow transplant recipients, without demonstrating any benefit (CitationKelsey et al 1999; CitationTollemar et al 1993a, Citation1993b). One study in liver transplant patients showed a significant decrease in invasive Candida spp. infections in the liposome-treated patients, compared to the placebo-treated patients but the 1-month survival was identical in both groups. However, long-term survival was increased in patients who received liposomal AMB (CitationTollemar et al 1995). Recently, a pharmacokinetic study of once-weekly high-dose liposomal AMB as fungal prophylaxis for immunocompromised children undergoing stem cell transplantation suggested that this dosage may provide useful protection against fungal infections (CitationMehta et al 2006).
Tolerability
Patients treated with liposomal AMB at 3 mg/kg/day had less infusion-related adverse events, needed less premedication, and had less nephrotoxicity than patients treated with Fungizone® at 0.6 mg/kg/day (CitationPrentice et al 1997; CitationWalsh et al 1999). The tolerability of high doses up to 7.5–15 mg/kg/day appeared satisfactory (CitationWalsh et al 2001). These results justified comparing a liposomal AMB loading dose regimen versus a standard liposomal AMB regimen (CitationCornely 2005). Higher rates of hypokalemia and nephrotoxicity were seen compared with the standard dose regimen with no better efficacy. In neonates, high doses (5–7 mg/kg/day) of liposomal AMB for a median of 18 days seem to be much better tolerated than in adults (CitationJuster-Reicher et al 2003). Compared with AmB lipid complex at 5 mg/kg/day, liposomal AMB at 3 and 5 mg/kg/day showed less infusion-related reactions and nephrotoxicity in febrile neutropenic patients (CitationWingard et al 2000). This study clearly indicates that liposomal AMB was better tolerated than AMB lipid complex.
Conclusion
Liposomal AMB has been shown to be at least as efficacious as Fungizone® and has a dramatically improved safety profile compared with the traditional form. The recommended dose is 3–5mg/kg/day for demonstrated fungal infection and 3 mg/kg/day for empirical therapy, and doses up to 3 mg/kg/day of liposomal AMB are well tolerated. Higher doses have not shown any therapeutic benefit in invasive aspergillosis whereas they increased renal toxicity. For some rare filamentous fungus infections such as those due to zygomycetes and Fusarium spp, the liposomal formulation may be considered as first-line therapy because of the absence of an effective alternative, although new azoles may be effective towards some of these fungi. The in vitro models and the experimental data in animals show that combination therapy may improve outcome, but these experimental results remain to be confirmed with clinical trials.
Lipid formulations of AMB in the treatment of leishmaniasis
Leishmaniasis is a family of protozoal infections transmitted by sand-fly bites, which affects about 12 million people in warm regions throughout the world. Visceral leishmaniasis, in which the parasite – Leishmania donovani in India and Bangladesh, L. infantum in the rest of Asia, Africa and Europe and L. chagasi in the Americas – develops within tissue macrophages in the liver, spleen and bone marrow, is the most serious manifestation (CitationHerwaldt 1999). It is endemic in India, Bangladesh and Sudan where it represents a major public health problem and is also becoming increasingly prevalent as an opportunistic infection in Western countries, among individuals who are infected with the HIV virus or are immunocompromised for other reasons. Cutaneous leishmaniasis, characterized by skin lesions, is more common but less serious. Mucocutaneous and disseminated cutaneous manifestations also occur (CitationHerwaldt 1999). Early treatment options were pentavalent antimonials and pentamidine, which have shown problems of toxicity and resistance (CitationMurray, 2001). AMB was found to be an effective treatment for visceral leishmaniasis in the 1990s (CitationMurray 2004; CitationSingh and Sivakumar 2004). Lipid formulations of AMB are a particularly attractive alternative in this context because they are accumulated in the same cells as the parasite. Thus AmBisome® was approved by the FDA in 1997 (CitationMeyerhoff 1999). Despite the reduced toxicity of the lipid formulations, their high cost is prohibitive in the zones in which visceral leishmaniasis is endemic. A comparative study by CitationSundar et al (2004) showed that although the higher doses that could be given with the lipid formulations reduced the total time for cure and therefore the cost of hospitalization, this only partly offset the high purchase price of the drug. On the other hand, in a European situation, when cost is not such a preponderant issue, lipid formulations of AMB have become the treatment of choice (CitationGradoni et al 2004).
Cutaneous leishmaniais can also be treated effectively with lipid formulations of AMB (CitationAmato et al 2004, CitationYardley and Croft 2000). In this case, the smaller formulations (AmBisome®, Amphocil™) are the most effective, because of their small size.
Recently, a new drug, miltefosine (hexadecylphosphocholine) has been shown to be effective against visceral leishmaniasis by the oral route (CitationMurray 2001; CitationSundar et al 2002). This is a definite breakthrough and shows an obvious advantage over the current formulations of AMB, which are administered intravenously. Associations of AMB and miltefosine may have some therapeutic advantage (CitationSeifert and Croft 2006).
Other formulations of AMB and new trends in their administration
Lipid-based formulations
An adhoc solution to the problem of AMB toxicity is to mix Fungizone® with Intralipid®, a preparation for parenteral nutrition, which consists of an oil-in-water emulsion stabilized with lecithin. The AMB is complexed by the phospholipids on the surface of the oil globules and its toxicity is reduced compared with Fungizone® alone. However, this method does not give reproducible results (CitationTomii 2002).
More recently, AMB has also been mixed with another proprietary lipid emulsion formulation, Lipofundin®. AMB was added as a powder, and the use of a high-pressure homogenizer promoted its dissolution in the interfacial layer, according to the patented SolEmuls® technology. However, no toxicity data are available for this formulation (CitationMüller et al 2004).
De novo emulsion formulations of AMB have also been described, for example, those studied by CitationEgito and collaborators (1996a). The emulsion form reduced the toxicity of AMB considerably, compared with Fungizone®, although less than AmBisome®, and allowed higher doses to be given, allowing a better cure rate of infections with Candida albicans. Circular dichroism studies showed that the AMB remained in the monomeric form within the emulsions, over a wide range of dilutions (CitationEgito et al 1996b).
Seki and co-workers have formulated AMB into nano-sized emulsions called “Lipid Nano Spheres” which attempt to imitate lipoproteins. These small particles (25–50 nm) reduced AMB toxicity compared with Fungizone®, and showed similar activity against Candida albicans. Like AmBisome®, these small particles showed reduced uptake by macrophages and persistence in the circulation (CitationFukui et al 2003).
Heat-treatment of AMB is a simple method of reducing toxicity. Heating the Fungizone® formulation to 70° C provokes the formation of superaggregates, as detected by spectrophotometric methods, rather than aggregates, which are less toxic to mammalian cells while retaining almost equivalent antifungal activity (CitationGaboriau et al 1997). Cryotransmission electron microscopy revealed that while the native product was composed mainly of micelles of about 4 nm in diameter, with some threadlike aggregated micelles, the heated formulation contained much larger networks of about 300 nm (Citationvan Etten et al 2000).
Another approach to modulating the solubility, and therefore the toxicity of AMB was the use of ions from the Hofmeister series which alter water properties. While kosmotropes increased AMB aggregation, the chaotrophic ions thiocyanate and trichloroacetate were found to allow solubilization of AMB as monomers (CitationGrijalba et al 2006).
Larabi et al developed a lipid complex system with a similar composition to Abelcet®, but prepared by a different method known as nanoprecipitation. This led to the formation of thin discs of about 250 nm in diameter, in which the lipids were probably in an interdigitated form rather than a bilayer (CitationLarabi et al 2004a). This change in size and morphology reduced the toxicity, both towards macrophages in vitro and in vivo after both acute and chronic administration to mice, compared to both Fungizone® and Abelcet® (CitationLarabi et al 2003, CitationLarabi et al 2004b). The activity of the complexes against visceral leishmaniasis in mice was higher than that of Abelcet® but not as high as that of AmBisome® (CitationLarabi et al 2003). This illustrates the importance of nanosystem morphology in determining the biological effect.
Another disc-like formulation was developed by CitationLincopan et al (2005, Citation2006) using the cationic lipid dioctadecyldimethylammonium bromide (DODAB). This lipid formed bilayer fragments of about 65 nm in diameter with AMB at a low drug-to-lipid ratio. This formulation reduced nephrotoxicity and hepatotoxicity compared with Fungizone®, but spleen toxicity due to the cationic lipid was observed. At higher drug-to-lipid ratios, drug particles surrounded by a lipid bilayer are formed. The toxicity and therapeutic activity of these formulations have not yet been investigated.
Another group (CitationOda et al 2006) has tried to imitate lipoprotein particles as an original delivery system for AMB. The specific apolipoprotein from high density lipoprotein, ApoA-I, was added to mixtures of dimyristoylphosphatidylcholine, dimyristoylphosphatidylglycerol and AMB. After sonication and dialysis, a limpid preparation was obtained, consisting of disc-like particles of 8–10 nm in diameter. The circular dichroism spectra indicated that AMB was associated with lipid in the formulation. This preparation had much lower toxicity than Fungizone® in vitro and in vivo, which allowed higher doses to be given to mice, leading to effective treatment of Candida albicans infection. These systems also showed a good activity against Leishmania major in Balb/C mice, although no comparison with any other formulation was made (CitationNelson et al 2006).
Lipid cochleates are an interesting system for delivering AMB. These are formed mainly from phosphatidylserine and calcium, which associate by electrostatic interaction to form cylindrical structures consisting of a rolled-up bilayer. They are particularly appropriate for entrapping small hydrophobic or amphiphilic molecules like AMB (CitationZarif 2005). Like other lipid-based systems, AMB cochleates reduce the toxicity of the antibiotic. They are effective against murine candidasis and aspergillosis after i.p. and oral administration (CitationSantangelo et al 2000; CitationDelmas et al 2002).
Polymer-based formulations
AMB has been conjugated to a number of macromolecules with the aim of improving its solubility. Many of these have been derived from polysaccharides. For example, AMB has been conjugated to arabinogalactan (CitationEhrenfreund-Kleinman et al 2002). Polymers of about 30 kDa with about 20% of AMB by weight were obtained. The maximum tolerated dose of AMB was greatly increased by conjugation, while the antifungal activity against Candida albicans remained comparable to that of Fungizone®. A similar approach used dextran as the polysaccharide carrier. In particular, the preparation of a conjugate in which the free aldehyde groups were blocked showed very low toxicity towards mammalian cells while conserving antiparasitic activity (CitationSokolsky-Papkov et al 2006). AMB has also been conjugated to poly (ethylene glycol) (PEG). Attachment of AMB to a PEG of 40 kDa led to a highly water-soluble product which was only hydrolyzed slowly in rat plasma. It was 6 times less toxic than Fungizone® in rats and showed equal or superior antifungal activity (CitationConover et al 2003).
A number of groups have incorporated AMB into micelles prepared from amphiphilic polymers. Diblock copolymers consisting of poly (ethylene oxide) and poly (aspartic acid) substituted with various hydrophobic groups have been extensively studied in the laboratory of Kwon. In particular, poly (ethylene oxide)-block-poly (N-hexyl-L-aspartamide)-stearic acid ester micelles allow the antibiotic to be incorporated in its non aggregated form, as shown by spectrophotometric measurements and to be released in a sustained fashion. Such micelles show similar activity to Fungizone® in a mouse model of disseminated candidiasis (CitationAdams and Kwon 2004). A similar system based on partially benzylated poly (aspartic acid) without a PEG block has been investigated by CitationYoo et al (2006). This polymer formed “nanoparticular” micelles of 20 nm in diameter, in which AMB aggregation was reduced compared with Fungizone®, as judged by its spectral properties. The acute toxicity in mice was reduced, as was damage to kidney cells after intravenous administration to rats, while the in vitro activity against Candida albicans was similar to that of Fungizone®. Vandermeulan et al (2006) have used poly (ethylene glycol)-block-poly (ɛ-caprolactone-co-trimethylenecarbonate) micelles to encapsulate AMB. These micelles are easy to prepare and although they reduce the antifungal activity they also reduce the amount of hemolysis.
There have been a few reports of nanoparticulate forms of AMB. A study by CitationVenier-Julienne et al (1995) used AMB incorporated into poly (D, L-lactide-co-glycolide) nanoparticles. When their activity was tested against cultures of promastigotes of L. donovani within peritoneal macrophages, unloaded nanoparticles had a high an effect as loaded ones. This could be attributed to reactive oxygen intermediate generation following phagocytosis of the nanoparticles.
Espuelas et al attempted to incorporate AMB into poly (ɛ-caprolactone) nanoparticles (CitationEspuelas et al 1998a; CitationEspuelas et al 1998b). In fact, AMB was adsorbed onto the surface of the particles and was released on dilution, but despite this limitation, the acute toxicity of AMB in mice was reduced compared with Fungizone® (Espuelas et al 1997). During this work, it was noted that AMB also formed mixed micelles with the poloxamer 188 surfactant used to stabilize the nanoparticles (CitationEspuelas et al 1998b). These micelles were found to have activity against clinical isolates of Candida albicans in vitro and also, interestingly, to reverse the resistance of Leishmania donovani parasites which had been rendered resistant to the drug by in vitro pressure, by a synergistic effect of AMB and the poloxamer (CitationEspuelas et al 2000). However, the results obtained with Candida albicans-infected macrophages and in mice were disappointing, since the LD50 was increased compared with Fungizone® (CitationEspuelas et al 2003).
More recently, AMB was incorporated into nanoparticles formed by a complex of two polysaccharides of opposing charge: chitosan and dextran sulfate. A high encapsulation rate for AMB was obtained, but spectral analysis showed that it was aggregated. A reduction in renal toxicity was observed but the large size of these particles (600–800 nm) suggests that they would only be useful for liver delivery (CitationTiyaboonchai et al 2006).
Microsphere formulations of AMB have also been tested for therapy of leishmaniasis. Albumin microspheres reduced the toxicity and increased the therapeutic efficiency of AMB against Leishmania infantum in hamsters. As might be expected, the microparticulate form increased drug accumulation in the liver and spleen (CitationSanchez-Brunete et al 2004). High doses of AMB administered in these particles deactivated expression of anti-inflammatory cytokines and increased pro-inflammatory ones, which probably contributed to the therapeutic effect (CitationRama Iniguez et al 2006). Different microsphere formulations were tested (CitationSanchez-Brunete et al 2005). Poly (lactide-co-glycolide) and poly-anhydride microspheres were less effective than albumin ones in reducing liver and spleen parasite load, and albumin microspheres also induced a significant antibody response to parasite antigens.
Carbon nanotubes (CNT) have been attracting much attention lately as potential drug delivery systems. AMB has been linked covalently to functionalized CNT at the same time as fluorescein and uptake of the resulting particles into Jurkat cells was demonstrated (CitationWu et al 2005). Interestingly, the minimal inhibitory concentrations for several fungal species were reduced by this association, while “empty” CNT were without effect.
Administration of AMB by other routes: oral and pulmonary
One major disadvantage of AMB is its very low bioavailability by the oral route. This is essentially due to its very low solubility in aqueous media and its relatively high molecular weight (CitationDangi et al 1998). A number of different lipid-based systems have been used in attempts to improve the intestinal absorption of AMB. The presentation of the drug in the monomeric form could be expected to facilitate its dissolution and other components of the formulations may have absorption promoting effects.
Thus, ternary mixed micelle systems of AMB, deoxycholate and oleic acid, monoolein or soy lecithin were found to enhance the permeability of AMB in isolated intestinal loops (CitationDangi et al 1998). These systems have not been tested in vivo, however. Another system in which AMB was mixed with Peceol, a glyceride-rich vehicle for oral administration, also gave promising results, increasing lymphatic transport of AMB after oral administration to rats (CitationRisovic et al 2004).
AMB cochleates (see above) have also proved to be efficient in the treatment of fungal infections by the oral route. In a model of Balb/C mice infected with Candida albicans, oral administration of these lipid particles was able to eradicate the infection from the lungs and prolong survival as effectively as Fungizone® given i.p. at a similar dose (CitationSantangelo et al 2000). Efficacy was also demonstrated in a murine model of systemic aspergillosis (CitationDelmas et al 2002).
Recently, AMB was associated with another lipidic anti-leishmanial agent, miltefosine (hexadecylphosphocholine or HePC). As described above, this molecule has been shown to be active by the oral route (CitationMurray 2001; CitationSundar et al 2002) and has the interesting property of opening tight junctions in the Caco2 intestinal cell model (CitationMénez et al 2006a). Its alkylphospholipid structure suggested that it might be able to associate with AMB and in fact, spectroscopic studies showed that this can be the case (CitationMénez et al 2006b). However, rather than promoting absorption of AMB, the association led to a reduction of both cellular uptake and transepithelial transport in the Caco2 model (CitationMénez et al 2006b).
CitationKayser et al (2003) formulated a nanosuspension of AMB for administration by the oral route. The particles were prepared by high pressure homogenization of the drug with a mixture of surfactants. A reduction in parasite load in the liver was observed when the formulation was administered orally to mice infected with Leishmania donovani. This result may be related to the presence in the formulation of Tween 80®, which is known to promote passage across biological membranes.
Pulmonary infections with Aspergillus spp. are a major clinical problem in immunocompromised patients. In consequence, there has been much interest in the use of aerosolized AMB formulations for treatment, and for prophylaxis in patients undergoing transplant surgery (CitationDrew 2006). Animal studies have shown that liposomal formulations lead to higher concentrations of AMB in the lungs than Fungizone® (CitationRuijgrok et al 2000) or AMB directly solubilized in fluorocarbons (CitationVyas et al 2005). Clinical trials have shown that the lipid formulations are easier to aerosolize and better tolerated than Fungizone® (CitationPerfect et al 2004; CitationDrew 2006).
Specific targeting of AMB-loaded nanosystems
The studies described above have mainly taken advantage of the uptake of colloidal particles by the mononuclear phagocyte system and thus reach micro-organisms within these cells, or on the small size (for example AmBisome®) and surface properties which allow the carriers to remain in the circulation and reach other tissues in a non specific function. However, there are a few reports of attempts to deliver AMB to particular sites using nanosystems bearing specific targeting ligands. The ligands used have been sugars, antibodies or small peptides, as described in the following paragraphs.
Although the “natural” target of colloidal drug carriers is phagocytic cells, their uptake by macrophage, and particularly the Küpffer cells of the liver, can be greatly increased by modifying the surface with mannose residues which are recognized by the mannose-fucose receptor on these cells (CitationBarratt and Schuber 1993). This strategy has been applied to the delivery of AMB to macrophages for treatment of leishmanias (CitationVyas et al 2000). Similarly, liposomes loaded with AMB and coated with mannan or pullanan, a glucose-containing polysaccharide have been administered to rats as an aerosol to target alveolar macrophages. Drug concentrations were higher than those delivered by unmodified liposomes and were sustained for 24h (CitationVyas et al 2005).
Galactose receptors are expressed by liver cells and some micro-organisms. Polylactide microspheres containing AMB have been functionalized with galactose residues and have been shown to bind to Kluyveromyces bulgaricus yeast cells (CitationKassab et al 2002). Heparin is a negatively charged polysaccharide with many interesting biological properties, including bioadhesion. CitationClemons et al (2001) encapsulated AMB within small (105 nm) hydrophilic nanoparticles bearing heparin at their surface. Their retention in the lungs was increased compared with Fungizone®, leading to a better therapeutic index against pulmonary blastomycosis in mice.
The coupling of antibodies to the surface of a liposome can theoretically give a delivery system targeted to a specific cell type. Small liposomes bearing a monoclonal antibody to Cryptococcus neoformans bound specifically to the yeast and, when administered intravenously to infected mice, prolonged survival longer than AMB in solution in dimethyl-sulfoxide/phosphate-buffered saline, non-targeted liposomes or liposomes targeted with an irrelevant antibody (CitationDromer et al 1990). However, antibody-bearing systems will still be accumulated within mononuclear phagocytes unless their surface is modified to avoid opsonization. Thus, the concept of sterically stabilized liposomes has emerged, in which the surface is covered with end-grafted PEG chains. Targeting can be achieved by coupling the antibody or other ligand to the distal end of a proportion of these chains (CitationMercadal et al 1999). Thus, sterically stabilized liposomes containing AMB and bearing an antibody specific for pulmonary endothelium at the end of the PEG chains have been prepared (CitationOtsubo et al 1998). Accumulation of antibiotic in the lungs was observed, as opposed to its remaining in the blood in the case of non-targeted PEG-bearing liposomes or accumulating in the liver in the case of conventional liposomes. This was accompanied by increased efficacy against experimental aspergillosis in mice.
One smaller ligand which has been to direct AMB-loaded liposomes is the tetrapeptide tuftsin (Thr-Lys-Pro-Arg, CitationAgrawal et al 2002), which binds to a specific receptor on macrophages. This peptide has the advantage of being both a targeting element promoting liver accumulation and a macrophage activator. The anti-leishmanial activity of the drug is thus reinforced by macrophage-mediated effects. CitationZhang et al (2003) have used a targeting strategy to deliver AMB across the blood-brain barrier. A peptide analogue of bradykinin, RMP-7, was coupled to PEG on sterically stabilized liposomes. This peptide interacts with the B2 receptor on brain capillary endothelial cells and increases the permeability of the vessels. By this means, AMB accumulation in the brain can be improved.
Conclusion
AMB is a good example of how an appropriate delivery system can improve the therapeutic index of a drug. The advantage of the commercial lipid-based formulations is principally that they reduce toxicity compared with the conventional formulation, allowing higher doses to be given. A disadvantage is the high cost of these formulations, particularly with respect to their activity against parasitic diseases. CitationKleinberg (2006) has attempted to review the cost-effectiveness of these formulations compared with Fungizone®, taking into account all factors such as the time of hospitalization, in a North American setting, and concluded that in many cases, for example in the opportunistic infections in cancer patients, lipid formulations are a better choice than the conventional formulation. However, the cost of these formulations remains prohibitively high for the treatment of leishmaniasis in endemic areas (CitationSundar et al 2004).
Another disadvantage of AMB for mass treatment is its very low bioavailability by the oral route. Some studies reported above suggest that the use of nanosized formulations based on lipids or other amphiphilic molecules could be useful in overcoming this problem. Similarly, the delivery of drugs by the pulmonary route to combat respiratory infections is attracting much attention at the moment. New formulations of AMB could contribute in this area by increasing tolerability and ensuring delivery to the appropriate part of the lung. Another non parenteral route for which new AMB formulations could provide a therapeutic advance is in the eye, for example, in the treatment of fungal keratitis. Liposomal formulations have shown some advantages by this route, by reducing irritation and prolonging the residence time of drugs (CitationBochot et al 2000).
Finally, progress in the design of drug delivery systems has led to the development of carriers targeted to specific tissues and cells. Such technology applied to AMB would lead to a further increase in its therapeutic index. Therefore, as a result of innovative formulations, after almost 50 years on the market, AMB remains an extremely useful drug.
References
- AdamsMKwonGS2004Spectroscopic investigation of the aggregation state of amphotericin B during loading, freeze-drying and reconstitution of polymeric micellesJ Pharm Pharmaceut Sci7S116
- Adler-MooreJPProffittR2002AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experienceJ Antimicrob Ther49Suppl12130
- AgrawalAJAgrawalAPalA2002Superior chemotherapeutic efficacy of Amphotericin B in tuftsin-bearing liposomes against Leishmania donovani infection in hamstersJ Drug Target1041511996085
- AliffTBMaslakPGJurcicJG2003Refractory Aspergillus pneumonia in patients with acute leukemia: successful therapy with combination caspofungin and liposomal amphotericinCancer9710253212569602
- AmatoVSRabelloARotondo-SilvaA2004Successful treatment of cutaneous leishmaniasis with lipid formulations of amphotericin B in two immunocompromised patientsActa Trop921273215350864
- BarrattGSchuberF1993Targeting of liposomes with mannose terminated ligandsGregoriadisGLiposome TechnologyVol III2nd edBoca RatonCRC Press Inc199218
- BarrettJPVardulakiKAConlonC2003A systematic review of the antifungal effectiveness and tolerability of amphotericin B formulationsClin Ther25129532012867214
- BochotACouvreurPFattalE2000Intravitreal administration of antisense oligonucleotides: potential of liposomal deliveryProg Retin Eye Res191314710674705
- BratjburgJElbergSBolardJ1984Interaction of plasma proteins and lipoproteins with amphotericin BJ Infect Dis149986976376657
- BratjburgJElbergSMedoffJ1985Involvement of oxidative damage in erythrocyte lysis induced by amphotericin BAntimicrob Agents Chemother2717263985601
- BrezisMRosenPSilvaK1984Polyene toxicity in renal medulla: injury mediated by transport activityScience2246686322305
- BrittainHG1994Circular dichroism studies of the self-association of Amphotericin BChirality66659
- ChiaJKMcManusEJ1990In vitro tumor necrosis factor induction assay for analysis of febrile toxicity associated with amphotericin B preparationsAntimicrob Agents Chemother3490682360827
- ClemonsKVRanneyDFStevensDA2001A novel heparin-coated hydrophilic preparation of amphotericin B hydrosomesCurr Opin Investig Drugs24807
- ClemonsKVEspirituMParmarR2005Comparative efficacies of conventional amphotericin B, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosisAntimicrob Agents Chemother4948677516304147
- ConoverCDZhaoHLongleyCB2003Utility of poly(ethylene glycol) conjugation to create prodrugs of amphotericin BBioconjugate Chem146616
- CornelyO2005AmbiloadAmer Soc HematologyAtlanta, GA
- CybulskaBHerveMBorowskiE1986Effects of the polar head structure of polyene macrolide antifungal antibiotics on the mode of permeabilization of ergosterol- and cholsterol-containing lipidic vesicles studied by 31P-NMRMol Pharmacol2929383951434
- DangiJSVyasSPDixitVK1998Effect of various lipid-bile salt mixed micelles on the intestinal absorption of amphotericin B in ratDrug Devel Ind Pharm2463159876507
- De KruijffBDemelRA1974Polyene antibiotic-sterol interactions in membranes of Acoleplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic-cholestrol complexesBiochim Biophys Acta33957704546885
- DelmasGParkSChenZW2002Efficacy of orally delivered co-chleates containing amphotericin B in a murine model of aspergillosisAntimicrob Agents Chemother462704712121962
- DrewR2006Potential role of aerosolized amphotericin B formulations in the prevention and adjunctive treatment of invasive fungal infectionsInt J Antimicrob Agents27SS3644
- DromerFBarbetJBolardJ1990Improvement of amphotericin B activity experimental crytococcosis by incorporation into specific immunoliposomesAntimicrob Agents Chemother342055602073097
- EggimannPGarbinoJPittetD2003Epidemiology of Candida species infections in critically ill non-immunosuppressed patientsLancet Infect Dis368570214592598
- EllisMSpenceDde PauwB1998An EORTC international multicenter randomized trial (EORTC number 19923) comparing two dosages of liposomal amphotericin B for treatment of invasive aspergillosisClin Infect Dis271406129868651
- EgitoESTAppelMFessiH1996aIn-vitro and in-vivo evaluation of a new amphotericin B emulsion-based delivery systemJ Antimicrob Chemother3848597
- EgitoESTFessiHAppelM1996bA morphological study of an amphotericin B emulsion-based delivery systemInt J Pharm1451727
- Ehrenfreund-KleinmanTAzzamTFalkR2002Synthesis and characterization of novel water soluble amphotericin B-arabinogalactan conjugatesBiomaterials2313273511804288
- EspuelasMSLegrandPChéronM1998aInteractions of amphotericin B with polymeric colloids. A spectroscopic studyColloids Surfaces B: Biointerfaces1114151
- EspuelasMSLegrandPChéronM1998bInteractions of amphotericin B with polymeric colloids. 2. Effect of poloxamer on the adsorption of amphotericin B onto poly(ɛ-caprolactone) nanospheresColloids Surfaces B: Biointerfaces1120312
- EspuelasMSLegrandPLoiseauPM2000In vitro reversion of amphotericin B resistance in Leishmania donovani by poloxamer 188Antimicrob Agents Chemother442190210898700
- EspuelasMSLegrandPLoiseauPM2002In vitro antileishmanial activity of amphotericin B loaded in poly(epsilon-caprolactone) nano-spheresJ Drug Target10593812683663
- EspuelasMSLegrandPCampaneroMA2003Polymeric carriers for amphotericin B: in vitro activity, toxicity and therapeutic efficacy against systemic candidiasis in neutropenic miceJ Antimicrob Chemother524192712888593
- FukuiHKoikeTNakagawaT2003Comparison of LNS-AmB, a novel low-dose formulation of amphotericin B with lipid nano-sphere (LNS®), with commerical lipid-based formulationsInt J Pharm2671011214602388
- GaboriauFChéronMPetitC1997Heat-induced superaggregation of amphotericin B reduces its in vitro toxicity: a new way to improve its therapeutic indexAntimicrob Agents Chemother412345519371331
- GeorgopapadakouNHWalshTJ1996Antifungal agents: chemotherapeutic targets and immunologic strategiesAntimicrob Agents Chemother40279918834867
- GradoniLGramicciaMScaloneA2004Change in human visceral leishmaniasis treatment in Italy: retrospective study of 630 patientsParassitologia4619920115305716
- GraybillJRCravenPCTaylorRL1982Treatment of murine Cryptococcus with liposome-associated amphotericin BJ Infect Dis145748527077097
- GrijalbaMTChéronMBorowskiE2006Modulation of mpolyene antibiotics self-association by ions from the Hofmeister seriesBiochim Biophys Acta1760973916563634
- GuoLSS2001Amphotericin B colloidal dispersion: an improved anti-fungal therapyAdv Drug Deliv Rev471496311311990
- HartselSBolardJ1996Amphotericin B: new life for an old drugTIPS1744599014498
- HartselSCWeilandTR2003Amphotericin B binds to amyloid fibrils and delays their formation: a therapeutic mechanismBiochemistry4262283312755626
- HeathSChanceMLNewRR1984Quantitative and ultrastructural studies on the uptake of drug loaded liposomes by mononuclear phagocytes infected with Leishmania donovani. Mol Biochem Parasitol1249606087139
- HerbrechtRDenningDWPattersonTF2002Voriconazole versus amphotericin B for primary therapy of invasive aspergillosisN Engl J Med3474081512167683
- HerbrechtRNatarajan-AmeSNivoixY2003The lipid formulations of amphotericin BExpert Opin Pharmacother412778712877636
- HerrmannJLDuboisNFourgeaudM1994Synergic inhibitory activity of amphotericin-B and gamma interferon against intracellular Cryptococcus neoformans in murine macrophagesJ Antimicrob Chemother34105187730221
- HerwaldtBL1999LeishmaniasisLancet541191910513726
- JanoffASPerkinsWRSaletanSL1993Amphotericin B lipid complex (ABLC™): a molecular rationale for the attenuation of amphotericin B-related toxicitesJ Liposome Res345171
- JohnsonMDMacDougallCOstrosky-ZeichnerL2004Combination antifungal therapyAntimicrob Agents Chemother4869371514982754
- Juster-ReicherAFlidel-RimonOAmitayM2003High-dose liposomal amphotericin B in the therapy of systemic candidiasis in neonatesEur J Clin Microbiol Infect Dis22603713680398
- KassabRParrot-LopezHFessiH2002Molecular recognition by Kluyveromyces of amphotericin B-loaded, galactose-targeted, poly(lactic acid) microspheresBioorg Med Chem1017677511937335
- KayserOOlbrichCYardleyV2003Formulation of amphotericin B as nanosuspension for oral administrationInt J Pharm25473512615413
- KelseySMGoldmanJMMcCannS1999Liposomal amphotericin (AmBisome) in the prophylaxis of fungal infections in neutropenic patients: a randomised, double-blind, placebo-controlled studyBone Marrow Transplant23163810197802
- KirkpatrickWRCocoBJPattersonTF2006Sequential or combination antifungal therapy with voriconazole and liposomal amphotericin B in a Guinea pig model of invasive aspergillosisAntimicrob Agents Chemother501567916569887
- KleinbergM2006What is the current and future status of conventional amphotericin B?Int J Antimicrob Agents27SS1216
- KontoyiannisDPHachemRLewisRE2003Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignanciesCancer98292912872348
- LarabiMLegrandPAppelM2001Reduction of NO synthase expression and TNF alpha production in macrophages by amphotericin B lipid carriersAntimicrob Agents Chemother455536211158754
- LarabiMYardleyVLoiseauPM2003Toxicity and antileishmanial activity of a new stable lipid suspension of amphotericin BAntimicrob Agents Chemother473774914638481
- LarabiMGulikADedieuJ-P2004aNew lipid formulation of amphotericin B : spectral and microscopic analysisBiochim Biophys Acta16641728115328049
- LarabiMPagesNPonsF2004Study of the toxicity of a new formulation of amphotericin BJ Antimicrob Chemother5381814657087
- LeendersACReissPPortegiesP1997Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitisAids111463719342068
- LeendersACDaenenSJansenRL1998Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infectionsBr J Haematol103205129792309
- LegrandPVertut-DoïABolardJ1996Comparative internalization and recycling of different amphotericin B formulations by a macrophage-like cell lineJ Antimicrob Chemother37519339182109
- LincopanNMamizukaEMCarmona-RibeiroAM2005Low nephrotoxicity of an effective amphotericin B formulation with cationic bilayer fragmentsJ Antimicrob Chemother557273415761070
- LincopanNBorelliPFockR2006Toxicity of an effective amphotericin B formulation at high cationic lipid to drug molar ratioExp Toxicol Pathol581758316982179
- Lopez-BeresteinGMehtaRHopferR1983Effects of sterols on the therapeutic efficacy of liposomal amphotericin B in murine candidiasisCancer Drug Deliv137426544116
- MaertensJVrebosMBoogaertsM2001Assessing risk factors for systemic fungal infectionsEur J Cancer Care (Engl)10566211827268
- MangéANishidaNMilhavetO2000Amphotericin B inhibits the generation of the scrapie isoform of the prion protein in infected culturesVirology74313540
- MarrKABoeckhMCarterRA2004Combination antifungal therapy for invasive aspergillosisClin Infect Dis3979780215472810
- MazerskiJBolardJBorowskiE1982Self association of some polyene macrolide antibiotics in aqueous mediaBiochim Biophys Acta7191117
- MehtaPVinksAFilipovichA2006High-dose weekly AmBisome antifungal prophylaxis in pediatric patients undergoing hematopoietic stem cell transplantation: a pharmacokinetic studyBiol Blood Marrow Transplant122354016443521
- MénezCBuyseMChacunH2006aModulation of intestinal barrier properties by miltefosineBiochem Pharmacol7148696
- MénezCBuyseMBesnardM2006bInteraction between miltefosine and amphotericin B: consequences for their activity towards intestinal epithelial cells and L. donovani promastigotes in vitroAntimicrob Agents Chemother503793800
- MercadalMDomingoJCPetrizJC1999A novel strategy affords high-yield coupling of antibody to extremities of liposomal surface grafted PEG chainsBiochim Biophys Acta1418232810209227
- MeyerhoffA1999U.S. Food and Drug Administration approval of AmBi-some (liposomal amphotericin B) for treatment of visceral leishmaniasisClin Infect Dis28428 discussion 49–5110028069
- MozaffarianNBermanJWCasadevallA1997Enhancement of nitric oxide synthesis by macrophages represents an additional mechanism of action for amphotericin BAntimicrob Agents Chemother41182599257771
- MüllerRHSchmidtSButtleI2004SolEmuls® - novel technology for the formulation of i.v. emulsions with poorly soluble drugsInt J Pharm26929330214706241
- MurrayHW2001Clinical and experimental advances in treatment of visceral leishmaniasisAntimicrob Agents Chemother4521859711451673
- MurrayHW2004Progress in the treatment of a neglected infectious disease: visceral leishmaniasisExpert Rev Anti-infect Ther22799215482193
- NelsonKGBishopJVRyanRO2006Nanodisk-associated amphotericin B clears Leishmania major cutaneous infections in susceptible BALB/c miceAntimicrob Agents Chemother5012384416569834
- NewRRChanceMLHeathS1981Antileishmanial activity of amphotericin and other antifungal agents entrapped in liposomesJ Antimicrob Chemother8371817319979
- OdaMNHargreavesPLBecksteadJA2006Reconstituted high-density lipoprotein enriched with the polyene antibiotic amphotericin BJ Lipid Res472607 errata p 111416314670
- OlsonJAAdler-MooreJPSmithPJ2005Treatment of Candida glabrata infection in immunosuppressed mice by using a combination of liposomal amphotericin B with caspofungin or micafunginAntimicrob Agents Chemother49489590216304150
- OtsuboTMaruyamaKMaesakiS1998Long-circulating immunoliposomal amphotericin B against invasive pulmonary aspergillosis in miceAntimicrob Agents Chemother424049449258
- PappasPGRexJHLeeJ2003A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patientsClin Infect Dis376344312942393
- PerfectJRDodds AshleyEDrewR2004Design of aerosolized amphotericin B formulations for prophylaxis trials among lung transplant recipientsClin Infect Dis39Suppl 4S2071015546119
- PolakA1999The past, present and future of antimycotic combination therapyMycoses423557010536428
- PrenticeHGHannIMHerbrechtR1997A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patientsBr J Haematol98711189332329
- Rama IniguezSDea-AyuelaMASánchez-BruneteJA2006Real-time reverse transcription-PCR quantification of cytokine mRNA expression in golden Syrian hamster infected with Leishmania infantum and treated with a new amphotericin B formulationAntimicrob Agents Chemother50119520116569829
- RingdenOMeunierFTollemarJ1991Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patientsJ Antimicrob Chemother28Suppl B73821778894
- RisovicVSachs-BarrableKBoydM2004Potential mechanisms by which Peceol increases the gastrointestinal absorption of amphotericin BDrug Dev Ind Pharm307677415491054
- RuijgrokRJFensMHBakker-WoudenbergIA2005Nebulization of four commercially available amphotericin B formulations in persistently granulocytopenic rats with invasive pulmonary aspergillosis: evidence of long-term biological activityJ Pharm Pharmacol5712899516259757
- Sánchez-BruneteJADeaMARamaS2004Treatment of experimental visceral leishmaniasis with amphotericin B in stable albumin microspheresAntimicrob Agents Chemother4832465215328080
- Sánchez-BruneteJADeaMARamaS2005Influence of the vehicle on the properties and efficacy of microparticles containing amphotericin BJ Drug Target132253316051534
- SantangeloRPaderuPDelmasG2000Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasisAntimicrob Agents Chemother4423566010952579
- SeifertKCroftSL2006In vitro and in vivo interactions between miltefosine and other antileishmanial drugsAntimicrob Agents Chemother5073916377670
- SinghSSivakumarR2004Challenges and new discoveries in the treatment of leishmaniasisJ Infect Chemother103071515614453
- Sokolsky-PapkovMDombAJGolenserJ2006Impact of aldehyde content on amphotericin B-dextran imine conjugate toxicityBiomacromolecules715293516677035
- SundarSMehtaHSureshAV2004Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulationsClin Infect Dis383778314727208
- SzokaFCMilhollandDBarzaM1987Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome-intercalated amphotericin BAntimicrob Agents Chemother3142193579259
- TaylorRLWilliamsDMCravenPC1982Amphotericin B in liposomes: a novel therapy for histoplasmosisAm Rev Respir Dis125610117081822
- TiyaboonchaiWLimpeanchobN2006Formulation and characterization of amphotericin B-chitosan-dextran sulphate nanoparticlesInt J Pharm doi:10.1016/j.ijpharm.2006.08.013
- TollemarJRingdenOAnderssonS1993aRandomized double-blind study of liposomal amphotericin B (Ambisome) prophylaxis of invasive fungal infections in bone marrow transplant recipientsBone Marrow Transplant12577828136741
- TollemarJRingdenOAnderssonS1993bProphylactic use of liposomal amphotericin B (AmBisome) against fungal infections: a randomized trial in bone marrow transplant recipientsTransplant Proc25149578442163
- TollemarJHockerstedtKEriczonBG1995Prophylaxis with liposomal amphotericin B (AmBisome) prevents fungal infections in liver transplant recipients: long-term results of a randomized, placebo-controlled trialTransplant Proc27119587878847
- TokudaYTsujiMYamazakiM1993Augmentation of murine tumor necrosis factor production by amphotericin B in vitro and in vivoAntimicrob Agents Chemother372228308257149
- TomiiY2002Lipid formulation as a drug carrier for drug deliveryCurr Pharmaceut Design846774
- UtzJPTregerAMcCulloughNB1958–1959Amphotericin B: intravenous use in 21 patients with systemic fungal diseasesAntibiot Annu1958195962834
- Van EttenEWMVan VianenWRooversP2000Mild heating of Amphotericin B-desoxycholate: effects on ultrastructure, in vitro activity and toxicity, and therapeutic efficacy in severe candidiasis in leukopenic miceAntimicrob Agents Chemother44159860310817715
- VanderputteJWachtelJLStillerET1955–1956Amphotericins A and B, antifungal antibiotics produced by a streptomycete. II The isolation and properties of the crystalline amphotericinsAntibiot Annu1955195658791
- VandermeulenGRouxhetLArienA2006Encapsulation of amphotericin B in poly(ethylene glycol) -block-poly(e-caprolactone-co-tri-methylenecarbonate) polymeric micellesInt J Pharm3092344016406402
- Venier-JulienneMCVouldoukisIMonjourL1995In vitro study of the anti-leishmanial activity of biodegradable nanoparticlesJ Drug Target32397655817
- VyasSPKatareYKMishraV2000Ligand directed macrophage targeting of amphotericin B loaded liposomesInt J Pharm21011411163983
- VyasSPQuraishiSGuptaS2005Aerosolized liposome-based delivery of amphotericin B to alveolar macrophagesInt J Pharm296122515885451
- WalshTJFinbergRWArndtC1999Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study GroupN Engl J Med3407647110072411
- WalshTJGoodmanJLPappasP2001Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose studyAntimicrob Agents Chemother4534879611709329
- WilsonEThorsonLSpeertDP1991Enhancement of macrophage superoxide anion production by amphotericin BAntimicrob Agents Chemother357968001649568
- WingardJRWhiteMHAnaissieE2000A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study GroupClin Infect Dis3111556311073745
- WuWWieckowskiSPastorinG2005Targeted delivery of amphotericin B to cells by using functionalized carbon nanotubesAngew Chem Int Ed44635862
- YardleyVCroftSL2000A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasisInt J Antimicrob Agents13243810755238
- YooBKJalil MiahMALeeE-S2006Reduced renal toxicity of nanoparticular amphotericin B micelles prepared with partially benzylated poly-L-aspartic acidBiol Pharm Bull291700516880628
- ZarifL2005Drug delivery by lipid cochleatesMeth Enzymol3913142915721389
- ZhangXXieJLiS2003The study on brain targeting of the amphotericin B liposomesJ Drug Target111172212881198