Abstract
The effect of SLN incorporation on transdermal delivery and in vitro antiherpetic activity of Artemisia arborescens essential oil was investigated. Two different SLN formulations were prepared using the hot – pressure homogenization technique, Compritol 888 ATO as lipid, and Poloxamer 188 and Miranol Ultra C32 as surfactants. Formulations were examined for their stability for two years by monitoring average size distribution and zeta potential values. The antiviral activity of free and SLN incorporated essential oil was tested in vitro against Herpes Simplex Virus-1 (HSV-1) by a quantitative tetrazolium-based colorimetric method (MTT), while the effects of essential oil incorporation into SLN on both the permeation through and the accumulation into the skin strata was investigated by using in vitro diffusion experiments through newborn pig skin and an almond oil Artemisia essential oil solution as a control.
Results showed that both SLN formulations were able to entrap the essential oil in high yields and that the mean particle size increased only slightly after two years of storage, indicating a high physical stability. In vitro antiviral assays showed that SLN incorporation did not affect the essential oil antiherpetic activity. The in vitro skin permeation experiments demonstrated the capability of SLN of greatly improving the oil accumulation into the skin, while oil permeation occurred only when the oil was delivered from the control solution.
Introduction
Herpes simplex virus (HSV) is one of the most common viral diseases in humans. HSV exists as two types, herpes simplex virus 1 (HSV-1) and herpes simplex virus 2 (HSV-2) which have been distinguished by clinical manifestations, biological and serological criteria. A characteristic property of herpes viruses is their ability to establish and maintain latent infections that can be frequently reactivated by several different stimuli such as stress, ultraviolet light, hormones, transient hyperthermia, and immunosuppression. Infections of these viruses can cause a wide range of signs and symptoms varying from mucocutaneous lesions to life-threatening encephalitis (CitationWhitley and Lakeman 1995; CitationSimmons 2002).
Several drugs have proven to be useful in the treatment of many specific infections, but viral strains resistant to these drugs have been increasingly identified and several cases of toxicity have been encountered, particularly in immunocompromised patients (CitationNugier et al 1992). According to the type and severity of the infection, antiviral agents may be administered via several routes: orally for the treatment of primary oral or genital infections, parenterally for serious infections such as neonatal herpes and herpes infections of the central nervous system, in drops for ocular herpetic infections and topically on lips and mucosae for mild recurrent orolabial lesions (CitationBrady and Berstein 2003).
In order to find less toxic antiviral agents much research has focused on plant products. In a previous paper we demonstrated the in vitro antiviral activity of the essential oil extracted from Artemisia arborescens L. Our results showed that the incorporation of this essential oil in multilamellar liposomes greatly improved its activity against intracellular HSV-1 (CitationSinico et al 2005).
Introduced at the beginning of the 1990s, solid lipid nanoparticles (SLN) have been intensively investigated for parenteral, peroral (CitationMüller et al 1995; CitationLai and Wissing 2003) and ocular delivery. Furthermore, because of their ability to enhance the penetration of drugs into the skin SLN are considered a promising drug carrier for topical application (CitationMehnert and Mader 2001). In particular, SLN are well suited for use in damaged or inflamed skin because the lipids used for their preparation are often non toxic and non irritative (CitationWissing and Müller 2001; CitationWissing and Müller 2002; CitationWissing and Müller 2003). The aim of our study was the incorporation of Artemisia arborescens L essential oil into SLN as topical delivery systems against Herpes simplex virus 1. The antiviral activity of free and SLN incorporated essential oil was tested in vitro against HSV-1 by a quantitative tetrazolium-based colorimetric method (MTT), while the effects of Artemisia arborescens L essential oil incorporation into SLN on both the permeation through the skin and the accumulation into the skin strata was investigated by using in vitro diffusion experiments through newborn pig skin.
Experimental
Materials
Compritol 888 ATO® which was a gift from Gattefossé (Milan, Italy) is declared as glyceryl behenate with a melting point of 72 ºC. It is a mixture of 12%–18% mono-, 52%–54% di- and 28%–32% triglycerides. The fatty acid fraction consists of >87% behenic acid (docosan acid). The surfactant Pluronic F68® (Poloxamer 188) was obtained from BASF AG (Ludwigshafen, Germany), Miranol Ultra C32® (sodium cocoamphoacetate) was a gift from Rhodia Gerozzano S.p.A. (Ospiate Bollate, Italy). Camphor, a + β-thujone and Nonidet P40 were from Fluka (Milan, Italy). 3-(4, 5-dimethylthiazol-2-yl)–2, 5-diphenyl tetrazolium bromide (MTT) was Sigma (Milan, Italy).
Virus and cells
African green monkey kidney cells (Vero) were obtained from the Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia (Brescia, Italy). Cells were grown in RPMI 1640 (Gibco Life Technologies, Rockville, MD, USA) supplemented with 10% foetal bovine serum (FBS, Gibco) and penicillin, streptomycin and amphotericin B (100 U/ml, 100 and 2.5 μg/ml, respectively). The strain of Herpes Simplex virus type 1 (HSV-1 strain F) was obtained from the American type culture collection (ATCC), Rockville, MD, USA, and was propagated in Vero cells. Virus titer was determined by plaque assay in Vero cells and stored at −70 °C until used.
Preparation and characterization of essential oil
The aerial parts of Artemisia arborescens were collected from the countryside around Usellus, Sardinia, Italy, during full blossom. Plants were identified and voucher specimens deposited in the Herbarium of the Institute of Botany and Botanical Garden, University of Cagliari, Italy. 5 Kg of fresh aerial parts were distilled in a steam apparatus (Albrigi Luigi S.r.l. Verona, Italy) with an aqueous phase recycling system for 3 h. Briefly the system is divided into three components: a 250 liter stainless steel cylinder which holds the plant material and water; a condenser, which the oil-steam mixture travels into to cool and condense into an oil-water mixture; a separator, which separates the essential oils from the water. The essential oil was dried over anhydrous sodium sulphate and stored at 4 °C. The quali-quantitative analysis of the essential oil was carried out by gas chromatographyion trap mass spectrometry (GC/ITMS). A Varian CP 3800 gas chromatograph (Varian, Inc., Palo Alto, CA, USA) coupled with a Saturn 2000 ITMS detector, a Varian CP 7800 autosampler, a split-splitless injector, and a MS ChemStation, was used. The column was a fused silica capillary DB-5MS (5% phenylmethylpolysyloxane, 30 m × 0.25 mm; film thickness 0.25 μm) (J and W Scientific Fisons, Folsom, CA). The injector and interface were at 150 °C and 280 °C, respectively. The oven temperature was programmed as follows: from 60 °C to 180 °C (3 °C/min), and isothermally held for 15 min. Helium was the carrier gas at 1 ml/min; the sample (1 μl) was injected in the split mode (1:20). MS conditions were as follows: ionization mode EI from 50–450 amu. The oil compounds were identified by comparison of their relative retention times with those of authentic samples or by comparison of their retention index (RI) relative to the series of n-hydrocarbons, and computer matching against commercial library (CitationAdams 1995, CitationNist Mass Spectral Search Program 1999) and homemade library mass spectra made up from pure substances and component of known oils and MS literature data.
The oil content was also assayed by HPLC at several wavelengths (209, 245 and 284 nm), using a Waters 2690 liquid chromatograph, equipped with a Photodiode Array detector 996 (Waters Corp, Milford, MA). The mobile phases were methanol and water. Separations were performed using 75% methanol and 25% water as eluent at a flow rate of 1.0 ml/min. The column was an Xbridge C18 5 μm (4.6 × 150 mm, Waters). Appropriate standard methanolic solutions of camphor and α + β-thujon were prepared and tested. All experiments were carried out in triplicate. Because of complexity of the essential oil composition, the permeation study was carried out by quantifying the most abundant essential oil components. To this purpose camphor, β-thujon and chamazulene were used as “lead” components as previously reported (CitationSinico et al 2005). In particular, the camphor was determined at 284 nm and the β-thujon at 209 nm. Retention times for camphor and β-thujon were respectively 1.74 and 2.67.
SLN preparation and characterization
SLN were prepared as previously reported (CitationLai et al 2006). Briefly, Artemisia arborescens L. essential oil was dissolved in the melted Compritol 888 ATO® at 75 ºC and the essential oil-loaded lipid dispersed in a hot aqueous surfactant solution. The mixtures were stirred with a T 25 Ultra Turrax (Janke und Kunkel GmbH and Co KG Staufen, Germany) for 1 minute at 8000 rpm. The obtained pre-emulsion was then homogenized at high pressure (three cycles, 500 bar) using an Emulsiflex C5 (Avestin Ottawa, Canada) thermostated at 75 ºC (CitationMüller and Lucks 1996). Details of SLN formulations are given in .
Table 1 Sample composition % (w/w)
The SLN dispersions were purified from non-incorporated Artemisia arborescens L. essential oil by gel chromatography on Sephadex G50. The encapsulation efficiency was calculated using the following equation: [(T-S)/T] × 100. T is the total quantity of incorporated and non-incorporated essential oil in the SLN dispersion and S is the non-incorporated oil quantity separated with gel chromatography. Quantitative determination of incorporated essential oil was carried out spectrophotometrically using the HPLC method previously described after extraction and dilution with methanol for 1 hour in an ultrasonic bath.
The average diameter (Z-AVE) and polydispersity index (PI) of SLN were determined by Dynamic Laser Light Scattering (DLLS) using a (N4 Plus, Beckman Coulter) at 25 °C. The aqueous SLN dispersions were diluted with distilled water before analysis. Samples were scattered (633 nm) at an angle of 90°. Data were fitted by the method of inverse “Laplace transformation” and CONTIN (CitationProvencher 1982a, Citation1982b). Each value is the average of ten measurements. The laser diffraction particle size analysis (LD) was performed by a Coulter LS 230 (Beckmann-Coulter Electronics, Germany). The LD data were evaluated using the volume distribution method to detect even few large particles. Characterization parameters were the diameters LD 50, LD 90, LD 99, eg, a diameter LD 90 of 1 μm means that 90% of all particles have a diameter of 1 μm or less.
The particle charge was quantified as zeta potential (ZP) using a Zetasizer 4 (Malvern Instr., UK) at 25 ºC. Measurements were performed in bidistilled water adjusted with sodium chloride to a conductivity of 50 μS/cm. The pH values of the samples were always between 6.2 ± 0.9. Zeta potential was calculated from the electrophoretic mobility after Helmholtz-Smoluchowski equation (CitationSmoluchowski 1918).
Antiviral activity
The antiviral activity of free and SLN essential oil formulations was evaluated against HSV-1. Vero cells 5 × 104 in 50 μl of RPMI 1640 containing FBS 5% were seeded in 96 multiwell microtiter plates (Falcon 353872, Becton Dickinson, Franklin Lakes, NJ, USA). The essential oil or SLN formulations in 50 μl of RPMI 1640 were added to cells and infected with 20 μl of HSV-1 1 × 104 pfu/ml to obtain final concentrations of essential oil ranging between 100 and 0.39 μg/ml and a multiplicity of infection (MOI) of 0.02. Plates were incubated at 37 °C in 5% CO2 incubator until the viral cytopathic effect (CPE) was observed in untreated virus control wells (usually 48–72 h). The reduction of the viral CPE in the essential oil containing wells in relation to cell controls and virus controls was determined by a quantitative tetrazolium-based colorimetric method. 30 μl of a 5 mg/ml solution of 3-(4, 5-dimeth-ylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) in phosphate-buffered saline (PBS) were added to each well to obtain a final concentration of MTT of 1 mg/ml. Plates were incubated at 37 °C for 4 h and the formazan product was dissolved with a mix solution consisting of 0.1 N HCl and 5% Nonidet P40 in isopropanol. After a few minutes at room temperature to ensure that all crystals were dissolved, the plates were read using an automatic plate reader (Sunrise Tecan, Grödig/Salzburg, Austria) at 570 nm test wavelength with a reference wavelength at 690 nm. The 50% inhibitory dose values (ID50), defined as the concentration of essential oil inhibiting 50% of the viral CPE, were calculated by regression analysis of the dose-response curves generated from the data (CitationDenizot and Lang 1986).
Skin permeation studies
In vitro experiments were performed in the dark in triplicate using skin fragments excised from newborn pigs (CitationManconi et al 2006). The skin, previously frozen at −18 °C, was placed at + 4 °C 1 day before the experiments. The subcutaneous fat was carefully removed and the skin was cut into squares of 3 × 3 cm2 and randomized. One day before the start of the experiments the skin was pre-equilibrated in PBS solution at +25 °C. Circular pieces of this skin were fixed on vertical Franz diffusion cells the receptor compartment of which (volume of 7 cm3 and an effective diffusion area of 0.636 cm2) were filled with a hydroalcoholic solution (ethanol:water 40:60) which was constantly stirred with a small magnetic bar and thermostated at 37 °C. 100 μl of nonpurified SLN formulation (0.1 mg/g) was applied to each of the skin pieces (CitationFranz 1975). Because of the high lipophilicity of the essential oil components, an almond oil solution of the essential oil (0.1 mg/g) was also studied as a reference. Samples of the receiving solution were withdrawn after elapsed times of 1, 2, 4, 6, and 8 h, replaced with an equal volume of hydroalcoholic solution to ensure sink conditions and analyzed by HPLC to determine the amount of permeated essential oil. At the end of the experiment the skin surface was washed 3 times with distilled water and then removed and dried with filter paper. The skin was crumbled with a scissors and soaked separately in 10 ml of ethanol for 24 h and then exposed to four sonication cycles of 30 min each in an ultrasound bath. Finally, the obtained solutions were concentrated and the essential oil content quantified by HPLC.
Statistical analysis of data
Data analysis was carried out with the software package Microsoft Excel version 2001. Results were expressed as a mean ± standard deviation. Statistically significant difference was determined using the analysis of variance (ANOVA) with p = 0.05 as a minimal level of significance.
Results and discussion
A blue essential oil (EO) was obtained in good yield (0.8%) distilling the fresh aerial part of Artemisia arborescens in a steam apparatus with an aqueous phase recycling system for 3 h. The oil slowly became green when stored in the presence of air and/or light. The analysis of EO confirmed the literature data (CitationSacco et al 1983), that is that the oil composition is a complex mixture of organic compounds among which mono-terpene ketones β-thujone and camphor represent more than 50% of the essential oil. Chamazulene, which is responsible for the blue color of the volatile oil, is also one of the main components. In the composition of the most abundant molecules of the essential oil is given.
Table 2 Main components of Artemisia arborescens essential oil as determined by GC and GC-ITMS
Two different SLN formulations were prepared using the hot high-pressure homogenization technique, Compritol 888 ATO as a lipid and Poloxamer 188 and Miranol Ultra C32 as surfactants.
One day after production, the SLN 1 had a size of 223 nm (0.243 PI) while the particle size of SLN 2 prepared using Miranol Ultra C32® as surfactant were 219 nm (0.301 PI) ().
Figure 1 Mean particle size and polydispersity index (PI) of Artemisia arborescens essential oil loaded SLN 1 and SLN 2 formulations stored at 4 ºC for 1 day (D1), 60 days (D60) and 2 years after production.
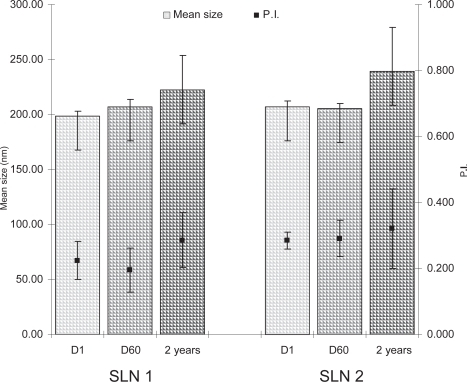
The mean particle size of the formulations increased only slightly after two years of storage, indicating a high physical stability of both SLN 1 and SLN 2 formulations (). In particular, 2 years after production, SLN 1 and SLN 2 formulations showed a mean size of 242 nm (0,285 PI) and 239 nm (0,321 PI). The PI values were always smaller than 0.350 indicating a fairly narrow size distribution of the particles.
The absence of particles in the micrometer range and aggregation was confirmed by laser diffraction particle size analysis (LD). For both Artemisia arborescens L. essential oil loaded formulations SLN 1 and SLN 2, the obtained data showed a LD 99 smaller than 700 nm 2 years after production.
The use of negatively charged surfactant Miranol Ultra C32® leads to SLN formulation (SLN 2) characterized by high zeta potential. Generally it is accepted that ZP values of −30 mV and above characterize a stable formulation (CitationFreitas and Müller 1998). The SLN 2 formulation possessed a high zeta potential at day 1 (−36.2 ± 0.5 mV) which did not change during the two investigational years () indicating a high long-term stability of this formulation.
Table 3 Zeta potential measurements (in mV) of Artemisia arborescens loaded SLN formulations in bidistilled water (50 μS/cm) stored at 4 ºC 1 day (D1), 60 days (D60) and 2 years after production
At day 1 the SLN 1 formulation prepared using the steric non-ionic surfactant Poloxamer 188 showed a ZP value of −15.6 ± 0.5 mV that decreased slightly during the investigational two years. The threshold of particle agglomeration is indicated at a zeta potential range between −20 mV and −11 mV. Energy input, such as high storage temperature, in a SLN dispersion with ZP values in this range or below can cause an increase in kinetic energy that could potentially promote the aggregation of particles (CitationFreitas and Müller 1998). However, for the steric stabilizer Poloxamer, it should be pointed out that its stabilizing efficiency is not reflected by the absolute ZP as demonstrated by the slightly increase of mean size during the investigated two years. The amount of entrapped and non-entrapped essential oil was determined at the wavelength of 284 nm using the HPLC method described in the experimental section after purification by gel chromatography on Sephadex G50. Both SLN formulations showed a high capability of entrapping the essential oil. In particular the E% of SLN 1 and SLN 2 were 87% and 92% respectively. The high incorporation capability of Compritol 888 ATO® SLN is achieved because of a high lipophilicity of the essential oil.
The antiviral activity of A. arborescens essential oil was determined by the reduction of viral CPE in Vero cells infected with HSV-1 using a MTT colorimetric method. For appropriate comparison, the antiviral activity of free and SLN loaded essential oil was determined and compared with that of empty SLN. Using the MTT test the empty SLN did not show any detectable antiviral activity. Results are shown in .
Figure 2 Antiviral activity of A. arborescens essential oil as determined by the reduction of viral CPE. Vero cells were infected with HSV-1 (MOI 0.02) and incubated in the presence of serial dilutions of free essential oil (upward triangle), SLN 1 (solid square), SLN 2 (open square), in RPMI 1640 until the viral cytophatic effect was observed in untreated virus control wells and then processed as described. The data represent the mean for six replicates of four separate experiments.
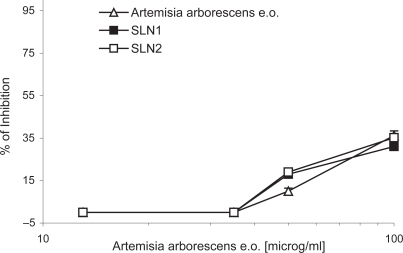
At the highest employed concentration (100 μg/ml) the essential oil induced a 36.20% of inhibition. No significant differences in the antiviral activity were observed by loading the essential oil in SLN 1 (31.10%) and SLN 2 (35.20%). Therefore, the encapsulation in lipid particles did not negatively affect the in vitro antiviral activity of the oil, although the high lipophilicity of the studied carrier systems.
In order to evaluate the effects of incorporation in SLN on the amount of Artemisia arborescens essential oil accumulated into the skin and the permeated quantities, we carried out in vitro permeation studies using the whole newborn pig skin and vertical Franz diffusion cells, as reported in the experimental section. During this study we compared the permeation data obtained from the two SLN 1 and SLN 2 formulations with those obtained from an almond oil Artemisia arborescens essential oil solution. We used the almond oil solution as a reference because of the excellent penetrating, moisturing and restructuring properties of this oil, which is one of the most popular components of several dermatological and cosmetic products. Moreover, it is rich in oleic and linoleic essential fatty acids that may act as penetration enhancers without inducing detectable cellular damage. Therefore, we believe that this solution is a good control in the permeation study. Results of the permeation experiments are reported in , where we list, for each of the vehicles, the amount of the essential oil accumulated into and delivered through the skin.
Figure 3 Comparison between the amount of essential oil accumulated into and delivered through the skin for the studied SLN 1 and SLN 2 formulations and the almond oil solution (control).
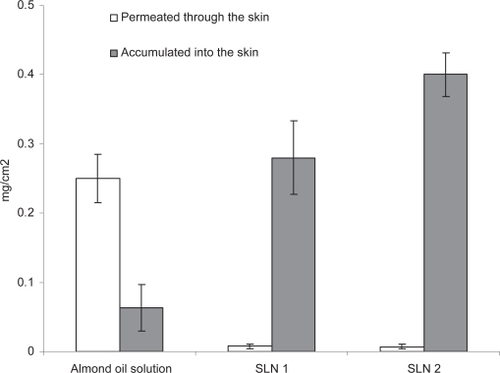
As can be seen from this figure, essential oil permeation occurred only when the oil was delivered from the control solution, while a significant amount of essential oil released by SLN formulations was found into the skin strata. Such relevant differences can be explained as a consequence of the SLN behavior on the skin surface. As reported previously (CitationMüller et al 2002), SLN seem to stick to the skin surface, forming an adhesive film that increase skin hydration and promote penetration of active compounds as well as the carrier into the stratum corneum. Here, lipid particles form a depot from which the oil is slowly released. Because of its physico-chemical properties (high lipophilicity, solid state at physiological temperature) the carrier can not improve essential oil diffusion through the inner more hydrophilic skin layers.
On the contrary, the control oil solution promotes essential oil permeation probably by increasing skin hydration that in case favors almond oil components to diffuse through the skin together the essential oil components.
Therefore, results obtained during this work clearly indicate that SLN are a good carrier for the cutaneous delivery of the antiviral Artemisia arborescens essential oil. In fact, SLN have proved to incorporate the essential oil in good yield and to possess a long-term stability. Moreover, they greatly improve skin accumulation of the phytocomplex, avoiding its permeation through the skin. However, further in vivo studies are needed to confirm these results.
Acknowledgements
This work was supported by a grant from MIUR (PRIN 2004).
References
- AdamsRP1995Identification of the Essential Oil Components by Gas-Chromatography\Mass SpectroscopyCarol Stream IL, USAAllured Publishing Corporation
- BradyRCBersteinDI2003Treatment of herpes simplex virus infectionsAntiviral Res61738114670580
- DenizotFLangR1986Rapid colorimetric assay for cell growth and survival. Modification to the tetrazolium dye procedure giving improved sensitivity and reliabilityJ Immunol Methods8927173486233
- FranzTJ1975Percutaneous absorption: on the relevance of in vitro dataJ Invest Dermatol641905123263
- FreitasCMüllerRH1998Effect of light and temperature on zeta potential and physical stability in Solid Lipid Nanoparticles (SLN) dispersionsInt J Pharm1682219
- LaiFWissingSA2003Peroral administration of SLNActa Technologiae et legis medicamentiXIV23
- LaiFWissingSAMüllerRH2006Artemisia arborescens l. essential oil loaded SLN for potential agricultural application: preparation and characterizationAAPS Pharm Sci Tech7E2
- ManconiMSinicoCValentiD2006Niosomes as carriers for tretinoin. III. A study into the in vitro cutaneous delivery of vesicle-incorporated tretinoinInt J Pharm3111–2111916439071
- MehnertWMaderK2001Solid lipid nanoparticle. Production, characterisation and applicationsAdv Drug Deliv Rev471659611311991
- MüllerRHMehnertWLucksJS1995Solid lipid nanoparticles (SLN) – An alternative colloidal carrier system for controlled drug deliveryEur J Pharm Biopharm41629
- MüllerRHLucksJS1996Arzneistofftrager aus festen Lipid-teilchen, Feste Lipidnanospharen (SLN)European Patent0605497
- MüllerRHRadtkeMWissingSA2002Solid lipid nanoparticles (SLN) and nanostuctured lipid carriers (NLC) in cosmetic and dermatological preparationsAdv Drug Deliv Rev5413155
- NugierFCollinsJNAyamardM1992Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a two-year sensitivity screening surveyJ Med Virol361121315366
- ProvencherSW1982aA constrained regularization method for inverting data represented by linear algebraic or integral equationsComput Phys Commun2721327
- ProvencherSW1982bContin: a general purpose constrained regularization program for inverting noisy linear algebraic and integral equationsComput Phys Commun2722942
- SaccoTFrattiniCBicchiC1983Constituents of essential oil of Artemisia arborescensPlanta Med47495117405093
- SimmonsA2002Clinical manifestations and treatment considerations of herpes simplex virus infectionsJ Infect Dis186717
- SinicoCDe LoguALaiF2005Liposomal incorporation of artemisia arborescens l. essential oil and in vitro antiviral activityEur J Pharm Biopharm59161815567314
- SmoluchowskiM1918Z Phys Chem93129
- The Nist Mass Spectral Search Program for the NIST\EPA\NIM Mass Spectral Library version 1.7, build 11\05\1999.
- WhitleyRJLakemanF1995Herpes virus simplex infections of the central nervous system: therapeutics and diagnostic considerationsClin Infect Dis20414207742450
- WissingSAMüllerRH2001A novel sunscreen system based on tocopherol acetate incorporated into solid lipis nanoparticlesJ Cosmet Sci2323343
- WissingSAMüllerRH2002Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetrationJ Control Release812253312044563
- WissingSAMüllerRH2003The influence of solid lipid nanoparticles (SLN) on skin hydration and viscoelasticity: in vivo studyEur J Pharm Biopharm56677212837483