Abstract
Cancer is one of the most complex diseases that has resulted in multiple genetic disorders and cellular abnormalities. Globally, cancer is the most common health concern disease that is affecting human beings. Great efforts have been made over the past decades in biology with the aim of searching novel and more efficient tools in therapy. Thus, small interfering RNAs (siRNAs) have been considered one of the most noteworthy developments which are able to regulate gene expression following a process known as RNA interference (RNAi). RNAi is a post-transcriptional mechanism that involves the inhibition of gene expression through promoting cleavage on a specific area of a target messenger RNA (mRNA). This technology has shown promising therapeutic results for a good number of diseases, especially in cancer. However, siRNA therapeutics have to face important drawbacks in therapy including stability and successful siRNA delivery in vivo. In this regard, the development of effective siRNA delivery systems has helped addressing these issues by opening novel therapeutic windows which have allowed to build up important advances in Nanomedicine. In this review, we discuss the progress of siRNA therapy as well as its medical application via nanoparticle-mediated delivery for cancer treatment.
Introduction
Cancer has become one of the most complex diseases due to numerous genetic disorders and cellular abnormalities. Cancer disease continues to be a major health concern worldwide being the second leading cause of death in the world. According to the World Health Organization (WHO), nearly 10 million people are estimated to die of cancer by the year 2020. Cancer has been known as the number one cause of deaths in developed countries in the current century.Citation1–Citation3 While remarkable efforts have been developed during the past few decades in the detection, prevention, and treatment,Citation4,Citation5 signaling pathway complexities that regulate cancer progression together with its tumor microenvironment heterogeneity and metastasis remain as serious obstacles to find efficient cancer treatments.Citation6,Citation7 Nowadays, treatments based on single chemotherapeutic drugs generally face lack effectiveness in cancer therapy, whereas two or more combined therapeutic methods involving various mechanisms of action are needed to achieve certain effectiveness in cancer therapy.
The currently used therapies are non-selective leading to side effects responsible for prolonged and expensive recovery, often followed by relapse at later time points. In this context, the selective targeting may provide a platform for the development of novel, more effective diagnostic tools and/or less harmful treatments.Citation8 Targeted therapies using monoclonal antibodies (mAb) against overexpressed receptors (eg, herceptin) have improved clinical outcomes.Citation9 Moreover, nanomaterials have also become an interesting alternative approach for the administration of drugs reducing the side effects (eg, biodegradable nanoparticles loaded with docetaxel).Citation10 The impact of these nanomedicines in human health may be enormous whether the right combination of targeting molecules and drugs become available with defined chemical structures. This has been demonstrated with the explosion of the antibodies-drug conjugates field.Citation11 However, the control of the number and position of the drugs on the antibodies, as well as the design of autoimmolative linkers to help trigger drug release after internalization is still far to be solved although this is an active field of development.Citation12
Recently, much attention has been paid to the potential application of RNA interference (RNAi) for cancer treatment.Citation8,Citation13 RNAi is the term given to the ability of a double-stranded RNA (dsRNA) containing a homologous sequence to a specific gene leading to sequence-specific gene silencing. RNAi is an endogenous post-transcriptional regulation process that consists of small regulatory RNAs including microRNAs (miRNAs) or small interfering RNAs (siRNAs) which are able to silence target messenger RNAs (mRNAs) in a sequence-specific procedure.Citation14 After the discovery of RNAi in Caenorhabditis elegansCitation15,Citation16 and subsequent demonstration of siRNA activity in mammalian cells, RNAi has received considerable attention as an effective therapy for multiple diseases like cancer and viral infections, particularly for those diseases with “undruggable” molecular targets.Citation17,Citation18
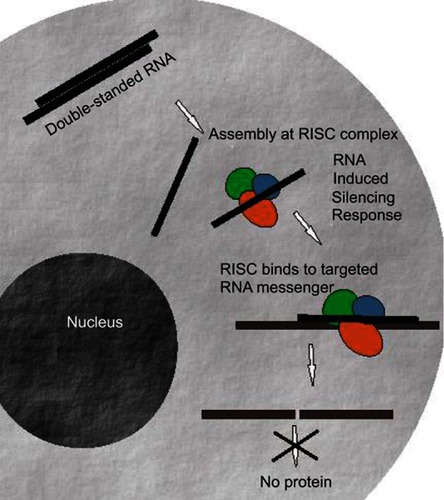
Today, RNAi has become a powerful technology for gene function studies and has been recently used in therapeutic applications.Citation18–Citation20 The main role of the RNAi in cells is the down-regulation of specific proteins (). RNAi mechanism is triggered initially by the enzyme Dicer which cleaves double-stranded RNAs (dsRNAs) into short double-stranded siRNAs of 21–25 nt. The siRNA passenger strand is then unwound, and the siRNA guide strand is loaded into the RNA-induced silencing (RISC) complex leading to cleavage of target mRNAs by Argonaute 2 (Ago2) when the guide strand sequence is paired with an mRNA complementary sequence.Citation21–Citation23 This important mechanism has allowed to open novel therapeutic approaches by designing oligonucleotide molecules through using mRNA transcripts sequences found in the existing human genomic data. Therefore, a careful sequence selection and synthesis of tailored siRNAs may have enormous repercussion in therapy as almost all genes might be down-regulated as well as splice variants, separate transcripts, or mutations might also be specifically targeted. As a consequence, this powerful approach might help circumvent the limitations exhibited by small molecule drugs in conventional cancer therapy treatments leading to drug development processes based on gene functionality.Citation21 Therefore, the development of this therapeutic strategy may have a high impact on modern medicine.Citation20,Citation24,Citation25
Figure 1 Mechanism of action of siRNA molecules. SiRNA duplexes are incorporated in the RNA-induced silencing complex (RISC). Then, siRNA are unwinded and the strand with lower thermodynamic stability at its 5’end remains in the complex and guides it to the complementary mRNA. The target mRNA is then cleaved and protein expression is abolished or reduced.
However, siRNA technology faces multiple obstacles regarding efficient delivery and effectiveness. To overcome this issue, siRNA intracellular delivery strategies should be nontoxic and stable into the site of action for succeeding therapeutic applications of RNAi.Citation26–Citation28 Currently, different methods including mechanical (ultrasound), physical methods,Citation29 electroporation,Citation30 hydrodynamic tail vein injections in mice,Citation31 and the use of a gene gun have been carried out for delivering siRNA in vivo. In addition, local administrations (eg, intraperitoneal, intravenous, subcutaneous injections) and chemical methods based on synthesizing non-viral vehicles (eg, polymers,Citation32–Citation34 cationic lipids,Citation35,Citation36 and peptidesCitation37,Citation38) have also been successfully used among others. To demonstrate the potential of RNAi-based therapeutics, several proof-of-concept studies including bio-distribution, efficiency of delivery, and toxicity caused by cationic delivery vehicles have been carefully analyzed.
siRNA technology in cancer therapy
siRNA has been investigated as an effective treatment for the viral disease as well as cancer extensively with the aim of blocking multiple disease-causing genes.Citation39 RNAi was discovered for the first time in the nematode Caenorhabditis elegans; however, this process has been also found in many different eukaryotes species including, vertebrates, plants, and insects. In mammalian cells, researchers reported that synthetic siRNAs were also able to promote RNAi and therefore silence the expression of altered proteins.Citation15–Citation17,Citation40
Numerous chemical modifications have been proposed in order to increase efficacy and potency in RNAi for in vivo use.Citation41 In this regard, rational chemical designs have allowed siRNA passenger strands to be more likely to modification than siRNA guide strands. These synthetic strategies have enabled to replace either non-bridging oxygen on the phosphate linkage with a sulfur atom, the 2′-hydroxyl group modification of the sugar ring with a methyl group (2′-OCH3) and ethyl group (2ʹ-OCH2CH3), among others.Citation42–Citation45 In addition, other strategies have been developed to deliver siRNAs safely in the cytoplasm. While most naked siRNAs have been effective for a good number of tumor cells in vitro, these siRNAs have unfortunately failed when have injected in vivo by systemic administrations.Citation46
Immunotherapy has gained increasing attention as a promising strategy for treating cancer by promoting the host immune system activation. This activation may occur by simply introducing cytokines, cancer vaccines, monoclonal antibodies, or using antigen-presenting cells (APC) through Toll-like receptors.Citation47 Dendritic cells (DCs) are the strongest antigen exhibiting cells that have the capacity to stimulate naïve T cells and stimulate differentiation and growth of B cells. Furthermore, they include a system of leukocytes broadly distributed in all tissues. DC therapy may offer a novel and promising immunotherapeutic method for prevention and treatment of advanced cancer as well as autoimmune disorders.Citation48,Citation49
DC immunization has been broadly studied in clinical trials involving several types of malignancies like melanoma, prostate cancer, and renal cell carcinoma (RCC), among others.Citation50–Citation54 The use of DC therapy has proved to be effective and a safe strategy in inducing antitumor immunity to patients containing advanced-stage cancer diseases.Citation51,Citation55 Several models have been proposed with the aim of isolating DCs in vitro. For example, Zhang et al developed a method to favor DC differentiation through contact circulating monocytes with natural killer (NK) cell subsets in order to find optimal protocols for DC vaccination.Citation56 According to in vivo studies, DCs can promote T cell-mediated tumor eradication and controlled tumor-bearing hosts when ex vivo isolation have been implemented with tumor antigens. These perceptions have prompted clinical trials to explore both immunologic and clinical impacts of antigen-loaded DCs regulated as therapeutic vaccines to patients containing specific tumors. Some promising outcomes reported from clinical trials in patients with melanoma and malignant prostate tumors showed that immunotherapeutic strategies involving the development of antigen presenting cells in DCs might demonstrate the effectiveness of this process to certain human tumors.Citation50 However, DC clinical effectiveness has not been as good as researchers expected in some specific cancers probably due to the heterogeneity and different methods to monocytes production as well as DC differentiation.
Interestingly, novel approaches have been implemented involving dendritic cell-based therapies and other therapies like RNAi in order to overcome these drawbacks and find a reliable vaccine for cancer therapy.Citation57 For example, Qian et al reported that siRNA may be combined to DC-based therapy in order to manipulate CD40 expression levels and therefore reduce the functional capability of DC to stimulate allogeneic T cells. In this regard, silencing of CD40 expression on DC might be used for generating tolerance-promoting DC with the aim of applying in autoimmunity and transplantation processes.Citation58
Recently, Liu et al prepared a specific cationic nanoparticle in order to deliver indoleamine 2, 3-dioxygenase (IDO) siRNA and tyrosine-related protein 2 (Trp2) to DCs.Citation59 The authors were able to inhibit IDO expression, trigger T cell immune reaction and consequently favor the secretion of cytokines like IL-6, IFN-ϒ and TNF-α as well as promote DC differentiation when compared to traditional control DC vaccines. Thus, in vitro and in vivo studies in B16-F10 mouse model proved this approach to be effective in reducing melanoma tumor growth and therefore enhancing immune response in mice.
siRNA-based gene silencing
Great efforts have been made to understand how dsRNAs, siRNAs, and microRNAs (miRNAs) are able to affect control expression in eukaryotic cells. As described previously, siRNA technology mediates specifically gene silencing at different levels like mRNA degradation, chromatin modification, and translational repression.Citation60–Citation64 Remarkable findings were also found and confirmed transcriptional gene silencing was conserved in mammalian cells when were mediated by siRNAs.Citation65 Despite advantages and interest showed by siRNA-based technology as an effective therapeutic approach, many obstacles like efficient cellular uptake, long-term stabilities, and off-target effects have been reported. These important issues have reduced siRNA effectiveness when used in vitro and/or injected in animal models as well.
While the enhancement of siRNA stability has been successfully solved by introducing chemical modifications at the level of sugar and phosphate groups, advances in efficient siRNA delivery systems to transport siRNAs safely into the cytoplasm of targeted cells is a vital element in cancer therapy.Citation66,Citation67 For this purpose, viral vectors (eg, lentivirus, retrovirus and adenovirus) have become potential vectors for siRNA delivery due to their ability to encapsulate and deliver genetic materials into cells.Citation68,Citation69 Despite such viral vectors having been shown highly transfection efficacies, their clinical usage is still limited due to possible risks of immune responses, mutation, and inflammation. To minimize such undesirable effects and exploit the potential of RNAi-based technology, a good number of synthetic non-viral vectors have been proposed to impart siRNA delivery safely and effectively.Citation22,Citation35
Off-target effects and stimulation of immune response
The specificity of action carried out by siRNA molecules depends upon the uniqueness of the selected sequences; however, many other factors may also have an impact on a lack of specificity.Citation70 Several types involving siRNA off-target effects have been described and thoroughly reviewed.Citation71 It is well known that mammalian immune cells tend to express a family of receptors so-called Toll-like receptors (TLRs) which have also the ability to recognize pathogens and molecules derived from microbes. In addition, some synthetic siRNA sequences as well as their non-viral vehicles have proved to promote the activation of immune system cells producing inflammatory cytokines and Type I interferon in vitro and in vivo by activating TLR7 and TLR8 in a sequence-dependent manner.Citation72
Other significant off-target effect associated to siRNA delivery falls into the miRNA-like off-target silencing. miRNAs are small non-coding endogenous RNA molecules which have the ability to regulate gene expression of several genes in the same way as siRNAs do. This undesirable sequence-specific activity is mainly produced via siRNA base-pairing with sequences located at the 3ʹ-UTR regions of mRNAs. As a consequence, many transcripts might be affected dealing with multiple-site cleavage translational blocks.Citation73,Citation74
Finally, the RNAi machinery saturation mediated by synthetic siRNAs is another source of off-target. Some data have shown that synthetic siRNAs when entered the RNAi pathway tend to compete with endogenous miRNAs for RISC. This process has been observed in certain model studies in which both transcript upregulation and target script downregulation have been observed. Efforts to mitigate or reduce such off-target effects have been effectively studied by designing effective modified siRNAs.Citation75–Citation79
Delivery systems
Lipid-based nanovectors for siRNA delivery
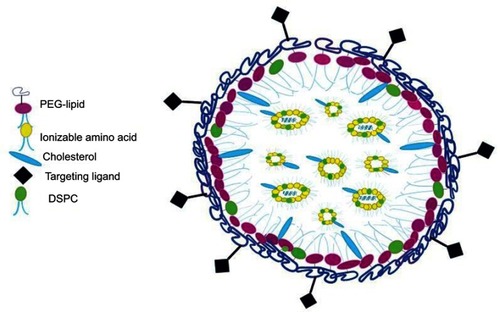
Various kinds of systems have been employed for delivering siRNA such as antibody conjugates, micelles, natural polysaccharides, peptides, synthetic cationic polymers, and microparticles among others. Nevertheless, some lipid-based formulations and other lipid-like materials such as liposomes, niosomes, and stable nucleic acid lipid particles (SNALPs) have proved to be effective drug delivery systems as promising strategies for in vivo siRNA deliveryCitation80–Citation85 ().
Liposomes and lipoplexes
As non-viral vectors, liposomes have become a powerful platform as a pharmaceutical carrier to facilitate the delivery of small molecule drugs and macromolecules.Citation86 Liposomes are made up of phospholipids which tend to form closed lipid bilayers in aqueous solvents dealing with particles formation of nanometric size. In addition to loading a good number of substances like active drugs, proteins, peptides, antibodies, and nucleic acids, liposomes have been also used in the transport of photosensitizers needed for promoting photo-dynamic therapy.Citation87 Second and third generation of liposomes have also been proposed by including certain ligands or loading polymers which have the ability to recognize specific receptors or enhance liposome stabilities, respectively.Citation88 These advances have allowed liposomes to be used in a good number of clinical applications and be also injected according to different administration routes.
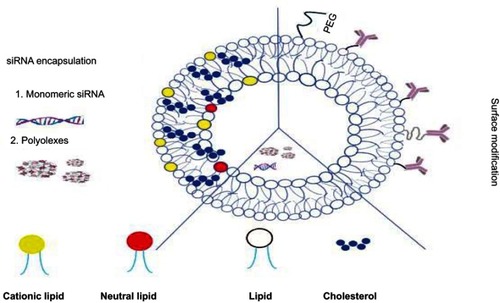
Nucleic acids in particular antisense oligonucleotides (ASOs) and siRNAs have been widely incorporated into liposomal nanocarriers ().Citation35,Citation62 This electrostatic combination has resulted in obtaining the corresponding lipoplexes which have helped nucleic acids increase both their long-term stabilities and cellular internalization. Positive and negatively charged together with neutral liposomes have been used as vehicles for transporting efficiently siRNA moleculesCitation89 (). In this regard, cationic lipids have attracted significant attention as appropriate non-viral vehicles. This interest has allowed to designing convenient synthetic strategies and characterizing a plethora of cationic lipids. Structurally, cationic lipids consist of three general components: i) the lipid tail(s); ii) the cationic head group and iii) the linkers and/or backbones that connect to both parts. Some studies have suggested a relationship between the efficiency and lipid structure; however, this involvement still remains a central goal in siRNA delivery.Citation82
Table 1 Summary of siRNA-loaded encapsulated liposomes utilized for siRNA delivery
While anionic liposomes are usually cleared from circulation, cationic liposomes (eg, DC-Cholesterol, DOTAP, and AtuFECT01 among others) in combination with helper lipids (eg, Cholesterol, DOPE, and DPhyPE) have proved efficient in siRNA delivery at optimized N/P ratios. However, reducing toxicity levels still remains crucial when forming these types of lipoplexes.Citation90–Citation92 To solve these issues, neutral liposomes [eg, 1,2-dioleoyl-sn-glycero-3-phosphatidyl-choline (DOPC)] have been prepared in order to reduce such toxicity and therefore increase in biocompatibility although their entrapment efficiency might be compromised.Citation93
The use of neutral lipid-based formulations has enabled the successful siRNA delivery in vivo in mouse models showing tumor growth inhibition and consequently the down-regulation of targeted genes.Citation67 Several siRNA-based treatments against ovarian cancer were suggested by Sood et al.Citation94 In this study, DOPC-encapsulated siRNA targeting the oncoprotein Ephrin Type-A receptor 2 was highly effective (65%) in reducing its expression after 48 hrs of being administered in a single dose in an orthotopic model. Interestingly, the authors found this liposomal formulation when injected both intraperitoneal and intravenous reduced the ovarian tumor size in mice xenograft models with the same efficiency. In addition, treatments with DOPC-based liposomes were also studied in order to target interleukin-8, β-2 adrenergic receptor, and focal adhesion-kinase by mediating intraperitoneal delivery of siRNAs in an ovarian cancer mouse model.Citation94
In spite of obtaining powerful and effective nanovehicles based on formulating lipid molecules and siRNAs, liposomes and niosomesCitation85 have been properly tuned in order to promote targeted delivery in specific organs and tissues. This strategy has enabled the preparation of unique lipid-based platforms bearing the arginine-glycine-aspartic acid (RGD) peptideCitation95 or decorated with an octa-arginine (R8) cell-penetrating peptide (CPPs).Citation96 These two strategies have confirmed increased particle stability, biocompatibility, and good siRNA therapeutic response without affecting remarkably cellular viability.
SNALPs
Stable nucleic acid lipid particles (SNALPs) has become a promising delivery platform for siRNA molecules in different animal models. This formulation is made up of a lipid bilayer based on a mixture of fusogenic and cationic lipids which enables endosomal release and therefore facilitates the siRNA cellular uptake. In addition, the SNALP surface is also coated during the formulation process with a PEG-lipid conjugate which provides hydrophilicity and a neutral layer with the aim of stabilizing these particles in the bloodstream when injected in vivo.Citation35 The first use of SNALPs was reported by Zimmermann et al to silence the apolipoprotein B (Apo B) gene expression in non-human primates with a single dose (2.5 mg/kg) of siRNA.Citation97 Optimized formulations based on modifying SNALPs chemically increased their siRNA efficacy in vivo by reducing doses until 0.01 mg·Kg−1 when targeted a hepatic endogenous gene.Citation82
Other important applications of SNALPs have been reported and reviewed in literature.Citation98 For example, McLachlan et al formulated a siRNA targeting the polymerase gene in vivo in order to fully protect guinea pigs against Ebola virus.Citation99 More recently, SNALP technology has been successfully employed to favor the silencing of mTTR expression.Citation100 Clinical results confirmed the suitability and therapeutic effect of this siRNA-SNALP combination in humans giving rise to the first RNAi therapeutic in the market.Citation68,Citation101
Polymeric nanoparticles
The last decades have been witnessed the large development on polymeric nanosized materials as drug carriers becoming some of the top selling drugs.Citation100,Citation102 Synthetic or naturally-occurring nanopolymers are colloidal solid materials specially designed to be degraded in vivo without producing toxic components.Citation103 Several polymeric nanoparticles have been approved as drug carriers for human use.Citation104 Due to their excellent properties, a variety of polymers including cationic polymers such as polyethylenimine (PEI), polysaccharides such as cyclodextrin (CD) and chitosan have been studied for siRNA delivery.Citation105,Citation106 As a general rule, polymer nanoparticles exhibit positively charged units to facilitate electrostatic binding of siRNA; however, the use of covalent strategies involving siRNA and polymers have been frequently prepared by using degradable linkers such as disulfide or thiol-maleimide bonds.Citation107
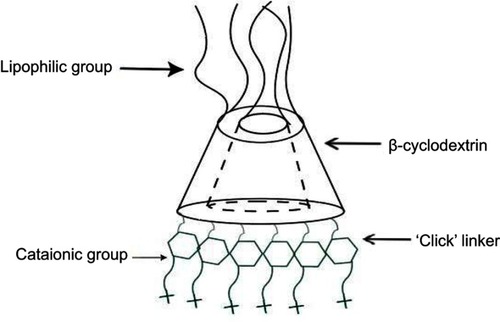
Cyclodextrins (CDs) nanoparticles
Cyclodextrins are naturally occurring oligosaccharides that are produced during the bacterial digestion of cellulose. They have been well studied and characterized as pharmaceutical excipients with a favorable toxicological profileCitation108–Citation110 (). Cyclodextrin-containing polycation nanoparticles are exciting polymer-based siRNA delivery systems, as they are able to self-assemble with siRNA dealing to shape colloidal particles of about 50 nm in diameter. In addition, their terminal imidazole groups help in the release and intracellular trafficking of nucleic acids. A complex system consisting of a siRNA, the human transferrin protein to engage transferrin receptors on the surface of the cancer cells, a cyclodextrin-based polymer, and polyethylene glycol (PEG) has been described to increase the stability of nanoparticles in biological fluids.Citation111,Citation112
An example of this system is CALAA-01, which is a targeted nanocomplex which includes an anti-R2 siRNA.Citation113 This targeted therapeutic system has been designed to inhibit tumor growth. The selected siRNA prevents the tumor from growing via RNAi to decrease expression of the M2 subunit of R2 (ribonucleotide reductase). This system was used in clinical studies in patients bearing solid tumors.Citation114
Chitosan and inulin nanoparticles
Chitosan and inulin are naturally occurring polysaccharides used for the preparation of siRNA formulations. Chitosan, that contains hydroxyl and amino groups, is widely used as a drug carrier due to its low cost, easy degradability and biocompatibility. Chitosan has positive charges under slightly acidic conditions allowing the formation of nanoparticles or complexes by interaction with siRNA molecules.Citation115,Citation116 Interestingly, optimized chitosan formulations containing folic acid as a targeting ligand resulted in increasing siRNA cellular uptake to tumor cells producing an enhancement in gene silencing properties in HeLa and OV-3 cell lines.Citation116 Furthermore, grafting chitosan with polyethylene glycolCitation117 and polyethylenenimineCitation118 enable them for in vivo use through intravenous or intraperitoneal administration.Citation117,Citation118 Chitosan is frequently used in combination with other carriers such as poly(L-lactide) (PLLA) porous microparticleCitation119,Citation120 or the triblock polymer poly(L-lactide)-poly(ethyleneglycol)-poly(L-lactide) (PLLA-PEG-PLLA) to increase storage stability and decrease immunogenicity.Citation121 These formulations, prepared by supercritical fluid technology,Citation122 allowed the addition of multiple polymers as well as other chemotherapeutic drugs such as paclitaxelCitation121 and doxorubicin.Citation120
Another polysaccharide used recently in siRNA delivery is inulin. This polysaccharide made up of fructose and glucose units is usually processed with oligoamines such as ethylenediamineCitation123 or diethylenetriamine and imidazoleCitation124,Citation125 in order to generate cationic groups for siRNA binding and therefore favor endosomal escape.
Polyethylenimine (PEI)
Polyethylenimine (PEI) is one of the most studied cationic polymers for transfection of oligonucleotides, siRNA, and plasmid DNA.Citation126 It is accessible either in branched or in a linear shape and many molecular weights.Citation127 The high charge density of PEI is considered a good property for complexation of siRNA and facilitates the endosomal escape by the proton sponge effect.Citation126 However, high molecular weight PEI has exhibited significant toxicity in many cell lines.Citation127 The use of PEI as a non-viral vehicle to deliver siRNAs was demonstrated to have an efficient antiviral effect in a guinea pig model of Ebola virus infectionCitation128 and a murine model of influenza infection.Citation129 The first successful applications of PEI delivery of siRNA in cancer was the inhibition of human EGF receptor 2 (HER2) in mouse models of ovarian cancer.Citation130 If provided the appropriate molecular weight polymer and structure to avoid toxicities, for systemic use, PEI appears to be a promising siRNA delivery system.Citation131
At present, research on PEI is directed towards the development of mixed polymers especially with PEG,Citation132 and polysaccharides such as chitosanCitation118 to reduce potential toxic effects and decrease the removal of nanoparticles by the reticuloendothelial system (RES). To direct PEI-siRNA complexes to specific target cells, PEI has been modified with receptor-mediated ligands such as folic acid, mannose, N-acetylgalactosamine, and others.Citation132
Anionic polymers
Poly-L-lactic-co-glycolic acid (PLGA) is a biocompatible, well-studied, and biodegradable polymer used for decades in pharmaceutical applications.Citation133,Citation134 PGLA polymer has the advantage of exhibiting lower toxicity when compared with cationic polymers and cationic lipids. On the other site, PLGA cannot form electrostatic complexes with siRNA as both are negatively charged. One way to overcome this issue is to use PLGA nanocapsules in order to encapsulate oligonucleotides bearing 5ʹ-lipophilic molecules. These resulting polymer microspheres have provided sustained release of the modified siRNAs and antisense oligodeoxynucleotides within 24 hrs when administered subcutaneously.Citation135 Another interesting approach is to react polyamines or positively charged dendrimers with some of the carboxyl groups of PLGA in order to generate additional positive charges that can be used for electrostatic binding.Citation134 In addition, siRNAs may also be covalently linked to PLGA via intracellular cleavable disulfide linkers.Citation136
Hyaluronic acid (HA) is also an anionic polymer that plays a pivotal role as a ligand against CD44 receptor which is highly overexpressed in tumor cells. The combination of PLGA and hyaluronic acid has also been used to co-deliver paclitaxel together with a siRNA against focal adhesion kinase (FAK) that is overexpressed in breast, colon and ovarian cancers.Citation137 The resulting PLGA nanoparticles were shown to possess a highly selective delivery of the paclitaxel and siRNA to CD44+ cells.
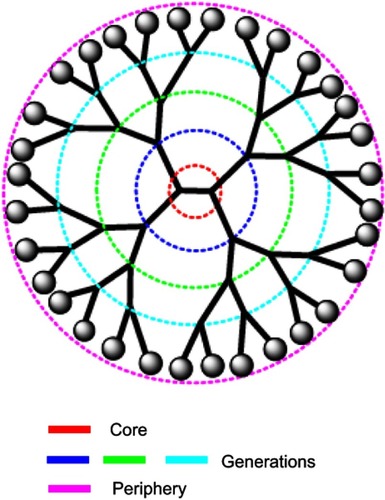
Cationic dendrimers
Cationic dendrimers with extremely branched peripheral chain ends are well-defined artificial macromolecules. These can be synthesized by adding several layer branches. Each branched layer represents a superior generation molecule.Citation133 The precise core-shell nanostructures of dendrimers enables drug loading by surface adsorption, interior encapsulation, or chemical conjugation (). The dendrimer surface can be functionalized to build a variety of functions for a good number of applications. Dendrimers have a well-defined chemical structure and size in comparison with alternative linear polymers with a spherical form. To deliver negatively charged plasmid DNA, siRNAs, and antisense oligonucleotides, dendrimers containing high density of positive charges on the surface have been used.Citation138,Citation139 Polycationic dendrimers including poly-propylenimine (PPI) and poly-(amidoamine) (PAMAM) dendrimers have been investigated for siRNA delivery in past years. PAMAM-mediated siRNA delivery is usually found at nucleolus and even perinuclear locations. In a study designed for increasing the siRNA loading capacity, dendrimers were additionally improved with magnetofluorescent nanoworms to create “dendriworms.”Citation140 After adding the dendriworms carrying siRNAs to human glioblastoma cells, the siRNA-dendrimers quickly internalized into the cells and escaped into the cytosol. The delivered siRNAs were demonstrated to silence expression of the targeted gene in vivo.Citation141
Carbon nanotubes (CNTs)
Carbon nanotubes (CNTs) have been considered as potential nucleic acid and drug delivery vehicles within the nanomedicine field.Citation142,Citation143 They can be either single-walled CNTs (a single layer of graphene sheets) or multiwalled CNTs which can be prepared using multiple layers. In addition, CNTs can be modified with additional functional groups and also offer a structural advantage due to their very large surface. This can be used for loading therapeutic drugs like proteins and nucleic acids, and they. In order to form complexes with siRNA, positively charged functionalized single-walled CNTs (SWCNTs+) have been prepared. In a model study, siRNA designed to inhibit telomerase reverse transcriptase (TERT) was complexed with SWCNTs+ and incubated with tumor cells. The complexes TERT siRNA:SWCNTs+ were effective in delivering siRNA and knockdown the expression of TERT in a variety of tumor cells.Citation144
Inorganic nanoparticles (INPs)
In the last decades, inorganic nanoparticles (INPs) have emerged as alternative nanomaterials to traditional lipid formulations for siRNA delivery.Citation145 Specifically, magnetic nanoparticles and gold nanoparticles have been extensively studied for siRNA delivery and in vivo imaging because of their biocompatibility and reduced toxicity.Citation145,Citation146 For example, mesoporous silica nanoparticles (MSNPs) have been used as interesting vehicles for delivering siRNAs in cancer cells. Recently, Ngamcherdtrakul et al successfully prepared 50 nm INPs decorated with PEI, PEG, and an antibody.Citation147 These authors were able to establish a lyophilization protocol for these modified INPs and therefore promote the formation of complexes with siRNAs. Interestingly, these siRNA nanoconstructs were stable, showed luciferase silencing inhibition, and were able to display anti-proliferative effect in vitro. Other INPs like carbonate apatite have also studied by Tiash et al. In this work, the authors used these NPs as vehicles for delivering siRNA targeting GFR genes simultaneously (egfr1 and erbb2) in mouse models showing the ability of these siRNA complexes to reduce tumor growth and reduce cell viability.Citation148
Magnetic nanoparticles (MNPs)
Magnetic nanoparticles (MNPs) have been extensively employed as magnetic resonance imaging (MRI) contrast agents for tumor imaging and drug delivery as well. Iron oxide nanoparticles can be synthesized to obtain particles of small nanometric size with narrow and high magnetization values. In addition, MNPs have shown to induce cancer cell apoptosis by magnetic heating because of their excellent contrast signal in MRI.Citation149 In addition, MNPs can be coated with siRNAs as well as other chemotherapeutic agents in order to promote delivery to the tumor site by applying external magnetic fields. Some representative examples inducing cellular death by magnetic heating have been reported.Citation150,Citation151
Gold nanoparticles (AuNPs)
Gold nanoparticles (AuNPs) have been used as effective nanomaterials for siRNA delivery applications for several reasons.Citation146,Citation152,Citation153 First, functional diversity can be easily obtained with the creation of multifunctional monolayers and second, AuNPs can be prepared in a scalable fashion with low size disparity. AuNPs have been widely utilized for gene therapy targets in preclinical animal model and in vitro studies because of their low toxicity, rapid endosomal escape, high payload, efficient uptake, increased half-life; specific, efficient, extensive transcriptional activation of the innate immune response, and selective gene silencing and transfection.Citation154 AuNPs were effectively employed as a platform for the delivery of Bcl-2 (B-cell lymphoma 2) or VEGF (vascular endothelial growth factor) siRNAs to a human cervical carcinoma cell line or into a human glioma cell line, for silencing of EGFP (enhanced green fluorescent protein) and LUC (luciferase) reporter genes.Citation155,Citation156
Limitations to the siRNA therapeutic approach
summarizes some of the limitations described for siRNA delivery.Citation157 Degradation in serum of siRNA therapeutic molecules is one of the major limitations for the therapeutic use of these molecules. Double-stranded siRNA molecules are more stable than single-stranded antisense oligonucleotides. For this reason, unmodified siRNA in saline buffer has been described to be successful for human treatments. However, these cases have been mostly focused for the treatment of ocular diseases by local administration.Citation158
Table 2 Intracellular and extracellular limitations of RNAi
Most of the siRNA molecules used for therapeutic uses are partially modified since a large number of modifications may block RISC binding to siRNA and therefore prevent RNA interference mechanism.Citation159 Frequently these modifications are located near the 3ʹ and 5ʹ-ends of the passenger and guide strands where it has been demonstrated that have a strong impact in the following properties: i) avoid exonuclease degradation action;Citation74,Citation159 ii) increase affinity to RISC;Citation76 iii) prevent the passenger strand loading;Citation76 iv) lower innate immunostimulation response;Citation79 and v) avoid miRNA-like effects.Citation75 Another interesting siRNA chemical modification is the preparation of siRNA conjugates covalently,Citation160 especially lipid conjugates such as cholesterol. Such lipid siRNA conjugates have exhibited exonuclease resistance have promoted cellular uptake and also increased serum circulation time by binding to serum proteins.Citation161,Citation162
Carbohydrate-siRNA conjugates and especially N-acetyl-galactosamine (GalNAc)-siRNA conjugates have also being used for in vivo targeting to hepatocytes. A large impact on cellular uptake was observed with siRNAs that were conjugated with triantennary GalNAc residues. Transfection experiments corroborated high specificity and gene silencing levels in liver.Citation163 Peptide-siRNA conjugates especially cell-penetrating peptides (CPPs) and fusogenic peptidesCitation160 have been also described to potentiate cellular uptake and facilitate endosomal escape being a good alternative for anti-cancer siRNA delivery.
Most of the clinical trials involving systemic delivery of siRNA molecules have used lipid formulations including liposomes, SNALPs, and other types of lipid formulations that contain cationic lipids to form complexes with siRNA molecules. One of the first human clinical trials for cancer treatment used two siRNA molecules formulated in lipid nanoparticles (LNP) to treat liver metastasis of colon cancer.Citation164 These formulations were actively taken up by the liver but treatment of cancer to other tissues was not demonstrated.
As shown in this review, the development of novel approaches based on nanomaterials is one of the most active areas of development for future medicines for cancer treatment. Polymeric nanoparticles are one of the most promising alternatives for the near future. The knowledge generated in the last decades for the delivery of chemotherapeutic agents using biocompatible nanopolymers will trigger the development of novel formulations that may include combinations of chemotherapeutic agents together with one or several siRNA molecules targeting overexpressed proteins for a more efficient and less toxic medicines. These nanomaterials as well as gold and SPION nanoparticles also allow the introduction of specific targeting molecules that will direct the accumulation of the therapeutic molecules near the tumors increasing the passive accumulation of nanoparticles by the enhanced permeability and retention (EPR) effect. The addition of acid responsive polymers such as PEI and peptides may also increase the efficacy by facilitating endosomal escape. A limitation on the use of nanomaterials is the potential in vivo aggregation with serum proteins and subsequent removal by the RES. This undesired effect may be prevented by the incorporation of PEG.Citation136
Finally, a novel approach with high potential is the use of DNA scaffolds for the delivery of siRNA.Citation165 For example, DNA dumbbell,Citation166 DNA nanoribbons,Citation167 and spherical nucleic acids (SNA)Citation168 have been described as alternative systems which are biodegradable, non-toxic, and also non-immunogenic. These DNA scaffolds have proved to be effective when have incorporated targeting ligands, siRNA drugs as well as chemotherapeutic drugs with high precision.Citation169,Citation170
siRNA in clinical trials for cancer therapy
Recently, RNAi-based treatment has been rapidly developed into clinical trials. In addition, it has been studied for treating diverse diseases, such as cancer, respiratory infection, AMD (age-related macular degeneration), glaucoma, and hypercholesterolemia, among others.Citation171 So far, at least 20 clinical trials have been initiated using siRNA- and miRNA-based therapeutics.Citation172 Although there are many ways to engage RNAi pathways, currently the majority of the clinical trials involve siRNA technology.Citation114,Citation173–Citation175
In a recent report from a phase I study on patients with refractory or relapsed solid cancer, Davis et al provided the first clinical evidence that RNAi could be achieved by administering siRNA against the M2 subunit of ribonucleotide reductase (RRM2).Citation109
Recently, the therapeutic potential of RNAi as a revolutionary modern class of medicine was demonstrated in cancer therapy for the treatment of inaccessible solid tumors via systemic administration by delivering Atu027Citation176 and CALAA-01Citation114 developed by Silence Therapeutics and Calando Pharmaceuticals Inc, respectively (). In 2008, CALAA-01 was employed for treating the first patient in a phase I clinical trial. In these studies, a siRNA nanoparticle targeting protein kinase N3 (PKN3) or RRM2 was administered intravenously for the treatment of solid tumors.Citation114 Recently Atu027, a siRNA targeting protein kinase N3 (PKN3), is being used in a clinical trial together with gemcitabine for the treatment of advanced or metastatic pancreatic cancer (clinicaltrials.gov Identifier: NCT01808638).
Table 3 Selection of clinical trials against cancer using siRNA drugs
An interesting therapeutic approach has been shown by Tabernero et al.Citation177 In this study, two different siRNAs targeting two genes (vascular endothelial growth factor, VEGF and kinesin spindle protein, KSP) involved in angiogenesis were formulated in the same lipid formulation. The authors showed that siRNAs were able to promote mRNA cleavage and antitumor activity in humans. This lipid formulation (ALN-RSV) (Alnylam Pharmaceuticals) demonstrated complete regression of liver metastases and endometrial cancer in some cases.
A similar lipid formulation is being used for silencing Myc oncoprotein which is deregulated in over half of human malignancies. In a recent dose-escalation work, the clinical activity of DCR-MYC was evaluated in patients with advanced solid tumors, multiple myeloma, or lymphoma showing good clinical and metabolic responses over various dose levels.Citation178
Recently, the use of 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine liposomes for systemic siRNA delivery was evaluated in phase 1 clinical trial to inhibit Ephrin type-A receptor2 (EphA2) gene.Citation179 The liposomal formulation was well tolerated, and clinical trials are ongoing.
In another approach, Silenseed has used the insertion of a specialized bio-polymeric “scaffold” containing the siRNA drug into the solid tumor core. The LODER® polymer matrix has been used in a clinical trial for silencing a mutated K-RAS oncogene relevant for pancreatic ductal adenocarcinoma.Citation180
Although there are not a large number of siRNAs in advanced clinical trials, RNAi is a noteworthy mechanism for advanced novel therapeutics because in cancer cells, the functionality and expression of overexpressed damaging genes are significantly reduced by siRNAs. Nonetheless, once applying RNAi techniques in vivo, the most obstacle to achieving gene silencing is the delivery of therapeutic siRNA molecules.Citation178 Thus, the clinical application of siRNA therapy faces several challenges to achieve effective dosages in target cells at safe and maintaining oligonucleotide stability in circulation.
The strategies for the observation of the distribution and therapeutic effects as well as techniques for enhancing cellular uptake also are essential.Citation18,Citation155 First clinical trials have shown encouraging results. An interesting direction is the use of combinations of chemotherapeutic agents and siRNA as well as the combination of several siRNA targeting overexpressed proteins involved in different metabolic routes such as oncogenes, control of the nucleotide pool, angiogenesis, telomeres, antiapoptotic genes, and so on. Finally, the use of exosomes derived from mesenchymal stromal cells will be evaluated in a clinical trial for the delivery KrasG12D siRNA in patients of pancreatic cancer with KrasG12D mutation (clinicaltrials.gov Identifier: NCT03608631). Exosomes are natural lipid-particles secreted by cells that play a crucial role in protecting and transporting macromolecules such as endogenous microRNA and mRNA. These interesting properties have triggered the interest in the use of such exosomes as potential nanovehicles for internalization of therapeutic molecules.
Conclusions
Cancer is accounted for one of the most prevalent diseases with high mortality rates in the world. It is considered one of the major challenges of medical treatment and health.Citation181 For instance, colorectal cancer with more than 1.2 million new cases causes 600 thousand deaths per year and is the fourth cause of deaths worldwide.Citation182 Nearly 50,000 people will die in the United States per year and nearly 135,000 new cases will be diagnosed.Citation183
In addition to surgery and classical chemotherapeutic and radiation methods, there is a need for novel and less aggressive treatments. RNA interference can be considered a promising alternative for cancer therapy as it is less toxic than classical chemotherapy. In addition, siRNAs have been studied for the treatment of various human diseases such as genetic disorders, ocular conditions, cardiovascular diseases, viral infections, and cancers. The ability to target virtually any gene(s) is one of the most attractive aspects of siRNA therapeutics when treatments involving protein-based drugs or small molecules cannot be properly used.
However, a major restriction within the therapeutic applications of siRNA is the low cellular uptake of unmodified siRNAs as they cannot penetrate the cells with high efficiency. For this reason, siRNA molecules need to be complexed or conjugated with an appropriate carrier system. In addition, their fast degradation in cellular cytoplasm and plasma lead to short half-lives. For this reason, numerous strategies involving the combination of biocompatible and versatile non-viral carriers with siRNAs containing modifications and optimized sequences have resulted in potential RNAi-based drugs as efficient medicines into the clinic.
Although a large number of excellent works have been described, future studies are required to concentrate on the in vivo safety profiles of the nanoparticle-based delivery systems like polymers, cationic lipids, dendrimers, and inorganic nanoparticles, including undesirable cytotoxicity and immune stimulation. For the clinical usage of siRNA-based cancer therapeutics, the development of biocompatible, biodegradable, and safe biodegradable nanoparticle delivery systems is still necessary together with the development of simple and reproducible protocols for the production of batches for regulatory assessment and clinical trials.
Disclosure
The authors report no conflicts of interest in this work.
References
- Ebrahimi M, Moazzen F, Marmari V, et al. In silico analysis, cloning and expression of recombinant CD166 in E. coli BL21 (DE3) as a marker for detection and treatment of colorectal cancer. J Med Microbiol Diagnosis. 2017;06(01):1–6. doi:10.4172/2161-0703.1000249
- Vries RGJ, Huch M, Clevers H. Stem cells and cancer of the stomach and intestine. Mol Oncol. 2010;4(5):373–384. doi:10.1016/j.molonc.2010.05.00120598659
- Dana H. An overview of cancer stem cell. J Stem Cell Res Ther. 2017;1(4):169–174. doi:10.15406/jsrt.2016.01.00029
- Zhang X, Li X, You Q, Zhang X. Prodrug strategy for cancer cell-specific targeting: a recent overview. Eur J Med Chem. 2017;139:542–563. doi:10.1016/j.ejmech.2017.08.01028837920
- Hassanpour SH, Dehghani M. Review of cancer from perspective of molecular. J Cancer Res Pract. 2017;4(4):127–129. doi:10.1016/j.jcrpr.2017.07.001
- Saraswathy M, Gong S. Recent developments in the co-delivery of siRNA and small molecule anticancer drugs for cancer treatment. Mater Today. 2014;17(6):298–306. doi:10.1016/j.mattod.2014.05.002
- Endoh T, Ohtsuki T. Cellular siRNA delivery using cell-penetrating peptides modified for endosomal escape. Adv Drug Deliv Rev. 2009;61(9):704–709. doi:10.1016/j.addr.2009.04.00519383521
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.2120824399786
- Spano J-P, Azria D, Gonçalves A. Patients’ satisfaction in early breast cancer treatment: change in treatment over time and impact of HER2-targeted therapy. Crit Rev Oncol Hematol. 2015;94(3):270–278. doi:10.1016/j.critrevonc.2015.01.00725682223
- Wee Gan C, Chien S, Feng -S-S. Nanomedicine: enhancement of chemotherapeutical efficacy of docetaxel by using a biodegradable nanoparticle formulation. Curr Pharm Des. 2010;16(21):2308–2320. doi:10.2174/13816121079192048720618152
- Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. MAbs. 2013;6(1):34–45. doi:10.4161/mabs.27022
- Leal M, Sapra P, Hurvitz SA, et al. Antibody–drug conjugates: an emerging modality for the treatment of cancer. Ann N Y Acad Sci. 2014;1321(1):41–54. doi:10.1111/nyas.1249925123209
- Jain S, Pathak K, Vaidya A. Molecular therapy using siRNA: recent trends and advances of multi target inhibition of cancer growth. Int J Biol Macromol. 2018;116:880–892. doi:10.1016/j.ijbiomac.2018.05.07729782974
- Mahmoodzad H, Ardaneh M, Zeinalinia E, et al. Microrna a new gate in cancer and human disease: a review. J Biol Sci. 2017;17(6):247–254. doi:10.3923/jbs.2017.247.254
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806. doi:10.1038/358889486653
- Caplen NJ, Parrish S, Imani F, Fire A, Morgan RA. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci. 2001;98(17):9742–9747. doi:10.1073/pnas.17125179811481446
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi:10.1038/3507810711373684
- Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8(3):173–184. doi:10.1038/nrg200617304245
- Dana H, Chalbatani GM, Mahmoodzadeh H, et al. Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci. 2017;13(2):48–57.28824341
- Yang J. Patisiran for the treatment of hereditary transthyretin-mediated amyloidosis. Expert Rev Clin Pharmacol. 2019;12(2):95–99. doi:10.1080/17512433.2019.156732630644768
- Lorenzer C, Dirin M, Winkler AM, Baumann V, Winkler J. Going beyond the liver: progress and challenges of targeted delivery of siRNA therapeutics. J Control Release. 2015;203:1–15. doi:10.1016/j.jconrel.2015.02.00325660205
- Hong CA, Nam YS. Functional nanostructures for effective delivery of small interfering RNA therapeutics. Theranostics. 2014;4(12):1211–1232. doi:10.7150/thno.849125285170
- Lee S-Y, Lee SJ, Oh Y-K, et al. Stability and cellular uptake of polymerized siRNA (poly-siRNA)/polyethylenimine (PEI) complexes for efficient gene silencing. J Control Release. 2009;141(3):339–346. doi:10.1016/j.jconrel.2009.10.00719836427
- Sjouke B, Balak DMW, Beuers U, Ratziu V, Stroes ESG. Is mipomersen ready for clinical implementation? A transatlantic dilemma. Curr Opin Lipidol. 2013;24:4. doi:10.1097/MOL.0b013e328362dfd923298958
- Burnett J, Rossi J. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19(1):60–71. doi:10.1016/j.chembiol.2011.12.00822284355
- Biswas S, Deshpande PP, Navarro G, Dodwadkar NS, Torchilin VP. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials. 2013;34(4):1289–1301. doi:10.1016/j.biomaterials.2012.10.02423137395
- Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi:10.1038/nrd274219180106
- Rettig GR, Behlke MA. Progress toward in vivo use of siRNAs-II. Mol Ther. 2012;20(3):483–512. doi:10.1038/mt.2011.26322186795
- Tsunoda S, Mazda O, Oda Y, et al. Sonoporation using microbubble BR14 promotes pDNA/siRNA transduction to murine heart. Biochem Biophys Res Commun. 2005;336(1):118–127. doi:10.1016/j.bbrc.2005.08.05216125678
- Kishida T, Asada H, Gojo S, et al. Sequence-specific gene silencing in murine muscle induced by electroporation-mediated transfer of short interfering RNA. J Gene Med. 2004;6(1):105–110. doi:10.1002/jgm.45614716682
- Morrissey DV, Blanchard K, Shaw L, et al. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology. 2005;41(6):1349–1356. doi:10.1002/hep.2070215880588
- Zhang S, Zhao B, Jiang H, Wang B, Ma B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Release. 2007;123(1):1–10. doi:10.1016/j.jconrel.2007.07.01617716771
- Kim WJ, Kim SW. Efficient siRNA delivery with non-viral polymeric vehicles. Pharm Res. 2009;26(3):657–666. doi:10.1007/s11095-008-9774-119015957
- Gary DJ, Puri N, Won YY. Polymer-based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J Control Release. 2007;121(1–2):64–73. doi:10.1016/j.jconrel.2007.05.02117588702
- Tseng YC, Mozumdar S, Huang L. Lipid-based systemic delivery of siRNA. Adv Drug Deliv Rev. 2009;61(9):721–731. doi:10.1016/j.addr.2009.03.00319328215
- Hassani Z, Lemkine GF, Erbacher P, et al. Lipid-mediated siRNA delivery down-regulates exogenous gene expression in the mouse brain at picomolar levels. J Gene Med. 2005;7(2):198–207. doi:10.1002/jgm.65915515135
- Simeoni F, Morris MC, Heitz F, Divita G. Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA into mammalian cells. Nucleic Acids Res. 2003;31(11):2717–2724. doi:10.1093/nar/gkg38512771197
- Simeoni F, Morris MC, Heitz F, Divita G. Peptide-based strategy for siRNA delivery into mammalian cells BT - RNA silencing: methods and protocols In: Carmichael GG, Totowa NJ, editors. Vol. 309. Humana Press; 2005:251–260. doi:10.1385/1-59259-935-4:251.
- Farrow B, Evers BM, Iwamura T, Murillo C, O’Connor KL, Rychahou P. Inhibition of pancreatic cancer cell growth and induction of apoptosis with novel therapies directed against protein kinase A. Surgery. 2003;134(2):197–205. doi:10.1067/msy.2003.22012947318
- Schwarz DS, Hutvágner G, Haley B, Zamore PD. Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol Cell. 2002;10(3):537–548. doi:10.1016/S1097-2765(02)00651-212408822
- Behlke MA. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18(4):305–320. doi:10.1089/oli.2008.016419025401
- Chiu Y, Rana TM. siRNA function in RNAi: a chemical modification analysis. Rna. 2003;9:1034–1048. doi:10.1261/rna.5103703.200012923253
- Hall AHS, Wan J, Shaughnessy EE, Shaw BR, Alexander KA. RNA interference using boranophosphate siRNAs: structure-activity relationships. Nucleic Acids Res. 2004;32(20):5991–6000. doi:10.1093/nar/gkh93615545637
- Dowler T, Bergeron D, Tedeschi AL, Paquet L, Ferrari N, Damha MJ. Improvements in siRNA properties mediated by 2′-deoxy-2′-fluoro-β-D-arabinonucleic acid (FANA). Nucleic Acids Res. 2006;34(6):1669–1675. doi:10.1093/nar/gkl03316554553
- Watts JK, Deleavey GF, Damha MJ. Chemically modified siRNA: tools and applications. Drug Discov Today. 2008;13(19–20):842–855. doi:10.1016/j.drudis.2008.05.00718614389
- Xu C, Wang J. Delivery systems for siRNA drug development in cancer therapy. Asian J Pharm Sci. 2015;10(1):1–12. doi:10.1016/j.ajps.2014.08.011
- Ghafouri-Fard S, Ghafouri-Fard S. siRNA and cancer immunotherapy. Immunotherapy. 2012;4(9):907–917. doi:10.2217/imt.12.8723046235
- Klippstein R, Pozo D. Nanotechnology-based manipulation of dendritic cells for enhanced immunotherapy strategies. Nanomedicine Nanotechnology Biol Med. 2010;6(4):523–529. doi:10.1016/j.nano.2010.01.001
- Banchereau J, Steinman RM. Dendritic cells and the control of immunology. Nature. 1998;392:245–252. doi:10.1038/325889521319
- Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Pathology. 2000;245–273. doi:10.1146/annurev.immunol.21.120601.14104011186419
- Constantino J, Gomes C, Falcão A, Neves BM, Cruz MT. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol Res. 2017;65(4):798–810. doi:10.1007/s12026-017-8931-128660480
- Verra N, De Jong D, Bex A, et al. Infiltration of activated dendritic cells and T cells in renal cell carcinoma following combined cytokine immunotherapy. Eur Urol. 2005;48(3):527–533. doi:10.1016/j.eururo.2005.03.03116115526
- Van Poppel H, Joniau S, Van Gool SW. Vaccine therapy in patients with renal cell carcinoma. Eur Urol. 2009;55(6):1333–1344. doi:10.1016/j.eururo.2009.01.04319201522
- Asemissen AM, Brossart P. Vaccination strategies in patients with renal cell carcinoma. Cancer Immunol Immunother. 2009;58(7):1169–1174. doi:10.1007/s00262-009-0706-719360405
- Anguille S, Smits EL, Lion E, Van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15(7):257–267. doi:10.1016/S1470-2045(13)70585-024534291
- Zhang AL, Purath U, Tarner IH, et al. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood. 2007;110(7):2484–2493. doi:10.1182/blood-2007-02-07636417626840
- Wang Z, Rao DD, Senzer N, Nemunaitis J. RNA interference and cancer therapy. Pharm Res. 2011:2983–2995. doi:10.1007/s11095-011-0604-5
- Li M, Qian H, Ichim TE, et al. Induction of RNA interference in dendritic cells. Immunol Res. 2004;30(2):215–230. doi:10.1385/IR:30:2:21515477662
- Liu S, Liu D, Ji M, et al. An indoleamine 2, 3-dioxygenase siRNA nanoparticle-coated and Trp2-displayed recombinant yeast vaccine inhibits melanoma tumor growth in mice. J Control Release. 2018;273:1–12. doi:10.1016/j.jconrel.2018.01.01329355622
- Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol. 2005;15:331–341. doi:10.1016/j.sbi.2005.05.00615925505
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi:10.1038/nrg137915211354
- Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. doi:10.1038/nature0775819158789
- Tatiparti K, Sau S, Kashaw S, Iyer A. siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials. 2017;7(4):77. doi:10.3390/nano7040077
- Massadeh S, Al Aamery M. Nano-materials for gene therapy: an efficient way in overcoming challenges of gene delivery. J Biosens Bioelectron. 2016;07(01):1–12. doi:10.4172/2155-6210.1000195
- Morris KV, Chan SW-L, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Cell. 2004;305(1):1289–1293.
- Li J, Xue S, Mao ZW. Nanoparticle delivery systems for siRNA-based therapeutics. J Mater Chem B. 2016;4(41):6620–6639. doi:10.1039/c6tb01462c
- Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev. 2009;61(10):850–862. doi:10.1016/j.addr.2009.04.01819422869
- Chen M, Du Q, Zhang H-Y, Wahlestedt C, Liang Z. Vector-based siRNA delivery strategies for high-throughput screening of novel target genes. J RNAi Gene Silencing. 2005;1(1):5–11.19771198
- Lee NS, Dohjima T, Bauer G, et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat Biotechnol. 2002;20(5):500–505. doi:10.1038/nbt0502-50011981565
- Moffatt S. siRNA-based nanoparticles for cancer therapy: hurdles and hopes. MOJ Proteomics Bioinforma. 2017;4(6):4–6. doi:10.15406/mojpb.2016.04.00142
- Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9(1):57–67. doi:10.1038/nrd301020043028
- Marques JT, Williams BRG. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399. doi:10.1038/nbt116116273073
- Alagia A, Eritja R. siRNA and RNAi optimization. Wiley Interdiscip Rev RNA. 2016;7(3):316–329. doi:10.1002/wrna.133726840434
- Bramsen JB, Pakula MM, Hansen TB, et al. A screen of chemical modifications identifies position-specific modification by UNA to most potently reduce siRNA off-target effects. Nucleic Acids Res. 2010;38(17):5761–5773. doi:10.1093/nar/gkq34120453030
- Suter SR, Ball-Jones A, Mumbleau MM, et al. Controlling miRNA-like off-target effects of an siRNA with nucleobase modifications. Org Biomol Chem. 2017;15(47):10029–10036. doi:10.1039/c7ob02654d29164215
- Alagia A, Jorge AF, Aviñó A, et al. Exploring PAZ/3′-overhang interaction to improve siRNA specificity. A combined experimental and modeling study. Chem Sci. 2018;9(8):2074–2086. doi:10.1039/c8sc00010g29719684
- Kang HS, Lee D, Li CJ, et al. Asymmetric shorter-duplex siRNA structures trigger efficient gene silencing with reduced nonspecific effects. Mol Ther. 2009;17(4):725–732. doi:10.1038/mt.2008.29819156133
- Sioud M. Advances in RNA sensing by the immune system: separation of siRNA unwanted effects from RNA interference. Methods Mol Biol. 2010;629:33–52. doi:10.1007/978-1-60761-657-3_320387141
- Eberle F, Peter M, Richert C, et al. Modifications in small interfering RNA that separate immunostimulation from RNA interference. J Immunol. 2014;180(5):3229–3237. doi:10.4049/jimmunol.180.5.3229
- Slobodkin G, Wilkinson L, Pence C, et al. Versatile cationic lipids for siRNA delivery. J Control Release. 2011;158(2):269–276. doi:10.1016/j.jconrel.2011.11.00622100441
- Tam YYC, Chen S, Cullis PR. Advances in lipid nanoparticles for siRNA delivery. Pharmaceutics. 2013;5(3):498–507. doi:10.3390/pharmaceutics503049824300520
- Semple SC, Akinc A, Chen J, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172. doi:10.1038/nbt.160220081866
- Love KT, Mahon KP, Christopher G, et al. Correction for Love et al., Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci. 2010;107(21):9915. doi:10.1073/pnas.1005136107
- Abrams MT, Koser ML, Seitzer J, et al. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: effect of dexamethasone co-treatment. Mol Ther. 2010;18(1):171–180. doi:10.1038/mt.2009.20819738601
- Grijalvo S, Puras G, Zárate J, et al. Cationic niosomes as non-viral vehicles for nucleic acids: challenges and opportunities in gene delivery. Pharmaceutics. 2019;11:2. doi:10.3390/pharmaceutics11020050
- Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115(19):10938–10966. doi:10.1021/acs.chemrev.5b0004626010257
- Derycke A. Liposomes for photodynamic therapy. Adv Drug Deliv Rev. 2003;56(1):17–30. doi:10.1016/j.addr.2003.07.014
- Minko T, Pakunlu RI, Wang Y, Khandare JJ, Saad M. New generation of liposomal drugs for cancer. Anticancer Agents Med Chem. 2014;6(6):537–552. doi:10.2174/187152006778699095
- Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–116. doi:10.1016/j.addr.2013.12.00824384374
- Duchemin A, Evrard B, Piel G, Sanna V, Lechanteur A, Mottet D. Cationic liposomes carrying siRNA: impact of lipid composition on physicochemical properties, cytotoxicity and endosomal escape. Nanomaterials. 2018;8(5):270. doi:10.3390/nano8050270
- Santel A, Aleku M, Keil O, et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;13(16):1222–1234. doi:10.1038/sj.gt.330277716625243
- Aleku M, Schulz P, Keil O, et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68(23):9788–9798. doi:10.1158/0008-5472.CAN-08-242819047158
- Wu SY, McMillan NAJ. Lipidic systems for in vivo siRNA delivery. Aaps J. 2009;11(4):639–652. doi:10.1208/s12248-009-9140-119757082
- Landen CN, Merritt WM, Mangala LS, et al. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5(12):1708–1713. doi:10.4161/cbt.5.12.346817106249
- Cheng Y, Ji Y. RGD-modified polymer and liposome nanovehicles: recent research progress for drug delivery in cancer therapeutics. Eur J Pharm Sci. 2019;128:8–17. doi:10.1016/j.ejps.2018.11.02330471410
- Mattern-Schain SI, Fisher RK, West PC, et al. Cell mimetic liposomal nanocarriers for tailored delivery of vascular therapeutics. Chem Phys Lipids. 2019;218:149–157. doi:10.1016/j.chemphyslip.2018.12.00930582896
- Zimmermann TS, Lee ACH, Akinc A, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441(1):111–114. doi:10.1038/nature0468816565705
- Ho W, Zhang XQ, Xu X. Biomaterials in siRNA delivery: a comprehensive review. Adv Healthc Mater. 2016;5(21):2715–2731. doi:10.1002/adhm.20160041827700013
- Sood V, Honko AN, MacLachlan I, et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375(9729):1896–1905. doi:10.1016/s0140-6736(10)60357-120511019
- Nikam RR, Gore KR. Journey of siRNA: clinical developments and targeted delivery. Nucleic Acid Ther. 2018;28(4):209–224. doi:10.1089/nat.2017.071529584585
- Lin PJC, Tam YK. Chapter 9 - controlling protein expression by delivery of RNA therapeutics using lipid nanoparticles In: Filice M, Ruiz-Cabello JBT-NA-N editors. Micro and Nano Technologies. Amsterdam, the Netherlands: Elsevier; 2019:277–310. doi:10.1016/B978-0-12-814470-1.00009-5.
- Zhao J, Weng G, Li J, Zhu J, Zhao J. Polyester-based nanoparticles for nucleic acid delivery. Mater Sci Eng C. 2018;92:983–994. doi:10.1016/j.msec.2018.07.027
- Rozema DB, Lewis DL, Wakefield DH, et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci. 2007;104(32):12982LP–12987. doi:10.1073/pnas.070377810417652171
- Wagner E. polymers for sirna delivery: inspired by viruses to be targeted, dynamic, and precise. Acc Chem Res. 2012;45(7):1005–1013. doi:10.1021/ar200223222191535
- Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release. 2011;152(1):2–12. doi:10.1016/j.jconrel.2011.01.03021295087
- Farra R, Musiani F, Perrone F, et al. Polymer-mediated delivery of siRNAs to hepatocellular carcinoma: variables affecting specificity and effectiveness. Molecules. 2018;23(4). doi:10.3390/molecules23040777
- Parmar RG, Poslusney M, Busuek M, et al. Novel endosomolytic poly(amido amine) polymer conjugates for systemic delivery of siRNA to hepatocytes in rodents and nonhuman primates. Bioconjug Chem. 2014;25(5):896–906. doi:10.1021/bc400527e24742200
- Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–977. doi:10.1038/nmat376524150415
- Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6(3):659–668. doi:10.1021/mp900015y19267452
- O’Mahony AM, Godinho BMDC, Ogier J, et al. Click-modified cyclodextrins as nonviral vectors for neuronal siRNA delivery. ACS Chem Neurosci. 2012;3(10):744–752. doi:10.1021/cn300037223077718
- Díaz-Moscoso A, Le Gourriérec L, Gómez-García M, et al. Polycationic amphiphilic cyclodextrins for gene delivery: synthesis and effect of structural modifications on plasmid DNA complex stability, cytotoxicity, and gene expression. Chem A Eur J. 2009;15(46):12871–12888. doi:10.1002/chem.200901149
- Miele E, Spinelli GP, Miele E, et al. Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. Int J Nanomedicine. 2012;7:3637–3657. doi:10.2147/IJN.S2369622915840
- Sica G, Chen Z, Wang Z, et al. RRM2 regulates Bcl-2 in head and neck and lung cancers: a potential target for cancer therapy. Clin Cancer Res. 2013;19(13):3416–3428. doi:10.1158/1078-0432.ccr-13-007323719266
- Davis ME, Seligson D, Tolcher A, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi:10.1038/nature0895620305636
- Hovgaard MB, Rahbek UL, Glud SZ, et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14(4):476–484. doi:10.1016/j.ymthe.2006.04.01016829204
- Dai K, Winnik F, Qiu X, et al. Low molecular weight chitosan conjugated with folate for siRNA delivery in vitro: optimization studies. Int J Nanomedicine. 2012;7:5833. doi:10.2147/ijn.s3556723209368
- De Smedt SC, Nysten B, Le Duff CS, et al. Chitosan nanoparticles for siRNA delivery: optimizing formulation to increase stability and efficiency. J Control Release. 2014;176:54–63. doi:10.1016/j.jconrel.2013.12.02624389132
- Kim YK, Minai-Tehrani A, Lee JH, Cho CS, Cho MH, Jiang HL. Therapeutic efficiency of folated poly(ethylene glycol)-chitosan-graft-polyethylenimine-Pdcd4 complexes in H-ras12V mice with liver cancer. Int J Nanomedicine. 2013;8:1489–1498. doi:10.2147/IJN.S4294923620665
- Kankala RK, Lin XF, Song HF, et al. Supercritical fluid-assisted decoration of nanoparticles on porous microcontainers for codelivery of therapeutics and inhalation therapy of diabetes. ACS Biomater Sci Eng. 2018;4(12):4225–4235. doi:10.1021/acsbiomaterials.8b00992
- Xu P-Y, Kankala RK, Pan Y-J, Yuan H, Wang S-B, Chen A-Z. Overcoming multidrug resistance through inhalable siRNA nanoparticles-decorated porous microparticles based on supercritical fluid technology. Int J Nanomedicine. 2018;13:4685–4698. doi:10.2147/IJN.S16939930154654
- Wang S-B, Chen A-Z, Tang N, Su X-Q, Kang Y-Q. Preparation and antitumor effect evaluation of composite microparticles co-loaded with siRNA and paclitaxel by a supercritical process. J Mater Chem B. 2015;3(31):6439–6447. doi:10.1039/c5tb00715a
- Kankala RK, Chen B-Q, Liu C-G, Tang H-X, Wang S-B, Chen A-Z. Solution-enhanced dispersion by supercritical fluids: an ecofriendly nanonization approach for processing biomaterials and pharmaceutical compounds. Int J Nanomedicine. 2018;13:4227–4245. doi:10.2147/IJN.S16612430087558
- Licciardi M, Li Volsi A, Sardo C, Mauro N, Cavallaro G, Giammona G. Inulin-ethylenediamine coated SPIONs magnetoplexes: a promising tool for improving siRNA delivery. Pharm Res. 2015;32(11):3674–3687. doi:10.1007/s11095-015-1726-y26085039
- Sardo C, Farra R, Licciardi M, et al. Development of a simple, biocompatible and cost-effective Inulin-Diethylenetriamine based siRNA delivery system. Eur J Pharm Sci. 2015;75:60–71. doi:10.1016/j.ejps.2015.03.02125845631
- Sardo C, Craparo EF, Porsio B, Giammona G, Cavallaro G. Improvements in rational design strategies of inulin derivative polycation for siRNA delivery. Biomacromolecules. 2016;17(7):2352–2366. doi:10.1021/acs.biomac.6b0028127238382
- Aigner A. Delivery systems for the direct application of siRNAs to induce RNA interference (RNAi) in vivo. J Biomed Biotechnol. 2006;2006:1–15. doi:10.1155/JBB/2006/71659
- Akhtar S, Benter IF. Review series nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117(12):3623–3632. doi:10.1172/JCI33494.following18060020
- Judge A, Fritz EA, Phelps JR, et al. Postexposure protection of guinea pigs against a lethal ebola virus challenge is conferred by RNA interference. J Infect Dis. 2006;193(12):1650–1657. doi:10.1086/50426716703508
- Bai A, Chen J, Filip L, Nguyen T, Ge Q, Eisen HN. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci. 2004;101(23):8676–8681. doi:10.1073/pnas.040248610115173599
- Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2004;12:461. doi:10.1038/sj.gt.3302425
- Akhtar S, Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev. 2007;59(2–3):164–182. doi:10.1016/j.addr.2007.03.01017481774
- Pandey AP, Sawant KK. Polyethylenimine: A versatile, multifunctional non-viral vector for nucleic acid delivery. Mater Sci Eng C. 2016;68:904–918. doi:10.1016/j.msec.2016.07.066
- Wang J, Lu Z, Wientjes MG, Au JL-S. Delivery of siRNA therapeutics: barriers and carriers. Aaps J. 2010;12(4):492–503. doi:10.1208/s12248-010-9210-420544328
- Fornaguera C, Grijalvo S, Galán M, et al. Novel non-viral gene delivery systems composed of carbosilane dendron functionalized nanoparticles prepared from nano-emulsions as non-viral carriers for antisense oligonucleotides. Int J Pharm. 2015;478(1):113–123. doi:10.1016/j.ijpharm.2014.11.03125448573
- Khan A, Benboubetra M, Sayyed PZ, et al. Sustained polymeric delivery of gene silencing antisense ODNs, siRNA, DNAzymes and ribozymes: in vitro and in vivo studies. J Drug Target. 2004;12(6):393–404. doi:10.1080/1061186040000385815545089
- Svenson S, Case RI, Cole RO, et al. Tumor selective silencing using an RNAi-conjugated polymeric nanopharmaceutical. Mol Pharm. 2016;13(3):737–747. doi:10.1021/acs.molpharmaceut.5b0060826835715
- Byeon Y, Lee J-W, Choi WS, et al. CD44-targeting PLGA nanoparticles incorporating paclitaxel and FAK siRNA overcome chemoresistance in epithelial ovarian cancer. Cancer Res. 2018;78(21):6247LP–6256. doi:10.1158/0008-5472.CAN-17-387130115698
- Winkler J. Nanomedicines based on recombinant fusion proteins for targeting therapeutic siRNA oligonucleotides. Ther Deliv. 2011;2(7):891–905. doi:10.4155/tde.11.5622318893
- Kaneshiro TL, Lu ZR. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials. 2009;30(29):5660–5666. doi:10.1016/j.biomaterials.2009.06.02619595449
- Bhatia S, Zhu H, Birjiniuk A, et al. Functional delivery of siRNA in mice using dendriworms. ACS Nano. 2009;3(9):2495–2504. doi:10.1021/nn900201e19673534
- Zhou J, Shum KT, Burnett JC, Rossi JJ. Nanoparticle-based delivery of RNAi therapeutics: progress and challenges. Pharmaceuticals. 2013;6(1):85–107. doi:10.3390/ph601008523667320
- Cheung W, Pontoriero F, Taratula O, Chen AM, He H. DNA and carbon nanotubes as medicine. Adv Drug Deliv Rev. 2010;62(6):633–649. doi:10.1016/j.addr.2010.03.00720338203
- Lynn Kirkpatrick D, Weiss M, Naumov A, Bartholomeusz G, Bruce Weisman R, Gliko O. Carbon nanotubes: solution for the therapeutic delivery of siRNA? Materials (Basel). 2012;5(2):278–301. doi:10.3390/ma502027828817045
- Zhang Z, Yang X, Zhang Y, et al. Delivery of telomerase reverse transcriptase small interfering RNA in complex with positively charged single-walled carbon nanotubes suppresses tumor growth. Clin Cancer Res. 2006;12(16):4933–4939. doi:10.1158/1078-0432.CCR-05-283116914582
- Jiang Y, Huo S, Hardie J, Liang X-J, Rotello VM. Progress and perspective of inorganic nanoparticle-based siRNA delivery systems. Expert Opin Drug Deliv. 2016;13(4):547–559. doi:10.1517/17425247.2016.113448626735861
- Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6(8):865–878. doi:10.1517/1742524090293290819637974
- Sangvanich T, Bejan D, Gu S, Yantasee W, Reda M, Ngamcherdtrakul W. Lyophilization and stability of antibody-conjugated mesoporous silica nanoparticle with cationic polymer and PEG for siRNA delivery. Int J Nanomedicine. 2018;13:4015–4027. doi:10.2147/ijn.s16439330022824
- Tiash S, Kamaruzman NIB, Chowdhury EH. Carbonate apatite nanoparticles carry siRNA(s) targeting growth factor receptor genes egfr1 and erbb2 to regress mouse breast tumor. Drug Deliv. 2017;24(1):1721–1730. doi:10.1080/10717544.2017.139638529119846
- Courty J, Latorre A, Villanueva A, et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. 2015;17(1):1–17. doi:10.1186/s13058-015-0576-125567532
- Cai D, Long R, Liu Y, et al. Bacterial magnetosomes-based nanocarriers for co-delivery of cancer therapeutics in vitro. Int J Nanomedicine. 2018;13:8269–8279. doi:10.2147/ijn.s18050330584299
- Wu Z, Shen J, Zhang L-M, et al. Magnetic cationic amylose nanoparticles used to deliver survivin-small interfering RNA for gene therapy of hepatocellular carcinoma in vitro. Nanomaterials. 2017;7(5):110. doi:10.3390/nano7050110
- Roca M, Aj H. Probing cells with noble metal nanoparticle aggregates. Nanomedicine. 2008;3(4):555–565. doi:10.2217/17435889.3.4.55518694317
- Xu ZP, Zeng QH, Lu GQ, Yu AB. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Eng Sci. 2006;61(3):1027–1040. doi:10.1016/j.ces.2005.06.019
- Wong JKL, Habib N, Seifalian AM, Mohseni R, Hamidieh AA, MacLaren RE. Will nanotechnology bring new hope for gene delivery? Trends Biotechnol. 2017;35(5):434–451. doi:10.1016/j.tibtech.2016.12.00928108036
- Mendes R, Fernandes AR, Baptista PV. Gold nanoparticle approach to the selective delivery of gene silencing in cancer-The case for combined delivery? Genes (Basel). 2017;8(3):94. doi:10.3390/genes8030094
- Hou W, Wei P, Kong L, Guo R, Wang S, Shi X. Partially PEGylated dendrimer-entrapped gold nanoparticles: A promising nanoplatform for highly efficient DNA and siRNA delivery. J Mater Chem B. 2016;4(17):2933–2943. doi:10.1039/c6tb00710d
- Tokatlian T, Segura T. siRNA applications in nanomedicine. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology. 2010;2(3):305–315. doi:10.1002/wnan.8120135697
- Benitez-Del-Castillo JM, Moreno-Montañés J, Jiménez-Alfaro I, et al. Safety and efficacy clinical trials for SYL1001, a novel short interfering RNA for the treatment of dry eye disease. Investig Ophthalmol Vis Sci. 2016;57(14):6447–6454. doi:10.1167/iovs.16-2030327893109
- Perales JC, Marquez VE, Eritja R, Ocampo SM, Terrazas M. Effect of north bicyclo[3.1.0]hexane 2′-Deoxy-pseudosugars on RNA Interference: a novel class of siRNA modification. ChemBioChem. 2011;12(7):1056–1065. doi:10.1002/cbic.20100079121452187
- Grijalvo S, Alagia A, Jorge AF, Eritja R. Covalent strategies for targeting messenger and non-coding RNAs: an updated review on siRNA, miRNA and antimiR conjugates. Genes (Basel). 2018;9:2. doi:10.3390/genes9020074
- Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):173–178. doi:10.1038/nature0312115538359
- Manoharan M, Andree C, Amaya P, et al. Identification of siRNA delivery enhancers by a chemical library screen. Nucleic Acids Res. 2015;43(16):7984–8001. doi:10.1093/nar/gkv76226220182
- Nair JK, Willoughby JLS, Chan A, et al. Multivalent N-Acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–16961. doi:10.1021/ja505986a25434769
- Meyers RE, Weiss GJ, Clausen VA, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406–417. doi:10.1158/2159-8290.cd-12-042923358650
- Donahoe JS, Nahrendorf M, Langer R, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7(6):389–393. doi:10.1038/nnano.2012.7322659608
- Salvans C, Brun-Heath I, Ivani I, et al. Rational design of novel N-alkyl-N capped biostable RNA nanostructures for efficient long-term inhibition of gene expression. Nucleic Acids Res. 2016;44(9):4354–4367. doi:10.1093/nar/gkw16926975656
- He C, Weizmann Y, Lin W, Liu D, Gannett TR, Chen G. Enzymatic synthesis of periodic DNA nanoribbons for intracellular pH sensing and gene silencing. J Am Chem Soc. 2015;137(11):3844–3851. doi:10.1021/ja512665z25622178
- Ruan W, Zheng M, An Y, et al. DNA nanoclew templated spherical nucleic acids for siRNA delivery. Chem Commun. 2018;54(29):3609–3612. doi:10.1039/c7cc09257a
- Jorge AF, Eritja R. Overview of DNA self-assembling: progresses in biomedical applications. Pharmaceutics. 2018;10:4. doi:10.3390/pharmaceutics10040268
- Li H, Wang L, Fan C, Gu H, Hu Q. DNA nanotechnology-enabled drug delivery systems. Chem Rev. 2018. doi:10.1021/acs.chemrev.7b00663
- Ni Z, Hui P. Emerging pharmacologic therapies for wet age-related macular degeneration. Ophthalmologica. 2009;223(6):401–410. doi:10.1159/00022892619622904
- Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee S-S. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids. 2017;8:132–143. doi:10.1016/j.omtn.2017.06.00528918016
- Xin Y, Huang M, Guo WW, Huang Q, Zhang L, Jiang G. Nano-based delivery of RNAi in cancer therapy. Mol Cancer. 2017;16(1):1–9. doi:10.1186/s12943-017-0683-y28093071
- Shim MS, Wong S, Kwon YJ. SiRNA as a conventional drug in the clinic? Challenges and current technologies. Drug Discov Today Technol. 2012;9(2):e167–e173. doi:10.1016/j.ddtec.2012.01.003
- Ferrari M. Vectoring siRNA therapeutics into the clinic. Nat Rev Clin Oncol. 2010;7:485. doi:10.1038/nrclinonc.2010.13120798696
- Santel A, Kaufmann J, Strumberg D, et al. First-in-human phase I Study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol. 2014;32(36):4141–4148. doi:10.1200/jco.2013.55.037625403217
- Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406LP–417. doi:10.1158/2159-8290.CD-12-042923358650
- Tolcher AW, Papadopoulos KP, Patnaik A, et al. Safety and activity of DCR-MYC, a first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. J Clin Oncol. 2015;33(15_suppl):11006. doi:10.1200/jco.2015.33.15_suppl.11006
- Wagner MJ, Sood AK, Lopez-Berestein G, et al. Preclinical mammalian safety studies of EPHARNA (DOPC Nanoliposomal EphA2-Targeted siRNA). Mol Cancer Ther. 2017;16(6):1114–1123. doi:10.1158/1535-7163.mct-16-054128265009
- Golan T, Khvalevsky EZ, Hubert A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6(27):24560–24570. doi:10.18632/oncotarget.418326009994
- Mahmoodzadeh H, Moazzen F, Mehmandoost N, et al. Cloning and expression of C2 and V Domains of ALCAM Protein in E. coli BL21 (DE3). Clin Microbiol Open Access. 2017;06(01):1–6. doi:10.4172/2327-5073.1000271
- Dana H, Marmari V, Mazraeh A, Ghamari A, Forghanifard MM. Cloning and expression of the V-domain of the CD166 in prokaryotic host cell. Int J Cancer Ther Oncol. 2017;5(1). http://ijcto.org/index.php/IJCTO/article/view/ijcto51.10.
- Dana H. CD166 as a stem cell marker? A potential target for therapy colorectal cancer? J Stem Cell Res Ther. 2017;1(6):6–9. doi:10.15406/jsrt.2016.01.00041