Abstract
Background:
Titanium (Ti) implant-associated infection, which is mostly caused by bacterial adhesion and biofilm formation, may result in implant failure and secondary surgery. Thus it is an urgent issue to prevent bacterial infections at the earliest step.
Purpose:
To develop a novel surface strategy of polydopamine (PDA) and silver (Ag) nanoparticle-loaded TiO2 nanorods (NRDs) coatings on Ti alloy.
Materials and methods:
Ag-TiO2@PDA NRDs was fabricated on Ti alloy by hydrothermal synthesis. The antibacterial activity of Ag-TiO2@PDA NRDs against Escherichia coli and methicillin-resistant Staphylococcus aureus were tested by FE-SEM, Live/Dead staining, zone of inhibition, bacteria counting method and protein leakage analysis in vitro. In addition, an implant infection model was conducted and the samples were tested by X-ray, Micro-CT and histological analysis in vivo. Besides, cell morphology and cytotoxicity of Mouse calvarial cells (MC3T3-E1) were characterized by FE-SEM, immunofluorescence and CCK-8 test in vitro.
Results:
Our study successfully developed a new surface coating of Ag-TiO2@PDA NRDs. The selective physical puncture of bacteria and controlled release of Ag+ ions of Ag-TiO2@PDA NRDs achieved a long-lasting bactericidal ability and anti-biofilm activity with satisfied biocompatibility.
Conclusion:
This strategy may be promising for clinical applications to reduce the occurrence of infection in the implant surgeries
Introduction
With the development of modern medical implants, titanium (Ti) alloy has been commonly applied in the field of orthopedics and dentistry, because of their excellent mechanical properties, biocompatibility, and chemical stability.Citation1 However, two major concerns of bacterial infections and poor osseointegration have been remained to be effectively resolved.Citation2,Citation3 Implant infection, which is mostly caused by the bacterial adhesion and biofilm formation, may result in implant failure and secondary surgery.Citation4 Since the biofilms are the physical barriers, which is composed by a three-dimension extracellular polymeric matrix, to protect the inside bacteria from attack by host defense and antibiotics.Citation5 As a result, 1,000-fold higher antibiotic dosage and extended antibiotic therapy may be required to kill the bacteria in biofilm than the common planktonic bacteria, especially for the antibiotics-resistant bacteria strain like methicillin-resistant Staphylococcus aureus (MRSA), etc.Citation6,Citation7 Therefore, it is an urgent issue to inhibit the formation of biofilm at the earliest step by using physical and/or chemical methods.Citation8 By far, there are three main categories of antimicrobial surfaces: (a) bactericidal element content releasing from coatings, such as the bactericide ion release; (b) contact-killing coatings, such as the surface nanostructure of the cicada wing; and (c) anti-adhesion coatings, such as PEG, zwitterion and physical surface modifications.Citation9 While inhibiting bacterial adhesion, Ti implants may not promote the spreading and attachment of osteoblasts, resulting in poor osseointegration and implant loosening.Citation10
Silver (Ag) with the properties of vast spectrum bactericidal activity and anti-biofilm effect has been widely applied from ancient times.Citation6 However, the occurrence of burst release remains one of the biggest concerns of the biomaterial, which invokes a considerable level of cytotoxicity.Citation11 Besides, in order not to compromise the material biocompatibility, the appropriate concentration range for Ag+ ions was reported between 100 ppb and 10 ppm in a biological solution, which was tough for the control of ion releasing.Citation12 These challenges have prompted researchers to develop new strategies for the adequate antibacterial ability and proper biocompatibility in implant applications.
Polydopamine (PDA) has been commonly used to modify the surface characteristics of biomaterial scaffolds as an important platform and stabilization agent, with excellent biocompatibility, biodegradability, and hydrophilicity.Citation13,Citation14 Meanwhile, as the desirable adhesion agent for biological reagents,Citation15 the o-diphenol and amine groups of PDA may modify the Ag+ ions burst release pattern to long-lasting release pattern by the chelation bonding of Ag nanoparticles.Citation16,Citation17 Moreover, PDA has been reported to promote the spreading and attachment of pre-osteoblast by engaging and activating cell membrane receptors.Citation18,Citation19
It has been reported that the specific surface topographies with patterns smaller than bacterial cells possessed a broad-spectrum bactericidal ability.Citation20 For example, the particular surface nanostructure of cicada wings was reported to have the outstanding bactericidal effect by mechanically rupturing of attached cells within 3 mins.Citation21,Citation22 Therefore, based on hydrothermal synthesis, TiO2 nanorods (NRDs) coatings fabricated on Ti implants may provide a new strategy to combat implant infection.
Our current research aims to produce a new surface strategy, namely the Ag-TiO2@PDA NRDs, with the properties of selective physical puncture of bacteria and long-lasting Ag+ ions release for the balance of the bactericidal activity and cytotoxicity. Through a series of in vitro and in vivo tests, it resulted that the Ag-TiO2@PDA NRDs coatings possessed the long-term anti-bacterial and anti-biofilm capacities.
Materials and methods
Specimen preparation
As illustrated in , Ti foils (10 mm×10 mm×1 mm) and Ti rods (diameter: 1 mm; length: 10 mm) were purchased and polished by SiC sandpapers, followed by degreasing sequentially with acetone, ethanol, and distilled water, and then dried by nitrogen gas. Later, the samples were loaded in a Teflon-lined autoclave with adequate 1 M NaOH and heated for 10 h in a sealed autoclave (200°C) to fabricate sodium titanate NRDs on the Ti foil, followed by ultrasonic washing. Then, the sodium titanate NRDs were subjected to a hydrogen ion exchange process in diluted hydrochloric acid (HCl) for 1 hr to obtain the titanic acid NRDs. After washed by deionized water and dried at 60°C, the products were annealed at 400°C for 2 hrs in the air to fabricate TiO2 NRDs specimens.
To prepare the PDA-coated TiO2 NRDs (denoted as TiO2@PDA NRDs), the as-prepared TiO2- NRDs specimens were immersed into a Tris-buffer solution (10 mM, 100 mL), and then 100 mg of dopamine hydrochloride was dispersed into the solution with magnetic stirring for 6 hrs. After washed with deionized water three times, the resultant TiO2@PDA NRDs were dried at 60°C. Then, the TiO2@PDA NRDs specimens were soaked in the AgNO3 solution (100 mM) for 15 mins and irradiated with ultraviolet light by a high-pressure Hg lamp for 30 mins to obtain Ag nanoparticles–loaded TiO2@PDA NRDs (denoted as Ag-TiO2@PDA NRDs). For comparison, the Ag nanoparticles–loaded TiO2 NRDs (denoted as Ag-TiO2 NRDs) were prepared in the same procedure except for PDA coatings.
All the samples were cleaned by sequential ultrasonic, dried by air, and sterilized by ultraviolet light in sequence before antibacterial tests.
Sample surface characterization
The surface morphology of samples was measured by a field emission scanning electron microscopy (FE-SEM; FEI Nova 400 Nano, USA) and the microstructure of Ag-TiO2@PDA NRDs was measured by a transmission electron microscopy (TEM; Tecnai G20, FEI, USA). The surface chemical composition and elemental analysis were evaluated by energy dispersive X-ray (EDX) spectrum and X-ray photoelectron spectroscopy (XPS; ESCALAB MK-II, VG, USA). The hydrophilicity of the samples was measured by the water contact angle (DSA1 System, Kruss, Germany).
Ag+ ions release into PBS
The Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs samples were immersed in 6 mL of PBS for 1, 3, 6, 10, and 14 days, and PBS was refreshed every 24 hrs. Then, the liquid was collected and evaluated by inductively coupled plasma atomic emission spectrometry (ICP-AES; IRIS Advantage ER/S) with a specific emission line at 328.1 nm for Ag.Citation6
Antibacterial activity in vitro
The antibacterial activities against gram-positive MRSA and gram-negative Escherichia coli (E. coli) were measured by FE-SEM, Live/Dead staining, zone of inhibition (ZOI), bacteria counting method, and protein leakage analysis in vitro.
Firstly, the bactericidal and antibiofilm formation ability was tested by FE-SEM and fluorescence observation. We placed the samples on a 24-well plate and immersed them in 1 mL bacteria suspension (1.5×104 colony-forming units [CFUS]/mL) in the standard Luria-Bertani culture medium each well for 24 hrs culturing at 37°C. Then, the samples were washed by PBS three times to remove non-adhesion bacteria, fixed with 2.5% glutaraldehyde solution at 4°C for 4 hrs, and dehydrated in ethanol series (30%, 45%, 75%, 95%, and 100%) for 15 mins sequentially. After coated with Au in a sputter coater, the dried samples were captured by FE-SEM eventually. Similarly, the specimens were stained with the Live/Dead BacLight Bacterial Viability Kit (cat# L7007; Invitrogen) for 15 mins after co-cultured with MRSA for 24 and 48 hrs and then imaged by the fluorescence microscope. Thus, intact cell membranes would be stained fluorescent green by SYTO 9 and damaged cell membranes would be stained fluorescent red by propidium iodide.
Secondly, the ZOI was conducted to test the anti-planktonic bacteria ability. We placed respective samples onto M-H plates, which was inoculated by 20 μL bacterial suspension (1.5×108 CFUS/mL) at 37°C for 24 hrs of incubation to observe zone inhibition diameter.
Thirdly, the bacteria counting method was conducted to evaluate the anti-adhesion bacterial rate. After co-cultured with 1 mL bacterial suspension (1.5×104 CFUS/mL−1) at 37°C for 24 hrs, the samples were collected in 1 mL of sterile PBS. The samples were dislodged by sonication at 50 Hz for 2 mins to remove the adhered bacteria. Then, the dissociated bacterial suspension was gradient diluted with sterile PBS and plated on M-H agar at 37°C. After 24 hrs of incubation, the active bacteria were counted carefully and the anti-adherent bacteria effect was represented by the antibacterial ratio based on the following formulas: antibacterial ratio (%)=(B–A)/B×100%, where A is the mean number of active bacteria inoculated with the experimental groups (CFU/sample), and B is the mean number of active bacteria inoculated with the pure Ti group (CFU/sample). The bacterial counting method was conducted among Ti, TiO2 NRDs and TiO2@PDA NRDs groups for 1 day and Ti, Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs groups for 1, 7 and 14 days to analyze the long-term anti-adherent bacterial ability.
Lastly, the protein concentration of bacterial suspension was measured by an Enhanced BCA Protein Assay Kit to test the potential protein leakage of bacterial cytoplasm.
Cell morphology and cytotoxicity evaluation in vitro
An mouse calvarial cells line (MC3T3-E1) was obtained from the Chinese Academy of Sciences Shanghai cell bank. MC3T3-E1 cells (5×104 cells/cm2) were cultured in a 24-well plate each well. The cell morphology was imaged by the fluorescence microscopy and FE-SEM. After incubation for 1 day, the samples were washed with sterile PBS three times and fixed in 2.5% glutaraldehyde solution at 4°C for 4 hrs. Then, the samples were stained by phalloidin (Sigma, USA) for 50 mins in the dark environment and DAPI for 10 mins and then imaged by the fluorescence microscope. The same FE-SEM procedures described above were adopted to observe the morphology of bacteria.
A CCK-8 kit (Boster Biological Technology Co., CA, USA) was used to test the cytotoxicity level for cell cultures on various samples. MC3T3-E1 cells (3×104 cells/cm2) were seeded on the samples and cultured for 24 hrs in a 24-well plate each well at 5% CO2 and 37°C. Then, the CCK-8 solution was added to each well for 1–4 hrs of incubation and the supernatant was collected in a 96-well plate. The absorbance was measured by a spectrophotometer (Bio Tek Instruments Inc., VT, USA) at 450 nm.
Biological evaluation in vivo
Surgical implantation of animal study
A total of 20 male Sprague Dawley rats (5–6 weeks old, average weight: 150 g) were used in our study, obtained from the Laboratory Animal Center of Tongji Medical College, Huazhong University of Science and Technology. The Ethics Committee of animal experimentation of Huazhong University of Science and Technology approved and reviewed the protocol on rats. Besides, the experimental rats were treated in adherence to the Guide for the Care and Use of Laboratory Animals (8th edition). The surgical procedure was based on our previous studies.Citation6,Citation23 Briefly, the rats were randomly divided into five subgroups: group Ⅰ (Ti), group Ⅱ (TiO2 NRDs), group Ⅲ (TiO2@PDA NRDs), group Ⅳ (Ag-TiO2 NRDs), and group Ⅴ (Ag-TiO2@PDA NRDs). Before surgery, the rats were anesthetized with pentobarbital (50 mg per 1,000 g body weight) by intraperitoneal injection. An incision was made along the right knee to expose the proximal metaphysis of the anterior tibia, after shaving and disinfecting. Then, a sterilized 20 g syringe needle was used to drill a hole and create a channel through the cortex from the tibia plateau to the medullary cavity. Subsequently, 30 μL bacteria suspension (MASA, 1.5×106 CFUs/mL) was injected into the exposed tibia hole using a micro-syringe. Few minutes later, the rod samples of relative groups were implanted into the cavities and the drilled hole was sealed by the sterilized bone wax to prevent leakage of the bacteria suspension. The skin and muscle fascias were sutured firmly at last. In the early stage of recovery, the health conditions of rats including body weight, temperature, and activity were monitored every 24 hrs.Citation24
X-ray and micro-CT evaluation
For the sake of evaluating the long-term infection symptoms of the rats, the X-ray examination (Kodak, DirectView, DR3000) with the human hand imaging parameter was scanned at 2 and 4 weeks post-surgery after anesthetized. High-resolution micro-computed tomography (I-CT 80 scanner Scanco Medical, Bassersdorf, Switzerland) was used to scan and evaluate the microstructure of the right tibiae at 300 ms, 70 kV, and 114 mA at 2 and 4 weeks after surgery.
Histopathological analysis
All rats were euthanized after 4 weeks. The right tibias with implants were removed and fixed in 4% buffered formalin for 24 hrs. Then, specimens were decalcified in 10% EDTA for one month, embedded in paraffin and further cut into 10-mm sections. The H&E staining and Gram’s staining methods were chosen for the histopathological analysis and the images were captured on a light microscopy (Nikon E600, Japan).
Statistical analysis
The experiments were repeated in triplicate using independent samples, and the results were represented as mean ± standard deviation. A one-way ANOVA combined with a Turkey’s post hoc test was used to assess the considered statistical significance; a value of p<0.05 was considered significant.
Results
Morphology, structure, and surface characterization
displays the FE-SEM images of Ti, TiO2 NRDs, TiO2@PDA NRDs, Ag-TiO2 NRDs, and Ag-TiO2@PDA NRDs, respectively. As shown in , the quasi-aligned TiO2 NRDs with diameters of about 50–100 nm and lengths of 1–2 μm are fabricated by a simple hydrothermal reaction with Ti foils. Followed by the self-polymerization of PDA, the NRDs surface represented an increased thickness of approximately 5 nm which was shown in the TEM image, while the needle-shaped morphology did not appear deformed (). An average size of embedded Ag nanoparticles nearly 30 nm was confirmed by using TEM (). The corresponding EDX spectrum () and the XPS spectra () showed that C, O, Ti, N, and Ag existed in the sample of Ag-TiO2@PDA NRDs, indirectly demonstrating the formation of TiO2 NRDs incorporated with PDA and Ag nanoparticles. The XPS spectra of Ag3d peaks were detected at 368.3 and 374.3 eV, respectively, suggesting the presence of Ag on the layer of NRDs. Besides, the release kinetics of Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs are shown in that the concentrations of released Ag+ ions were 0.50, 0.19, 0.17, 0.12, and 0.07 ppm for Ag-TiO2 NRDs and 0.31, 0.24, 0.23, 0.22, and 0.19 ppm for Ag-TiO2@PDA NRDs at 1, 3, 6, 10, and 14 days, respectively. Because of refreshing the soaking liquid every 24 hrs, we observed a burst-release pattern of Ag+ ions in Ag-TiO2 NRDs group by ICP-AES in the first 3 days. On the contrary, the release pattern of Ag+ ions in Ag-TiO2@PDA NRDs group was much slower and more gentle. The contact angles of Ti, TiO2 NRDs, TiO2@PDA NRDs, Ag-TiO2 NRDs, and Ag-TiO2@PDA NRDs were 62.9°, 144.4°, 22.7°, 68.1°, and 36.4°, respectively (). The nitrogen-to-carbon (N/C) ratio of 12.56% (8.1% for nitrogen; 64.48% for carbon) of TiO2@PDA NRDs determined by XPS was same to the theoretical N/C for dopamine,Citation13,Citation25 further indicating that TiO2 NRDs were modified with PDA. As the high-resolution spectra shown in Fig. 2F1 and F2, the C 1s peaks of TiO2@PDA NRDs and Ag-TiO2@PDA NRDs can be deconvoluted into subpeaks at 284.6, 285.1, and 286.3 eV corresponding to C-C, C-N, and C-O, respectively.Citation26
Figure 2 Sample surface characterization and Ag+ ions release. (A) FE-SEM images of various surfaces (scale bars =3 μm); (B) corresponding EDS spectrum of various samples; (C) TEM images of Ag-TiO2@PDA NRDs (scale bars =100 [left panel] and 20 [right panel] nm); (D) Ag-release profiles from Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs into PBS; (E) water contact angles and photographs of water droplets on various surfaces; (F) XPS spectra of various samples; (F1) C 1s peak of Ag-TiO2 NRDs; (F2) C 1s peak of Ag-TiO2@PDA NRDs.
![Figure 2 Sample surface characterization and Ag+ ions release. (A) FE-SEM images of various surfaces (scale bars =3 μm); (B) corresponding EDS spectrum of various samples; (C) TEM images of Ag-TiO2@PDA NRDs (scale bars =100 [left panel] and 20 [right panel] nm); (D) Ag-release profiles from Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs into PBS; (E) water contact angles and photographs of water droplets on various surfaces; (F) XPS spectra of various samples; (F1) C 1s peak of Ag-TiO2 NRDs; (F2) C 1s peak of Ag-TiO2@PDA NRDs.](/cms/asset/e3c30da0-fbc2-4f19-b2a4-52021431ced4/dijn_a_12190890_f0002_c.jpg)
Antibacterial activity in vitro
The antibacterial activities against MRSA and E. coli were measured by FE-SEM, Live/Dead staining, ZOI, bacteria counting method, and protein leakage analysis in vitro.
After incubation for 24 hrs, the morphology of the adherent MRSA and E. coli was measured by FE-SEM in , respectively. MRSA presented spherical on pure Ti, but irregular and lysed on Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs surfaces. Similarly, E. coli became corrugated, distorted, and lysed on Ag-TiO2 NRDs and Ag-TiO2@PDA NRD surfaces.
Figure 3 Antibacterial activity in vitro. (A) FE-SEM morphology of methicillin-resistant Staphylococcus aureus (MRSA) seeded on the various surfaces (scale bars =1 μm); (B) FE-SEM morphology of Escherichia coli (E. coli) seeded on the various surfaces (scale bars =2 μm); (C) fluorescent images of stained MRSA and bacterial suspension (insert in part C) after co-cultured with various surfaces for 24 hrs (scale bars =50 μm); (D) fluorescent images of stained MRSA and bacterial suspension (insert in part D) after co-cultured with various surfaces for 48 hrs (scale bars =50 μm); (E) zone of inhibition (ZOI) test of the various coatings against MRSA; (F) ZOI test of the various coatings against E. coli.
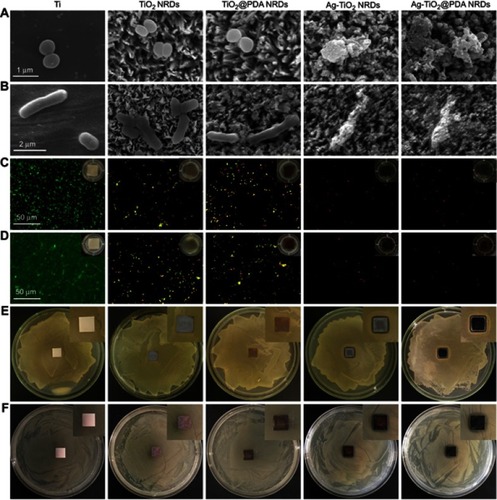
The live/dead staining assay is shown in . The biofilm was formed on the complete coverage of pure Ti group at 48 hrs. However, the amount of bacteria coverage on NRDs samples was considerably lower than that on pure Ti group. Moreover, many yellow and red spots can be observed from TiO2 NRDs and TiO2@PDA NRDs group, indicating significantly physical punctured of bacteria membrane. Almost all red spots can be observed from Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs, indicating more dead bacteria and admirable antibacterial ability.
As shown in , the ZOI diameters of Ag-TiO2 NRDs for MRSA and E. coli were 13.2±0.2 mm and 15.9±1.6 mm, respectively. The ZOI diameters of Ag-TiO2@PDA NRDs for MRSA and E. coli were 13.5±0.5 mm and 16.1±1.3 mm, respectively.
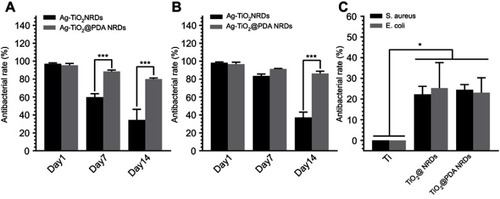
The anti-adherent bacterial activity against MRSA and E. coli was evaluated by the bacteria counting method. Compared to the pure Ti group, Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs showed adequate bactericidal effect. Ag-TiO2 NRDs showed the antibacterial rates of 97.1±1.0%, 59.8±3.9%, and 34.3±12.0% against MRSA () and 98.2±1.0%, 83.5±2.1%, and 37.2±5.9% against E. coli at 1, 7, and 14 days (), respectively. However, Ag-TiO2@PDA NRDs showed longer-term antibacterial effects, with antibacterial rates of 95.4±2.0%, 88.6±1.5%, and 80.1±1.1% against MRSA () and 96.7±2.1%, 91.3±0.5%, and 86.2±2.6% against E. coli at 1, 7, and 14 days (), respectively. Interestingly, TiO2 NRDs (22.3±3.9% for MRSA and 25.4±12.3% for E. coli) and TiO2@PDA NRDs (24.5±2.4% for MRSA and 23.1±7.2% for E. coli) group also have the anti-adherent bacterial ability compared with the pure Ti group ().
Figure 4 Anti-adherent bacterial activity in vitro. (A) Methicillin-resistant Staphylococcus aureus (MRSA) recultured on agar after dissociation from Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs at day 1, day 7, and day 14; (B) Escherichia coli (E. coli) recultured on agar after dissociation from Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs at day 1, day 7, and day 14; (C) MRSA and E. coli recultured on agar after dissociation from Ti, TiO2 NRDs, and TiO2@PDA NRDs at day 1. The error bars indicate means ± standard deviations: *p<0.05 and ***p<0.001.
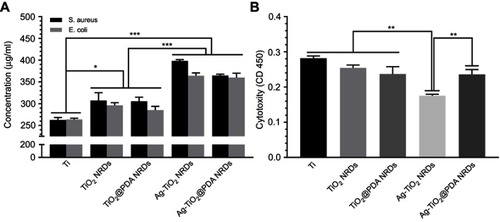
As shown in , the protein leakage concentration of Ti, TiO2 NRDs, TiO2@PDA NRDs, Ag-TiO2 NRDs, and Ag-TiO2@PDA NRDs was 262.5±5.5, 307.5±17.8, 305.7±9.5, 398.2±2.8, and 364.9±2.8 μg/mL for MRSA and 263.8±2.8, 296.4±5.9, 285.3±8.7, 364.3±6.4, and 359.9±10.2 μg/mL for E. coli, respectively.
Cell adhesion, morphology, and cytotoxicity in vitro
As shown in , the pure Ti had a low affinity to osteoblasts with the spherical morphology. In contrast, the polygonal shape and distinct pseudopodia of MC3T3-E1 were observed on TiO2 NRDs and TiO2@PDA NRDs reflecting the adequate cellular adhesion and extension. However, the different degrees of cell shrinkage were observed on the Ag-TiO2 NRDs surfaces, probably because of the burst release of Ag+ ions. With the PDA coatings, the extensive morphology of cells was detected on Ag-TiO2@PDA NRDs samples, confirming that the cytotoxicity of Ag+ ions can be sharply reduced through the combination by PDA.
Figure 6 Cell morphology in vitro. (A, B) FE-SEM cell morphology of mouse calvarial cells (MC3T3-E1) cells cultured on various surfaces for 24 hrs (scale bars =200 [A] and 10 [B] μm); (C) fluorescent images of MC3T3-E1 cells cultured on various surfaces for 24 hrs with phalloidion (green) and DAPI (blue) (scale bars =100 μm).
![Figure 6 Cell morphology in vitro. (A, B) FE-SEM cell morphology of mouse calvarial cells (MC3T3-E1) cells cultured on various surfaces for 24 hrs (scale bars =200 [A] and 10 [B] μm); (C) fluorescent images of MC3T3-E1 cells cultured on various surfaces for 24 hrs with phalloidion (green) and DAPI (blue) (scale bars =100 μm).](/cms/asset/05ce9d27-93eb-4eb8-b789-6674e377332e/dijn_a_12190890_f0006_c.jpg)
Similar to FE-SEM morphology, the cell morphology, adhesion, and spreading activity were measured by immunofluorescence to visualize the cytoskeleton and nuclei, respectively (). Compared to the pure Ti and Ag-TiO2 NRDs group, TiO2 NRDs, TiO2@PDA NRDs, and Ag-TiO2@PDA NRDs promoted cell extension and cell spreading. The shrinking cells on the Ag-TiO2 NRD surfaces implied the inevitable cytotoxicity of Ag+ ions.
Furthermore, we used a CCK-8 assay kit to investigate the cytotoxicity level for cell cultures on various samples (). Compared with Ti, TiO2 NRDs, TiO2@PDA NRDs, and Ag-TiO2@PDA NRDs, the Ag-TiO2 NRDs samples exhibited the highest cytotoxicity (** p<0.01). Besides, the absorbance of Ag-TiO2@PDA NRDs group showed no significant difference with the absorbance of TiO2 NRDs and TiO2@PDA NRDs groups (p>0.05).
In vivo antibacterial assay
As shown in , X-ray images were taken at 2 and 4 weeks after surgery. Two weeks after surgery, a classic symptom of implant infection like osteolysis (red arrows), architectural deformation, periosteal reactions (white arrows), and soft tissue deformation was visible in group Ⅰ, group Ⅱ, and group Ⅲ. Four weeks after surgery, extensive osteolysis (red arrows) and fibrosis of bone marrow cavity was observed in group Ⅰ, group Ⅱ, and group Ⅲ. In contrast, no signs of infection were observed in groups Ⅳ and Ⅴ.
Figure 7 Implant infection model in vivo. (A) X-ray examination of the osteomyelitis model implanted with Ti rod 1 hr after surgery (left panel) and different kinds of rods at 2 weeks and 4 weeks (right panel), red arrows indicate osteolysis, white arrows indicate periosteal reaction; (B) cross-sectional images of micro-CT of different samples at 2 weeks and 4 weeks, white arrows indicate sequestrum; (C) H&E stain of different samples at 4 weeks (scale bars =50 μm); (D) gram stain of different samples at 4 weeks (scale bars =100 μm), blue arrows indicate MRSA colonies.
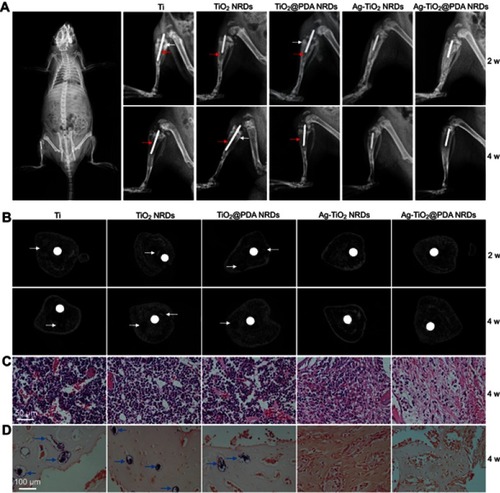
Micro CT scanning was used to analyze the samples (). Two weeks after surgery, the obvious sequestrum (white arrows) was detected in group Ⅰ, group Ⅱ, and group Ⅲ. Four weeks after surgery, the involucrum was formed and the sequestrum (white arrows) was partially resolved in group Ⅰ, group Ⅱ, and group Ⅲ. Similar to the X-ray results, no sign of infection was detected in groups Ⅳ and Ⅴ.
In , histological analysis showed that abundant and excessive neutrophil infiltration accompanied with abscess and necrotic cells was observed at 4 weeks in groups Ⅰ, Ⅱ, and Ⅲ. Gram staining in showed that the stained bacteria (blue arrows) were gathering around sequestrum or micro-abscesses. However, negligible inflammatory infiltration and absence of bacteria were observed in group Ⅳ or Ⅴ at 4 weeks.
Discussion
The bacterial infection, mainly caused by gram-positive S. aureus and gram-negative E. coli, usually becomes a devastating blow for the orthopedic and dental surgery, leading to inevitable implant failure and secondary surgery.Citation4,Citation6,Citation7 In our study, we successfully utilized the method of hydrothermal synthesis to produce Ag-TiO2@PDA NRDs coatings on Ti substrates, which possessed an adequate long-term antibacterial ability and anti-biofilm activities with satisfied biocompatibility in vitro and in vivo. Therefore, this novel surface strategy may be expected for the biomedical applications in the future clinical implants.
The potential synergistic anti-bacterial mechanisms of Ag-TiO2@PDA NRDs might have three aspects. Firstly, the needle-like NRDs can selective physical puncture the bacteria cell wall because of fewer anchoring sites, less contact area, and higher contact pressure between the NRDs and the bacteria, leading to the severely damaged of membrane (FE-SEM and Live/Dead Bacterial Viability fluorescence) and the significant cytoplasm leakage (the quantitative protein analysis test) of bacteria cells.Citation21,Citation22,Citation26 In our Live/Dead Bacterial Viability fluorescence test, the intact and damaged bacterial membranes could be stained by SYTO 9 (green), while propidium iodide (red) only penetrated and stained cells with damaged membranes, causing a reduction of SYTO 9 stain. Thus, NRDs have been proved to have the ability of selectively physically puncturing the bacterial membrane according to more yellow and red spots observed from TiO2 NRDs and TiO2@PDA NRDs group. In addition, the quantitative protein analysis test showed that the protein leakage concentration of TiO2 NRDs, TiO2@PDA NRDs, Ag-TiO2 NRDs, and Ag-TiO2@PDA NRDs groups was significantly higher than that of Ti group, which added another evidence of physical bacteria membrane puncture. However, MC3T3-E1 exhibited good adherence and spreading behavior with the polygonal shape and distinct pseudopodia on NRDs surfaces. This is probably due to an approximate order of magnitude difference in the size of morphology.26
Secondly, Ag+ ions released from the Ag nanoparticles have the excellent bactericidal effect (ZOI and the bacteria counting method). The results of ZOI showed that Ag-TiO2 NRDs and Ag-TiO2@PDA NRDs both exhibited high-efficient antibacterial activity (13.2±0.2 mm for MRSA and 15.9±1.6 mm for E. coli in Ag-TiO2 NRDs; 13.5±0.5 mm for MRSA and 16.1±1.3 mm for E. coli in Ag-TiO2@PDA NRDs). The contributing bactericidal mechanisms may include the inhibition and disruption of bacterial cell wall synthesis, reactive oxygen species forming, protein denaturation, and DNA damaging.Citation27,Citation28 Aside from its bactericidal actions, Ag can also completely restrict the growth of resistant strains.Citation29,Citation30
Thirdly, the topography of submicron and microsized surface can prevent the biofilm formation (FE-SEM, Live/Dead Bacterial Viability fluorescence, and the bacteria counting method).Citation31–Citation33 In comparison to the patterned surface, it was reported that bacteria were more likely to attach on smoother ones.Citation31,Citation34–Citation36 Thus, the results of Live/Dead Bacterial Viability fluorescence showed that the amount of bacteria coverage on NRDs samples was significantly lower than that on the pure Ti group on which the typical biofilm was formed after 48 hrs.
In our study, the self-polymerization of PDA was coated on the NRDs to modulate the Ag+ ions release kinetics, improve the hydrophilicity of the surface, and promote the biocompatibility of Ag-TiO2@PDA NRDs. A large concentration of Ag+ ions can generate a considerable level of cytotoxicity and affect tissue formation. Unlike the burst-release pattern of Ag+ ions observed by ICP-AES in Ag-TiO2 NRDs group in the first 3 days, the PDA of Ag-TiO2@PDA NRDs coatings can bind to Ag+ ions more stably via the effect of chelation and modify the release kinetics of Ag+ ions to reduce the cytotoxicity and improve the biocompatibility. In addition, the antibacterial property of Ag-TiO2@PDA NRDs was significantly more durable and efficient than that of Ag-TiO2 NRDs, according to the results of the bacteria counting method (88.6±1.5% vs 59.8±3.9% on day 7 and 80.1±1.1% vs 34.3±12.0% on day 14 against MRSA; 86.2±2.6% vs 37.2±5.9% on day 14 against E. coli). It is implied that the controlled Ag+ ions release by the chelation effect of PDA almost completely preserved the long-term bactericidal ability. Meanwhile, owing to the particular surface nanostructure and lower surface free energy, the NRDs surface with the water contact angle of 144.4° exhibited the considerable hydrophobic property.Citation37 After coating with PDA, Ag-TiO2@PDA NRDs showed the hydrophilicity of 36.4°, which improved the wettability of NRDs and was more conducive to cell adhesion, proliferation, osteoblast differentiation, and osteogenesis.Citation38,Citation39 In addition, the micro- or nano-textured topography and roughness of implant surface have been also reported to affect the cell morphology, proliferation, and differentiation of stem cell.Citation40,Citation41 Well-spread pre-osteoblasts with a mature cell shape and larger surface area are more accessible to ossification differentiation.Citation42,Citation43 Thus, the special ability of the NRDs coatings to promote cell adherence and spreading may eventually enhance the bone regeneration. In general, considering the balance of cytotoxicity and long-term antibacterial effects, we demonstrate that the abilities of PDA coatings will highlight the promising potential for the application in Ag-based tissue engineering.
Modern medical imaging technologies, such as X-ray and CT, have been widely used in the diagnosis of bone infection.Citation44 In order to further test the bactericidal ability of Ag-TiO2@PDA NRDs in vivo, we successfully conducted a rat model of osteomyelitis following a previously established protocol, which subsequently observed the radiographic signs of osteomyelitis by X-ray and micro-CT at 2 and 4 weeks and analyzed the histopathology by H&E and Gram’s staining at 4 weeks. The consistent results of imaging and histopathology demonstrated that the injected MRSA was killed by the Ag+ ions–loaded coatings. Consequently, the Ag-TiO2@PDA NRDs coatings possessed a convincing antibacterial ability, which was highly recommended for the developing clinical applications.
One limitation needs to be mentioned. The different sizes, shapes, and concentrations of Ag nanoparticles may lead to the change of dissolution kinetics.Citation45 For example, a decrease in the size of Ag nanoparticles may increase the speed of Ag+ ions dissolution.Citation46 Thus, finding the proper size, shape, and concentration of Ag nanoparticles requires further research.
Conclusion
We successfully fabricated the Ag-TiO2@PDA NRDs coatings on Ti substrates by the method of hydrothermal synthesis. The Ag-TiO2@PDA NRDs coatings with the synergistic effect of selective physical punctual and controlled Ag+ ions release achieved an adequate long-term antibacterial and anti-biofilm activities against MRSA and E. coli with satisfied biocompatibility in vitro and in vivo. Meanwhile, with relatively simple, economical, and suitable fabrication method, the Ag-TiO2@PDA NRDs coatings may be hopeful for the applications of orthopedic and dental implants.
Disclosure
There are no potential conflicts of interest for the authors to disclose in this work.
Acknowledgments
This work is supported by National Natural Science Foundation of China (No. 81571816 and 81601611). Ming Guan is also supported by a fellowship from the China Scholarship Council.
References
- Sidambe AT. Biocompatibility of advanced manufactured titanium implants: a review. Materials (Basel, Switzerland). 2014;7(12):8168–8188. doi:10.3390/ma7128168
- Raphel J, Holodniy M, Goodman SB, Heilshorn SC. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials. 2016;84:301–314. doi:10.1016/j.biomaterials.2016.01.01626851394
- Tobin EJ. Recent coating developments for combination devices in orthopedic and dental applications: a literature review. Adv Drug Delivery Rev. 2017;112:88–100. doi:10.1016/j.addr.2017.01.007
- Vielgut I, Sadoghi P, Wolf M, et al. Two-stage revision of prosthetic hip joint infections using antibiotic-loaded cement spacers: when is the best time to perform the second stage? Int Orthop. 2015;39(9):1731–1736. doi:10.1007/s00264-015-2751-525870167
- Cheng H, Xiong W, Fang Z, et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016;31:388–400. doi:10.1016/j.actbio.2015.11.04626612413
- Xu N, Cheng H, Xu J, et al. Silver-loaded nanotubular structures enhanced bactericidal efficiency of antibiotics with synergistic effect in vitro and in vivo. Int J Nanomed. 2017;12:731–743. doi:10.2147/IJN.S123648
- Darley ES, MacGowan AP. Antibiotic treatment of gram-positive bone and joint infections. J Antimicrob Chemother. 2004;53(6):928–935. doi:10.1093/jac/dkh19115117932
- Anselme K, Davidson P, Popa AM, Giazzon M, Liley M, Ploux L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010;6(10):3824–3846. doi:10.1016/j.actbio.2010.04.00120371386
- Cloutier M, Mantovani D, Rosei F. Antibacterial coatings: challenges, perspectives, and opportunities. Trends Biotechnol. 2015;33(11):637–652. doi:10.1016/j.tibtech.2015.09.00226463723
- Amin Yavari S, van der Stok J, Chai YC, et al. Bone regeneration performance of surface-treated porous titanium. Biomaterials. 2014;35(24):6172–6181. doi:10.1016/j.biomaterials.2014.04.05424811260
- Lee YH, Cheng FY, Chiu HW, et al. Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials. 2014;35(16):4706–4715. doi:10.1016/j.biomaterials.2014.02.02124630838
- Kumar R, Munstedt H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials. 2005;26(14):2081–2088. doi:10.1016/j.biomaterials.2004.05.03015576182
- Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–430. doi:10.1126/science.114724117947576
- Liu Y, Ai K, Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev. 2014;114(9):5057–5115. doi:10.1021/cr400407a24517847
- Ho Cc, Ding Sj. Structure, properties and applications of mussel-inspired polydopamine. J Biomed Nanotechnol. 2014;10(10):3063–3084.25992429
- Lynge ME, van der Westen R, Postma A, Stadler B. Polydopamine – a nature-inspired polymer coating for biomedical science. Nanoscale. 2011;3(12):4916–4928. doi:10.1039/c1nr10969c22024699
- Ye Q, Zhou F, Liu W. Bioinspired catecholic chemistry for surface modification. Chem Soc Rev. 2011;40(7):4244–4258. doi:10.1039/c1cs15026j21603689
- Ku SH, Park CB. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials. 2010;31(36):9431–9437. doi:10.1016/j.biomaterials.2010.08.07120880578
- Ku SH, Lee JS, Park CB. Spatial control of cell adhesion and patterning through mussel-inspired surface modification by polydopamine. Langmuir. 2010;26(19):15104–15108. doi:10.1021/la102825p20806924
- Meng J, Zhang P, Wang S. Recent progress in biointerfaces with controlled bacterial adhesion by using chemical and physical methods. Chem Asian J. 2014;9(8):2004–2016. doi:10.1002/asia.20140220024866966
- Ivanova EP, Hasan J, Webb HK, et al. Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small (Weinheim an Der Bergstrasse, Germany). 2012;8(16):2489–2494. doi:10.1002/smll.201200528
- Ivanova EP, Hasan J, Webb HK, et al. Bactericidal activity of black silicon. Nat Commun. 2013;4:2838. doi:10.1038/ncomms383824281410
- Cheng H, Li Y, Huo K, Gao B, Xiong W. Long-lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles. J Biomed Mater Res Part A. 2014;102(10):3488–3499. doi:10.1002/jbm.a.35019
- Nicklas W, Baneux P, Boot R, et al. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Labor Animals. 2002;36(1):20–42. doi:10.1258/0023677021911740
- Lee H, Rho J, Messersmith PB. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv Mater. 2009;21(4):431–434. doi:10.1002/adma.20080122219802352
- Li J, Tan L, Liu X, et al. Balancing bacteria-osteoblast competition through selective physical puncture and biofunctionalization of ZnO/polydopamine/arginine-glycine-aspartic acid-cysteine nanorods. ACS Nano. 2017;11(11):11250–11263. doi:10.1021/acsnano.7b0562029049874
- Franci G, Falanga A, Galdiero S, et al. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20(5):8856–8874. doi:10.3390/molecules2005885625993417
- Duran N, Duran M, de Jesus MB, Seabra AB, Favaro WJ, Nakazato G. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomed Nanotechnol Biol Med. 2016;12(3):789–799. doi:10.1016/j.nano.2015.11.016
- Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28. doi:10.1016/j.jare.2015.02.00726843966
- Panacek A, Smekalova M, Kilianova M, et al. Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect. Molecules. 2015;21(1):E26. doi:10.3390/molecules2101002626729075
- Zhang B, Luo Y, Pearlstein AJ, et al. Fabrication of biomimetically patterned surfaces and their application to probing plant-bacteria interactions. ACS Appl Mater Interf. 2014;6(15):12467–12478. doi:10.1021/am502384q
- Burgers R, Gerlach T, Hahnel S, Schwarz F, Handel G, Gosau M. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin Oral Implants Res. 2010;21(2):156–164. doi:10.1111/j.1600-0501.2009.01815.x19912269
- Crawford RJ, Webb HK, Truong VK, Hasan J, Ivanova EP. Surface topographical factors influencing bacterial attachment. Adv Colloid Interf Sci. 2012;179-182:142–149. doi:10.1016/j.cis.2012.06.015
- Ge X, Leng Y, Lu X, et al. Bacterial responses to periodic micropillar array. J Biomed Mater Res Part A. 2015;103(1):384–396. doi:10.1002/jbm.a.35182
- Valle J, Burgui S, Langheinrich D, et al. Evaluation of surface microtopography engineered by direct laser interference for bacterial anti-biofouling. Macromol Biosci. 2015;15(8):1060–1069. doi:10.1002/mabi.20150010725914260
- Vasudevan R, Kennedy AJ, Merritt M, Crocker FH, Baney RH. Microscale patterned surfaces reduce bacterial fouling-microscopic and theoretical analysis. Colloids Surf B Biointerf. 2014;117:225–232. doi:10.1016/j.colsurfb.2014.02.037
- Xiang Y, Li J, Liu X, Cui, Z., Yang, X., Yeung, K.W.K., Pan, H., Wu, S. Construction of poly(lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater Sci Eng C. 2017;79:629–637. doi:10.1016/j.msec.2017.05.115
- Wu S, Liu X, Hu T, et al. A biomimetic hierarchical scaffold: natural growth of nanotitanates on three-dimensional microporous Ti-based metals. Nano Lett. 2008;8(11):3803–3808. doi:10.1021/nl802145n18950232
- Liao H, Andersson AS, Sutherland D, Petronis S, Kasemo B, Thomsen P. Response of rat osteoblast-like cells to microstructured model surfaces in vitro. Biomaterials. 2003;24(4):649–654.12437959
- Xu JY, Chen XS, Zhang CY, Liu Y, Wang J, Deng FL. Improved bioactivity of selective laser melting titanium: surface modification with micro-/nano-textured hierarchical topography and bone regeneration performance evaluation. Mater Sci Eng C Mater Biol Appl. 2016;68:229–240. doi:10.1016/j.msec.2016.05.09627524017
- Huang Q, Elkhooly TA, Liu X, et al. Effects of hierarchical micro/nano-topographies on the morphology, proliferation and differentiation of osteoblast-like cells. Colloids Surf B Biointerf. 2016;145:37–45. doi:10.1016/j.colsurfb.2016.04.031
- Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA. 2010;107(11):4872–4877. doi:10.1073/pnas.090326910720194780
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Develop Cell. 2004;6(4):483–495.
- Peltola H, Paakkonen M. Acute osteomyelitis in children. New Engl J Med. 2014;370(4):352–360. doi:10.1056/NEJMra121395624450893
- Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angewandte Chemie. 2013;52(6):1636–1653. doi:10.1002/anie.20120592323255416
- Liu J, Sonshine DA, Shervani S, Hurt RH. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;4(11):6903–6913. doi:10.1021/nn102272n20968290