Abstract
Our previous study showed that Gusuibu (Drynaria fortunei J. Sm.) can stimulate osteoblast maturation. This study was further designed to evaluate the effects of nanoparticles prepared from the water extract of Gusuibu (WEG) on osteoblast survival and maturation. Primary osteoblasts were exposed to 1, 10, 100, and 1000 μg/mL nanoparticles of WEG (nWEG) for 24, 48, and 72 hours did not affect morphologies, viability, or apoptosis of osteoblasts. In comparison, treatment of osteoblasts with 1000 μg/mL WEG for 72 hours decreased cell viability and induced DNA fragmentation and cell apoptosis. nWEG had better antioxidant bioactivity in protecting osteoblasts from oxidative and nitrosative stress-induced apoptosis than WEG. In addition, nWEG stimulated greater osteoblast maturation than did WEG. Therefore, this study shows that WEG nanoparticles are safer to primary osteoblasts than are normal-sized products, and may promote better bone healing by protecting osteoblasts from apoptotic insults, and by promoting osteogenic maturation.
Introduction
Nanomedicine has developed into a new scientific field.Citation1,Citation2 For example, changes in the resonance of a localized nanoparticle surface plasmon upon particle aggregation are constructive to optically detect biomolecular interactions.Citation3,Citation4 When Danshen (Salvia miltiorrhiza) was prepared as nanoparticles, the nanoproducts enhanced antioxidant bioactivity.Citation5 In addition, nano-Ganoderma spores are more easily taken up and have better antitumor activity.Citation6 Thus, nanoparticles prepared from traditional medicines possess new pharmacokinetic and pharmacodynamic characteristics and can be applied to develop new therapeutic strategies.
Bone fractures are a common accident. During the healing process of bone fractures, osteoblast differentiation and maturation sequentially occur and ultimately stimulate bone formation.Citation7,Citation8 Meanwhile, bone fracture-triggered inflammation can elevate oxidative or nitrosative stress and usually impairs bone healing.Citation9 Our previous studies showed that oxidative and nitrosative stress can reduce alkaline phosphatase activity and induce apoptotic insults to rat calvarial osteoblasts through a mitochondrial-dependent pathway.Citation10,Citation11 A variety of biomolecules participate in regulating inflammation-induced osteoblast injury.Citation12–Citation14 Reducing such stress-caused damage can promote osteoblast maturation and bone healing. Our previous studies showed that Gusuibu, a traditional Chinese herb, can protect osteoblasts from oxidative stress- induced insults and promotes osteogenic differentiation.Citation15,Citation16
Drynaria fortunei J. Sm. (Pteridophyta) is a variety of the traditional Chinese herb Gusuibu, which is frequently applied to prevent or treat bone-related diseases. Wang et al reported that the components of Gusuibu exhibited proliferative activity in osteoblast-like UMR106 cells.Citation17 In addition, the flavonoid fractions of Gusuibu can prevent nephrotoxicity and excite regeneration of primary kidney epithelial tubular cells.Citation18 Our previous study showed that the water extract of Gusuibu can protect rat calvarial osteoblasts from hydrogen peroxide-induced insults.Citation16 In an animal study, crude extracts from Gusuibu were also shown to have systemic effects on bone formation in mice.Citation19 Recently, we provided supplementary evidence to show that the water extract of Gusuibu can promote osteoblast differentiation through regulating osteogenic differentiation-related gene expression.Citation15 Nanomedicine is a growing scientific field which may be broadly applicable to develop novel strategies to prevent and treat bone diseases. Thus, the purposes of this study were to evaluate the effects of nanoparticles prepared from the water extract of Gusuibu on the cytotoxicity and cytoprotection against stress-induced insults to rat osteoblasts and the promotion of osteogenic maturation.
Materials and methods
Preparation of the water extract of Gusuibu (WEG)
Gusuibu (D. fortunei) was grown in Chengdu, Sichuan Province, China. The herb was provided by the Brion Research Institute, Sun Ten Group (Taipei, Taiwan), and identified by institutional experts according to macroscopic and microscopic approaches. In addition, the chemical and physical characteristics of D. fortunei are routinely analyzed, and the product was commercialized (CAS no. 6005, Sun Ten Pharmaceutical, Sun Ten Group). Dry pieces of D. fortunei () were ground into a fine powder. WEG was prepared by decocting 50 g of herb powder with 400 mL water for 1 hour, as previously described.Citation16 After filtration, the filtrate was frozen at −70°C and then concentrated by a Freeze Dryer FD24 (Kingmech, Taiwan) into a dry powder. WEG was stored at room temperature and protected from light and moisture.
Figure 1 Preparation of the water extract of Gusuibu (WEG) and nanoparticles of the WEG (nWEG). Dry pieces of Gusuibu (A), Drynaria fortunei, were ground into a fine powder. WEG (B) and nWEG were prepared as described in Materials and Methods. The distribution of nWEG particles was analyzed using an atomic force microscope (C).
Preparation of WEG nanoparticles
Nanoparticles of WEG (nWEG) were prepared following a previously described method.Citation20 Briefly, WEG was carefully ground up using an auto grinder-polisher (EcoMet® 250, Buehler, Lake Bluff, IL) for 10 minutes in an aluminum oxide mortar. Fractions of particles were passed through a series of sieves. The mass percentage of each particle size range was detected. The distribution of particles of different sizes was observed and analyzed using an atomic force microscope (Mobile S, Nanosurf, Liestal District, Switzerland).
Isolation of rat osteoblasts and drug treatment
Rat osteoblasts were prepared from 3-day-old Wistar rat calvarias according to a previously described method.Citation21 All procedures were performed according to the National Institutes of Health Guidelines for the Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Taipei Medical University (Taipei, Taiwan). Rat osteoblasts were seeded in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum, L-glutamine, penicillin (100 IU/mL), and streptomycin (100 μg/mL) in 75-cm2 flasks at 37°C in a humidified atmosphere of 5% CO2. These bone cells were grown to confluence prior to drug treatment. Only the first passage of rat osteoblasts was used in this study. WEG and nWEG were dissolved in deionized distilled water and filtrated through 0.25-mm filters. Rat osteoblasts were treated with different concentrations of WEG and nWEG for various time intervals in independent experiments. In protection assays, rat osteoblasts were exposed to 100 μM hydrogen peroxide, 10 μg/mL WEG, 10 μg/mL nWEG, and a combination of hydrogen peroxide with WEG or nWEG for 24 hours. Cell viability, DNA fragmentation, and cell apoptosis were quantified. Sodium nitroprusside was administered to rat osteoblasts, and cell viability was assayed.
Toxicity assays
Toxicities of WEG and nWEG were evaluated by observing cell morphologies using a light microscope and assaying cell viability by a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay, as per a previously described method.Citation13 After drug treatment, morphologies of osteoblasts were observed and photographed using a light microscope. Rat osteoblasts (2 × 104 cells per well) were seeded in 96-well tissue culture plates overnight. After treatment with WEG and nWEG, rat osteoblasts were cultured with new medium containing 0.5 mg/mL MTT for a further 3 hours. The blue formazan products in osteoblasts were dissolved in dimethyl sulfoxide and spectrophotometrically measured at a wavelength of 550 nm.
Quantification of DNA fragmentation
DNA fragmentation in rat osteoblasts was quantified by the cellular DNA fragmentation enzyme-linked immunosorbent assay kit (Boehringer Mannheim, Indianapolis, IN) as described previously.Citation22 Briefly, rat osteoblasts (2 × 105 cells) were subcultured in 24-well tissue culture plates and labeled with BrdU overnight. Cells were harvested and suspended in the culture medium. One hundred microliters of cell suspension was added to each well of 96-well tissue culture plates. Rat osteoblasts were cocultured with WEG or nWEG for another 8 hours at 37°C in a humidified atmosphere of 5% CO2. Amounts of BrdU-labeled DNA in the cytoplasm were quantified using an Anthos 2010 microplate photometer (Anthos Labtec Instruments, Lagerhausstrasse, Wals/Salzburg, Austria) at a wavelength of 450 nm.
Analysis of apoptotic cells
Apoptotic cells were determined using propidium iodide to detect DNA fragments in nuclei, according to a previously described method.Citation23 After drug treatment, medium containing floating cells was collected, and attached cells were washed and trypsinized. Floating and trypsinized osteoblasts were collected in the same centrifuge tubes. After centrifugation and washing, cell pellets were fixed in cold 80% ethanol. Fixed cells were stained with propidium iodide. Apoptotic cells were quantified by detecting the proportion of rat osteoblasts arrested at the sub-G1 phase using flow cytometry (FACS Calibur, Becton Dickinson, San Jose, CA).
Assays of osteoblast mineralization
Osteoblast maturation was determined by evaluating cell mineralization using the von Kossa and Alizarin red S dye-staining protocols.Citation24,Citation25 Rat osteoblasts were treated with 10 μg/mL of WEG, nWEG, or a combination of 10 nM dexamethasone, 100 μg/mL ascorbic acid, and 10 mM β-glycerophosphate for 21 days. After drug treatment, rat osteoblasts were washed with ice-cold phosphate-based saline buffer (0.14 M NaCl, 2.6 mM KCl, 8 mM Na2HPO4, and 1.5 mM KH2PO4) and then fixed in ice-cold 10% formalin for 20 minutes. For the von Kossa protocol, mineralized matrix was detected by treating fixed cells with 5% silver nitrate for 30 minutes, followed by subsequent washes with 5% sodium carbonate in 10% formalin for 1 minute and 5% sodium thiosulfate for 5 minutes. The reaction was stopped by washing cells twice with deionized water. For the Alizarin red S dye staining protocol, fixed osteoblasts were thoroughly rinsed and then incubated in 1% alcian blue (pH 2.5; Fisher Scientific, Pittsburgh, PA) for 12 hours. Sections were then incubated in Alizarin red S (Fisher Scientific) for 8 minutes, dehydrated briefly in xylene and covered with a coverslip in Permount (Fisher Scientific). Mineralized nodules were visualized and counted under an inverted microscope. Each experiment was performed in duplicate wells and repeated 3 times.
Statistical analyses
The statistical significance of differences between the control and drug-treated groups was evaluated using Student’s t-test, and differences were considered statistically significant at P values of <0.05. Differences between drug-treated groups were considered significant when the P value of Duncan’s multiple-range test was <0.05. Statistical analysis between groups over time was carried out by a 2-way analysis of variance (ANOVA).
Results
Preparation of WEG and nWEG
Dry pieces of Gusuibu (D. fortunei) () were ground into a fine powder, and the WEG was prepared (). nWEG was prepared, and the distribution of the particles with different sizes was analyzed (). Results revealed that >90% of nWEG particles were <1000 nm.
Toxicity of nWEG to rat osteoblasts
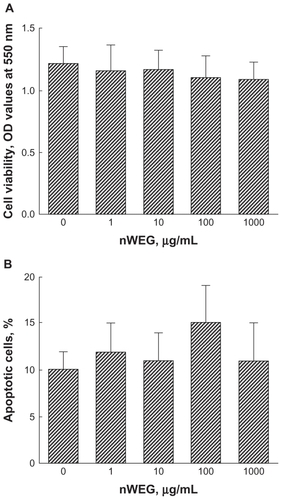
Cell morphologies, cell viability, and apoptotic cells were analyzed to determine the cytotoxicity of nWEG to rat osteoblasts. Treatment of rat osteoblasts with 1, 10, 100, and 1000 μg/mL nWEG for 72 hours did not affect cell morphologies (data not shown). The results of the viability analyses also showed that exposure to 1, 10, 100, and 1000 μg/mL nWEG for 72 hours did not cause the death of rat osteoblasts (). Furthermore, after treatment for 72 hours, nWEG at 1, 10, 100, and 1000 μg/mL nWEG did not induce osteoblast apoptosis ().
Figure 2 Effects of nanoparticles prepared from the water extract of Gusuibu (nWEG) on cell viability and apoptotic cells. Primary rat osteoblasts isolated from neonatal calvarias were exposed to 1, 10, 100, and 1000 μg/mL of nWEG for 72 hours. The viability of rat osteoblasts was assayed using a colorimetric method (A). Cell apoptosis was quantified by flow cytometry (B).
Note: Each value represents the mean ± SEM for n = 6.
nWEG is safer to primary rat osteoblasts than WEG
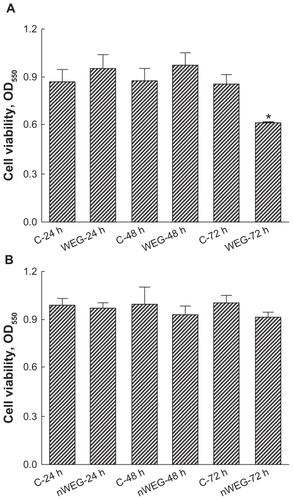
Cell viability was analyzed carried out to compare the toxicities of WEG and nWEG to primary rat osteoblasts (). Exposure of rat osteoblasts to 1000 μg/mL WEG for 24 and 48 hours did not influence cell viability (). Meanwhile, when the treatment time intervals reached 72 hours, WEG caused a significant 31% decrease in osteoblast viability. In comparison, exposure to 1000 μg/mL nWEG for 24, 48, and 72 hours did not affect the viability of rat osteoblasts ().
Figure 3 Effects of the water extract of Gusuibu (WEG) and nanoproducts of the WEG (nWEG) on cell viability. Primary rat osteoblasts isolated from neonatal calvarias were exposed to 1000 μg/mL of WEG (A) and nWEG (B) for 24, 48, and 72 hours. Cell viability was assayed according to a colorimetric method.
Notes: Each value represents the mean ± SEM for n = 6. *Indicates that a value significantly differs from the respective control, P < 0.05.
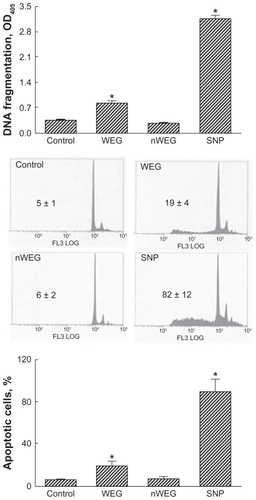
DNA fragmentation and apoptotic cells were analyzed to evaluate the mechanism of WEG-induced death of rat osteoblasts (). Treatment of rat osteoblasts with 1000 μg/mL WEG for 72 hours induced DNA fragmentation by 2.4-fold (). Following exposure to 1000 μg/mL nWEG for 72 hours, the genomic DNA of rat osteoblasts was not damaged. Treatment with 1000 μg/mL WEG for 72 hours induced apoptosis of rat osteoblasts by 20% (). nWEG did not cause osteoblast apoptosis. Sodium nitroprusside was administered to rat osteoblasts as a positive control (). Exposure of rat osteoblasts to sodium nitroprusside caused significant 9.1-fold and 89% increases in DNA fragmentation and cell apoptosis, respectively ().
Figure 4 Effects of the water extract of Gusuibu (WEG) and nanoproducts of the WEG (nWEG) on DNA fragmentation and cell apoptosis. Primary rat osteoblasts isolated from neonatal calvarias were exposed to 1000 μg/mL of WEG and nWEG for 72 hours. DNA fragmentation was assayed using an enzyme-linked immunosorbent assay kit (A). Cell apoptosis was analyzed and quantified by flow cytometry (B, C). Sodium nitroprusside (SNP) was administered to rat osteoblasts as a positive control.
Notes: Each value represents the mean ± SEM for n = 6. *Indicate that a value significantly (P < 0.05) differs from control and WEG-treated groups, respectively.
nWEG has better cytoprotection against hydrogen peroxide-induced insults
The effects of WEG and nWEG on stress-induced insults to primary rat osteoblasts were determined (). Exposure of rat osteoblasts to 100 μM hydrogen peroxide for 24 hours caused a significant 40% reduction in cell viability (). After treatment with 10 μg/mL WEG or nWEG alone, viability of rat osteoblasts did not change. Exposure to WEG decreased hydrogen-caused death of rat osteoblasts by 49%. However, nWEG completely alleviated the hydrogen peroxide-caused decrease in cell viability (). Treatment of rat osteoblasts with 100 μM hydrogen peroxide for 24 hours induced DNA fragmentation and cell apoptosis by 4.7-fold and 54%, respectively (). Neither WEG nor nWEG caused genomic DNA injury or cell apoptosis. Treatment of rat osteoblasts with WEG caused significant 57% and 45% decreases in hydrogen peroxide-induced DNA fragmentation and cell apoptosis, respectively (). In comparison, nWEG completely lowered hydrogen peroxide-induced DNA damage and caused a 65% attenuation of oxidative stress-triggered apoptosis ().
Figure 5 Effects of the water extract of Gusuibu (WEG) and nanoproducts of the WEG (nWEG) on hydrogen peroxide- (HP) and sodium nitroprusside (SNP)-induced cell insults. Primary rat osteoblasts isolated from neonatal calvarias were exposed to 100 μM HP, 10 μg/mL WEG, 10 μg/mL nWEG, and a combination of HP with WEG or nWEG for 24 hours. Cell viability (A), DNA fragmentation (B), and cell apoptosis (C) were quantified. SNP was administered to rat osteoblasts, and cell viability was assayed (D).
Note: Each value represents the mean ± SEM for n = 6. *,#,+Indicate that a value significantly (P < 0.05) differs from control, and HP- and WEG-treated groups, respectively.
The defense by WEG and nWEG against nitrosative stress-induced insults to rat osteoblasts was further evaluated (). Exposure of rat osteoblasts to 2 mM sodium nitroprusside for 24 hours caused a 78% reduction in cell viability. Treatment with WEG or nWEG alone at 10 μg/mL did not influence the viability of rat osteoblasts. However, exposure to WEG and nWEG significantly attenuated sodium nitroprusside-induced death of rat osteoblasts by 36% and 56%, respectively ().
nWEG promotes osteoblast mineralization
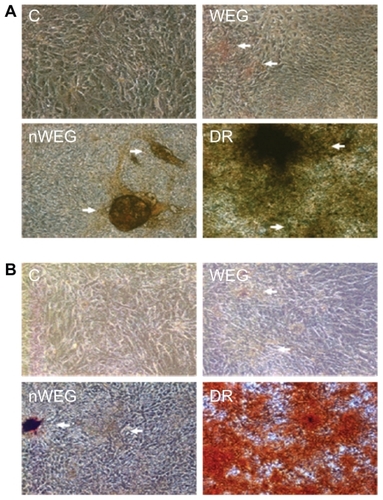
Effects of WEG and nWEG on osteoblast maturation were determined by analyzing cell mineralization (). Results of von Kossa staining revealed that no mineralized nodules were observed in control osteoblasts (, left-top panel). After repeated treatment of primary rat osteoblasts with 10 μg/mL WEG for 21 days, mineralized nodules were observed (right-top panel). Exposure of rat osteoblasts to nWEG at the same concentration of 10 μg/mL for 21 days stimulated more mineralized nodules (left-bottom panel). Analysis with the Alizarin red S dye-staining protocol showed similar results as with the von Kossa method; that is, nWEG had better potential to promote osteoblast mineralization than WEG (, right-top and left-bottom panels). A combination of dexamethasone, ascorbic acid, and β-glycerophosphate was applied to rat osteoblasts as a positive control, and the results revealed such treatment massively stimulated osteoblast maturation (, right-bottom panels).
Figure 6 Effects of the water extract of Gusuibu (WEG) and nanoproducts of the WEG (nWEG) on osteoblast maturation. Primary rat osteoblasts isolated from neonatal calvarias were exposed to 10 μg/mL WEG, 10 μg/mL nWEG, or a differentiation agent, including dexamethasone, ascorbic acid, and β-glycerophosphate, for 21 days. Mineralized nodules were stained using the von Kossa (A) and Alizarin red S-staining protocols (B), and then photographed with a light microscope. 100×.
Discussion
Nanoparticles prepared from a Gusuibu water extract can promote osteoblast maturation and do not cause cytotoxicity to primary rat osteoblasts. In this study, we showed that treatment of rat osteoblasts with 1, 10, 100, and 1000 μg/mL nWEG for 24, 48, or 72 hours did not change cell morphologies or cell viability. Analysis of the cell cycle further showed that the nanosized Gusuibu did not induce osteoblast apoptosis. Thus, our present results show that Gusuibu nanoproducts even at a high concentration of 1000 μg/mL were safe to rat osteoblasts. In parallel, when primary rat osteoblasts were repeatedly treated with 10 μg/mL nanoparticles of Gusuibu water extracts for 21 days, cell maturation was significantly promoted. Liu et al used nanotechnology to prepare Danshen (S. miltiorrhiza) products and found that these nanoproducts possessed stronger antioxidant bioactivitives than the traditional ground particles.Citation5 In addition, nano-Ganoderma spores are more easily taken up and have better antitumor activity.Citation6 During bone healing, osteoblast maturation plays a key role in the process of bone formation.Citation26 Hence, the nanoparticles prepared from Gusuibu extracts could be better updated by rat osteoblasts than normal-sized WEG and may promote more bone formation by stimulating cell maturation.
Nanoparticles prepared from Gusuibu extracts were much safer to primary rat osteoblasts than was normal-sized WEG. The present results show that exposure of rat osteoblasts to 1000 μg/mL nWEG for 72 hours did not cause cell death. By comparison, treatment with 1000 μg/mL WEG for 72 hours decreased cell viability. Thus, this study shows that WEG induced greater cytotoxic to rat osteoblasts than nWEG. WEG induced DNA fragmentation and sub-G1 arrest of rat osteoblasts. DNA fragmentation and arrest at the sub-G1 phase are two typical characteristics of cells undergoing apoptosis.Citation10,Citation27 Hence, WEG at a high concentration caused the death of rat osteoblasts via an apoptotic mechanism. Nanoparticles were proposed as a good delivery system for low-molecular-weight heparin due to their low toxicity.Citation28 Li et al also reported that nanostructured hydroxyapatite pellets can enhance cell attachment and promote proliferation of osteoblast cells.Citation29 This study further showed that nanoparticles of Gusuibu extracts have lower cytotoxicity to primary rat osteoblasts.
Nanoparticles of Gusuibu water extracts can lessen hydrogen peroxide- and sodium nitroprusside-induced insults to primary rat osteoblasts. This study showed that hydrogen peroxide decreased cell viability and induced DNA fragmentation and cell cycle arrest. Sodium nitroprusside reduces the viability of rat osteoblasts. Exposure to hydrogen peroxide and sodium nitroprusside elevates cellular oxidative and nitrosative stress.Citation13 Thus, when rat osteoblasts were exposed to hydrogen peroxide and sodium nitroprusside, the augmented oxidative and nitrosative stress caused cell death through an apoptotic pathway. At the same time, after treatment with the nanoproducts of Gusuibu extracts, the hydrogen peroxide-induced apoptotic insult to rat osteoblasts was significantly alleviated. Wang et al reported that 11 flavonoids are found in the extracts of Gusuibu, which have osteoprotective effects.Citation17 Our previous study also demonstrated that naringin, a flavonoid, may be one of the bioactive components in Gusuibu water extracts.Citation15 Therefore, the flavonoid components in nanoparticles of Gusuibu extracts may contribute to the osteoprotection against oxidative or nitrosative stress-induced apoptosis of rat osteoblasts.
Gusuibu nanoproducts have better antioxidant activity than normal-sized particles. This study showed that both of nWEG and WEG could decrease hydrogen peroxide-induced death, DNA fragmentation, and apoptosis. By comparison, the nanoparticles of the Gusuibu water extract significantly caused much greater alleviation of oxidative and nitrosative stress-induced insults to rat osteoblasts than did normal-sized products. In human intestinal Caco-2 cells, nanoparticles of apoferritin-encapsulated platinum were shown to possess better catalytic efficiency and long-term stability, and reduced hydrogen peroxide-induced oxidative injury.Citation30 In addition, nanoparticles prepared from Danshen showed stronger antioxidant bioactivitives.Citation5 During bone fracture-induced inflammation, enhanced oxidative stress can decrease osteoblast differentiation and bone formation.Citation31 Our previous study also showed that sodium nitroprusside-caused nitrosative stress induced apoptosis of osteoblasts, but the water extract of Gusuibu attenuated such damage.Citation15,Citation16 Consequently, our previous and present studies demonstrate that Gusuibu has the potential to promote bone healing by attenuating nitrosative and oxidative stress-induced apoptotic insults to osteoblasts. In particular, nanoproducts of Gusuibu water extracts have much better osteoprotective effects.
Nanoparticles prepared from Gusuibu water extracts can stimulate osteoblast maturation. The present results analyzed by the von Kosa and Alizarin red staining protocols revealed that Gusuibu nanoproducts promoted osteoblast mineralization. The succession of osteoblast development includes osteoprogenitor proliferation, matrix maturation, and cell mineralization.Citation26 There are varieties of differentiation-related proteins, such as insulin-like growth factor-1, bone morphogenetic proteins, alkaline phosphatase, osteopontin, and osteocalcin, which are involved in regulating osteogenic differentiation.Citation32–Citation34 Our previous study reported that Gusuibu water extracts can promote osteoblast maturation by regulating these bone differentiation-related gene expressions.Citation15 As a result, one of the major reasons explaining Gusuibu nanoproducts’ promotion of osteoblast maturation is the effects of these nanoparticles on regulating these osteogenesis-related gene expressions. By comparison with normal-sized products, nanoparticles of Gusuibu extracts had improved osteogenic mineralization. De Oliveira and Nanci reported that the nanotexturing of titanium-based surfaces upregulated the early expression of bone sialoprotein and osteopontin in osteogenic cell cultures.Citation34 By changing the resonance, the localized nanoparticle surface plasmon is conducive to optically detect biomolecular interactions.Citation4 Thus, nanotechnology can be applied to the nanopreparation of traditional Chinese medicines in order to enhance bone formation by stimulating osteoblast maturation.
Conclusion
In conclusion, this study shows that nanoparticles prepared from the Gusuibu water extract did not induce DNA fragmentation or apoptotic death of primary rat osteoblasts. By comparison, products of Gusuibu extracts of normal sizes at a high concentration caused the death of rat osteoblasts via an apoptotic mechanism. Both nano-and normalsized particles of Gusuibu water extracts could lessen hydrogen peroxide- and sodium nitroprusside-induced DNA fragmentation and apoptosis of rat osteoblasts. Meanwhile, the nanoproducts had stronger antioxidant activities of scavenging oxidative and nitrosative stress. As with normalsized products, nanoparticles of Gusuibu water extracts could also promote osteoblast mineralization. However, Gusuibu nanoproducts stimulated greater osteoblast maturation than products of normal sizes. Therefore, our present results suggest that nanoparticles prepared from the water extract of Gusuibu have stronger osteoprotection against stress-induced apoptotic insults to primary rat osteoblasts and possess better promotion of bone cell maturation. The pharmacokinetics and pharmcodynamics of nanoproducts of Gusuibu water extract will be further evaluated in our future study.
Acknowledgments
This study was supported by the National Taipei University of Technology-Taipei Medical University Joint Research Program (NTUT-TMU-98-08; NTUT-TMU-100-06) and Wan Fang Medical Center (100 swf08), Taipei, Taiwan.
Disclosure
The authors report no conflicts of interest in this work.
References
- FarokhzadOCLangerRimpact of nanotechnology on drug deliveryACS Nanol200931620
- ChenQLiDYOiwaKThe coordination of protein motors and the kinetic behavior of microtubule – a computational studyBiophys Chem2007129606917566632
- HsiehBYChangYFNgMYLocalized surface plasmon coupled fluorescence fiber-optic biosensor with gold nanoparticlesAnal Chem2007793487349317378542
- FaunceTAWhiteJMatthaeiKIIntegrated research into the nanoparticle– protein corona: a new focus for safe, sustainable and equitable development of nanomedicinesNanomedicine2008385986619025459
- LiuJRChenGFShihHNKuoPCEnhanced antioxidant bioactivity of Salvia miltiorrhiza (Danshen) products prepared using nanotechnologyPhytomedicine200815233018077145
- LiuXWangJHYuanJPPharmacological and anti-tumor activities of Ganoderma spores processed by top-down approachesJ Nanosci Nanotechnol200552001201316430134
- SeemanEDelmasPDBone quality – the material and structural basis of bone strength and fragilityN Engl J Med20063542250226116723616
- YelerHTahtabasFCandanFInvestigation of oxidative stress during fracture healing in the ratsCell Biochem Funct20052313713915651080
- ShapiroFBone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblastsEur Cells Mater2008155376
- ChenRMChenTLChiuWTChangCCMolecular mechanism of nitric oxide-induced osteoblast apoptosisJ Orthop Res20052346246815734263
- ChangCCLiaoYSLinYLChenRMNitric oxide protects osteoblasts from oxidative stress-induced apoptotic insults via a mitochondria-dependent mechanismJ Orthop Res2006241917192516917919
- TaiYTCherngYGChangCCHwangYPChenJTChenRMPretreatment with low nitric oxide protects osteoblasts from high nitric oxide-induced apoptotic insults through regulation of c-Jun N-terminal kinase/c-Jun-mediated Bcl-2 gene expression and protein translocationJ Orthop Res20072562563517262823
- HoWPChanWPHsiehMSChenRMRunx2-mediated Bcl-2 gene expression contributes to nitric oxide protection against oxidative stress-induced osteoblast apoptosisJ Cell Biochem20091081084109319746447
- ChenRMLinYLChouCWGATA-3 transduces survival signals in osteoblasts through upregulation of bcl-xL gene expressionJ Bone Miner Res2010252193220420499358
- HuangTYChenTLLiaoMHDrynaria fortunei J. Sm. promotes osteoblast maturation by inducing differentiation-related gene expression and protecting against oxidative stress-induced apoptotic insultsJ Ethnopharmacol2010131707720554009
- LiuHCChenRMJianWCLinYLCytotoxic and antioxidant effects of the water extract of traditional Chinese herb Gusuibu (Drynaria fortunei) on rat osteoblastsJ Formos Med Assoc200110038338811480247
- WangXLWangNLZhangYEffects of eleven flavonoids from the osteoprotective fraction of Drynaria fortunei (KUNZE) J. Sm. on osteoblastic proliferation using an osteoblast-like cell lineChem Pharm Bull200856651
- LongMQiuDLiFJohnsonFLuftBFlavonoid of Drynaria fortunei protects against acute renal failurePhytother Res20051942242716106396
- WongRWRabieABSystemic effect of crude extract from rhizome of Drynaria fortunei on bone formation in micePhytother Res20062031331516557621
- OuKLWuJLaiWFEffects of the nanostructure and nanoporosity on bioactive nanohydroxyapatite/reconstituted collagen by electrodepositionJ Biomed Mater Res201092906912
- ChenRMLinYLJeanWCChenJSWangJHLiuHCNitric oxide induces osteoblast apoptosis through the de novo synthesis of Bax proteinJ Orthop Res20022029530211918309
- ChangHCChenTGTaiYTChenTLChiuWTChenRMResveratrol attenuates oxidized LDL-evoked Lox-1 signaling and consequently protects against apoptotic insults to cerebrovascular endothelial cellsJ Cerebr Blood Flow Met201131842854
- LinYLChangHCChenTLResveratrol protects against oxidized LDL-induced breakage of the blood-brain barrier by lessening disruption of tight junctions and apoptotic insults to mouse cerebrovascular endothelial cellsJ Nutr20101402187219220980646
- CooperLFYliheikkilaPKFeltonDAWhitsonSWSpatiotemporal assessment of fetal bovine osteoblast culture differentiation indicates a role for BSP in promoting differentiationJ Bone Min Res199813620632
- ReselandJESyversenUBakkeILeptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralizationJ Bone Miner Res2001161426143311499865
- AubinJEBone stem cellsJ Cell Biochem199830–31738219594448
- LeeSTWuTTYuPYChenRMApoptotic insults to human HepG2 cells induced by S-(+)-ketamine occurs through activation of a Bax-mitochondria-caspase protease pathwayBrit J Anaesth2009102808919001360
- EidiHJoubertOAttikGCytotoxicity assessment of heparin nanoparticles in NR8383 macrophagesInt J Pharm201039615616520542101
- LiHKhorKAChowVCheangPNanostructural characteristics, mechanical properties, and osteoblast response of spark plasma sintered hydroxyapatiteJ Biomed Mater Res200782296303
- ZhangLLaugLMünchgesangWReducing stress on cells with apoferritin-encapsulated platinum nanoparticlesNano Lett20101021922320017497
- ShouhedDKhaHTRichardsonJAAmanteaCMHahnTJParhamiFOsteogenic oxysterols inhibit the adverse effects of oxidative stress on osteogenic differentiation of marrow stromal cellsJ Cell Biochem2005951276128315880703
- Van LeeuwenJPvan DrielMvan den BemdGJPolsHAVitamin D control of osteoblast function and bone extracellular matrix mineralizationCrit Rev Eukar Gene Exp200111199226
- KochHJadlowiecJACampbellPGInsulin-like growth factor-I induces early osteoblast gene expression in human mesenchymal stem cellsStem Cells Dev20051462163116433617
- De OliveiraPTNanciANanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cellsBiomaterials20042540341314585688