Abstract
A porous poly(L-lactic acid)/β-tricalcium phosphate (PLLA/β-TCP) composite scaffold was fabricated using a novel technique comprising powder mixing, compression molding, low-temperature treatment, and particulate leaching without any organic solvent. The effect of this scaffold on osteoblast proliferation and differentiation was evaluated in vitro. The fabricated scaffold had a homogeneously interconnected porous structure with a porosity of 70% and compressive strength of 1.35 MPa. The methylthiazol tetrazolium values and alkaline phosphatase (ALP) activity of osteoblasts seeded on the solvent-free scaffold were significant higher than those of the control. Using real-time PCR, gene expressions of ALP, osteocalcin, and type 1 collagen were shown to be upregulated. As the method does not use any organic solvent, it eliminates problems associated with organic solvent residue and therefore improves the cell compatibility. It has a promising potential for the preparation of porous scaffold for bone tissue engineering.
Introduction
The repair of bone defects is of key interest in orthopedics and in dental implant treatment. Although some ceramics such as hydroxyapatite and tricalcium phosphate (β-TCP) are currently available as bone graft substitutes, none has been demonstrated to be fully satisfactory.Citation1 The brittleness of calcium phosphate ceramics confines them to nonload or low-load bone repairs. Poly(L-lactic acid) (PLLA) is also extensively used for the regeneration of bone tissue, because of its biocompatibility and biodegradation.Citation2 However, the acid degradation products of PLLA can result in aseptic inflammation of tissues, and the hydrophobicity of PLLA may significantly affect cell attachment ability. An approach to overcome these limitations is the use of ceramic–polymer composite materials.Citation2–Citation4
Several methods have been reported to prepare porous composite scaffolds, such as porogen leaching,Citation5 thermally induced phase separation,Citation6 and freeze-extraction and freeze-gelation methods.Citation7 However, in these methods, a large number of organic solvents and surfactants are used which form residues in the composite scaffolds. These residual organic solvents and surfactants have the potential to harm cell and tissue.Citation8 Furthermore, in these methods, the polymer is usually dissolved in an organic solvent and then the ceramic particles are added. After the solvent is volatilized, the ceramic particles are usually coated by polymer, which leads to the hydrophobic surface of the scaffold.
To solve the problems of residual organic solvent and cell compatibility, we previously demonstrated a novel technique comprising powder mixing, compression molding, low-temperature treatment, and particulate leaching (PCLP) to prepare PLLA/β-TCP composite scaffolds without using any organic solvent.Citation9 But in the previous study, the in vitro biological evaluation of the scaffold prepared by this novel method was not comprehensively investigated. Therefore, we further studied the effect of this composite scaffold on in vitro osteoblast proliferation and differentiation, compared with that of scaffolds made by the conventional solvent casting method.
Experimental
Preparation of PLLA/β-TCP scaffold
The PLLA/β-TCP scaffold was prepared by the PCLP method reported previously with minor modification.Citation9 Briefly, PLLA and β-TCP (diameter: Dav = 1.31–2.34 μm) were mixed together (50:50, w/w), and further homogeneously mixed with sieved sodium chloride (porogen, diameter: 200–400 μm) by magnetic force stirring for 30 minutes before loading into a disk mold (inner diameter: 13 mm). PLLA (MW: 600 kDa) was purchased from Chengdu Organic Chemicals Co. Ltd., Chinese Academy of Sciences. The weight ratio of salt particulates to compounds (PLLA and β-TCP) used was 2.4:1, ie, the percentage of salt in the total mixture weight was 70.59%. After the mixture and stainless steel mold were heated at 170°C for 10 minutes, and the mixture in mold was quickly pressed at a pressure of 10 MPa for 5 minutes to yield a solid disc. Next, the prepared composite disc was further heated at 200°C for 30 minutes with conversion every 5 minutes during the heating period. The sodium chloride particulates were subsequently removed from the composites by leaching the composites in distilled water with shaking for 72 hours, with water changed every 6 hours until no precipitation occurred by adding silver nitrate into the changed water. The composites were then dried in a vacuum oven for further use. For the control experiments, composites were fabricated by conventional solution casting/film crushing/compression molding/particulate leaching method from 5% w/v chloroform solution.
Scaffold characterization
Scaffold porosity was measured using the Archimedes immersion technique as described previously.Citation10 Scaffold pore diameter and morphology were observed with a scanning electron microscopy (SEM) (FEI Inspect F, The Netherlands) after gold coating. The compressive strength of the scaffold was evaluated on an AG-10TA electronic apparatus (Shimadzu, Japan).
Primary culture of calvarial osteoblasts
Osteoblastic cells were enzymatically isolated from neonatal Sprague-Dawley rats as described preciously.Citation11 Briefly, dissected calvariae were sequentially digested for 10-minute intervals with 0.1% type I collagenase (Sigma-Aldrich, St Louis, Mo) and 0.25% trypsin (Sigma-Aldrich) at 37°C. Cells obtained from the second to the fourth digestions were incubated in DMEM (Gibco) with antibiotics and 10% fetal bovine serum (Gibco). At about 90% confluence, cells were trypsinized and subcultured. To confirm that cultures were osteoblast-enriched, mineralized bone nodule formation assay and alkaline phosphatase (ALP) staining were performed. All experiments were performed using the cells of third passage.
Scanning electron microscopy
Cell suspension (3 × 104 cells/mL, 1 mL/well) were pipetted on to scaffolds in the 24-well plates and incubated at 37°C. After 24 hours, cell morphology was visualized using an SEM (FEI Inspect F, The Netherlands). Scaffolds attached with cells were fixed in 2.5% glutaraldehyde, dehydrated, and sputter coated with gold/palladium prior to SEM observation.
Assessment of cell proliferation
Cell proliferation was assessed using a methylthiazol tetrazolium (MTT, Sigma-Aldrich) method. Briefly, 50 μL MTT solution (5 mg/mL) was added to each well, followed by incubation for 4 hours at 37°C. The medium and MTT were then replaced by 1 mL dimethyl sulfoxide to dissolve the formazan crystals. After 30 minutes, 200 μL of supernatant was transferred to microplate wells (Falcon), and the optical density was quantified at a wavelength of 492 nm (HTS 7000 p1us, PerkinElmer, USA).
Measurement of ALP activity
ALP activity was determined in cell homogenates prepared by ultrasonication, with an ALP diagnostic reagent of p-nitrophenyl phosphate (Merit Choice Co. Ltd. Beijing, China). The absorbance of p-nitrophenol formed by the hydrolysis of p-nitrophenyl phosphate was measured at 405 nm 3 times every 10 minutes. Total protein content was measured with the Bradford method. ALP activity was then normalized to its protein concentration and expressed as units/g protein.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from each sample by the TRIzol method strictly following the manufacturer’s protocol (Invitrogen, Carlsbad, CA.). The first-strand cDNA was synthesized from 1 μg RNA with murine leukemia virus reverse transcriptase (Takara, Japan), and used for quantitative real-time PCR. Expression levels of representative genes, including ALP, osteocalcin (OCN), and type 1 collagen (COL-1), were quantified with an ABI 7300 realtime PCR system (Applied Biosystems, Foster City, CA) and SYBR green PCR reaction mix (Infinigen, USA). Primers and annealing temperature for each gene are listed in . The program used was: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and annealing temperature for 1 minute. Melting analysis and agarose gel electrophoresis were performed to confirm the specificity of the PCR products. The relative expression levels of genes were analyzed using the 2−ΔΔCt method by normalizing with β-actin housekeeping gene expression, and presented as fold increase relative to the control group.
Table 1 Specific primers designed following the cDNA sequences of each gene in GenBank
Statistical analysis
Data are shown as means ± standard deviation, and were statistically compared using independent-samples t tests. Differences at P < 0.05 were considered statistically significant.
Results and discussion
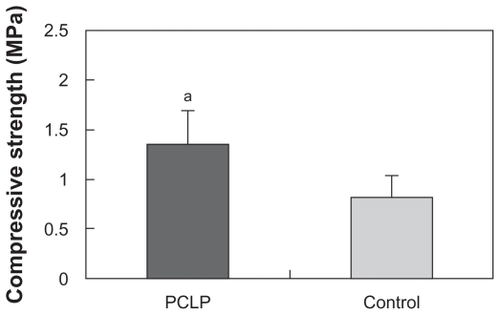
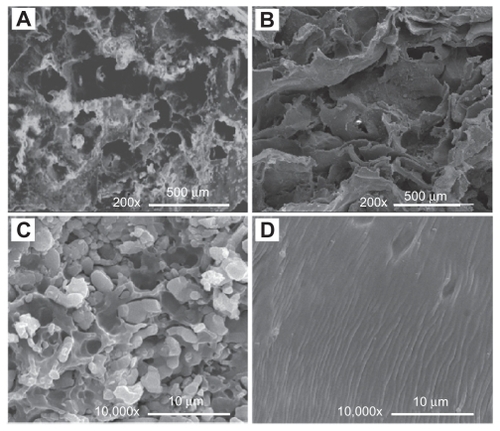
shows the interconnected pore structure of PCLP scaffold made by the organic solvent-free method. The Archimedes immersion technique showed the porosity of the PCLP scaffold to be around 70%. Scaffold porosity and pore size are important features for evaluating biomaterial properties for tissue engineering. Sufficient pore size allows maximum osteoconduction with sufficient nutrient transfer for optimal tissue growth, although there are conflicting reports on the pore size that favors new bone formation and growthCitation12–Citation14 The porosity and pore structure can be controlled by salt weight, particulate size, and polymer fraction. Our previous study indicated that scaffold porosity increases with increasing sodium chloride particulate size. However, the compressive strength of PLLA/β-TCP scaffold decreased with increasing porosity.Citation9 Therefore, we used the sieved sodium chloride with a particulate size of 200–400 μm and weight ratio of 70.59% to prepare the composites. As shown in , the compressive strength of PCLP scaffold was 1.35 MPa, significantly higher than that of solvent-casting composites. This result indicates that the conditions used in the current study could achieve a highly porous scaffold with uncompromised compressive strength.
Figure 1 SEM images of scaffolds made by PCLP method (A, C) and by conventional organic solvent casting method (B, D).
Abbreviations: PCLP, powder mixing, compression molding, low-temperature treatment, and particulate leaching; SEM, scanning electron microscopy.
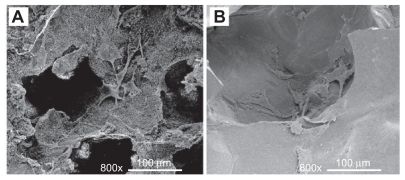
shows the morphological features of osteoblasts cultured on the scaffolds for 1 day. The osteoblasts attached and spread on the surface well. SEM images indicate that more cells were presented in the PCLP scaffolds together with more distinguishable and typical cell shape. Cells extend filopodia to the surface of PCLP scaffolds, and multiple point attachments were observed.
Figure 3 SEM images of osteoblasts cultured on the scaffolds for 1 day: (A) PCLP scaffold; (B) solvent casting scaffold.
Abbreviations: PCLP, powder mixing, compression molding, low-temperature treatment, and particulate leaching; SEM, scanning electron microscopy.
As shown in , PCLP composites made by the organic solvent-free method demonstrated superior cell proliferation compared with the composites prepared by solvent casting. Especially in the first 7 days, cells seeded on the PCLP scaffold grew rapidly while those on the solvent casting scaffold proliferated very slowly. This result may be due to the cytotoxicity of the residual organic solvent. These organic residues in the scaffolds may be harmful to adherent cells, growth factors, or nearby tissues.Citation15,Citation16 After 7 days of culture, cells seeded on PCLP scaffolds grew to a high confluence and their proliferation slowed. Meanwhile, the organic residues in the control group might have been removed after 2 changes of culture medium (the culture medium was changed every 3 days). Therefore, a delayed proliferation was observed in the control group after 7 days of culture.
Figure 4 Results of in vitro tests: cell proliferation assessed by MTT method (A), ALP activity (B), and expression of osteogenic differentiation associated genes quantified by real-time RT-PCR (C, D, and E).
Notes: aP < 0.05; bP < 0.01; n = 3–6.
Abbreviations: ALP, alkaline phosphatases; MTT, methylthiazol tetrazolium.
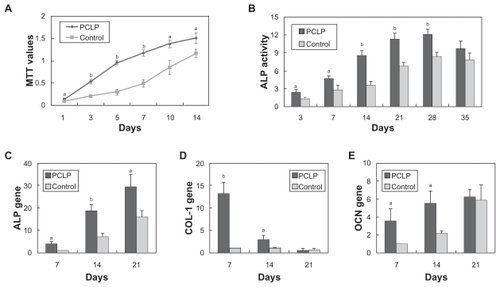
shows that the presence of PCLP scaffolds did increase ALP activity of osteoblasts. On day 28, ALP activity in PCLP scaffolds reached a peak of 12.099 ± 0.882 units/g protein, and slightly deceased to 9.762 ± 1.275 units/g protein on day 35. Using real-time quantitative PCR, we also noted that expression of osteogenic differentiation-associated genes was upregulated by PCLP scaffolds (). These results were consistent with the report of Kim et al.Citation8 There may be 2 reasons for this enhanced biological performance: first, the solvent-free methods we used during the fabricating process, and second, the refined surface structure of the scaffold. As shown in , β-TCP particles disperse homogenously in the PCLP scaffolds and become exposed directly to the cell. It has been proved that bioceramics enhance osteoblast differentiation as well as bone regeneration.Citation17,Citation18 In contrast, in the solvent casting process the mixing of ceramic with polymer solution followed by solvent evaporation resulted in coating of ceramic by a polymer layer (). This polymer layer may hinder the exposure of bioceramics and subsequently reduce the osteogenic induction of bioceramics.Citation16
In the conventional sintering method, the solvent-free process can overcome the coating of ceramic by the polymer layer.Citation19 Blending of polymer, ceramic, and salt followed by pressure with heating, a conventional heating method, can reduce the degree of ceramic coating compared with the solvent casting method.Citation20 But coating of ceramics by polymer melting can still occur, because the polymer swells during melting in the limited space of the mold, and polymer solution flows into the spaces within ceramics under pressure. To reduce this negative effect, Jung et alCitation16 introduced a method of separate pressing and thermal processes; however, this method may compromise the strength of the composite. In this study, we performed a number of experiments according to the properties of PLLA to determine the appropriate temperature and time of heat treatment.Citation9 Moreover, the fabricating process was divided into 2 steps: heating and pressing. First, we chose 170°C as the preheating temperature and quickly pressed at a pressure of 10 MPa for 5 minutes. Then the mixture was sintered at 200°C and pressed again. This process showed limited contact between β-TCP and polymer, leading to a minimum polymer coating, which results in superior osteoconduction to the osteoblast culture.
Another important factor affecting the ability of calcium phosphate-based materials to promote bone generation is grain size. It has been widely accepted that ceramics, metals, polymers, and composites with nanometer grain sizes can stimulate osteoblast activity, leading to faster bone formation, compared with micron grain sizes.Citation21–Citation23 In this study, the diameter of β-TCP particles used was around 1.31 μm to 2.34 μm. In future studies, using nanoparticles may further improve scaffold properties.
Conclusion
A novel PCLP method was successfully developed to prepare PLLA/β-TCP scaffolds without using any organic solvent. The resultant scaffolds had high porosity with interconnected porous structure and high compressive strength. In vitro tests showed that cell proliferation, ALP activity, and osteogenic genes expression were significantly better for PCLP than for solvent casting scaffolds. Such a superior outcomes for the PCLP scaffolds can be attributed to the fact that this new method does not use any organic solvent. Its advantage is that it avoids the problems associated with solvent residues. As β-TCP particles disperse homogeneously in PLLA continuous phase matrix, the direct exposure of these particles to osteoblasts also stimulates cell proliferation and osteogenic differentiation.
Acknowledgments
We would like to thank Dr Malcom Xing, University of Manitoba, for valuable suggestions and manuscript editing.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
- CanceddaRGiannoniPMastrogiacomoMA tissue engineering approach to bone repair in large animal models and in clinical practiceBiomaterials200728294240425017644173
- PiskinEBiodegradable polymeric matrices for bioartificial implantsInt J Artif Organs200225543444012074342
- KimSSSun ParkMJeonOYong ChoiCKimBSPoly(lactide-co- glycolide)/hydroxyapatite composite scaffolds for bone tissue engineeringBiomaterials20062781399140916169074
- KunzeCFreierTHelwigESurface modification of tricalcium phosphate for improvement of the interfacial compatibility with biodegradable polymersBiomaterials200324696797412504518
- ChenGUshidaTTateishiTPreparation of poly(L-lactic acid) and poly(DL-lactic-co-glycolic acid) foams by use of ice microparticulatesBiomaterials200122182563256711516089
- NamYSParkTGPorous biodegradable polymeric scaffolds prepared by thermally induced phase separationJ Biomed Mater Res199947181710400875
- HoMHKuoPYHsiehHJPreparation of porous scaffolds by using freeze-extraction and freeze-gelation methodsBiomaterials200425112913814580916
- KimHJKimUJVunjak-NovakovicGMinBHKaplanDLInfluence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cellsBiomaterials200526214442445215701373
- KangYYinGYuanQPreparation of poly(L-lactic acid)/beta-tricalcium phosphate scaffold for bone tissue engineering without organic solventMater Lett200862121320292032
- KangYQXuXJYinGFA comparative study of the in vitro degradation of poly(L-lactic acid)/beta-tricalcium phosphate scaffold in static and dynamic simulated body fluidEur Polym J200743517681778
- HinoiEFujimoriSNakamuraYYonedaYGroup III metabotropic glutamate receptors in rat cultured calvarial osteoblastsBiochem Biophys Res Commun2001281234134611181052
- GauthierOBoulerJMAguadoEPiletPDaculsiGMacroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowthBiomaterials199819131331399678844
- HingKABestSMBonfieldWCharacterization of porous hydroxyapatiteJ Mater Sci Mater Med199910313514515348161
- MikosAGSarakinosGLymanMDIngberDEVacantiJPLangerRPrevascularization of porous biodegradable polymersBiotechnol Bioeng199342671672318613104
- HileDDPishkoMVSolvent-free protein encapsulation within biodegradable polymer foamsDrug Deliv200411528729315742553
- JungYKimSSKimYHA poly(lactic acid)/calcium metaphosphate composite for bone tissue engineeringBiomaterials200526326314632215913759
- ChenYMakAFWangMLiJSWongMSIn vitro behavior of osteoblast-like cells on PLLA films with a biomimetic apatite or apatite/collagen composite coatingJ Mater Sci Mater Med20081962261226818058196
- LinkDPvan den DolderJvan den BeuckenJJEvaluation of the biocompatibility of calcium phosphate cement/PLGA microparticle compositesJ Biomed Mater Res A200887376076918200545
- WangMDeveloping bioactive composite materials for tissue replacementBiomaterials200324132133215112699650
- AmbrosioAMSahotaJSKhanYLaurencinCTA novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterizationJ Biomed Mater Res200158329530111319744
- RaimondoTPuckettSWebsterTJGreater osteoblast and endothelial cell adhesion on nanostructured polyethylene and titaniumInt J Nanomedicine2010564765220856840
- NelsonMBalasundaramGWebsterTJIncreased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite functionalized with KRSRInt J Nanomedicine20061333934917717974
- WebsterTJSmithTAIncreased osteoblast function on PLGA composites containing nanophase titaniaJ Biomed Mater Res A200574467768616035065