?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Solid lipid nanoparticles (SLNs) are colloidal carrier systems which provide controlled-release profiles for many substances. In this study, we prepared aqueous dispersions of lipid nanoparticles using a modified, pH-sensitive derivative of phosphatidylethanolamine.
Methods
SLNs were prepared using polysorbate 80 as the surfactant and tripalmitin glyceride and N-glutaryl phosphatidylethanolamine as the lipid components. Particle size, polydispersity index, and zeta potential were examined by photon correlation spectroscopy. Morphological evaluation was performed using scanning electron microscopy, atomic force microscopy, and differential scanning calorimetry.
Results
Photon correlation spectroscopy revealed a particle hydrodynamic diameter of 165.8 nm and zeta potential of −41.6.0 mV for the drug-loaded nanoparticles. Atomic force microscopy investigation showed the nanoparticles to be 50–600 nm in length and 66.5 nm in height. Differential scanning calorimetry indicated that the majority of SLNs possessed less ordered arrangements of crystals compared with corresponding bulk lipids, which is favorable for improving drug-loading capacity. Drug-loading capacity and drug entrapment efficiency values for the SLNs were 25.32% and 94.32%, respectively.
Conclusion
The SLNs prepared in this study were able to control the release of triamcinolone acetonide under acidic conditions.
Introduction
Solid lipid nanoparticles (SLNs) were developed in the early 1990s as an alternative carrier system to traditional colloidal systems, including emulsions, liposomes, and polymeric microparticles and nanoparticles.Citation1 Tremendous progress has been made in the treatment of disorders using new drug delivery systems, including SLNs, which are made from solid lipids (ie, lipids solid at room temperature as well as body temperature) and stabilized by surfactant(s).Citation2 They are generally composed of a solid hydrophobic core with a monolayer of phospholipids or phospholipid-derived coating. The solid core contains the active drug dissolved or dispersed in a solid fat matrix with the hydrophobic ends of the phospholipid chains embedded in the fat matrix. Thus, they have the potential to carry lipophilic or hydrophilic drug(s) for diagnostic purposes.Citation3–Citation5
A clear advantage of SLNs over polymeric nanoparticles is the fact that the lipid matrix is made from physiologically tolerated lipid components, which decreases the potential for acute and chronic toxicity.Citation5,Citation6 SLNs have fewer storage and drug leakage problems compared with systems such as liposomes and also have good stability for 2–3 years.Citation6–Citation9
It is noteworthy that tumors and inflamed tissues are often associated with leaky vascular architecture as a result of the poorly regulated nature of tumor angiogenesis. Furthermore, the acidity of these regions differs from physiological pH. Therefore, the use of acid-sensitive drug carriers has been one of the most extensively studied active triggering strategies.Citation10,Citation11 Dong and Hoffman prepared and reported pH-sensitive hydrogels for drug delivery.Citation12 Oh and Lee created a novel vector for delivery of anticancer therapy to solid tumors using diafiltration to synthesize pH-sensitive polymeric micelles, and observed that release of doxorubicin as micelles was triggered at pH 6.8.Citation13 Reddy and Low prepared a pH-sensitive lipid formulation from dioleylphosphatidylethanolamine and citraconic anhydride, and used this to prepare liposomes. They observed that the resulting liposomes were stable at neutral pH but fusogenic at pH 5.Citation11
The nature of tumor angiogenesis enables submicronsized particulate matter, such as SLNs, to penetrate preferentially into tumor tissue and be retained there. Consequently, tumor-specific drug delivery is attracting considerable attention in cancer therapy.Citation7,Citation14–Citation16 Moreover, with advances in surface engineering technology, the biodistribution of SLNs can be further manipulated by modifying the surface physicochemical properties of SLNs to target them to the tissue of interest, particularly to tumors and inflamed tissue.Citation7,Citation17 So far, researchers have prepared various derivatives of phospholipids and used them on the outer shells of SLNs.Citation18
The aim of the present study was to investigate the feasibility of the inclusion of triamcinolone acetonide into surface-modified solid lipid nanoparticles. For this purpose, nanoparticles were covered with modified phosphatidylethanolamine as an outer shell. Triamcinolone acetonide is usually administered via the parenteral route to treat inflammatory disorders and cancer of the lymph nodes. It is a drug with poor water solubility and high lipophilicity, which makes it suitable for SLN encapsulation and in vitro characterization. SLN release has been studied using lyophilized nanoparticles in different media. Modified high shear homogenization and ultrasound techniques have been used to produce low particle sizes and high encapsulation efficiency,Citation19 while photon correlation spectroscopy, differential scanning calorimetry, atomic force microscopy, and scanning electron microscopy have been used for their characterization.
Materials and methods
The following materials were obtained from the indicated sources and used in our study without further purification. Tripalmitin glyceride was purchased from Alfa Aesar (Germany), and phosphatidylethanolamine and triamcinolone acetonide from Sigma-Aldrich (St Louis, MO). Glutaric anhydride, polysorbate 80, and sucrose were obtained from Merck (Darmstadt, Germany). High-pressure liquid chromatography (HPLC) grade methanol and analytical grade chloroform and ethanol were also purchased from Merck. Other reagents were of analytical or HPLC grade. Double-distilled water was prepared in our laboratory.
Preparation of modified phosphatidylethanolamine
For preparation of modified phosphatidylethanolamine, 5 mg of lipid was dissolved in 10 mL of chloroform, and an approximately five-fold molar excess (3.85 mg) of glutaric anhydride was then added in the presence of 7.5 μL pyridine, and the mixture was incubated at 20°C for 5 hours.Citation20 Modified phosphatidylethanolamine was identified by iodine staining.Citation21 N-glutaryl phosphatidylethanolamine was isolated using plate chromatography and chloroform-methanol (65:35 v/v) as the solvent, with a yield of 90%:
1H NMR (200 MHz, CDCl3) δ 0.88 (6H), 1.30 (32 H), 1.59 (4H), 1.99 (2H), 2.16 (8H), 2.27 (6H), 2.61 (2H), 3.48 (2H), 3.95 (2H), 4.13 and 4.38 (2H), 4.20 and 4.51 (2H), 4.71 (1H), 5.33 (4H), and 7.38 (1H) that peaked in 7.38 ppm indicated amidic hydrogen.
Preparation of aqueous SLNs and lyophilization
Triamcinolone acetonide (0.75% w/v), tripalmitin glyceride (2% w/v), and N-glutaryl phosphatidylethanolamine (0.5% w/v) were dissolved in 10 mL of chloroform and methanol mixture (1:1). Organic solvents were completely removed using a rotoevaporator (Laborota 4003, Heidolph, Schwabach, Germany). The drug-embedded lipid layer was melted by heating at 70°C above the melting point of the lipid. An aqueous phase was prepared by dissolving polysorbate 80 (1% w/v) in double-distilled water and heated to the same temperature as the oil phase. The hot aqueous phase was added to the oil phase, and homogenization was achieved (at 10,000 rpm and 70°C) using a Diax 900 homogenizer (Heidolph) for 2 minutes. The resulting coarse hot oil in water emulsion was ultrasonicated using a Sonoplus ultrahomogenizer (Bandelin, Germany) for 15 minutes. Triamcinolone acetonide-SLNs were obtained by allowing the hot nanoemulsion to cool off at room temperature.
Sucrose was used in the freeze-drying process as a cryoprotector at a concentration of 3 wt%.Citation22 The SLN suspension was frozen in an aqueous sucrose solution at −70°C overnight, and the sample was subsequently transferred to the freeze-drier (ZiRBuS Technology VaCo 5, D-37539) at −50°C for 72 hours, and finally the SLN powder was collected for further experiments.
Determination of entrapment efficiency and drug-loading capacity
The percentage of incorporated triamcinolone acetonide (entrapment efficiency) was determined by spectrophotometric determination at 234 nm using an Agilent 8453-spectrophotometer, after ultracentrifugation of the aqueous dispersion (40,000 rpm for 45 minutes). The amount of free drug was detected in the supernatant and the amount of incorporated drug was calculated as the initial drug minus the free drug. The drug entrapment efficiency and drug-loading in the SLNs were calculated using Equationequations (1)(1) and Equation(2)
(2) :
where EE is entrapment efficiency, DL is drug-loading, and Wa, Ws, and WL are the weight of drug added into the system, analyzed weight of drug in the supernatant, and weight of lipid added into the system, respectively.Citation23
Particle diameter, polydispersity index, and zeta potential
Measurement of hydrodynamic diameter, polydispersity index, and zeta potential of the nanoparticles was accomplished using photon correlation spectroscopy (Nano ZS4700, Malvern Instruments, Worcestershire, UK). For size measurement, all formulations were diluted by deionized water to eliminate the effect of viscosity caused by the ingredients. Zeta potential measurements were conducted for samples prepared for size measurement. Hydrodynamic diameter and zeta potential were reported as the mean of three measurements at 25°C. The refractive index of the SLNs and water was set at 1.35 and 1.33, respectively.
Scanning electron microscopy
In order to verify the result, the morphology of the triamcinolone acetonide-SLNs was studied by scanning electron microscopy using a Philips XL30 microscope at an accelerating voltage of 10 kV. One droplet of SLN nanosuspension was placed on an aluminum specimen stub. After oven-drying for 12 hours, the sample was coated with a platinum layer using an SCDOOS sputter coater (BAL-TEC, Sweden) in an argon atmosphere. Subsequently, the sample was scanned and photomicrographs were obtained.
Atomic force microscopy
The surface properties of the drug-loaded SLNs were visualized using an atomic force microscope (Mobile S, Nanosurf, Switzerland). Explorer atomic force microscopy was in noncontact mode, using high resonant frequency (F0 170 kHz) pyramidal cantilevers with silicon probes having dynamic force. The sample was diluted with distilled water and then dropped onto a freshly cleaved mica plate, followed by vacuum drying for 24 hours at 25°C.
Differential scanning calorimetry
The thermal characteristics of the SLNs and pure drug were determined using a differential scanning calorimeter (Shimadzu DSC-60, single heating ramp 0°C–300°C, heated at 5°C/minute) under a dry nitrogen atmosphere. Samples (7–8 mg) were placed in an aluminum pan and heated at a rate of 5°C/minute. Empty aluminum pans were used as references, and the entire thermal behavior was studied under a nitrogen purge.
Assay for triamcinolone acetonide
A simple and sensitive HPLC method with ultraviolet detection was used for analysis of triamcinolone acetonide.Citation20 The triamcinolone acetonide concentration was determined using an HPLC system (LC-10AS Liquid Chromatograph, SCL- 10A System Controller, SIL-10AV UV-Vis Detector, C-R6A Chromatopac, Shimadzu, Japan). The analytical conditions were as follows: column C18, HPLC Pack column, Inertsil ODS, 5 μm, 4.6 × 250 mm (GL Sciences Inc, Japan); ultraviolet detection, 240 nm; mobile phase, methanol; water 50:50 (v/v); flow rate 1 mL/minute; and injection volume 25 μL.
In vitro release of triamcinolone acetonide from SLNs
This experiment was conducted using a static horizontal Franz diffusion cell to evaluate the amount of triamcinolone acetonide released from each formulation.Citation24 A cellulose acetate membrane with a molecular weight cutoff of 12,000 Da and a surface area of 2.0 cm2 was used and mounted on the Franz diffusion cell. The receptor medium was precisely 50 mL in volume and composed of an aqueous solution of physiological saline, phosphate-buffered saline, and 20% ethanol at four pH values of 4.0, 5.0, 6.0, and 7.4, stirred by a magnetic bar at 500 rpm to homogenize the medium. Each formulation, 10 mg in 1 mL volume, was loaded onto the membrane in the donor compartment. The temperature of the assay was controlled at precisely 37°C. At predetermined time intervals, 1 mL aliquots of the release medium were withdrawn using a syringe needle, and the same volumes of freshly prepared receptor medium were added. The samples were analyzed using an HPLC method described previously.Citation20 The experiments were repeated three times and the results were expressed as means ± standard deviation.
Results and discussion
Preparation of nanoparticles, drug entrapment efficiency, and loading capacity
In this study, homogenization was performed at above the melting point of the lipid followed by ultrasonication for preparation of modified SLN.Citation25,Citation26 To disperse the triamcinolone acetonide homogeneously in the lipid, a chloroform/methanol solvent system was used and homogenized for 2 minutes.Citation27 Subsequently, the prepared SLNs were subjected to further lyophilization. Our preliminary studies showed that reconstitution of freeze-dried SLNs was impossible when they were freeze-dried without addition of cryoprotectants. Therefore, a 1:1 dilution with 3% w/w of sucrose as a cryoprotectant was performed.Citation28
Many different drugs have been incorporated in the SLNs.Citation17 The prerequisite for obtaining a sufficient loading capacity is a sufficiently high solubility of the drug in the lipid melt. Relatively higher encapsulation efficiency constitutes one of the major advantages of SLNs.
For calculations of entrapment efficiency and loading capacity, calibration curves for the ultraviolet assays of triamcinolone acetonide were conducted on eight solutions in the concentration ranges of 1.5 × 10−3 to 1.25 × 10−4 mol/L; encapsulation efficiency and drug-loading were 94.32% and 25.32%, respectively. The high entrapment efficiency of the drug is thought to be the result of the lipophilic characteristics and high compatibility between the drug and the lipid. Furthermore, the high encapsulation efficiency and drug-loading in comparison with previous workCitation24 is a result of the method of preparation and mixing of the drug and lipid matrix in the organic solvents.
Nanoparticle characterization
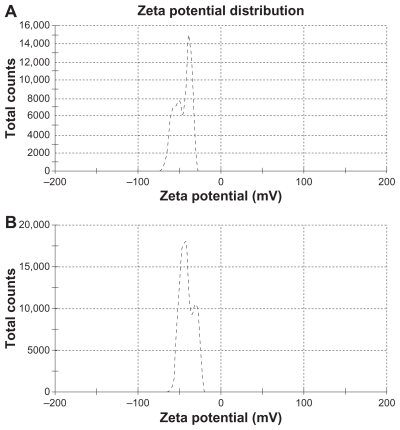
The mean particle size, polydispersity index and zeta potential of the SLNs with and without drug were measured by photon correlation spectroscopy, as summarized in . The mean particle sizes of 165.8 nm and 97.44 nm were obtained for drug-loaded and free SLNs, respectively (). The increase in size after drug loading must be due to incorporation of the drug in the nanoparticle matrix.Citation23 The polydispersity index is a ratio providing information about the homogeneity of particle size distribution in a given system, and ideally, this should be <0.3.Citation29 Triamcinolone acetonide-loaded and free SLN had polydispersity index values of 0.254 and 0.337, respectively, indicating that the nanoparticles had a narrow size distribution, and suggesting nanoparticle monodispersity.Citation30,Citation31 The zeta potential is a key factor for evaluation of the stability of a colloidal dispersion.Citation23 When the absolute value of the zeta potential is higher than 30 mV for a colloidal formulation, the particles are likely to be electrochemically stable under the investigated condition.Citation25 Zeta potential values of −41.6 mV and −43.0 mV were obtained for the drug-loaded and free SLNs, respectively (). As demonstrates, as time passes, increases are observed in size, polydispersity index, and zeta potential as a result of light and other factors that cause gelation.Citation32 However, it is obvious from these values that the prepared nanosuspension has an acceptable electrochemical and physical stability potential for 3 and 6 months of storage.
Table 1 Particle size polydispersity index and zeta potential for drug-loaded and free solid lipid nanoparticles in colloidal suspension
Figure 1 Zeta potential of (A) drug-loaded solid lipid nanoparticles and (B) free solid lipid nanoparticles.
Similarly, in order to evaluate the stability of SLNs lyophilized powder after 6 months, 10 mg of sample was dispersed in distilled water and characterized. The values for particle diameter, polydispersity index, and zeta potential were 223.3 nm, 0.321, and −30.1, respectively. These results indicate that, using sucrose as a cryoprotectant, the particle size remains in the nanometric range.
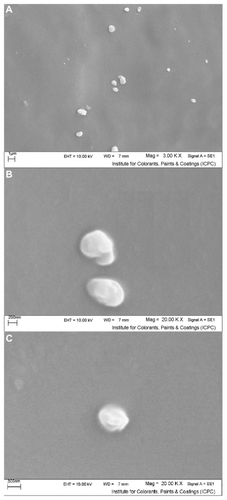
In order to obtain a scanning electron microscopic image, we used freshly prepared SLN loaded with triamcinolone acetonide, and micrographs of the samples containing drug-loaded SLNs are shown in . The micrographs demonstrated a spherical shape and a smooth surface, with a particle size in the nanometric range.Citation25
Figure 2 Scanning electron micrographs of freshly prepared solid lipid nanoparticles loaded with triamcinolone acetonide; (A) 3.00 K× resolution, (B and C) 20.00 K× resolution.
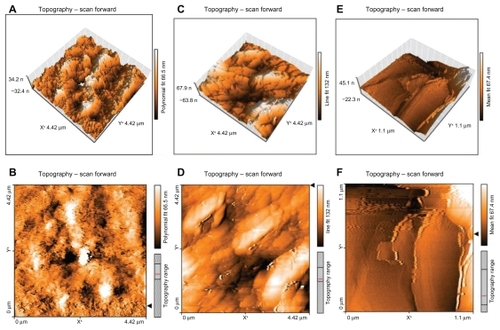
The atomic force microscopy technique has been widely used to obtain size, shape, and surface morphological information about nanoparticles. It is capable of resolving surface details down to 0.01 nm and producing a contrasted and three-dimensional image of the sample.Citation23 A drop of diluted aqueous suspension (without preliminary ultrafiltration) was placed on a mica slide and dried out at room temperature for 24 hours. gives the tapping mode atomic force microscopic images of freshly prepared SLN containing triamcinolone acetonide after 24 hours (A and B), 48 hours (C and D), and 1 week (E and F). The nanoparticle images demonstrated spherical particles of 50–600 nm in diameter, but only 66.5 nm in height. After 48 hours, the nanoparticles were uniformly grown on mica slides, as illustrated in . One week after spreading SLNs onto the mica plates, no particles could be distinguished in the atomic force microscopic images, which showed homogeneous and fiat shapes, as illustrated in .Citation33 This result is not surprising, considering the well known ability of particles to fuse during evaporation of water consequent to the molecular diffusion on the surface of the particles. Moreover, the presence of free surfactant in the suspension may increase the characteristic size.Citation33
Figure 3 Atomic force microscopy of solid lipid nanoparticles loaded with triamcinolone acetonide. (A) and (B) are solid lipid nanoparticles loaded with triamcinolone acetonide after 24 hours. (C) and (D) are solid lipid nanoparticles loaded with triamcinolone acetonide after 48 hours remaining on a mica slide. (E) and (F) are solid lipid nanoparticles loaded with triamcinolone acetonide after 1 week remaining on a mica slide. A, C, and D are three-dimensional images of the multiparticles and B, D, and F are the two-dimensional pictures of the multiparticles.
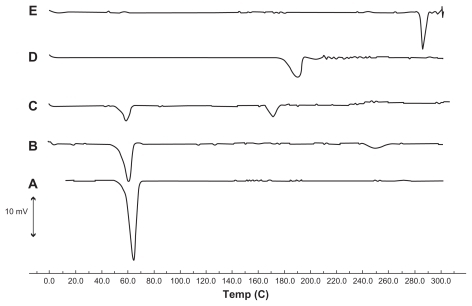
Differential scanning calorimetry thermograms for triamcinolone acetonide, tripalmitin glyceride, a physical mixture of triamcinolone acetonide, tripalmitin glyceride, modified phosphatidylethanolamine, and lyophilized SLNs are shown in . A sucrose thermogram is also shown in this figure to demonstrate peaks of lyophilized SLNs. The triamcinolone acetonide powder showed a sharp melting process with an onset temperature of 284.72°C and a peak of 286.04°C, with a melting enthalpy of 64.73 J/g. This was confirmed by the presence of a melting peak for triamcinolone acetonide in the physical mixture. However, no melting process was observed for the lyophilized SLN suspension. This suggested that there was no crystalline triamcinolone acetonide in the SLN suspension, and triamcinolone acetonide might be present in an amorphous state. Similar results have been reported by previous researchers, stating that rapid quenching of the nanoemulsion does not allow the drug to crystallize.Citation26,Citation27 An endothermic peak of the sucrose used as a cryoprotectant was observed at 170.21°C in the triamcinolone acetonide- SLN curve. Meanwhile, the melting peak temperature of tripalmitin glyceride in lyophilized SLN (57.73°C) was diminished when compared with the temperature of the bulk lipid (64.38°C). The decrease in melting point can be attributed to the small size (nanometer range), high specific surface area, and presence of a surfactant.Citation15,Citation30 This can also be attributed to the Kelvin effect and is described by the Thomson equation.Citation3 Moreover, the lower melting enthalpy value of 35.72 J/g indicates a less ordered lattice arrangement of lipids within the nanoparticles compared with the bulk materials.Citation9
In vitro drug release
In this study, the in vitro release of triamcinolone acetonide from SLNs was investigated for 60 hours at 37°C using a Franz diffusion cell (). Due to the low water solubility of triamcinolone acetonide, 20% ethanol was added to the phosphate-buffered solution at four pH values of 4.0, 5.0, 6.0, and 7.4.Citation24 Our results showed that up to 50% was released over the test period. The prolonged and lower release of SLNs after 12 hours can be explained by slower diffusion of triamcinolone acetonide from the inner solid core matrix of the formulations. However, the initial fast release was detected at a lower pH. In this experiment, we studied the release profile of pure drug compared with nanoparticles in acidic pH, and the release half-life of drug release was significantly diminished compared with nanoparticles (about 25 minutes).
Figure 5 Drug release from nanoparticles after 60 hours in four different types of medium, each value represents the mean of three experiments ± standard deviation.
Abbreviation: TA, triamcinolone acetonide.
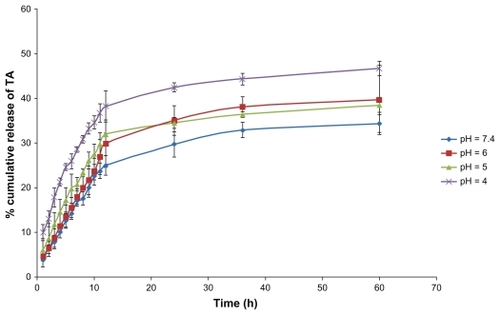
The release data were analyzed using the following Higuchi kinetic equation:Citation25 Qt = k·t0.5, where Qt is the percentage of drug released at time t, and k is the release rate constant. Regarding the release model of all formulations, it was found that the prolonged release characteristic of triamcinolone acetonide was well fitted to Higuchi’s square root model, as has been reported for drug-loaded SLN systems.Citation25,Citation34,Citation35 Linear fits were obtained, indicating that the release profile of triamcinolone acetonide from homogenous and granular matrix systems is diffusion-controlled. The R2 values from in vitro release kinetics and the K values or release rate constant obtained from the Higuchi model plot are presented in . Furthermore, as shown in and the in vitro release data, we found that the burst release was increased with a decline in pH that demonstrates a change in structure of the phospholipids and release of the terminal anhydride. Several previous studies have been conducted to identify a pH-sensitive carrier preparation, and often the amount of drug release from SLNs was small compared with our results, and the values for common SLNs without modification were below 25%.Citation8,Citation25,Citation27 Subedi et al investigated pH-sensitive SLNs and used them for doxorubicin delivery; in their work, 30% of the drug was released after 24 hours.Citation8
Table 2 R2 and K for drug-release experiment in four types of receptor medium
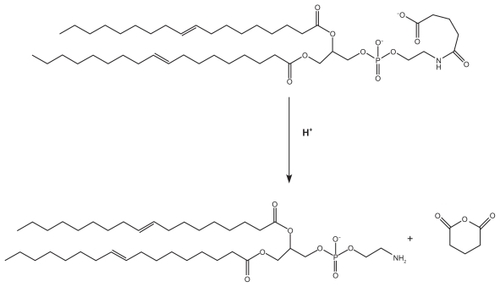
Venkateswarlo and Manjunath investigated SLNs of tripalmitin and used them as clozapine carriers, and in their work, up to 20% of drug was released after 48 hours.Citation25 Liu et al investigated SLNs containing triamcinolone acetonide in mixtures of monoglycerides, diglycerides, and triglycerides of palmitic acid and stearic acid, and found that the drug was released more slowly compared with our prepared carrier.Citation24 The simple mechanism for terminal release of anhydride from modified polyethylene is shown in . This pathway was faster at an acidic pH of 4. Nevertheless, it is certain that this carrier requires further in vitro and in vivo studies.
Conclusion
As mentioned above, SLNs offer an attractive means of drug delivery, particularly for poorly water-soluble drugs. They combine the advantages of polymeric nanoparticles, fat emulsions, and liposomes. In this study, modified N-glutaryl- phosphatidylethanolamine was prepared, used for SLN preparation, and characterized. The results indicate that the carrier has suitable stability in colloidal form and may increase drug release under acidic conditions. Homogenization followed by ultrasonication is suitable for producing small SLNs with an average diameter of 165.8 nm. Using a Zetasizer, atomic force microscopy, and SEM, the diameter and morphology of the particles was investigated. Zeta potential data indicate the stability of the nanosuspension. After 6 months, Zetasizer data did not indicate any obvious changes in diameter, polydispersity index, and zeta potential. An in vitro study indicated an increase in burst release and a pH decrement as well. In vitro release kinetics coincide with the Higuchi equation. These observations suggest that the present system offers an exciting mode of target delivery for potent lipophilic anticancer drugs and anti-inflammatory agents.
Acknowledgments
The authors acknowledge the instructors of the School of Pharmacy at Kermanshah University of Medical Sciences, with special thanks to Miss Farnaz Ahmadi and Miss Marjan Mohebbi from the analysis laboratory.
Disclosure
The authors report no conflicts of interest in this work.
References
- MullerRHMaderKGohlaSSolid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the artEur J Pharm Biopharm20005016117710840199
- WissingSAKayserOMullerRHSolid lipid nanoparticles for parenteral drug deliveryAdv Drug Deliv Rev2004561257127215109768
- KauraIPBhandariRBhandariSKakkarVPotential of solid lipid nanoparticles in brain targetingJ Control Release20081279710918313785
- ChenDBYangTZLuWLZhangQIn vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxelChem Pharm Bull2001491444144711724235
- HeiatiHDevelopment and characterization of solid lipid nanoparticles for delivery of bioactive materialsPhD thesisMontrealUniversity of Montreal1996
- TorchilinVPNanoparticulates as Drug CarriersLondon, UKImperial College Press2006
- WongHLBendayanRRauthAMLiYWuXYChemotherapy with anticancer drugs encapsulated in solid lipid nanoparticlesAdv Drug Deliv Rev20075949150417532091
- SubediRKKangKWChoiHKPreparation and characterization of solid lipid nanoparticles loaded with doxorubicinEur J Pharm Sci20093750851319406231
- LaiFSinicoCDe LoquAZaruMMüllerRHFaddaAMSLN as a topical delivery system for Artemisia arborescens essential oil: In vitro antiviral activity and skin permeation studyInt J Nanomed20072419425
- AndresenTLJensenSSJorgensenKAdvanced strategies in liposomal cancer therapy: Problems and prospects of active and tumor specific drug releaseProg Lipid Res200544689715748655
- ReddyJALowPSEnhanced folate receptor mediated gene therapy using a novel pH-sensitive lipid formulationJ Control Release200064273710640643
- DongLHoffmanASA novel approach for preparation of pHsensitive hydrogels for enteric drug deliveryJ Control Release199115141152
- OhKTLeeESCancer-associated pH-responsive tetracopolymeric micelles composed of poly (ethylene glycol)-b-poly (L-histidine)-b-poly (L-lactic acid)-b-poly(ethylene glycol)Polym Adv Technol20081919071913
- MatsumuraYMaedaHA new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCSCancer Res19866193210
- DerakhshandehKSoheiliMDadashzadehSSaghiriRPreparation and in vitro characterization of 9-nitrocamptothecin loaded long circulating nanoparticles for delivery in cancer patientsInt J Nanomedicine2010515920161983
- DadashzadehSDerakhshandehKHoseinishiraziF9-Nitrocamptothecin polymeric nanoparticles: Cytotoxicity and pharmacokinetic studies of lactone and total forms of drug in ratsAnticancer Drugs20081980581118690092
- MehnertWMaderKSolid lipid nanoparticles: Production, characterization and applicationsAdv Drug Deliv Rev20014716519611311991
- Garcia-FuentesMAlonsoMJTorresDDesign and characterization of a new drug nanocarrier made from solid-liquid lipid mixturesJ Colloid Interface Sci200528559059815837476
- HouDZXieCSHuangKJZhuCHThe production and characteristics of solid lipid nanoparticles (SLNs)Biomaterials2003241781178512593960
- ZhangNPingQNHuangGHXuaWFInvestigation of lectin-modified insulin liposomes as carriers for oral administrationInt J Pharm200529424725915814248
- DrummondDCDalekeDLSynthesis and characterization of N-acylated, pH-sensitive ‘caged’ aminophospholipidsChem Phys Lipids19957527417697781
- LinXLiXZhengLQYuLZhangQLiuWPreparation and characterization of monocaprate nanostructured lipid carriersColloids Surf A Physicochem Eng Asp2007311106111
- HuFQJiangSPDuYZYuanHYeYQZengSPreparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous systemColloids Surf B Biointerfaces20054516717316198092
- LiuWHuMLiuWXueCXuHYangXLInvestigation of the carbopol gel of solid lipid nanoparticles for the transdermal iontophoretic delivery of triamcinolone acetateInt J Pharm200836413514118778760
- RuktanonchaiUBejraphaPSakulkhuUPhysicochemical characteristics, cytotoxicity, and antioxidant activity of three lipid nanoparticulate formulations of alpha-lipoic acidAAPS Pharm Sci Tech200910227234
- BhaskarKAnbuJRavichandiranVVenkateswarluVRaoYMLipid nanoparticles for transdermal delivery of flurbiprofen: Formulation, in vitro, ex vivo and in vivo studiesLipids Health Dis2009811519128464
- VenkateswarluVManjunathKPreparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticlesJ Control Release20049562763815023472
- JoshiMMisraADry powder inhalation of liposomal ketotifen fumarate: Formulation and characterizationInt J Pharm2001223152711451628
- PathakPNagarsenkerMFormulation and evaluation of lidocaine lipid nanosystems for dermal deliveryAAPS Pharm Sci Tech200910985992
- AliHEl-SayedKSylvesterPNazzalSMolecular interaction and localization of tocotrienol-rich fraction (TRF) within the matrices of lipid nanoparticles: Evidence studies by differential scanning calorimetry (DSC) and proton nuclear magnetic resonance spectroscopy (1H NMR)Colloids Surf B Biointerfaces20107728629720189780
- DerakhshandehKErfanMDadashzadehSEncapsulation of 9-nitrocamptothecin, a novel anticancer drug, in biodegradable nanoparticles: Factorial design, characterization and release kineticsEur J Pharm Biopharm200766344117070678
- FreitasCMullerRHEffect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN) dispersionsInt J Pharm1998168221229
- IgartuaMSaulnierPHeurtaultBDevelopment and characterization of solid lipid nanoparticles loaded with magnetiteInt J Pharm200223314915711897419
- JenningVThunemannAFGohlaSHCharacterization of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipidsInt J Pharm200019916717710802410
- TiyaboonchaiWTungpraditWPlianbangchangPFormulation and characterization of curcuminoids loaded solid lipid nanoparticlesInt J Pharm200733729930617287099