 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Over the last decade, nanotechnology has provided researchers with new nanometer materials, such as nanoparticles, which have the potential to provide new therapies for many lung diseases. In this study, we investigated the acute effects of polystyrene nanoparticles on epithelial ion channel function.
Methods
Human submucosal Calu-3 cells that express cystic fibrosis transmembrane conductance regulator (CFTR) and baby hamster kidney cells engineered to express the wild-type CFTR gene were used to investigate the actions of negatively charged 20 nm polystyrene nanoparticles on short-circuit current in Calu-3 cells by Ussing chamber and single CFTR Clchannels alone and in the presence of known CFTR channel activators by using baby hamster kidney cell patches.
Results
Polystyrene nanoparticles caused sustained, repeatable, and concentration-dependent increases in short-circuit current. In turn, these short-circuit current responses were found to be biphasic in nature, ie, an initial peak followed by a plateau. EC50 values for peak and plateau short-circuit current responses were 1457 and 315.5 ng/mL, respectively. Short-circuit current was inhibited by diphenylamine-2-carboxylate, a CFTR Cl− channel blocker. Polystyrene nanoparticles activated basolateral K+ channels and affected Cl− and HCO3 − secretion. The mechanism of short-circuit current activation by polystyrene nanoparticles was found to be largely dependent on calcium-dependent and cyclic nucleotide-dependent phosphorylation of CFTR Cl− channels. Recordings from isolated inside-out patches using baby hamster kidney cells confirmed the direct activation of CFTR Cl− channels by the nanoparticles.
Conclusion
This is the first study to identify the activation of ion channels in airway cells after exposure to polystyrene-based nanomaterials. Thus, polystyrene nanoparticles cannot be considered as a simple neutral vehicle for drug delivery for the treatment of lung diseases, due to the fact that they may have the ability to affect epithelial cell function and physiological processes on their own.
Introduction
Cystic fibrosis is an inheritable disease caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene located on chromosome 7.Citation1 The cftr gene encodes a cAMP-regulated Cl− channel, CFTR, located on the apical membrane of epithelial cells.Citation2 Structural analysis of the CFTR protein shows that it consists of a 1480 amino acid backbone containing two nucleotide-binding domains, 12 transmembrane domains, and a unique cytoplasmic regulatory domain.Citation3 Phosphorylation of the regulatory domain by cAMP-dependent protein kinase A is a prerequisite for channel opening.Citation4 ATP-induced dimerization of nucleotide-binding domains also plays an important role in this process.Citation5
There are over 1800 recognized mutations of the cftr gene which give rise to the disease known as cystic fibrosis.Citation6 Lung disease in cystic fibrosis patients is the principal cause of morbidity and mortality associated with the condition, and is characterized by impaired mucus clearance due to altered ion transport by airway epithelial cells.Citation7 Submucosal glands of the respiratory system have been proposed as the primary site for the pathology of cystic fibrosis lung disease.Citation8 CFTR Cl− channels located on the apical membrane of lung epithelial cells are involved in the regulation of physiological processes, such as cell volume control and transepithelial fluid transport, as well as modulating the function of other ion channels, eg, epithelial Na+ channels, outwardly rectifying Cl− channels, and K+ channels, and thus the transport of Na+, K+ and Cl− ions, and H2O.Citation9
Nanotechnology is providing science with a new platform in medicine which has the potential to provide disciplines such as diagnostics and clinical medicine, as well as basic research, with new materials in the nanometer range that have many far reaching applications. Nanomaterials, such as nanoparticles, differ from other materials due to a number of special characteristics, including small particle size, large surface area, shape, chemical composition, and charge.Citation10 Together these characteristics give nanoparticles numerous advantages over other delivery systems, and the targeted delivery of drugs using nanocarriers for the treatment of respiratory diseases is a major focus of interest.Citation10 Many approaches have been undertaken for the delivery of nano-structures, such as micelles, liposomes, and nanoparticles to the lungs via the use of nebulization for suspensions and dry powder carriers.Citation10
In 2007, Yacobi et al investigated the effects of ultrafine ambient particulate suspensions, polystyrene nanoparticles, quantum dots, and single-walled carbon nanotubes on transmonolayer resistance (Rt) and equivalent short-circuit current on rat alveolar cell epithelia monolayers. They found that Rt was reduced after apical exposure of rat alveolar cell epithelia monolayers to a variety of nanomaterials, including ultrafine ambient particulate suspensions, positively charged quantum dots, and single-walled carbon nanotubes at varying concentrations.Citation11 In turn, other research groups have investigated the interaction of silver nanoparticles on voltage-activated Na+ currents in hippocampal CA1 neurons, with results indicating that silver nanoparticles may alter the action potential of these neurons by reducing voltage-gated sodium currents.Citation12
Even though there have been many advances in the area of bionanoscience, there is still very little known about the complex interaction of nanoparticles with the cell membrane on airway epithelial cells, and the effect that this interaction can have on many diverse cellular processes. Nanoparticles at the cell membrane have the potential to interact with numerous cell signaling receptors, ion channels, transporters, and cytoskeleton machinery which work to control and regulate basic cellular and physiological processes. Recent studies have shown that gold nanoparticles coated with antibodies have the ability to alter signaling processes and regulate membrane receptor internalization in human breast cancer cells.Citation13 Furthermore, titanium dioxide nanoparticles, upon contact with BEAS-2B human bronchial cells, can induce programmed cell death via the mitochondrial apoptosis pathway.Citation14
Taken together, our research group was interested in studying the interactions of nanoparticles with ion channels in human airway epithelial cells, with a specific focus on the CFTR Cl− channel. We hypothesized that nanoparticles could modulate epithelial ion channel function. To this end, we wanted to investigate if nanoparticles could affect apically located CFTR Cl− channels and/or other ion channels (basolateral K+ channels) and cotransporters, either directly or indirectly. To test this hypothesis, we used commercially available 20 nm negatively charged polystyrene nanoparticles (N20). We chose to use polystyrene nanoparticles because this specific type of nanoparticle is being increasingly characterized for use in nanosensors and drug nanocarrier investigations.Citation15,Citation16 Polystyrene nanoparticles have also been shown to interact with proteins to form a “protein corona” upon interaction with biological fluids,Citation17 and more recently studies by Salomon and Ehrhardt have found that polystyrene nanoparticles can affect the function of P glycoprotein/ MDR1 membrane transporters in A549 human alveolar epithelial cells.Citation18
We used the Ussing chamber technique to study the effects of N20 on ion fluxes in human airway submucosal cells, ie, Calu-3. Any findings with the Ussing chamber were then verified with the single channel patch clamp technique using baby hamster kidney cells stably transfected with a wild-type CFTR Cl− channel. We discovered that acute apical exposure of lung epithelial cells to N20 promoted transepithelial ion transport, affecting not only apically located CFTR Cl− channels but also basolateral K+ channels. The mechanism of short-circuit current activation by nanoparticles was found to be mainly dependent on calcium-dependent and cyclic nucleotide-dependent phosphorylation of CFTR Cl− channels. Our patch clamp data reveal an exciting new phenomenon that nanoparticles can interact and activate CFTR Cl− channels directly. No other study to our knowledge has shown the direct activation of airway ion channels by polystyrene nanoparticles.
Materials and methods
Chemicals
Polystyrene latex beads were purchased from Invitrogen (carboxyl-modified 20 nm, F-8787; Carlsbad, CA) and corelabeled with fluorophore according to the manufacturer’s specifications. Before all experiments, test solutions of polystyrene nanoparticles were dispersed using a sonicator (8890-MTH; Cole–Parmer, Vernon Hills, IL) to prevent aggregation. Forskolin (10 mM; LC Laboratories, Woburn, MA) was made as a 1000-fold stock solution in ethanol. Diphenylamine-2-carboxylate 1 M was dissolved in dimethyl sulfoxide and was prepared fresh for each experiment. Furosemide 100 mM was sourced from Sigma-Aldrich (St Louis, MO) and was dissolved in distilled H2O with one drop of concentrated NaOH 5 N. XE991 (10 mM) was a generous gift from Dr BS Brown (DuPont, Wilmington, DE) and was dissolved in HCl 0.1 N. Nystatin was prepared as a 180 mg/mL stock solution in dimethyl sulfoxide and sonicated for 30 seconds just before use. Clotrimazole 30 mL was sourced from Sigma-Aldrich and was made as a 1000-fold stock solution in ethanol. Carbachol 100 mM and S-nitrosoglutathione 100 mM were purchased from Sigma-Aldrich and dissolved in distilled H2O. NG-nitro-l-arginine methyl ester (l-NAME) 100 mM was purchased from Alexis Biochemicals (San Diego, CA). 1H-[1, 2, 4] oxadiazolol-[4, 3-a] quinoxalin-1-one (ODQ, 10 mM; Tocris Cookson, St Louis, MO) and thapsigargin 1 mM (Sigma-Aldrich) were prepared as 1000-fold stock solutions in dimethyl sulfoxide. All other items were purchased from Sigma-Aldrich.
Cell lines and culture
A Calu-3 cell line was obtained from the American Type Culture Collection (ATCC HBT-55) and maintained as a monolayer culture in plastic T-75 cm2 tissue culture flasks. The cells were grown in Dulbecco’s Modified Eagle’s Medium, a low glucose media 1 g/L also containing sodium pyruvate 110 mg/L and supplemented with 10% fetal bovine serum, gentamicin sulfate 5 μg/mL, penicillin G 6 μg/mL, and streptomycin 10 μg/mL. Cells were maintained at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. When confluent, the cell line was detached enzymatically with trypsin-ethylenediamine tetra-acetic acid and subcultured into a new cell culture flask. The medium was replaced every 2 days. Cells were used for experiments between passages 21–45. For transepithelial measurements, Calu-3 cells were seeded at a density of 2 × 105 cells/cm2 onto Snapwell™ inserts (0.45 μm, 1 cm2; Corning, Cambridge, MA). For the first 7 days, cells were grown in liquid-covered culture, with the basolateral and apical media being changed every 2 days (2 mL media basolaterally, 500 μL apically). After day 7, from initial seeding on Snapwell inserts, all cells were grown using air interface culturing in which medium was added only to the basolateral side of the inserts (2 mL). Cells were used in transepithelial experiments on days 12–22.
Size and zeta potential determination for polystyrene nanoparticles
The size and zeta potential for N20 was determined using a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK). Zeta potential and size was measured at 37°C, three repeats per sample. Polystyrene nanoparticles were diluted in Krebs–Henseleit solution containing (in mM): NaCl, 116.4; KCl, 4.7; CaCl2, 1.8; MgCl2, 1.2; NaH2PO4, 0.78; NaHCO3, 25.0; and 10 glucose (pH was 7.4 ± 0.1 when bubbled with 95% O2 and 5% CO2). Measurements were conducted using a concentration of 100 μg/mL. The dielectric constant of the dispersant was set at 78.5, viscosity as for water at 0.8872 cP, and refractive index at 1.333.
Cytotoxicity
Lactate dehydrogenase assay and polystyrene nanoparticles
To evaluate the cytotoxicity of N20 alone on Calu-3 cells, 104 × cells/100 μL of cell culture medium were plated into 96-well round-bottom plates, and the lactate dehydrogenase assay was performed. Extracellular lactate dehydrogenase release was measured using a colorimetric CytoTox 96® non-radioactive cytotoxicity assay kit from Promega (Madison, WI) following the manufacturer’s instructions, with absorbance recorded at 492 nm (FLUO-star, OPTIMA; BMG Labtech, Offenburg, Germany). N20 was diluted in serum-free medium and was added to cells (in triplicate) at concentrations of 1, 10, 25, 50, 100, and 200 μg/mL. Cell survival was determined 24 hours posttreatment with N20 by the lactate dehydrogenase assay described above. To determine the percentage cytotoxicity, the average absorbance of the triplicate was calculated. As control, extracellular release of lactate dehydrogenase was obtained from unexposed cells (low control), and maximum release of lactate dehydrogenase was obtained by lysis of cells with the supplied lysis buffer (high control). The following equation was applied to the experimental values obtained: percent cytotoxicity = release of lactate dehydrogenase from exposed cells – release of lactate dehydrogenase (low control)/ release of lactate dehydrogenase (high control) – release of lactate dehydrogenase (low control) × 100.
MTT assay and polystyrene nanoparticles
Cytotoxicity was measured using a CellTiter 96® nonradioactive cell proliferation assay kit from Promega following the manufacturer’s instructions. Calu-3 cells were seeded in triplicate at a density of 104 × cells/100 μL of cell culture medium into 96-well plates. The following day, Calu-3 cells were treated with N20 under serum-free conditions at the following concentrations; 1, 10, 25, 50, 100, and 200 μg/mL for 24 hours. The 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay evaluates mitochondrial activity (cell growth and cell death) and is performed by adding a premixed optimized dye solution to culture wells. Absorbance is recorded at 570 nm (FLUO-star). The recorded absorbance is directly proportional to the number of live cells. To determine the percentage cytotoxicity, the average of the triplicates was calculated for each concentration tested. Results were calculated as a percentage of the control values (unexposed cells), where percent cytotoxicity = (experimental abs570 nm of exposed cells/abs570 nm of unexposed cells) ×100.
Transepithelial measurements
Ussing chamber studies were carried out using the apparatus and methods described by Duta et alCitation19 and Duszyk.Citation20 Prior to use in transepithelial measurement studies, all Calu-3 cell inserts were fed with complete fresh media for 1 hour and were washed for 30 minutes in Krebs–Henseleit solution (2 mL basolaterally, 500 μL apically). Calu-3 cell monolayers were grown on Snapwell inserts for at least 12 days prior to mounting into modified Ussing chambers. The cell monolayers were bathed at a temperature of 37°C in Krebs– Henseleit solution. Chemicals were added from concentrated stocks and all chambers were continuously mixed by bubbling the Krebs–Henseleit solution with 95% O2 and 5% CO2 to maintain a constant pH of 7.4. The transepithelial potential difference was clamped to zero using a DVC 1000 voltage/ current amplifier (World Precision Instruments, Sarasota, FL), and the resulting short-circuit current was recorded by Ag-AgCl2 electrodes, using 3 M KCl agar bridges. The short-circuit current was allowed to stabilize for up to approximately 10 minutes or more before the application of nanoparticles or other tested chemicals. Nanoparticles were always added apically. The transepithelial resistance was calculated using Ohm’s law, by measuring current changes in response to 0.5 mV pulses.
Basolateral membrane K+ currents
The effects of the nanoparticles on basolateral membrane K+ channels were measured after permeabilization of the apical membrane with nystatin 180 μg/mL and establishment of an apical-to-basolateral K+ concentration gradient. Apical NaCl was replaced by equimolar amounts of potassium gluconate and basolateral NaCl with sodium gluconate. The concentration of calcium gluconate was increased from 2.5 mM to 5 mM. Under such conditions, the contribution of apical Cl− channels to the short-circuit current is eliminated and the measured short-circuit current represents K+ currents as these ions move down the concentration gradient through basolateral K+ channels.
Anion substitution studies
HCO3 − free transepithelial measurements and polystyrene nanoparticles
The effects of nanoparticles on the short-circuit current were assessed in the absence of bicarbonate ions. A bicarbonate-free Krebs–Henseleit solution was used with apical and basolateral HCO3 − ions exchanged for HEPES 10 mM. The overall pH was adjusted to 7.4 and the solutions were mixed with O2.
Low Cl− transepithelial measurement and polystyrene nanoparticles
The effects of nanoparticles on the short-circuit current were measured under low chloride conditions. A low chloride Krebs–Henseleit solution was used with apical and basolateral NaCl being exchanged for sodium gluconate 116 mM and KCl for potassium gluconate 4.7 mM. The concentration of calcium gluconate was increased to 5 mM to compensate for the Ca2+-buffering capacity of the gluconate.
Patch clamp experiments
These experiments were carried out using baby hamster kidney cells stably transfected with wild-type human CFTR, as previously described.Citation21 The experiments were performed in the excised, inside-out configuration of the patch clamp technique. In all experiments, the pipette solution contained (in mM) sodium gluconate 150, MgCl2 2, and HEPES 10, and the bath solution NaCl 150, MgCl2 2, and HEPES 10. All solutions were adjusted to pH 7.4 with NaOH 5 N. The membrane potential was held at 0 mV and the junction potential was compensated. Following patch excision, channel activity was assessed by the addition of N20 50 μg/mL to the bath solution. Controls (untransfected baby hamster kidney cell patches) were treated with N20 50 μg/mL, as described earlier. This concentration was chosen based on Ussing chamber studies showing that N20 50 μg/mL resulted in maximal stimulation of transepithelial anion fluxes. In the second set of experiments, the patch excision was exposed to N20, as already discussed, in the presence of a 10–20 nM protein kinase as a catalytic subunit and MgATP 0.2 to 1 mM in the bath. Au(CN)2 500 μM was used as a blocker of the CFTR channel. Patch clamp recordings were analyzed by the Clampfit program, and open probability (Po) was calculated using standard techniques.
Data analysis
Nanoparticle concentration-response was fitted with the following equation:
The data points show the mean ± the standard error of the mean. The short-circuit current is expressed in units of μA/cm2. All data are presented as group means ± standard error of the mean for the individual experiments. Statistical analysis of the mean difference between multiple groups was determined by one-way analysis of variance, followed by Bonferroni post hoc tests or between two groups by paired/ unpaired t-tests as appropriate. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism (v 5.00 for Windows; GraphPad Software, San Diego, CA).
Results
Nanoparticle characterization
N20 nanoparticles according to manufacturer’s specifications were 20 nm in size and negatively charged. Our own measurements have shown that N20 had a Z average (nm) of 45.2 ± 0.01, a zeta potential of −15.1 ± 0.8 mV, and a polydispersity index of 0.22 ± 0.002 in Krebs–Henseleit solution. Furthermore, our lactate dehydrogenase cytotoxicity studies showed that N20 1–200 μg/mL did not exert any cytotoxic effects on Calu-3 cells for up to 24 hours (n = 4, data not shown). Also, we did not observe any significant changes in Calu-3 cell viability by MTT analysis after cells were exposed to N20 1–200 μg/mL over a 24-hour period (cell viability at the highest concentration of N20 tested was 92.5% ± 5.7% vs control, P > 0.05, n = 3).
Nanoparticles activate anion secretion in Calu-3 cells
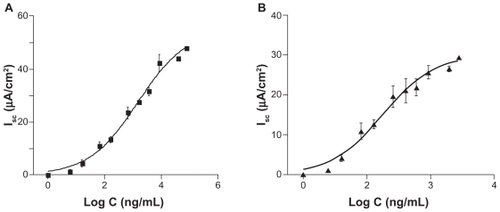
Acute apical exposure of Calu-3 cells to N20 activated the short-circuit current in a concentration-dependent manner. shows the fitting of Equationequation (1)(1) to the experimental data. The current activated by N20 showed a biphasic response, ie, an initial peak followed by a plateau. Both peak and plateau values were used to determine the EC50 and Hill coefficient (,) with both values working at low concentrations (1457 ng/mL for peak short-circuit current and 315.5 ng/mL for plateau short-circuit current, respectively). Therefore, in our remaining anion transport studies, plateau and peak short-circuit current values are reported where appropriate to determine the effect of N20 on the short-circuit current.
Figure 1 Concentration-response curves for short-circuit current activation by N20 in Calu-3 cells. A) Peak EC50 = 1457 ng/mL and a Hill coefficient of 0.52 ± 0.035 (n = 4). B) Plateau EC50 = 315.5 ng/mL and a Hill coefficient of 0.54 ± 0.045 (n = 4).
Apically located CFTR Cl− channels serve as the primary conductive pathway for anion secretion in Calu-3 cells and are the main contributors to the short-circuit current in transepithelial studies. To determine the mechanism(s) of action of nanoparticles on short-circuit current activation, a number of pharmacological agents were used to probe the actions of nanoparticles on ion channels. N20 10 μg/mL increased short-circuit current by 12.4 ± 0.4 μA/cm2 (plateau Isc, ). The subsequent addition of forskolin, an activator of adenylyl cyclase (10 μM), further increased the short-circuit current by 15.8 ± 2.9 μA/cm2 (plateau Isc, ) and this effect was blocked by diphenylamine-2-carboxylate, a blocker of CFTR (1 mM).Citation22 In the absence of nanoparticles, forskolin activated the short-circuit current by 16.2 ± 1.0 μA/cm2 (plateau Isc, ). However, the subsequent addition of N20 after forskolin resulted in a significantly lower short-circuit current response (4.7 ± 2.4 μA/cm2) when compared with control responses (P < 0.05, n = 3, ), showing that there is in fact an overlap between the mechanism of short-circuit current activation by N20 and forskolin. A summary of the statistical analysis of is shown in .
Figure 2 The effect of N20 on transepithelial anion secretion in Calu-3 cells. A) Activation of the short-circuit current by N20 10 μg/mL apical. Forskolin 10 μM both sides further increased short-circuit current in N20 pretreated cells. B) Activation of the short-circuit current by N20 was significantly reduced when cells had been prestimulated with forskolin. C) Pretreatment of cells with diphenylamine-2-carboxylate 1 mM apical reduced the effects of N20. Furosemide 1 mM basolaterally inhibited the effect of N20 on the short-circuit current. D) Statistical analysis of the effects of N20 on the short-circuit current in the presence and absence of forskolin. Note: *P < 0.05 (n = 3, one-way analysis of variance).
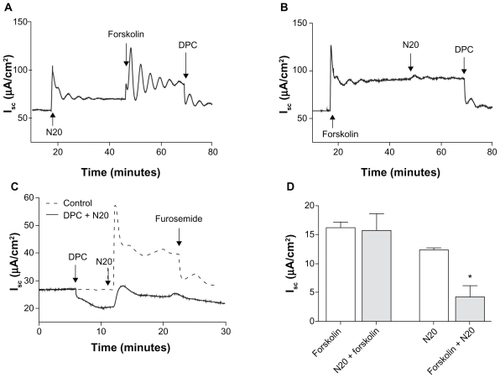
As shown on , diphenylamine-2-carboxylate 1 mM reduced the basal short-circuit current by 9.2 ± 1.6 μA/cm2 prior to the addition of N20 10 μg/mL. However, this pretreatment with diphenylamine-2-carboxylate only partially inhibited the activation of short-circuit current by N20 (5.0 ± 2.3 μA/cm2) when compared with control responses of 28.72 ± 3.5 μA/cm2 (peak Isc, P < 0.05, unpaired t-test, n = 3, ). Furosemide, a blocker of the NKCC cotransporter located on the basolateral membrane, exerted little effect on inhibition of the short-circuit current caused by N20 in the presence of diphenylamine-2-carboxylate ().
Nanoparticles activate K+ channels in Calu-3 cells
Observations made in previous experiments () which showed that N20 was capable of increasing the short-circuit current in the presence of a blocked CFTR and also that furosemide had minimal effect on the blocking of short-circuit current in the presence of diphenylamine-2-carboxylate, suggested that N20 in addition to CFTR has other ion channel and/or cotransporter targets. Thus, we began to investigate the interactions between basolateral K+ channels and nanoparticles, because these channels can also act as a driving force for anion secretion in Calu-3 cells.
Nystatin was used to permeabilize the apical membrane in the presence of an established transepithelial ion gradient to measure K+ currents. In our experiments, permeabilization of the apical membrane with nystatin 180 μg/mL led to an increase in the short-circuit current of 97.3 ± 13.4 μA/cm2. N20 10 μg/mL apical, activated K+ current in Calu-3 cells by a further 56.2 ± 6.7 μA/cm2 (). The activation of K+ currents by N20 was inhibited by XE991, a potent and selective inhibitor of K+ channels,Citation23 with an average reduction in the short-circuit current of 57.6 ± 9.0 μA/cm2.
Figure 3 The effects of N20 on basolateral membrane K+ currents. A) N20 10 μg/mL apical activated K+ currents. The effect of N20 was inhibited by XE991 10 μM basolateral. B) Prestimulation of cell monolayers with forskolin 10 μM both sides abolished the effects of N20 on K+ currents; effect of forskolin was inhibited by XE991. C) Clotrimazole 30 μM basolateral did not block the effects of N20 on K+ currents.
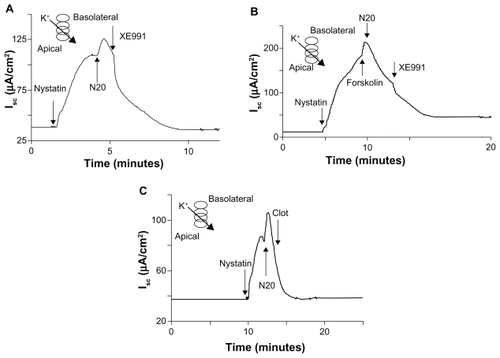
To investigate if this effect of nanoparticles on K+ channels was cAMP-dependent, Calu-3 cells were pretreated with forskolin 10 μM both sides. Forskolin increased the short-circuit current by 39.3 ± 9.2 μA/cm2 and abolished the effects of N20 10 μg/mL on K+ currents (). Moreover, forskolin-induced activation of K+ channels was XE991-sensitive, decreasing the short-circuit current by 114.2 ± 17.6 μA/cm2, thus again showing that N20 activates K+ currents in a cAMP-dependent manner (). Further confirmation that N20 acts through the cAMP-pathway came from data showing that N20-activated K+ currents in Calu-3 cells were not sensitive to clotrimazole, a known inhibitor of Ca2+-regulated K+ channels ().
Anion substitution studies
In airway epithelial cells such as Calu-3, the primary basolateral entry pathways for Cl− and HCO3 − anions are through the NKCC and Na+-HCO3 − cotransporters, respectively. Both anions have been shown to leave the cell via CFTR Cl− channels. We wanted to examine if either one of these two anions (Cl− or HCO3 −) is preferentially secreted by Calu-3 cells upon stimulation by N20. In these experiments, we decided to use forskolin short-circuit current responses as a point for comparison with N20-induced short-circuit current responses.
When we compared the responses of forskolin and N20 on the short-circuit current in normal Krebs–Henseleit solution, we found no significant differences between their stimulation of short-circuit current; 36.3 ± 1.5 vs 29.8 ± 4.8 μA/ cm2, respectively (peak Isc, P > 0.05, n = 3). However, when we compared the response of N20 (11.7 ± 2.0 μA/cm2) on short-circuit current under conditions free of HCO3 − with that of forskolin (35.7 ± 8.8 μA/cm2) under the same conditions, we found a significant decrease in N20-induced short-circuit current responses (peak Isc, P < 0.05, n = 4). Similarly, we observed a reduction in N20-induced short-circuit current responses under low Cl− conditions (26.1 ± 3.5 μA/cm2) when compared with forskolin (58.8 ± 0.7 μA/cm2, peak Isc, P < 0.05, n = 4). A summary of these results is shown in . In all experiments, the addition of diphenylamine-2-carboxylate 1 mM apical reduced the activated short-circuit current to baseline levels (data not shown).
Figure 4 Effects of anion substitution on forskolin and N20 short-circuit current responses. A summary of the effects of anion substitution on forskolin and N20 short-circuit current responses in Calu-3 cell monolayers. Note: *P < 0.05 (n = 4, one-way analysis of variance).
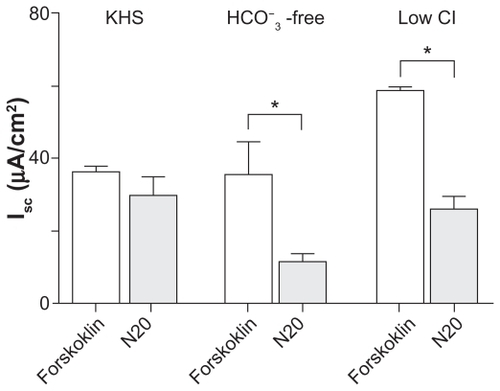
For each set of experiments carried out using either normal KH, HCO3 − free, or low Cl− solutions, the ratio for the N20-activated short-circuit current as a factor of the forskolin-activated short-circuit current was calculated (N20-induced short-circuit current/forskolin induced short-circuit current). The ratio value under normal Krebs–Henseleit solution conditions was found to be 0.82 ± 0.1. The ratio value we obtained under HCO3 − free and low Cl− conditions was 0.32 ± 0.06 and 0.42 ± 0.08, respectively. Therefore, N20-induced short-circuit current responses are dependent on both HCO3 − and Cl− transport by airway cells.
Nanoparticles affect Ca2+-mediated anion secretion in Calu-3 cells
In these studies, we used carbachol and thapsigargin (an endoplasmic reticulum Ca2+-ATPase inhibitor), both known agonists of Ca2+-dependent signaling, to probe the actions of nanoparticles on Ca2+-mediated short-circuit current responses in Calu-3 cells. Carbachol, a cholinergic agonist which causes the release of Ca2+ ions from intracellular stores (100 μM basolateral), increased short-circuit current by 12.4 ± 0.6 μA/cm2 (peak Isc, data not shown). The subsequent addition of N20 10 μg/mL apical further increased the short-circuit current by 57.3 ± 1.8 μA/cm2 (peak Isc), and this effect was blocked by BaCl2 5 mM, basolateral a blocker of K+ channels, by 30.8 ± 1.0 μA/cm2 (data not shown). On the other hand, in the absence of carbachol, N20 activated the short-circuit current by 37.9 ± 5.8 μA/cm2 (peak Isc, ). Furthermore, the addition of carbachol after N20 significantly increased the short-circuit current by 123 ± 22.6 μA/cm2 (peak Isc, ). As expected, BaCl2 5 mM, basolateral reduced the short-circuit current by 29.7 ± 3.8 μA/cm2 (). A summary of the statistical analysis is shown in .
Figure 5 Effect of N20 on calcium-mediated anion secretion in Calu-3 cells. A) Carbachol-induced Isc (CBL) 100 μM basolateral was significantly increased by prestimulation with N20 10 μg/mL apical, and BaCl2 5 mM basolateral as shown inhibited Isc. B) Statistical analysis of the effects of N20 on Isc in the presence and absence of carbachol. *P < 0.05 (n = 3, one-way analysis of variance). C) Activation of Isc by thapsigargin (Thap) 1 μM both sides in Calu-3 cells. N20 further increased Isc in thapsigargin pretreated cells. D) Thapsigargin-induced Isc was significantly increased by prestimulation with N20. Furosemide 1 mM basolateral and BaCl2, as shown in C and D, inhibited Isc responses. E) Statistical analysis of the effects of N20 on Isc in the presence and absence of thapsigargin. Note: *P < 0.05 (n = 3, one-way analysis of variance).
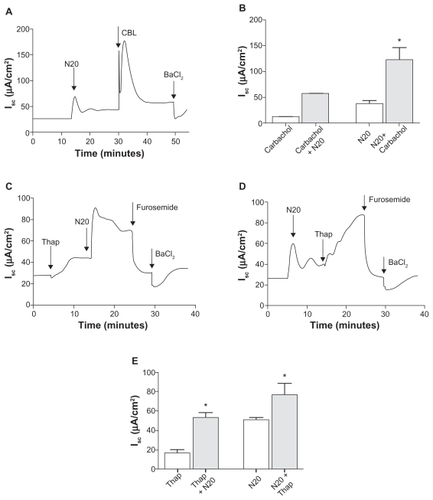
To elucidate further the mechanisms by which N20 synergistically increased short-circuit current responses in the presence of carbachol, we used thapsigargin to investigate if N20 was affecting Ca2+-dependent cell signaling directly via the involvement of intracellular Ca2+ stores. Thapsigargin 1 μM bilaterally was found to increase the short-circuit current by 16.5 ± 3.6 μA/cm2 in Calu-3 cells (peak Isc, ). The subsequent addition of N20 10 μg/mL apical further increased the short-circuit current by 53.5 ± 5.2 μA/cm2 (peak Isc, ) and this effect was blocked by furosemide 1 mM basolateral, decreasing the stimulated short-circuit current by 44.5 ± 4.8 μA/cm2, and BaCl2 5 mM basolateral transiently reduced the short-circuit current by a further 13.3 ± 0.5 μA/cm2. In the absence of thapsigargin, N20 activated the short-circuit current by 49.4 ± 3.4 μA/cm2 (peak Isc, ). Furthermore, the addition of thapsigargin after N20 increased the short-circuit current by 65.8 ± 3.6 μA/cm2 (peak Isc, ). Furosemide 1 mM basolateral and BaCl2 5 mM basolateral both reduced the short-circuit current by 49.4 ± 11.4 and 10.5 ± 0.1 μA/cm2, respectively (Figure D). The statistical analysis of results in is shown in . Overall, these results indicate that N20 can act upon Ca2+-dependent cell signaling to drive anion secretion, and that intracellular calcium stores may be the source of this cation in Calu-3 cells.
Nanoparticles affect soluble guanylyl cyclase-cGMP cell signaling involved in anion secretion
The nitric oxide-soluble guanylyl cyclase-cGMP pathway is an important pathway for anion secretion in respiratory cells.Citation20,Citation24 Therefore, we wanted to investigate the effect of N20 on the short-circuit current in the presence and absence of the nitric oxide donor, S-nitrosoglutathione. In the absence of S-nitrosoglutathione, N20 10 μg/mL apical activated the short-circuit current by 11.8 ± 3.3 μA/cm2 (peak Isc). In the presence of S-nitrosoglutathione 100 μM both sides, N20 increased the short-circuit current by only 2.4 ± 0.7 μA/cm2 (peak Isc, data not shown). S-nitrosoglutathione-induced short-circuit current responses (peak Isc), in the presence and absence of N20, were 39.1 ± 1.6 and 26.1 ± 2.5 μA/cm2, respectively. These responses were found to be significantly different from each other (peak Isc, P < 0.05, n = 3, data not shown).
Next, we examined the response of N20 in the presence of L-NAME, a nitric oxide synthase inhibitor which inhibits the generation of endogenous nitric oxide. The application of L-NAME 1 mM both sides caused a transient reduction in the basal short-circuit current (). The subsequent addition of N20 10 μg/mL apical increased the short-circuit current by 9.4 ± 1.2 μA/cm2. Finally, the addition of diphenylamine-2-carboxylate 1 mM apical and BaCl2 5 mM basolateral reduced the short-circuit current by 6.6 ± 0.4 and 1.3 ± 0.4 μA/cm2, respectively (). This increase in short-circuit current as a result of N20 was found not to be significantly different from the effects detected in the absence of L-NAME (control, P > 0.05, unpaired t-test, n = 3). In controls, the short-circuit current was inhibited by the addition of diphenylamine-2-carboxylate 1 mM apical (5.4 ± 0.2 μA/cm2) and BaCl2 5 mM basolateral (1.01 ± 0.3 μA/cm2, ).
Figure 6 Effect of N20 on cGMP-mediated transepithelial anion secretion in Calu-3 cells. A) Prestimulation of cells with S-nitrosoglutathione 100 μM both sides resulted in a significant decrease in N20-induced responses. *P < 0.05 (n = 3, one-way analysis of variance). B) Pretreatment of cells with NG-nitro-l-arginine methyl ester 1 mM both sides did not inhibit the effects of N20 10 μg/mL apical on short-circuit current activation. C) Pretreatment of cells with 1H-[1, 2, 4] oxadiazolol-[4, 3-a] quinoxalin-1-one 10 μM both sides inhibited the effects of N20 on short-circuit current. As shown in B) and C), short-circuit current was significantly reduced by the application of diphenylamine-2-carboxylate 1 mM apical and BaCl2 5 mM basolateral.
![Figure 6 Effect of N20 on cGMP-mediated transepithelial anion secretion in Calu-3 cells. A) Prestimulation of cells with S-nitrosoglutathione 100 μM both sides resulted in a significant decrease in N20-induced responses. *P < 0.05 (n = 3, one-way analysis of variance). B) Pretreatment of cells with NG-nitro-l-arginine methyl ester 1 mM both sides did not inhibit the effects of N20 10 μg/mL apical on short-circuit current activation. C) Pretreatment of cells with 1H-[1, 2, 4] oxadiazolol-[4, 3-a] quinoxalin-1-one 10 μM both sides inhibited the effects of N20 on short-circuit current. As shown in B) and C), short-circuit current was significantly reduced by the application of diphenylamine-2-carboxylate 1 mM apical and BaCl2 5 mM basolateral.](/cms/asset/27aaa4a2-e521-4c6d-b3cf-c05d2034e53e/dijn_a_21145_f0006_b.jpg)
The effects of nitric oxide on short-circuit current in Calu-3 cells are known to be mediated by the activation of soluble guanylyl cyclase enzymes through increases in cGMP.Citation20,Citation25 To determine whether this pathway was involved in short-circuit current activation by N20, Calu-3 cells were pretreated with a selective inhibitor of soluble guanylyl cyclase, ODQ 10 μM both sides. ODQ caused a transient reduction in short-circuit current and prevented the activation of the short-circuit current by N20 10 μg/mL apical (P < 0.05, unpaired t-test, n = 3, ). As expected, the short-circuit current was inhibited by the addition of diphenylamine-2-carboxylate 1 mM apical (6.9 ± 1.8 μA/cm2) and BaCl2 5 mM basolateral (3.1 ± 0.5 μA/cm2, ). These results indicate that soluble guanylyl cyclase and downstream cGMP signaling plays a crucial role in N20-mediated short-circuit current activation in Calu-3 cells.
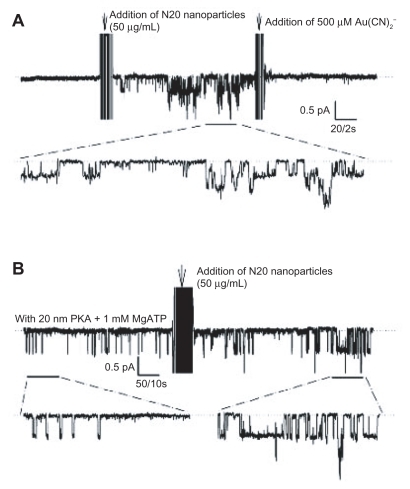
Excised inside-out patch clamp recordings of N20-stimulated CFTR activity
shows that the addition of N20 50 μg/mL to the bath of the membrane patch activated CFTR Cl− channels with a Po value of 0.22 ± 0.031 (n = 3). The channel activity could be blocked by 500 μM Au(CN)2 −, ie, a blocker of the CFTR channel. shows the stimulation of CFTR channels by 20 nM protein kinase A and 1 mM MgATP. The subsequent addition of N20 50 μg/mL to the bath further increased the channel activity. In other studies, stimulation of the cAMP pathway by protein kinase A/MgATP resulted in the activation of CFTR channels with Po = 0.07 ± 0.05 (n = 3). Subsequent addition of N20 50 μg/mL to the bath further increased CFTR activity to Po = 0.34 ± 0.09 (P < 0.05, paired t-test, n = 3). Control baby hamster kidney cell patches lacking expression of wild-type CFTR Cl− channels showed no response when stimulated with N20 50 μg/mL (data not shown).
Figure 7 Patch clamp studies of the effects of N20 on single CFTR Cl− channel function. A) Silent CFTR Cl− channels were activated by N20 50 μg/mL and blocked by Au(CN)2 − 500 μM in excised, inside-out patches. B) Stimulation by N20 of CFTR Cl− channels preactivated with protein kinase A 20 nM and MgATP 1 mM in excised inside-out patches (n = 3, dashed lines indicate closed CFTR state).
Discussion
We investigated a hypothesis that polystyrene nanoparticles have the ability to act as modulators of ion channel function in human airway epithelial cells. The main novel findings of this study were that acute exposure of epithelial cells to N20 led to the activation of transepithelial anion transport, an effect inhibited by diphenylamine-2-carboxylate, a CFTR Cl− channel blocker. The effectiveness of N20 to activate CFTR Cl− channels was comparable with that of the cAMP elevating agonist, forskolin. N20 activated cAMP-dependent basolateral K+ channels and affected three distinct cell signaling systems concerned with ion channel activation in respiratory cells, ie, the cAMP, Ca2+, and soluble guanylyl cyclase-cGMP pathways. Our patch clamp studies also show the direct activation of single CFTR Cl− channels by N20.
We know that nanoparticle characteristics, including size, surface modification, and charge, are major factors influencing biological interactions with nanomaterials. Such characteristics can exert a profound effect on respiratory tissues, and thus be an important criterion for short-circuit current activation in Calu-3 cells. Nanoparticle surface modifications could influence the affinity of N20 to the plasma membrane. Wang et al examined the effects of polystyrene nanoparticles on single-component phospholipid bilayers using fluorescence and calorimetry experiments after mixing together either positively or negatively charged nanoparticles of approximately 20 nm in size in suspension with liposomes.Citation26 The findings of their study showed that there was surface reconstruction where the nanoparticles absorbed onto the bilayer membrane, with negatively charged nanoparticles inducing local gelation in fluid bilayers and positively charged nanoparticles inducing gelled membranes to fluid locally.Citation26 In 2010, Yang and Ning investigated the interactions between charged nanoparticles (positive, negative, and uncharged) and charge-neutral phospholipid membranes by coarse-grained molecular dynamic simulations.Citation27 Their results were discussed in terms of free energy, entropy, and enthalpy, where they describe an energy barrier existing between lipids and charged nanoparticles. They concluded that electrostatic attractions help to improve the adhesion of charged nanoparticles to phospholipid membranes, and that increases in electrostatic energy can result in charged nanoparticles being almost fully wrapped by membrane.Citation27 Chen et al showed using whole cell patches that cationic nanoparticles working at noncytotoxic concentrations can cause nanoscale defects in the plasma membrane of human embryonic kidney and human epidermoid carcinoma cells.Citation28 Thus, membrane fluidity may be affected by nanoparticle charge.
Polystyrene nanoparticles have been shown specifically to affect processes and membrane structure dynamics on airway epithelial cells. Salomon and Ehrhardt showed that small carboxylated and sulfated polystyrene nanoparticles reduced P glycoprotein-mediated effluxes of Rh123 from alveolar airway cells, A549.Citation18 They hypothesized that this effect was due to the direct interaction and interference of polystyrene nanoparticles with P glycoprotein function. Brandenberger et alCitation29 exposed human pulmonary epithelial cells to fluorescent polystyrene nanospheres (41 nm) without surface charge modifications. They found that these particles induced changes in the apical plasma membrane surface area as measured by design-based stereology. They concluded that this observed enlargement was dependent on particle surface area dose.Citation29 Interestingly, our own data show that short-circuit current activation by N20, working at noncytotoxic concentrations, occurs in a concentration-dependent manner in Calu-3 cells. Therefore, nanoparticle– membrane interactions with subsequent secondary activation of cell signaling pathways may play an important role in our observed effects.
We found that the effects of N20 on CFTR-driven short-circuit current responses in Calu-3 cells were abolished by ODQ, a selective inhibitor of soluble guanylyl cyclase,Citation30 implicating the soluble guanylyl cyclase-cGMP pathway and cGMP-dependent phosphorylation of CFTR Cl− channels in the actions of N20. Pretreatment of epithelial cells with an inhibitor of nitric oxide synthase, ie, l-NAME, did not modify the activator effects of N20. Because nitric oxide synthase is present and active in Calu-3 cells regulating anion secretion,Citation20 these data together indicate that soluble guanylyl cyclase, but not nitric oxide synthase-dependent responses, are targeted by N20. Our experiments using agonists of adenylate (forskolin) and guanylyl (S-nitrosoglutathione) cyclase showed that prestimulation of either system largely abolishes the effects of the nanoparticles. Similarly, the inhibition of soluble guanylyl cyclase by ODQ abolished nanoparticle-driven short-circuit current responses.
There are a number of levels where the cAMP and cGMP systems can cross-talk with each other. This includes activation of respective protein kinases (A and G) involved in channel gating, as well as cGMP-mediated inhibition of cAMP breakdown by the family of phosphodiesterases.Citation31 In turn, nanoparticle-stimulated increases in cyclic nucleotide levels will increase protein kinase-controlled protein phosphorylation and the subsequent phosphorylation of sites on the regulatory domain of CFTR Cl− channels, which in turn will facilitate the exit of Cl− through apically located CFTR Cl− channels, an effect supported by an increased bioavailability of Ca2+.Citation20,Citation32
Other groups have also found that nanomaterials can affect Ca2+ and cAMP-signaling systems in neuronal and fibroblast cell models. Tang et al showed that nanoparticles can affect intracellular Ca2+ levels in mammalian cells.Citation33 They demonstrated that unmodified cadmium selenium quantum dots elevate cytoplasmic calcium levels in primary cultures of rat hippocampal neurons. The mechanism for this activation is still unknown, although the group did identify that the increase in cytoplasmic calcium involved both extracellular Ca2+ influx and internal Ca2+ release. Also, extracellular influx of Ca2+ could only be partially inhibited by a Ca2+ channel antagonist (eg, verapamil), whereas internal Ca2+ release was abolished by treatment of cells with clonazepam, a specific inhibitor of mitochondrial Na+-K+ exchangers, and with antrolene, an antagonist of ryanodine receptors in the endoplasmic reticulum.Citation33
Many research groups are interested in the manipulation of magnetic nanoparticles, such as magnetic tweezers or ligand-coated magnetic nanoparticles which are capable of mechanical activation of cell receptors.Citation34 Magnetic particles used to investigate mechanotransduction, such as integrin-bound collagen-coated ferric oxide beads, have revealed that tension applied on human fibroblasts can cause Ca2+ spikes which can modulate cellular functions.Citation35 Meyer et al, using suspended bovine endothelial cells and a magnetic twisting device demonstrated that, when a controlled twisting (shear) stress of 15.6 dyne per cm−2 was applied to ligand-coated magnetic microbeads in contact with these cells, cAMP production was increased due to adenylyl cyclase activation.Citation36 Thus, the literature shows that mechanical stress applied to the cell surface can alter both Ca2+ and cAMP signaling.
N20 had distinct targets other than CFTR Cl− channels. K+ ions are recycled across the basolateral membrane by K+ channels which work to maintain the negative potential difference of the cell interior. The opening of basolateral K+ channels in epithelial cells is an important process for anion secretion because it causes the cell to become hyperpolarized which, in turn, increases the electrical gradient for Cl− ions to exit across the apical surface of the epithelium.Citation37 Our studies show that N20 can also activate cAMP-dependent basolateral K+ channels. The evidence for this comes from experiments which show that N20-activated K+ currents were inhibited by XE991, an inhibitor of cAMP-sensitive basolateral K+ channels, and that the effects of N20 on basolateral K+ currents were abolished by pretreating cells with forskolin. It is likely that stimulation of cAMP, along with significant cross-talk between membrane systems,Citation38 can lead to indirect activation of cAMP-regulated K+ channels upon apical exposure of Calu-3 cells to N20.
The NKCC1 cotransporter isoform is expressed on the basolateral membrane of secretory epithelia, where it acts in concert with other transporters and ion channels, such as CFTR, basolateral K+ channels, and Na+-K+ pumps, to produce transepithelial Cl− secretion. NKCC is expressed on virtually all mammalian cells, where it functions to maintain cell volume.Citation39 The observed effects of N20 on Cl− and HCO3 − transport are most likely indirect consequences of short-circuit current activation, eg, the NKCC cotransporter is activated to restore intracellular chloride levels due to the efflux of Cl− from cells by activated CFTR Cl− channels and, in turn, HCO3 − transport is more likely linked to the activation of basolateral K+ channels.Citation37 Finally, the cyclic forskolin response observed after stimulation of cells with N20 () could be the result of Cl− bursts out of the cell due to the unsynchronized effects of NKCC cotransporters and CFTR Cl− channels working to restore depleted intracellular chloride levels.
We used the inside-out configuration of the patch clamp technique because it allowed us to study the interaction of nanoparticles with the intracellular domains of CFTR Cl− channels. No other research group to our knowledge has investigated the effect of nanoparticles on ion channel function using this configuration of the technique. In 2009, Zhao et al showed using the whole cell patch clamp technique that zinc nanoparticles enhanced the current amplitudes of sodium and potassium by a mechanism mainly relying on increasing the opening number of sodium channels and delaying rectifier potassium channels.Citation40 Overall, that study showed that zinc nanoparticles have the ability to affect ionic homeostasis and physiological processes in rat hippocampal CA3 pyramidal neurons. We have shown in baby hamster kidney cells that N20 can directly activate CFTR Cl− channels both in the presence and absence of ATP and protein kinase A. Studies using nucleotide binding domain mutants of CFTR Cl− channels, such as G551D and G1349D, demonstrate that most CFTR activators have decreased affinity for activating mutant CFTR Cl− channels.Citation41 Therefore, putative binding sites on the nucleotide-binding domains of CFTR Cl− channels are proposed to be target(s) for many CFTR activators.Citation42,Citation43 However, our data indicate that the primary mechanism of single CFTR Cl− channel activation by N20 may not involve the nucleotide-binding domains or regulatory domain of CFTR, because these sites have been previously stimulated by ATP and protein kinase A. Therefore, we cannot rule out the possibility that N20 may in some way influence structural changes in the transmembrane domains of CFTR Cl− channels, resulting in channel activation.
Altogether, our data demonstrate that polystyrene nanoparticles can affect processes at the plasma membrane on airway epithelial cells specifically with regard to ion channel function and ionic homeostasis. Furthermore, once inside airway cells, our patch clamp data demonstrate that polystyrene nanoparticles could potentially affect CFTR Cl− channel function directly. This is an exciting observation because it gives us an insight as to how nanoparticles might behave once inside the cell. More experimental data is needed to clarify the precise sequence of molecular events following the exposure of epithelial cells to nanoparticles and also the interesting data that reveal the selectivity for the stimulation of cAMP-regulated K+ channels in comparison with Ca2+-regulated ones on the basolateral membrane. Finally, experimental validation with regard to other nanoparticle types, eg, silica, titanium, and zinc, would have to be done in future studies to establish if NP composition is an important factor underlying the ability of polystyrene nanoparticles to activate CFTR Cl− channels and affect ion transport in airway epithelial cells.
Conclusion
Our studies demonstrate that polystyrene nanoparticles cannot be considered as a simple neutral vehicle for drug delivery. Polystyrene nanoparticles in the respiratory system may influence cell signaling systems (cyclic nucleotide and calcium) as a result of nanoparticle-membrane interactions. Finally, after endocytosis by epithelial cells lining the airways, polystyrene nanoparticles have the potential to interact directly with ion channels such as CFTR.
Acknowledgments
This work was supported by a Strategic Research Cluster grant from Science Foundation Ireland, as well as support from FÁS Science Challenge Ireland and the Canadian Cystic Fibrosis Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- RiordanJRRommensJMKeremBIdentification of the cystic fibrosis gene: Cloning and characterisation of complementary DNAScience1989245106610732475911
- CollinsFSCystic fibrosis: Molecular biology and therapeutic implicationsScience19922567747791375392
- GadsbyDCVerganiPCsanádyLThe ABC protein turned chloride channel whose failure causes cystic fibrosisNature200644047748316554808
- OstedgaardLSBaldurssonOWelshMJRegulation of the cystic fibrosis transmembrane conductance regulator Cl− channel by its R domainJ Biol Chem20012767689769211244086
- VerganiPLocklessSWNairnACCFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domainsNature200543387688015729345
- US Cystic Fibrosis Foundation Available at: http://www.genet.sickkids.on.ca/cftrAccessed April 8, 2011
- LivraghiARandellSHCystic fibrosis and other respiratory diseases of impaired mucus clearanceToxicol Pathol20073511612917325980
- SalinasDHaggiePMThiagarajahJRSubmucosal gland dysfunction as a primary defect in cystic fibrosisFASEB J20041943143315596485
- McAuleyDFElbornJSCystic fibrosis: Basic sciencePaediatr Respir Rev200019310012531100
- AzarmiSRoaWHLöbenbergRTargeted delivery of nanoparticles for the treatment of lung diseasesAdv Drug Deliv Rev20086086387518308418
- YacobiNRPhuleriaHCDemaioLNanoparticle effects on rat alveolar epithelial cell monolayer barrier propertiesToxicol In Vitro2007211373138117555923
- LiuZRenGZhangTAction potential changes associated with the inhibitory effects on voltage-gated sodium current of hippocampal CA1 neurons by silver nanoparticlesToxicology200926417918419683029
- JiangWKimBYRutkaJTNanoparticle-mediated cellular response is size-dependentNat Nanotechnol2008314515018654486
- ShiYWangFHeJTitanium dioxide nanoparticles cause apoptosis in BEAS-2B cells through the caspase 8/t-Bid-independent mitochondrial pathwayToxicol Lett2010196212720362650
- BorisovSMMayrTMistlbergerGPrecipitation as a simple and versatile method for preparation of optical nanochemosensorsTalanta2009791322133019635366
- TsapisNBennettDJacksonBTrojan particles: Large porous carriers of nanoparticles for drug deliveryProc Natl Acad Sci U S A200299120011200512200546
- LundqvistMStiglerJEliaINanoparticle size and surface properties determine the protein corona with possible implications for biological impactsProc Natl Acad Sci U S A2008105142651427018809927
- SalomonJJEhrhardtCNanoparticles attenuate P-glycoprotein/ MDR1 function in A549 human alveolar epithelial cellsEur J Pharm Biopharm20117739239721093586
- DutaVDutaFPuttaguntaLRegulation of basolateral Cl− channels in airway epithelial cells: The role of nitric oxideJ Membr Biol200621316517417468957
- DuszykMRegulation of anion secretion by nitric oxide in human airway epithelial cellsAm J Physiol Lung Cell Mol Physiol2001281L45045711435220
- LinsdellPGongXMultiple inhibitory effects of Au(CN)2 − ions on cystic fibrosis transmembrane conductance regulator Cl− channel currentsJ Physiol2002540293811927666
- McCartyNAMcDonoughSCohenBNVoltage-dependent block of the cystic fibrosis transmembrane conductance regulator Cl− channel by two closely related arylaminobenzoatesJ Gen Physiol19931021238397274
- MacVinishLJGuoYDixonAKXe991 reveals differences in K(+) channels regulating chloride secretion in murine airway and colonic epitheliumMol Pharmacol20016075376011562437
- WatkinsDNPeroniDJBasclainKAExpression and activity of nitric oxide synthases in human airway epitheliumAm J Respir Cell Mol Biol1997166296399191464
- VaandragerASmolenskiATillyBCMembrane targeting of cGMP-dependent protein kinase is required for cystic fibrosis transmembrane conductance regulator Cl− channel activationProc Natl Acad Sci U S A199895146614719465038
- WangBZhangLBaeSCNanoparticle-induced surface reconstruction of phospholipid membranesProc Natl Acad Sci U S A2008105181711817519011086
- YangLiNingGuThermodynamics of charged nanoparticle adsorption on charge-neutral membranes: a simulation studyJ Phys Chem B20101142749275420146444
- ChenJHesslerJAPutchakayalaKCationic nanoparticles induce nanoscale disruption in living cell plasma membraneJ Phys Chem B2009113111791118519606833
- BrandenbergerCRothen-RutishauserBBlankFParticles induce apical plasma membrane enlargement in epithelial lung cell line depending on particle surface area doseRespir Res2009102219284624
- MoroMARusselRJCellekScGMP mediates the vascular and platelet actions of nitric oxide: Confirmation using an inhibitor of the soluble guanylyl cyclaseProc Natl Acad Sci U S A199693148014858643658
- ZaccoloMMovsesianMAcAMP and cGMP signalling cross-talk: Role of phosphodiesterases and implications for cardiac pathophysiologyCirc Res20071001569157817556670
- NamkungWFinkbeinerWEVerkmanASCFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell culturesMol Biol Cell2010212639264820554763
- TangMXingTZengJUnmodified CdSe quantum dots induce elevation of cytoplasmic calcium levels and impairment of functional properties of sodium channels in rat primary cultured hippocampal neuronsEnviron Health Perspect200811691592218629314
- SniadeckiNJMinireview: A tiny touch: Activation of cell signalling pathways with magnetic nanoparticlesEndocrinology201015145145720016028
- GlogauerMFerrierJMcCullochCAMagnetic fields applied to collagen-coated ferric oxide beads induce stretch-activated Ca2+ flux in fibroblastsAm J Physiol Cell Physiol1995269C10931104
- MeyerCJAlenghatFJRimPMechanical control of cyclic AMP signalling and gene transcription through integrinsNat Cell Biol2000266666810980709
- CowleyEALinsdellPCharacterization of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3J Physiol200253874775711826162
- DriesscheWVKreindlerJLMalikABInterrelations/cross talk between transcellular transport function and paracellular tight junctional properties in lung epithelial and endothelial barriersAm J Physiol Lung Cell Mol Physiol2007293L52052417601795
- MatthewsJBMolecular regulation of Na+-K+-2Cl− cotransporter (NKCC1) and epithelial chloride secretionWorld J Surg20022682683011948363
- ZhaoJXuLZhangTInfluences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neuronsNeurotoxicology20093022023019146874
- MoranOGaliettaLJZegarra-MoranOBinding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domainsCell Mol Life Sci20056244646015719171
- CaiZSheppardDNPhloxine B interacts with the cystic fibrosis transmembrane conductance regulator at multiple sites to modulate channel activityJ Biol Chem2002277195461955311904291
- AiTBompadreSGWangXCapsaicin potentiates wild-type and mutant cystic fibrosis transmembrane conductance regulator chloridechannel currentsMol Pharmacol2004651415142615155835