Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrotic interstitial pneumonia. And, oxidation/antioxidant imbalance plays an important role in the progress of IPF. Fullerene is considered to be a novel “structural” antioxidant. This study aimed to explore if water-soluble C60 (C60(OH)22) can exhibit antifibrotic activity in its antioxidant role.
Methods
Healthy C57BL/6J mice were randomly grouped and induced pulmonary fibrosis by intratracheal injection of bleomycin.
Results
The survival rate of mice was observed and found that 10mg/kg was the optimal dose of water-soluble C60 for pulmonary fibrosis. We observed that water-soluble C60 can alleviate the severity of pulmonary fibrosis by observing the chest computed tomography, pulmonary pathology, and content of collagen, alpha smooth muscle actin and fibronectin in lung. Compared with bleomycin group, ROS, the content of TNF-α in BALF, and the number of fibroblasts was significantly decreased and the number of type II alveolar epithelial cells was increased after treatment with C60.
Conclusion
Therefore, thanks to its powerful antioxidant action, water-soluble C60 can reduce the severity of pulmonary fibrosis induced by bleomycin in mice.
Introduction
Idiopathic pulmonary fibrosis (IPF) is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia with unknown causes. The median survival time of IPF patients is 2–3 years from diagnosis with a 5-year mortality of 30–50%. There is no effect therapy except lung transplantation.Citation1 Furthermore, potent new agents are urgently needed.
The pathogenic mechanisms of IPF remain unclear. It is currently believed that IPF originates from the abnormal repair of alveolar epithelium after repeated minor damage, which leads to scarring and lung tissue destruction.Citation2 For the past few years, it is discovered that oxidation/antioxidant imbalance plays an important role in the progress of IPF.Citation3 Reduced oxidative stress can reduce the degree of pulmonary fibrosis.Citation4
Oxidative stress is an imbalance between oxidants such as reactive oxygen species (ROS) and antioxidants, which may affect lipids, DNA, carbohydrates and proteins. ROS is free radical generated physiologically during oxidative phosphorylation, including superoxide anion, hydroxyl and hydrogen peroxide. It has various physiological roles such as causing cell dysfunction and death. Research has shown that oxidants and myeloperoxidase of cells in bronchoalveolar lavage fluid (BALF) increased to a higher concentration in IPF patients,Citation5 and the epithelial injury in IPF was related to the increasing activity of peroxidase. Daniil et al had determined oxidative burden in serum based on analysis of hydroperoxidesCitation6 and found that systemic oxidative stress level in IPF was significantly higher than that in control, and it was negatively correlated with dyspnea severity and lung function marked by forced vital capacity and diffuse lung capacity for carbon monoxide. In summary, the oxidation/antioxidant imbalance is related to the progression of idiopathic pulmonary fibrosis.
Fullerene was considered to be a novel “structural” antioxidant and characterized as a “radical sponge” by Krusic et alCitation7. Water-soluble fullerene C60 is nontoxic at low physiological concentrations,Citation8,Citation9 which is able to penetrate through the membrane of cellsCitation10,Citation11 and have strong antioxidant properties.Citation8 Moreover, it has been found that fullerene C60 possesses anticancer activity.Citation12,Citation13
In this study, we aimed to explore if water-soluble C60 (C60(OH)22) can exhibit antifibrotic activity in a murine model of bleomycin (BLM) induced pulmonary fibrosis by its antioxidant role.
Materials and Methods
Animals
Specific pathogen-free (SPF) C57BL/6J mice were purchased from the Animal Center of Peking University (Beijing, China), and fed under SPF conditions in the basic Medical Research Center of Beijing Chaoyang Hospital affiliated to Capital Medical University. Male mice aged 7–8 weeks and weighing 22–25g were used for the preparation of bleomycin-induced pulmonary fibrosis in mice. The care and use of laboratory animals in this study was according to the National Institutes of Health guide strictly. This study was approved by the Animal Care and Utilization Committee of Capital Medical University (AEEI-2014-034).
Preparation of Water-Soluble C60 and Pirfenidone
C60(OH)22 with purity of more than 99.9% was obtained from Suzhou Dade Carbon Nanotechnology Co., Ltd. C60(OH)22 was dissolved in sterilized and apyrogenic physiological saline (0.9%). Pirfenidone was purchased from Beijing Continent Pharmaceutical Co. Ltd.
Experimental Design
The animals were randomly divided into 10 groups in the following way.
For exploring the preventive effects are there 1) NS (normal saline) group–mice received saline only; 2) BLM group–mice received BLM, treatment–saline (per day); 3) 1 mg/kg group–mice received BLM, treatment–C60(OH)22 1 mg/kg/day; 4) 10 mg/kg group–mice received BLM, treatment–C60(OH)22 10 mg/kg/day; 5) 100 mg/kg group–mice received BLM, treatment–C60(OH)22, 100 mg/kg/day; 6) 500 mg/kg group–mice received BLM, treatment–C60(OH)22, 500 mg/kg/day.
For exploring the therapeutic effects are there 1) NS group – mice received saline only; 2) BLM group–mice received BLM, treatment–saline (per day); 3) BLM + C60 group–mice received BLM, treatment–C60(OH)22 10 mg/kg/day; 4) BLM + pirfenidone group–mice received BLM, treatment–pirfenidone 300 mg/kg/day.
All mice (except NS group) were treated with a single intratracheal injection of 3.5 (preventive) or 2.0 (therapeutic) mg/kg body weight BLM hydrochloride diluted by saline.Citation14 C60(OH)22 and pirfenidone were administered intraperitoneal injection beginning from the first day (preventive) and 14th day (therapeutic) until mice were sacrificed after 21 days (preventive) and 28 days (therapeutic) after intratracheal injection of BLM (). Moreover, blood, bronchoalveolar lavage fluid (BALF) and lung tissue were collected as previously described,Citation15 the left lung was fixed by 10% formaldehyde solution, the right lung was frozen at −80°C for using.
Lung Histological Assessment
The collection of mouse lungs for histology was performed as conventional treatment.Citation16 Briefly, the lung was fixed (by 10% formaldehyde solution), dehydrated, paraffin-embedded, and cut into 4-μm sections. Lung sections were stained with H&E and Masson’s trichrome stain for assessment of pathological changes.Citation17 Immunostaining was performed using antibodies against Collagen I (Abcam, Cambridge, USA), surfactant protein C (Abcam, Cambridge, USA) and alpha smooth muscle (α-SMA) antibody (Abcam, Cambridge, USA).
Hydroxyproline Assay
The content of hydroxyproline in the lung were measured using conventional methods.Citation18 The content of hydroxyproline was calculated based upon the lung weight, and the data were expressed as micrograms of hydroxyproline in each gram of lung tissue.
ELISA
Concentrations of TGF-β1 and TNF-α in lung tissue, BALF and plasma were determined using ELISA kits (Invitrogen, USA) according to the manufacturer’s instructions.
Protein Extraction and Western Blot Analysis
Frozen lung tissues were homogenized using RIPA buffer (Solarbio, Beijing, China) containing 1:100 phenylmethylsulfonyl fluoride (PMSF) and phosphatase inhibitors. The total protein concentration was resuspended in protein loading buffer containing 5% mercaptoethanol. The proteins were separated by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad, Hercules, CA, USA) using a Mini-Protean electrophoresis module assembly (Bio-Rad) at 80 mV and transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA) for 100 min using the Mini Trans-Blot electrophoresis transfer cell (Bio-Rad) at 300 mA. The membranes were treated with anti-rabbit or anti-mouse IgG (LI-COR, Lincoln, NE, USA). Positive bands were visualized, and the intensity of the bands was evaluated using a LI-COR Odyssey. The primary antibodies used were anti-fibronectin antibody (Abcam, Cambridge, USA), anti-α-SMA antibody (Abcam, Cambridge, USA) and anti-β actin antibody (Abcam, Cambridge, USA).
Survival Analysis
We performed survival analysis in each preventive group (n=9–10). Survival time of each mouse was recorded until 21st day after BLM or saline administration.
Measurement of ROS Concentration
The content of ROS was measured by using DCFH-DA as the manufacturer’s instructions, which was purchased from Nanjing Jiancheng Bioengineering Institute.
Statistical Analysis
All data were expressed as the mean ± SD. All experiments were conducted with three independent replications. GraphPad Prism 6 (GraphPad, La Jolla, CA, USA) was used for statistical analyses using t-test or one-way analysis of variance; P < 0.05 was considered to indicate statistically significant differences in all comparisons.
Results
The Optimal Dose of C60(OH)22 for Pulmonary Fibrosis Was 10 mg/kg/day
Survival Time
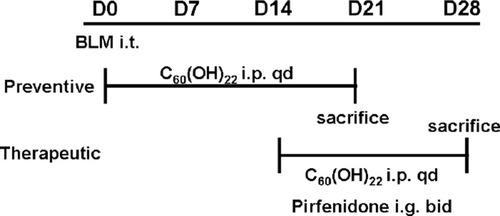
The mortality had a significant difference between preventive groups. All mice in NS group survived for 21 days. However, substantial mortality of mice was demonstrated in BLM group, and a significant change of median survival time existed between the group treated with C60(OH)22 10 mg/kg/day and the BLM group (). Up to the 21st day, 30% of mice survived in BLM group, 44.4% in C60(OH)22 1 mg/kg group and 100 mg/kg group, 66.7% in 10 mg/kg group, and no mice survived in 500 mg/kg group. These results indicated that C60(OH)22 could protect mice from death when mice were treated with the dose of 10 mg/kg/day and 1 mg/kg/day, but the mice treated with C60(OH)22 by the dose of 100 mg/kg/day had no difference with BLM group, what is more, C60(OH)22 with a dose of 500 mg/kg/day had injury but no advantage.
Figure 2 Effect of C60(OH)22 on survival time and body weight. The doses of 1, 10, 100 and 500 mg/kg of C60(OH)22 were administered intraperitoneal injection to the mice for 21 days after intratracheal injection of BLM. Kaplan–Meier survival curves (A) and body weight change (B) were noted.
Abbreviations: NS, no treatment; BLM, bleomycin.
Body Weight
Body weight of mice in preventive groups (except NS group) had a significant decrease. However, compared to BLM group, the mice in 10 mg/kg group had a slight decrease, but the difference was not significant ().
C60(OH)22 Had a Therapeutic Effect in the Advanced Stages of BLM-Induced Pulmonary Fibrosis
Computed Tomography Images of Mice Lung
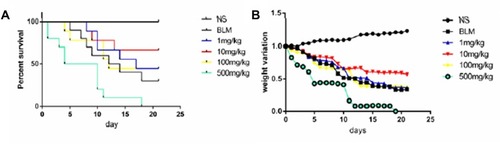
CT images of mice lung on the 28th day after BLM or saline administration are shown in . Lungs in the BLM groups demonstrated some consolidated shadows compared with the NS group (). However, compared with BLM group, the images of lungs in BLM+C60 group revealed decreased density and diffuse ground-glass opacities with or without areas of consolidation (), but quantitative evaluation was difficult.
Figure 3 Examination of the antifibrotic effects of C60(OH)22 and pirfenidone on BLM-induced pulmonary fibrosis in mice. Chest CT and H&E and Masson-stained sections were observed after BLM or saline administration in the NS, BLM, BLM+C60 and BLM+pirfenidone groups (A). Collagen deposition was monitored by immunohistochemical analysis (A), and date was reported as means ± SD (D). Fibronectin and α-SMA were quantified by Western blot (B). The content of hydroxyproline was determined in lung tissues, which is a marker of collagen deposition (C). *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: NS, no treatment; BLM, bleomycin; HYP, hydroxyproline; col Ι, collagen Ι.
H&E and MASSON
BLM-induced pulmonary injury and fibrosis in mice were monitored by histopathological analysis. It was shown that BLM instillation produced a significant increase of fibrosis in the lung by H&E-stained sections. BLM-induced fibrotic mice demonstrated increased pulmonary parenchymal distortion, showing thicker alveolar membrane, collapsed alveoli, and inflammatory cell infiltration (). Masson’s trichrome staining of collagen was used to demonstrate that BLM induced severe collagen deposition in mice. However, C60(OH)22 and pirfenidone administration markedly ameliorated lung injuries and evidently attenuated collagen deposition ().
Hydroxyproline
Hydroxyproline was concentrated in BLM-induced inflammatory response, and there was a positive correlation between the level of hydroxyproline and collagen. As illustrated (), the hydroxyproline was significantly increased after BLM administration while reversed after C60(OH)22 and pirfenidone treatment.
Collagen Ι, α-SMA and Fibronectin
We subsequently investigated the ability of C60(OH)22 to modulate the expression of collagen Ι, α-SMA and fibronectin which were key markers of pulmonary fibrosis. The results showed that the lung tissues from BLM treated mice were markedly up-regulated the expression of collagen Ι, α-SMA and fibronectin (). Impressively, levels of α-SMA and fibronectin were obviously reduced after C60(OH)22 and pirfenidone treatment (). The expression of collagen Ι was monitored by immunohistochemical analysis. Lung sections of mice in NS group showed weak positive staining of collagen Ι, while intratracheal instillation of BLM resulted in markedly increased expression of collagen Ι in lung tissues. The administration C60 or pirfenidone largely decreased the expression of collagen Ι compared with BLM group (). These results directly reflected the attenuation effect of C60(OH)22 on BLM-induced pulmonary fibrosis in mice.
Mechanisms for the Therapeutic Effect of C60(OH)22 on BLM-Induced Pulmonary Fibrosis
ROS
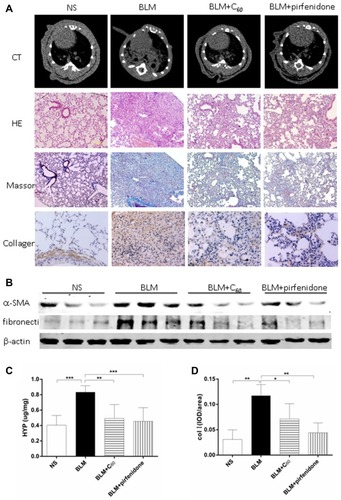
The levels of ROS were determined in the lung tissue in order to investigate the effect of C60(OH)22 against the oxidative stress in BLM-induced lung fibrosis (). A significant rise in the level of ROS in lung tissue was observed in the BLM treated animals, while it was reversed after C60(OH)22 administration (P<0.05).
Figure 4 Mechanisms for the therapeutic effect of C60(OH)22 on BLM-induced pulmonary fibrosis. The content of ROS was tested in lung tissues (A). AEC II was marked with SPC by immunohistochemistry (B), and date was reported as mean ± SD (C). α-SMA was monitored by immunohistochemical (B), as the marker of fibroblast, and was reported as mean ± SD (D). The expression of TGF-β1 and TNF-α in lung tissue, BALF and plasma were determined and reported as means ± SD (E and F). *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Abbreviations: NS, no treatment; BLM, bleomycin; SPC, surfactant protein C; AEC II, type 2 alveolar epithelial cells; ROS, reactive oxygen species.
TGF-β1 and TNF-α
TGF-β1 and TNF-α play important roles in the pathogenesis and exacerbation of pulmonary fibrosis,Citation19 and we assessed the effect of C60(OH)22 on their expression. As shown in ., the expression of TGF-β1 and TNF-α in lung tissue, BALF and plasma were significantly increased in BLM group compared with NS group while it was decreased after C60(OH)22 administration, which suggested that C60(OH)22 could inhibit the expression of TGF-β1 and TNF-α.
AEC II and α-SMA
Type 2 alveolar epithelial cells (AEC II) were marked with surfactant protein C (SPC) by immunohistochemistry (). Compared with NS group, lung sections of BLM group were characterized by a significant decrease in the number of AEC II, while, the number rose again in mice receiving C60 group. α-SMA was monitored by immunohistochemical to reflect the amount of fibroblast. The sections in the NS group showed weak positive staining of α-SMA, while increasing expression was showed in BLM group (). These results directly reflected that C60(OH)22 could decrease the apoptosis of AEC IIand the amount of fibroblast by easing oxidative stress.
Discussion
In this study, water-soluble C60 was found to serve as an antifibrotic agent for BLM-induced mouse pulmonary fibrosis, which has been used as a classic model for the evaluation of the antifibrotic effect.Citation20 The results showed that BLM could induce pulmonary fibrosis and cause mice death, body weight loss and exacerbated lung histopathology abnormalities with collagen deposition. However, these adverse consequences caused by BLM were effectively attenuated by water-soluble C60. Our study had shown that water-soluble C60 possessed significant anti-inflammation and anti-oxidant effects on BLM-induced lung damage in mice. To the best of my knowledge, it is the first time to detect the role of C60, a novel “structural” antioxidant, in pulmonary fibrosis mice induced by bleomycin.
It has been reported by many studies that oxidative stress is closely related to the development of IPF.Citation5,Citation6,Citation21 The excessive production of ROS plays a key role in oxidative stress, and ROS is also an important medium in the process of pulmonary fibrosis. It has been shown that ROS can make single-stranded DNA damage and fracture, resulting in AEC injury and necrosis. And the excessive production of ROS can induce AEC apoptosis by activating the death receptor pathway,Citation22 mitochondrial death pathwayCitation23 and endoplasmic reticulum-associated death.Citation24 Therefore, the activation of fibroblast and the deposition of collagen are promoted, and then, pulmonary fibrosis progresses. Our study showed that water-soluble C60 can significantly reduce the concentration of ROS in lung tissue. And we observed that water-soluble C60 could reduce the apoptosis and/or necrosis of AEC and decreased fibroblast activation. These results suggest that the development of IPF would be inhibited by water-soluble C60.
In addition, ROS could regulate signal transduction.Citation25 ROS could increase the expression of TGF-β1, TNF-α, interleukin, platelet-derived growth factor Chaudhary et al.Citation26 Among them, TGF-β1 plays a key role in pulmonary fibrosis which is called the “master switch” of organ fibrosis (including pulmonary fibrosis).Citation27 To a certain extent, the degree of inflammation and fibrosis is depended on the quantity of TGF-β1.Citation28,Citation29 Apoptosis of AEC was mediated by TNF-α. TNF-α which is an important factor in the process of pulmonary fibrosis was up-regulated the expression of TGF-β1.Citation30 The over-expression of these cytokines aggravated the damage and apoptosis of cells and advanced the development of pulmonary fibrosis. While ROS has been shown to activate these cytokines, and these cytokines also increase the production of ROS in human lung fibroblasts, so a vicious circle formed. In our study, we observed that the content of TGF-β1 and TNF-α significantly decreased in the lungs of mice after treated with C60(OH)22 compared with BLM group, suggesting water-soluble C60 could reduce the expression of TGF-β1 and TNF-α. Therefore, eventually, alleviate inflammation and inhibit fibrosis by reducing the content of ROS.
IPF is a chronic and fatal disease, and the management of IPF is highly debatable and no curative treatment has been developed except for pirfenidone and nintedanib. However, adverse events such as gastrointestinal symptoms, photosensitivity and fatigue might occur at pirfenidone administration.Citation31,Citation32 And nintedanib slowed disease progression with side-effect profiles such as hepatic enzymes increased, Mazzei et alCitation33 However, Gharbi et al have shown that fullerene C60 is a powerful antioxidant with no acute or subacute toxicity.Citation8,Citation9 Baati et al have found that the lifespan of rats would be prolonged by repeated oral administration of fullerene C60.Citation34 Therefore fullerene C60 is likely to be a new management of IPF.
Conclusion
It was demonstrated that water-soluble C60, a novel “structural” antioxidant, could reduce the severity of pulmonary fibrosis induced by BLM in mice. Therefore, water-soluble C60 probably has a great potential for IPF therapeutic applications.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China [Nos. 81430001, 81470258 and 81871328] and Beijing Natural Science Foundation [No. 7182149].
Disclosure
The authors declare no conflicts of interest in this work.
References
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi:10.1164/rccm.2009-040GL21471066
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134(2):136–151. doi:10.7326/0003-4819-134-2-200101160-0001511177318
- Wadsworth RM. Oxidative stress and the endothelium. Exp Physiol. 2008;93(1):155–157. doi:10.1113/expphysiol.2007.03868718165435
- Teixeira KC, Soares FS, Rocha LG, et al. Attenuation of bleomycin-induced lung injury and oxidative stress by N-acetylcysteine plus deferoxamine. Pulm Pharmacol Ther. 2008;21(2):309–316. doi:10.1016/j.pupt.2007.07.00617904883
- Cantin AM, North SL, Fells GA, et al. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest. 1987;79(6):1665–1673. doi:10.1172/JCI1130053034979
- Daniil ZD, Papageorgiou E, Koutsokera A, et al. Serum levels of oxidative stress as a marker of disease severity in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2008;21(1):26–31. doi:10.1016/j.pupt.2006.10.00517161968
- Krusic PJ, Wasserman E, Keizer PN, et al. Radical reactions of c60. Science. 1991;254(5035):1183–1185. doi:10.1126/science.254.5035.118317776407
- Gharbi N, Pressac M, Hadchouel M, et al. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005;5(12):2578–2585. doi:10.1021/nl051866b16351219
- Johnston HJ, Hutchison GR, Christensen FM, et al. The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol Sci. 2010;114(2):162–182. doi:10.1093/toxsci/kfp26519901017
- Qiao R, Roberts AP, Mount AS, et al. Translocation of C60 and its derivatives across a lipid bilayer. Nano Lett. 2007;7(3):614–619. doi:10.1021/nl062515f17316055
- Prylutska S, Bilyy R, Overchuk M, et al. Water-soluble pristine fullerenes C60 increase the specific conductivity and capacity of lipid model membrane and form the channels in cellular plasma membrane. J Biomed Nanotechnol. 2012;8(3):522–527. doi:10.1166/jbn.2012.140422764423
- Shi J, Wang L, Gao J, et al. A fullerene-based multi-functional nanoplatform for cancer theranostic applications. Biomaterials. 2014;35(22):5771–5784. doi:10.1016/j.biomaterials.2014.03.07124746227
- Panchuk RR, Prylutska SV, Chumakl VV, et al. Application of C60 Fullerene-doxorubicin complex for tumor cell treatment in vitro and in vivo. J Biomed Nanotechnol. 2015;11(7):1139–1152. doi:10.1166/jbn.2015.205826307837
- Li XX, Jiang DY, Huang XX, et al. Toll-like receptor 4 promotes fibrosis in bleomycin-induced lung injury in mice. Genet Mol Res. 2015;14(4):17391–17398. doi:10.4238/2015.December.21.826782380
- Jiang D, Liang J, Hodge J, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114(2):291–299. doi:10.1172/JCI1686115254596
- Jiang DY, Huang XX, Jing G, et al. Pulmonary fibrosis in a mouse model of sarcoid granulomatosis induced by booster challenge with Propionibacterium acnes. Oncotarget. 2016;7(23):33703–33714. doi:10.18632/oncotarget.939727203210
- Hübner RH, Gitter W, El MNE, et al. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques. 2008;44(4):507–11, 514–7. doi:10.2144/000112729
- Sisson TH, Mendez M, Choi K, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181(3):254–263. doi:10.1164/rccm.200810-1615OC19850947
- Willis BC, Borok Z. TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L525–34. doi:10.1152/ajplung.00163.200717631612
- Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med. 2011;17(5):355–361. doi:10.1097/MCP.0b013e328349ac2b21832918
- Gao F, Koenitzer JR, Tobolewski JM, et al. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J Biol Chem. 2008;283(10):6058–6066. doi:10.1074/jbc.M70927320018165226
- Liu G, Beri R, Mueller A, Kamp DW. Molecular mechanisms of asbestos-induced lung epithelial cell apoptosis. Chem Biol Interact. 2010;188(2):309–318. doi:10.1016/j.cbi.2010.03.04720380827
- Fu YQ, Fang F, Lu ZY, et al. N-acetylcysteine protects alveolar epithelial cells from hydrogen peroxide-induced apoptosis through scavenging reactive oxygen species and suppressing c-Jun N-terminal kinase. Exp Lung Res. 2010;36(6):352–361. doi:10.3109/0190214100367858220653470
- Jorgensen E, Stinson A, Shan L, et al. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer. 2008;8(1):229. doi:10.1186/1471-2407-8-22918694499
- Rees MD, Kennett EC, Whitelock JM, et al. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic Biol Med. 2008;44(12):1973–2001. doi:10.1016/j.freeradbiomed.2008.03.01618423414
- Chaudhary NI, Roth GJ, Hilberg F, et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007;29(5):976–985. doi:10.1183/09031936.0015210617301095
- Sime PJ, O’Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol. 2001;99(3):308–319. doi:10.1006/clim.2001.500811358425
- Cuzzocrea S, Genovese T, Failla M, et al. Protective effect of orally administered carnosine on bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1095–104. doi:10.1152/ajplung.00283.200617220373
- Kang HR, Lee CG, Homer RJ, et al. Semaphorin 7A plays a critical role in TGF-β1–induced pulmonary fibrosis. J Exp Med. 2007;204(5):1083–1093. doi:10.1084/jem.2006127317485510
- Failla M, Genovese T, Mazzon E, et al. Pharmacological inhibition of leukotrienes in an animal model of bleomycin-induced acute lung injury. Respir Res. 2006;7(1):137. doi:10.1186/1465-9921-7-13717118201
- Takeda Y, Tsujino K, Kijima T, et al. Efficacy and safety of pirfenidone for idiopathic pulmonary fibrosis. Patient Prefer Adherence. 2014;8:361–370. doi:10.2147/PPA24711695
- Valeyre D, Albera C, Bradford WZ, et al. Comprehensive assessment of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19(5):740–747. doi:10.1111/resp.1229724836849
- Mazzei ME, Richeldi L, Collard HR. Nintedanib in the treatment of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2015;9(3):121–129. doi:10.1177/175346581557936525862013
- Baati T, Bourasset F, Gharbi N, et al. The prolongation of the lifespan of rats by repeated oral administration of [60]fullerene. Biomaterials. 2012;33(19):4936–4946. doi:10.1016/j.biomaterials.2012.03.03622498298