?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The purpose of this study was to develop folate-poly (PEG-cyanoacrylate-co-cholesteryl cyanoacrylate) (FA-PEG-PCHL)-modified freeze-dried liposomes for targeted chemotherapy using docetaxel as a model drug.
Methods
FA-PEG-PCHL was synthesized and its cytotoxicity was evaluated by CCK-8 assay in L929. Docetaxel-loaded liposomes modified by FA-PEG-PCHL were prepared by an organic solvent injection method and lyophilized to obtain freeze-dried FA-PEG-PCHL-docetaxel liposomes (FA-PDCT-L). Two carcinoma cell lines (MCF-7 and A-549 cells) were cultured with docetaxel solution, conventional docetaxel-loaded liposomes, or FA-PDCT-L, and the cytotoxicity and apoptosis was evaluated for each preparation. The uptake of the docetaxel preparations into MCF-7 cells was studied by confocal laser scanning microscopy. Liquid chromatography-mass spectrometry was used to study the pharmacokinetics and tissue distribution characteristics of the preparations.
Results
The existence of an enlarged fixed aqueous layer on the surface of the liposomes was affirmed by zeta potential analysis. The entrapment efficiency and particle size distribution were almost the same as those of docetaxel-loaded liposomes. The drug release profile showed that the release rate was faster at higher molecular weight of the polymer. Compared with docetaxel solution and docetaxel-loaded liposomes, FA-PDCT-L demonstrated the strongest cytotoxicity against two carcinoma cell lines, the greatest intracellular uptake especially in the nucleus, as well as the most powerful apoptotic efficacy. In pharmacokinetic studies, the area under the plasma concentration-time curve of FA-PDCT-L was increased 3.82 and 6.23 times in comparison with the values for the docetaxel-loaded liposomes and docetaxel solution, respectively. Meanwhile, a lower concentration of docetaxel was observed for FA-PDCT-L in the liver and spleen, and a significantly higher concentration of FA-PDCT-L in tumors suggested that the presence of FA-PEG-PCHL on the liposomes resulted in greater accumulation of the drug in tumor tissue.
Conclusion
Liposomes modified by FA-PEG-PCHL could be one of the promising suspensions for the delivery of antitumor drugs in cancer.
Introduction
Nanotechnology has been extensively exploited to improve conventional cancer therapy recently.Citation1,Citation2 The nanocarriers currently under intensive investigation can be divided into two categories in general, ie, polymer-based and lipid-based polymeric nanoparticles and liposomes.Citation3 The use of polymeric nanoparticles using biodegradable polymers, such as poly (D,L-lactic acid), poly (D,L-lactic-co-glycolic acid) and poly (3-caprolactone), and their copolymers diblocked or multiblocked with polyethylene glycol (PEG), for drug delivery has shown promising therapeutic potential.Citation4–Citation6 These nanoparticles have been widely used because of their high drug-loading capacity especially for hydrophobic drugs, controlled drug release, acceptable biocompatibility, high cellular internalization, and a long circulation half-life.Citation2 However, polymeric nanoparticles have reported moderate circulation half-lives in comparison with their liposomal counterparts, even when coated with biocompatible polymers such as PEG.Citation7
Liposomes are spherical vesicles composed of single or multiple lipid bilayers, and have a number of appealing features, including high biocompatibility, high delivery efficiency, a favorable pharmacokinetic profile, and ease of surface modification.Citation8,Citation9 The performance of liposome drug delivery systems for tumor therapy could be further improved by using a ligand coupled to the surface of vesicles to achieve an active targeting effect. The selective overexpression of folate receptors in tumor cells and the high affinity of tumors for folic acid provide a unique opportunity for folic acid to be used as a targeting ligand to deliver therapeutic agents to cancer cells via folate receptor-mediated endocytosis.Citation10 Folate-mediated targeting liposomes has been obtained by the incorporation of a small amount of folate-polyethyleneglycol-distearoyl-phosphatidyl-ethanolamine.Citation11
In this study, we explored the potential use of a novel conjugate, folate-poly (PEG-cyanoacrylate-co-cholesteryl cyanoacrylate) (FA-PEG-PCHL) for liposome targeting by insertion into liposomal bilayers. Docetaxel is used as a model hydrophobic anticancer drug, and shows high anticancer efficacy in patients with ovarian carcinoma, lung cancer, advanced breast cancer, and head/neck cancer.Citation12 The incorporation of PEG can prolong the residence time of liposomes in the blood, and efficient folate receptor binding and endocytosis of folate-derived liposomes can be achieved by separating folate from the liposomal surface when a PEG spacer is used,Citation13 for the reason that folic acid has the flexibility to interact with the corresponding cell surface receptor.Citation14 As a result, PEG was selected as the hydrophilic group to achieve a long circulation effect and better connection performance from folic acid. Meanwhile, the hydrophobic domains are formed by the poly cholesteryl cyanoacrylate moieties. We modified this block copolymer with cholesterol in order to achieve better compatibility with the lecithin bilayer.Citation15 Cholesterol is often used to modify biomaterials hydrophobically due to its rigid and highly hydrophobic sterol skeleton.Citation16,Citation17
The two main goals of the present work were, firstly, to report a novel amphiphilic copolymer, ie, FA-PEG-PCHL, with a series of molecular weights (4374–12394 Da) and low cytotoxicity, and, secondly, to investigate the characteristics, in vitro cytotoxicity, intracellular uptake, apoptotic effect, in vivo pharmacokinetics, and tissue distribution of FA-PEG-PCHL- modified, freeze-dried docetaxel-loaded liposomes (FA-PDCT-L).
Materials and methods
Materials
α-Amino-ω-hydroxy-polyethyleneglycol (NH2-PEG-OH, molecular weights 2 kDa, 4 kDa, and 10 kDa) was purchased from Alfa Aesar (Heysham, UK). Dicyclohexylcarbodiimide, cyanoacetic acid, cholesterol, and 1, 4-(dimethylamino) pyridine (DMAP) were purchased from Acros Organics (Geel, Belgium). Cyanoacetic acid was dried over phosphorus pentoxide at 40°C for 48 hours. Docetaxel and paclitaxel were purchased from Ningbo Chemical Factory (Ningbo, China). Soybean lecithin was supplied by Shanghai Taiwei Pharmaceutical Industry (Shanghai, China). Docetaxel injection (Taxotere®) was from Aventis Pharma SA (Antony Cedex, France), and coumarin-6 was from Sigma-Aldrich (St Louis, MO). All other chemicals and solvents were of reagent grade and the solvents were dehydrated by molecular sieving.
Animals
Male Wistar rats (200 ±20 g) and male Kunming strain mice (20–25 g) were from the Shenyang Pharmaceutical University Animal Institution. The rats were housed under standard conditions and were fed on a commercial diet and had access to water ad libitum. All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Research Council. The protocol was approved by the local animal ethics committee.
Cell culture
For cell culture, mouse connective tissue fibroblast cells (L929), human breast adenocarcinoma cells (MCF-7), and human lung adenocarcinoma cells (A-549) were obtained from Nanjing Keygen Biotech Co Ltd (Nanjing, China). The reagents employed in this study were as follows: RPMI 1640, F-12k medium (Hyclone, Logan, UT), fetal bovine serum (Gibco, Grand Island, NY), trypsin (Sigma-Aldrich) and dimethyl sulfoxide (Sigma-Aldrich). All other materials were used without further purification. All cells were maintained in a medium supplemented with penicillin 10% (v/v) 100 U/mL and streptomycin 100 μg/mL. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Synthesis of copolymers of FA-PEG-PCHL
Preparation of cyanoacetate esters
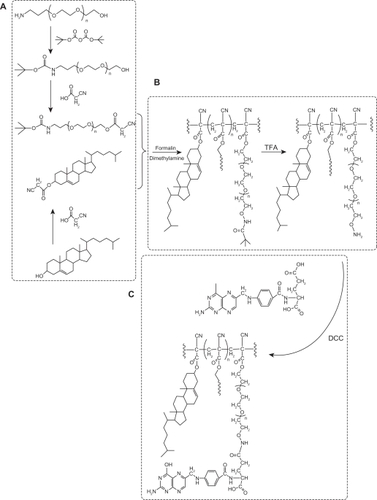
The amino group of NH2-PEG-OH was protected by making it into t-butyloxycarbonylamino-ω-hydroxyl-polyethyleneglycol (t-boc-NH2-PEG-OH).Citation18 As shown in , a mixture of 20 mmol triethylamine, 18 mmol di-t-butyl dicarbonate, and 20 mmol NH2-PEG2000-OH in 300 mL dichloromethane was stirred for 72 hours. HCl 0.5 M was then added to the mixture. The organic layer was separated and washed with saturated NaCl solution. The organic phase was separated again and dried over MgSO4. The solvent was evaporated and the residue was allowed to cool under vacuum. t-boc-NH-PEG cyanoacetate was prepared by esterification of the cyanoacetic acid with boc-NH-PEG-OH in dichloromethane at room temperature under nitrogen, in the presence of dicyclohexylcarbodiimide and DMAP as the catalyst. Cyanoacetic acid 10 mmol and boc-NH-PEG-OH 5 mmol (molar ratio 2:1) were placed into a glass flask and dissolved in dichloromethane 40 mL and dicyclohexylcarbodiimide 11 mmol, and a catalytic amount of DMAP were added. After stirring for six hours, the solid was filtered off and washed three times with CH2Cl2 15 mL. The combined filtrates were concentrated under reduced pressure to obtain viscous oil which was solidified after 12 hours. The crude boc-NH-PEG cyanoacetate obtained was used directly in the polymerization step without further purification. The cyanoacetate ester of cholesterol was prepared according to the same procedure as aforementioned ().
Condensation/polymerization
The reaction scheme for condensation of t-boc-NH2-PEG cyanoacetate with cholesteryl cyanoacetate in the presence of formalin and dimethylamine, as previously described was shown in . Briefly, t-boc-NH2-PEG cyanoacetate 1 mmol and cholesteryl cyanoacetate 5 mmol were dissolved in ethanol/dichloromethane 20 mL (1:1, v/v) in a glass flask, and formalin 10 mmol (37%, w/v) and dimethylamine 10 mmol (40%, w/v) were added.Citation18 The reaction was carried out at room temperature under nitrogen. After stirring for 12 hours, the reaction mixture was poured into water and extracted with dichloromethane. The combined organic phases were dried over MgSO4. The solvent was evaporated, and the residue was placed several hours under vacuum to cool down.
The amino group of t-boc-NH2-PEG was deprotected by trifluoroacetic acid in dichloromethane ().Citation19 Briefly, trifluoroacetic acid 1 mL was added to polymer 1 mmol solution, and the reaction was carried out for one hour at room temperature under magnetic stirring. The reaction mixture was then neutralized with 10% aqueous sodium bicarbonate solution and extracted with dichloromethane. The organic layer was dried over MgSO4 and evaporated under reduced pressure to give a pale yellow waxy material.
Conjugation of folic acid and NH2-PEG-PCHL
Folate-conjugated co-copolymer was synthesized by coupling the NH2-PEG-PCHL to an activated folic acid as described in the previous studies with a minor modification.Citation11,Citation20,Citation21 As shown in , NH2-PEG-PCHL 1 mmol dissolved in dimethyl sulfoxide 30 mL was mixed with folic acid 4 mmol and dicyclohexylcarbodiimide 1.2 mmol, and two drops of triethylamine was then added as a catalyst. The reaction was performed at room temperature for 12 hours, and the reaction solution was then filtered. The filtrate was dialyzed in dimethyl sulfoxide for 72 hours to remove free folic acid and then dialyzed for another 72 hours to eliminate dimethyl sulfoxide. The mixture was freeze-dried in a dialysis bag to give the final product.
Characterization of polymers
FTIR spectra, 1H-NMR spectra and MW of polymers
Fourier transform infrared (FTIR) spectra were obtained from a neat film cast from the dimethyl sulfoxide copolymer solution between KBr tablets on an FTIR spectrometer (IFS-55, Bruker Corporation, Switzerland). 1H-NMR spectra was recorded in deuterated pyridine for PEG-PCHL and in deuterated dimethyl sulfoxide for FA-PEG-PCHL by 1H-NMR spectroscopy (ARX-300, Bruker Corporation, Fällanden, Switzerland). Gel permeation chromatography of PEG-PCHL was performed in tetrahydrofuran (Fisher Scientific, Fair Lawn, NJ) of high-pressure liquid chromatography grade with a Waters Alliance 2690 separation module (Waters Corporation, Milford, MA) equipped with a set of two Waters Styragel columns having pore size diameters of 103 and 100Å, and a Waters 2410 refractive index detector (Waters Corporation). A series of narrow polystyrene (Polymer Laboratories, Amherst, MA) molecular weight standards (30–500 kDa) were used to calibrate the gel permeation chromatography system.
Cytotoxicity of polymers by CCK-8 assay
The L929 cell line was selected to evaluate cytotoxicity as a direct contact test, as recommended by USP 26. The experiment was carried out according to the method described for the Cell-Counting Kit 8 (Fluka, St Louis, MO).Citation22 The copolymers were added as free solutions. The relative cell viability compared with control cells containing cell culture medium without polymer was calculated as [A]test/[A]control.
Liposome preparation
Liposomes were prepared using an organic solvent injection method.Citation23 Briefly, in all formulations containing polymers of different molecular weight, a mixture of FA-PEG-PCHL 0.005 mmol, docetaxel 0.012 mmol, and soybean lecithin 0.25 mmol was dissolved in tetrahydrofuran 5 mL. The solution was injected into purified water at 25°C and the suspension obtained was stirred for 180 minutes to facilitate the removal of tetrahydrofuran. To obtain a uniform particle size distribution, the suspension was sonicated at a frequency of 20–25 kHz and power of 500 W for 2.5 minutes by a probe-type sonicator (JY92-II, Scientz, Ningbo, China) and then sterilized by filtering through a 0.22 μm cellulose acetate filter. Appropriate amounts of cryoprotectants (5%, w/v) were dissolved in the liposome dispersion. The samples were frozen at −85°C for eight hours before being lyophilized using a freeze drier (Eyela FDU-1100, Prkakikai, Tokyo, Japan). The drying time was 36 hours. The resulting solid matrix was collected for later experiments after rehydration with deionized water.
Characterization of liposomes
Transmission electron microscopy
FA-PDCT-L were viewed before and after lyophilization under an electron microscope (JEM-1200 EX, JEOL, Tokyo, Japan) by conventional negative staining methods using phosphotungstic acid buffer 0.3% (pH 6.0) as a staining agent.Citation23
Particle size distribution, zeta potentials, and fixed aqueous layer thickness
Particle size distribution and zeta potentials for FA-PDCT-L were determined by dynamic light scattering and electrophoretic light scattering, respectively, both using ELS 800 apparatus (Otsuka Electronics, Osaka, Japan) at 25°C after dilution of the dispersion to an appropriate volume with Millipore-filtered water. According to the Gouy-Chapmann theory, the zeta potentials of samples diluted with NaCl solutions at a series of concentrations (0 M, 0.01 M, 0.05 M, and 0.1 M) measured and plotted against the slope L gives the position of the slipping plane or fixed aqueous layer thickness FALT) in nm units. FALT was calculated by:
where ψ (L) is the electrostatic potential at the position of the slipping plane L (nm), A is the constant, and κ is the Debye-Huckel parameter which equals C−2/0.3 for univalent salts, where C is the molality of electrolytes.Citation24
Determination of entrapment efficiency
The liposome entrapment efficiency was determined using an ultrafiltration technique for separating the nonentrapped drug from liposomes.Citation25 Briefly, 5 mL of drug-loaded liposomes were placed in a stirred cell (Millipore 8010, Millipore Corporation, Bedford, MA) fitted with a filter membrane (molecular weight cutoff 50,000) under nitrogen. The ultrafiltrate was collected, and the drug content in the ultrafiltrate (Cfree) was determined by high-pressure liquid chromatography using an LC-ATvp pump and SPD-10 Avp ultraviolet light detector (Shimadzu, Kyoto, Japan) and a Diamonsil C18 analytical column 250 mm × 4.6 mm, 5 μm (Dikma, Tianjin, China).Citation26 The injection volume was 20 μL and docetaxel was detected at 228 nm. The mobile phase consisted of acetonitrile and water (53:47, v/v) delivered at a flow rate of 1.0 mL/minute. A liposomal suspension 1 mL was then diluted with absolute ethanol to determine the total docetaxel (Ctotal) by high-pressure liquid chromatography. The entrapment efficiency (EE%) was calculated as:
In vitro drug release
The drug release profile for the docetaxel-loaded liposomes was studied using a dialysis method in vitro. Briefly, an aliquot of FA-PDCT-L were diluted 50-fold with phosphate buffer (0.1 M, pH 7.4) containing Tween 80 (0.3%, w/v), thereby improving the solubility of docetaxel in phosphate-buffered saline, and incubated in buffer at 37°C under magnetic stirring in a dialysis bag (molecular weight cutoff 7000 Da). Aliquots of 1 mL were withdrawn from the release medium and replaced by an equal volume at each sampling time. The amount of docetaxel was determined by high-pressure liquid chromatography. All operations were carried out in triplicate.
Cytotoxicity of liposomes
A CCK-8 assay was performed to compare the cytotoxic effects of docetaxel solution, FA-PDCT-L, and empty FA-PEG-PCHL-modified liposomes (FA-P-L) against two cancer cell lines in vitro using a previously established method.Citation27–Citation29 Before the experiment, MCF-7 breast cancer cells and A-549 lung cancer cells were precultured until confluence was reached. After incubating the cells in docetaxel solution, FA-PDCT-L, and FA-P-L for 24, 48, and 72 hours, with docetaxel concentrations ranging from 0.5 nM to 40 nM, the CCK-8 assay was performed and the percentage cell viability was determined. Error bars were obtained from triplicate samples.
In vitro cellular uptake of liposomes
Confocal laser scanning microscopy was used to visualize the interaction of the colloidal particles with the cancer cells. For fluorescence imaging of cellular uptake, MCF-7 cells at a density of 1 × 106 cells/mL were cultivated for 24 hours on coverslips in six-well culture plates (1 mL/well). Coumarin-6 solution was prepared following the same prescription and preparation process as for Taxotere. Suspensions of coumarin-6-loaded liposomes (COU-L) and coumarin-6-loaded FA-PEG4000-PCHL-modified liposomes (FA-PCOU4000-L) were then added (200 μL/well) to the cell culture medium at a concentration of 100 μg/mL. Cells were washed three times after incubation for two hours and then fixed using 4% (v/v) paraformaldehyde aqueous solution. After 10 minutes of fixing at room temperature, followed by rinsing with phosphate-buffered saline, propidium iodide 10 μg/mL in phosphate-buffered saline was added to stain the nuclei for 30 minutes. The stained cells were then washed with phosphate-buffered saline and observed using a confocal laser scanning microscopy system (TCS SP2/AOBS, Leica, Germany).
Acridine orange/ethidium bromide staining
Morphological demonstration of apoptosis was done using acridine orange/ethidium bromide staining. MCF-7 cells were cultured and divided into control and test groups. Normal cells were treated with serum-free medium for 24 hours. Test groups were treated with docetaxel solution, docetaxel-loaded liposomes, and FA-PDCT-L, respectively, for 24 hours at a docetaxel concentration of 20 μg/mL. After removal of the incubation medium, MCF-7 cells were harvested with trypsin 0.25% solution and treated with dye mixture (a normal/apoptotic/necrotic cell detection kit, Nanjing KeyGen Biotech Co Ltd, China). Double-staining using these two agents provided the percentage of live, apoptotic, and necrotic cells. The cells were observed immediately using an Olympus B × 61 fluorescence microscope (Tokyo, Japan) and the appropriate fluorescein filter. Acridine orange exhibits green fluorescence and ethidium bromide exhibits red fluorescence when bound to DNA. In this way, it is possible to distinguish between viable (green fluorescence with intact nucleus), early apoptotic (green fluorescence with chromatin condensation), late apoptotic (orange fluorescence with chromatin condensation), and necrotic cells (orange fluorescence with intact nucleus).
AnnexinV/propidium iodide double staining
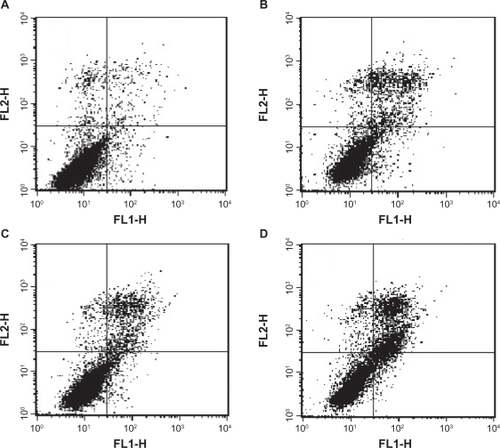
MCF-7 cells were divided and treated as aforementioned, then detached by trypsin and washed twice. Apoptotic cells were identified by binding of fluorescein-labeled annexin V to exposed phosphatidylserine on the cell surface and necrosis by nuclear staining with propidium iodide. Cell clusters were identified as described in the annexin V-FITC staining kit (Nanjing KeyGen Biotech Co Ltd, China) according to the manufacturer’s recommendations with flow cytometric analysis (FACSCalibur, BD Biosciences, San Jose, CA), and a dot plot of FL1 (annexin V) and FL2 (propidium iodide) was constructed to show the gated events. Quadrant markers were applied to identify the four cell populations (annexin V−/propidium iodide+, annexin V+/propidium iodide+, annexin V−/propidium iodide−, annexin V+/propidium iodide−). The results were expressed as a percentage of the gated events.Citation9
Pharmacokinetics
Twelve male Wistar rats were fasted overnight and randomly divided into two groups of six rats each for an in vivo pharmacokinetics study. A docetaxel solution as a reference and FA-PDCT-L were injected intravenously at a dose of 10 mg/kg. Blood samples were collected from the orbital plexus at predetermined time points into heparinized tubes and then centrifuged at 5000 rpm for five minutes to separate the plasma.
To analyze the plasma samples, paclitaxel (20 μL of a methanol 10 μg/mL solution) was added as the internal standard to 200 μL of plasma and the mixture was extracted with ether before being centrifuged. Docetaxel in the supernatant was dried under nitrogen and redissolved in methanol for liquid chromatography-mass spectrometry measurement. The assay was performed on a liquid chromatography-mass spectrometry system (2010A, Shimadzu). Liquid chromatographic separation was achieved on a Kromasil C18 column (150 mm × 4.6 mm, 5 μm) and preceded by a C18 guard column (4.0 mm × 2.0 mm, Phenomenex, Torrance, CA). The column and autosampler tray temperatures were kept constant at 25°C and 4°C, respectively. The mobile phase was methanol and water (75:25, v/v). The flow rate was set at 0.8 mL/min, with 25% of the eluent splitted into the inlet of the mass spectrometer. The injection volume was 10 μL.
The analytes were ionized by an ESI source in positive ion mode under the following source conditions: nebulizing gas 1.5 L/min; drying gas 2.0 L/min; curved desolvation line temperature 250°C; heat block temperature 200°C; detector voltage 1.70 kV; and other parameters fixed as for the tuning file. Analysis was carried out by selected ion monitoring at [M + Na]+ m/z 830.50 for docetaxel and at [M+Na]+ m/z 876.50 for paclitaxel. The internal standard ratio was calculated for each standard by dividing the analyte peak area by the peak area of the internal standard. Standard curves of docetaxel and paclitaxel were constructed by plotting the internal standard ratio versus the concentration of analyte in each sample. Standard curves were fit by linear regression with weighting by 1/y2, followed by back calculation of concentrations.
Tissue distribution of liposomes in sarcoma-180 solid tumor-bearing mice
Male Kunming mice, body weight 20–25 g, were used for biodistribution studies after sarcoma-180 cells were implanted intradermally into the armpit for one week. Twenty-seven tumor-bearing mice were fasted overnight and randomly divided into three groups of nine mice each. Docetaxel solution, docetaxel-loaded liposomes for reference, and reconstituted freeze-dried FA-PEG4000-PCHL-modified docetaxel liposomes (FA-PDCT4000-L) were injected intravenously at a dose of 20 mg/kg. Another three animals were sacrificed without treatment, and their tissues were used as blank controls and for preparation of spiked control samples.
The animals were decapitated in groups of three at seven, 120, and 240 minutes, and the heart, liver, spleen, lungs, kidneys, brain, and tumor tissue were removed, washed of residual blood, air-dried, and weighed. Tissue samples were homogenized with saline 0.2 mg/mL, and methanol solution 20 μL containing paclitaxel 10 μg/mL was then added as the internal standard. Tissue samples were extracted with ether and then centrifuged. The supernatants were dried under nitrogen and redissolved in methanol for liquid chromatography-mass spectrometry measurement as described earlier.
Statistical analysis
Statistical analysis of the cytotoxicity of FA-PEG2000-PCHL, FA-PEG4000-PCHL, and FA-PEG10000-PCHL, and the effects of the three polymers on particle size, zeta potentials, FALT, entrapment efficiency, and mean 24-hour cumulative release (%) of the various formulations were performed using the Kruskal–Wallis test. In all cases, post hoc comparisons of the means of individual groups were performed using the least significant difference test.
Plasma drug concentration-time data for docetaxel in individual rats were analyzed by noncompartmental estimations using 3p87 software (Chinese Society of Mathematical Pharmacology, Beijing, China). Plasma and tissue concentrations of docetaxel obtained from the rats were pooled to provide mean concentrations. Statistical analysis of the pharmacokinetic parameters (peak concentration [Cmax], clearance, mean residence time from time 0 extrapolated to infinity [MRT] and area under the plasma-concentration time curve from time 0 extrapolated to infinity [AUC]) for the docetaxel solution, docetaxel-loaded liposomes, and FA-PDCT4000-L were carried out using the one-way analysis of variance t-test. Differences in tissue drug concentrations at different time points were estimated using the Kruskal–Wallis test, and post hoc comparisons of the means of individual groups were performed using the least significant difference test. A significance level of P < 0.05 denoted significance in all cases. Statistical analysis was performed using SPSS version 17.0 (SPSS Inc, Chicago, IL). Each experiment was conducted three times to ascertain reproducibility.
Results and discussion
Characterization of polymers
FTIR spectra
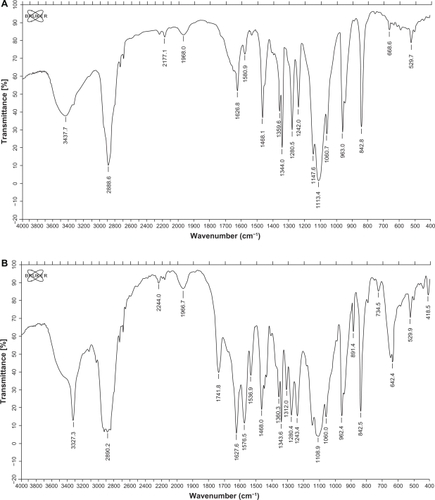
The FTIR spectrum of NH2-PEG-PCHL () showed absorption bands related to the CN stretching vibration at 2177.6 cm−1 and the ester carbonyl at 1741.6 cm−1. The C—O stretching of PEG appeared at 1112.0 cm−1. The FTIR spectrum of FA-PEG-PCHL () showed absorption bands related to CN stretching vibration at 2244.0 cm−1 and the ester carbonyl at 1741.8 cm−1. The C—O stretching of PEG appeared at 1108.9 cm−1. The C=C and =C—H stretching vibration of aromatic ring of folate showed at 1536.9 and 842.5 cm−1, respectively.
1H-NMR spectra
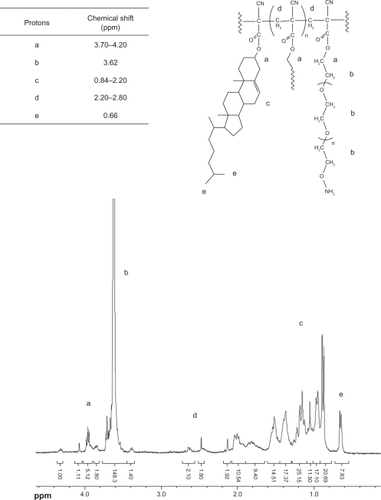
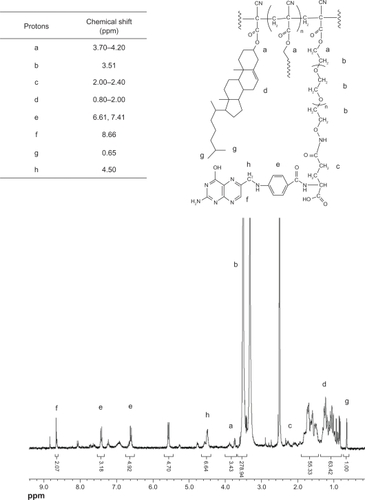
The 1H-NMR spectra were consistent with the structure of the expected polymers. shows the spectrum of PEG-PCHL. The peaks at 3.70–4.20 ppm were attributed to the methylene in the R-position of the ester groups. The signal at 2.20–2.80 ppm and the resonance at 3.62 ppm were assigned to the methylene protons of poly(cyanoacrylate) and the PEG backbone, respectively. Signals at 0.84–2.20 ppm were assigned to the methylene and methyl protons of the cholesteryl skeleton. The peak at 0.66 ppm was attributed to the methyl protons of the cholesteryl side chain. shows the spectrum of FA-PEG-PCHL. The peaks at 3.70–4.20 ppm were attributed to the methylene in the R-position of the ester groups. The resonance at 3.51 ppm was assigned to the methylene protons of the PEG backbone. The signal at 2.00–2.40 ppm and the resonance at 4.50 ppm were attributed to the methylene in the R-position for the amide groups of folate, respectively. The signals at 6.61 ppm, 7.41 ppm, and the resonance at 8.66 ppm were attributed to the protons of the aromatic ring of folate, separately. Signals at 0.80–2.00 ppm were assigned to the methylene and methyl protons of the cholesteryl skeleton. The peak at 0.65 ppm was attributed to the methyl protons of the cholesteryl side chain.
Molecular weights
The molecular weights of the obtained polymers, as well as the polydispersity indices, were calculated by gel permeation chromatography. reports the data for polymers with different chain lengths of PEG. A low molecular weight and low polydispersity index were achieved. The unimodal mass distribution excluded the presence of poly-(PEG acrylate) or poly-(cholesteryl acrylate). Because the condensation of alkyl cyanoacetates with formaldehyde is a method described for the synthesis of alkyl cyanoacrylate monomers, the formation of a poly-(alkyl cyanoacrylate) with a low molecular weight was expected.Citation18 Furthermore, the interest in using poly-(alkyl-cyanoacrylate) in controlled drug delivery is due to its rapid degradability by erosion after hydrolysis of the lateral ester chain and the formation of water-soluble poly-(cyanoacrylic acid), which is eliminated by renal excretion.Citation30 Thus, a low molecular weight is an important issue for the degradability properties of the obtained material. Finally, the molecular weight of the “hydrophobic” alkyl cyanoacrylate part of the copolymer was of the same order as that observed in nanoparticles prepared by emulsion/butyl cyanoacrylate, well-known to degrade rapidly in vivo.Citation31
Table 1 MWs and polydispersity index of polymers
Cytotoxicity by CCK-8 assay
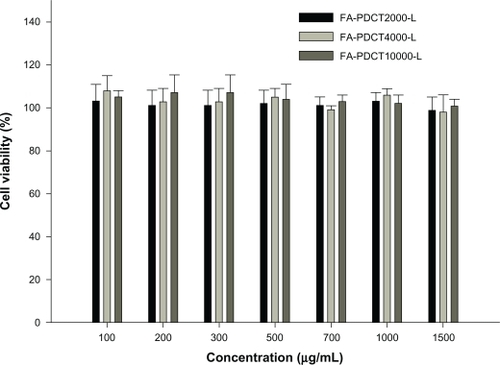
The relative cell viability compared with control cells containing cell culture medium without polymer was calculated by [A]test/[A]control. Based on the viability of L929 cells incubated together with various polymers, FA-PEG-PCHL showed no cytotoxicity with experimental concentrations up to 1500 μg/mL in cell culture (). No significant difference was found between the groups (P > 0.05).
Characterization of liposomes
Investigation of cryoprotectants
Liposomal lyophilization is one of the most promising procedures for keeping liposomes stable during long-term storage.Citation32 Therefore, different cryoprotectants, ie, lactose, dextran, sucrose, trehalose, and mannitol (%, w/v), were evaluated as candidates after preliminary experiments according to the appearance, morphology, and stability of the lyophilized liposomes after reconstitution.
In terms of the protective effect of lyoprotectants (lactose, sucrose, trehalose, and dextran), the size and encapsulation efficiency of FA-PDCT-L before and after lyophilization were similar (data not shown). In particular, the dried product obtained with trehalose was satisfactory in appearance and was easy to reconstitute, with an unchanged and uniform size distribution. As far as mannitol was concerned, the lyophilized product had a smooth and bright appearance, but was difficult to rehydrate, together with a significant increase in particle size. Therefore, we chose trehalose as the lyoprotectant (5%, w/v).
Morphology analysis
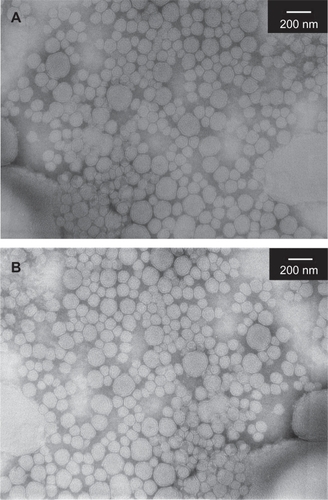
The morphological images of FA-PDCT-L before and after lyophilization are shown in . In all cases, spherical or ellipsoid-shaped particles were found.
PSD, zeta potentials, FALT, and entrapment efficiency
A unimodal particle size distribution was observed, and FA-PDCT-L with polymers of different molecular weight had a similar particle size distribution, ranging from 111 nm to 127 nm, as shown in . Meanwhile, the negativity of the zeta potential for the liposomes decreased dramatically with increasing PEG chain length (P < 0.05). Increasing NaCl concentration also led to reduction of the negative surface charge of the liposomes, indicating that the FALT of the liposomes increased dramatically with length of the PEG chain (P < 0.05). The data suggested that the surface structure of FA-PDCT-L fitted a “mushroom” structure, with FALT no more than 10 nm.Citation33 “Mushroom” structure theory postulates that PEG, folding on the surface of the liposomes due to its compressibility, is favorable for liposomes to prevent aggregation and improve its ability to evade uptake by macrophages. The entrapment efficiency of liposomes with or without modification was not significantly different, indicating that the loading of polymers did not change the encapsulation ability of the liposomes.
Table 2 Characteristics of FA-PDCT-L and FA-PCOU-L
In vitro release
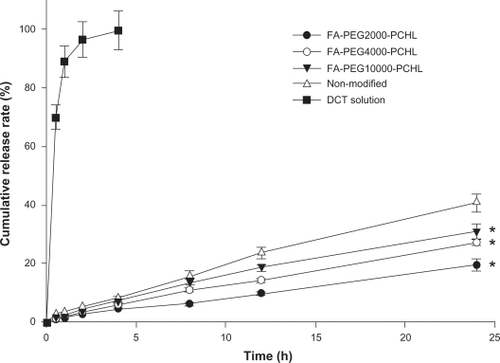
There was no obvious burst release of drug for up to 24 hours, and a prolonged release phenomenon was showed in all groups (). The release rate was faster with higher molecular weights of the polymer, showing a molecular-dependent release profile. The cumulative amount of docetaxel released within 24 hours was 40.8% for docetaxel-loaded liposomes, while a significant decreased amount of docetaxel in FA-PDCT-L was released over 24 hours, ie, 19.5%, 27.2%, and 31.1% for FA-PDCT2000-L, FA-PDCT4000-L, and FA-PDCT10000-L, respectively (P < 0.05). Polymer-modified liposomes released drug significantly more slowly than nonmodified liposomes because of the poly (cholesteryl cyanoacrylate) moieties in the polymer. Cholesterol is known to enhance the rigidity of the lethicin bilayer.Citation34 As a result, incorporation of the polymer possibly altered the flexibility of the liposomal bilayer, and reduced the rate of drug release. We also observed that the mass of the hydrophilic group of the polymer significantly influenced the rate of drug release. The higher the chain length of PEG, the faster the rate of drug release, and this may be attributable to the loose structure of FA-PDCT10000-L, leading to accelerating drug release.
Cytotoxicity of liposomes
The in vitro cytotoxic activity of docetaxel solution, docetaxel-loaded liposomes, and FA-PDCT-L is shown in . The IC50 of FA-PDCT2000-L, FA-PDCT4000-L, and FA-PDCT10000-L was significantly lower than that of docetaxel solution and docetaxel-loaded liposomes in both cancer cell lines for all incubation time periods. FA-PDCT4000-L showed significant advantages in inhibiting cancer cell proliferation when compared with the docetaxel solution and docetaxel-loaded liposomes. In comparison with docetaxel solution, the IC50 for FA-PDCT4000-L decreased to 83.6%, 71.1%, and 58.4% in the MCF-7 line, and to 76.3%, 65.3%, and 66.3% in the A-549 line after 24, 48, and 72 hours of treatment, respectively, while in comparison with docetaxel-loaded liposomes, the IC50 for FA-PDCT4000-L decreased to 82.6%, 75.9%, and 65. 5% in the MCF-7 line and to 74.6%, 75.9%, and 67.8% in the A-549 line after 24, 48, and 72 hours of treatment, respectively, showing a significant reduction in IC50 values. Compared with FA-PDCT2000-L and FA-PDCT4000-L, the IC50 of FA-PDCT10000-L was significantly higher in both cell lines. FA-P-L demonstrated no significant cytotoxic effect in either the MCF-7 line or in the A-549 line, even at concentrations as high as 1000 μg/mL (data not shown). IC50 values could not be determined accurately for FA-P-L.
Table 3 IC50 of DCT solution DCT-L and FA-PDCT-L in MCF-7 and A-549 cells after 24 h, 48 h, and 72 h incubation
In vitro cytotoxicity studies showed that FA-PDCT-L was significantly more cytotoxic in MCF-7 and A-529 lines compared with the docetaxel solution and docetaxel-loaded liposomes (). Higher dose and longer duration of exposure led to an improved effect. Thus, a lower amount of docetaxel associated with liposomes will be required to achieve the same effect as docetaxel solution alone, which is anticipated to reduce the side effects of docetaxel. The greater antiproliferative effect was attributed mainly to a higher intracellular drug level as a result of folate-mediated phagocytosis or reduced exocytosis involving a xenobiotic extrusion pump, or both.Citation35,Citation36 The IC50 of FA-PDCT10000-L was significantly higher than that of FA-PDCT2000-L and FA-PDCT4000-L, which could be explained by overfolding of the PEG10000 chain blocking folate, which is the key to mediation of phagocytosis for PEGylated liposomes.
In vitro cellular uptake of liposomes
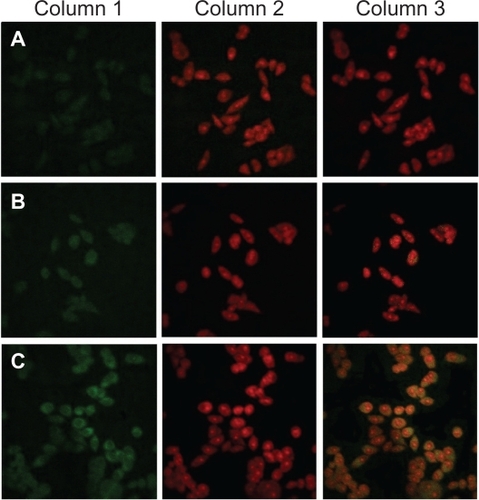
To investigate folate-mediated cellular uptake, a short-term particle endocytosis test was carried out visually using coumarin-6 solution, COU-L, and FA-PCOU4000-L. To have a reliable means of comparison, the physiochemical characteristics of COU-L are shown in , and there was no significant difference between the drug-loaded liposomes and COU-L (P > 0.05). shows that COU-L (green fluorescent dots) penetrated the cells and was mostly distributed in the cytoplasm around the nucleus. Furthermore, the green fluorescent dots in samples incubated with FA-PCOU4000-L were more concentrated than those incubated with coumarin-6 solution and COU-L. These images demonstrate the improved effect of folate on cellular uptake, which could be a possible explanation for the higher cytotoxicity of the FA-PEG-PCHL-modified liposomes.
Figure 8 Confocal laser scanning microscopy images showing the internalization of fluorescent preparations in MCF-7 cells (2 h incubation). Column 1: FITC channels showing green fluorescence from coumarin-6-loaded preparations distributed in the cytoplasm. Column 2: PI channels showing red fluorescence from propidium iodidestained nuclei. Column 3: Merged channels of FITC and PI. Rows A: coumarin-6 solution. Rows B: COU-L. Row C: FA-PCOU4000-L.
Acridine orange/ethidium bromide staining
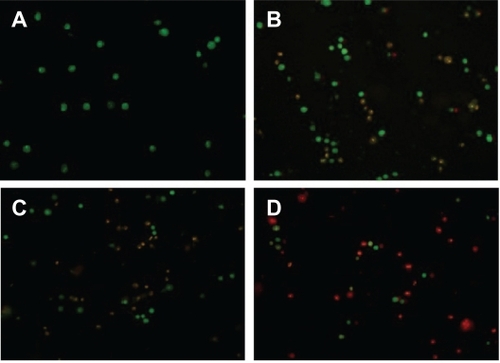
To investigate the enhanced effect of FA-PEG-PCHL on induction of apoptosis by the docetaxel-loaded liposomes, MCF-7 cells were stained after treatment with acridine orange/ethidium bromide, which combines the differential uptake of fluorescent DNA binding dyes, acridine orange, and ethidium bromide, allowing distinction between viable, apoptotic, and necrotic cells. shows late apoptotic cells (yellow stain) and necrotic cells (red stain) when exposed to docetaxel solution, docetaxel-loaded liposomes, and FA-PDCT4000-L. When treated with FA-PDCT4000-L, the number of apoptotic and necrotic cells was significantly increased, which confirmed the results for cytotoxicity of the liposomes.
AnnexinV/propidium iodide double staining
Flow cytometric analysis with annexin V and propidium iodide staining was performed to detect apoptosis by targeting the loss of phospholipid asymmetry in the plasma membrane. MCF-7 cells were analyzed by flow cytometry after staining of phosphatidylserine translocation with FITC-annexin V in combination with propidium iodide. Phosphatidylserine is a lipid found on the inner surface of the cell membrane. When undergoing apoptosis, cells externalize phosphatidylserine, which can then be labeled with fluorochrome-conjugated annexin V. The MCF-7 cells were divided into four groups, ie, mechanically injured cells (left upper part, annexin V−/propidium iodide+), healthy living cells (left lower part, annexin V−/propidium iodide−), cells at the stage of early apoptosis (right lower part, annexin V+/propidium iodide−), and necrotic cells or cells exhibiting late-stage apoptosis (right upper part, annexin V+/propidium iodide+). The cells within each cluster were then counted and represented as a percentage of the gated cells. Cells of both the right upper and right lower parts were considered to be apoptotic. There was a significant difference among the experimental groups. indicates that the percentage of early apoptotic cells and late apoptosis cells in the control group were 2.29% and 2.58%, while that of the FA-PDCT4000-L group were 5.60% and 23.09%, which were significantly higher than for both the docetaxel solution group (3.91% and 11.46%) and the docetaxel-loaded liposomes group (4.50% and 12.01%), thus showing a significant increase in apoptosis.
Pharmacokinetics
The results of the characterization analysis and in vitro cytotoxicity assays for the docetaxel preparations suggest that FA-PDCT4000-L has a better potential for tumor targeting. The research reported by Gref et al also suggest that a PEG chain with a molecular weight around 5000 Da possesses a better and longer circulation effect, and that a PEG content in the nanoparticles above 5% (w/w) could build a good steric barrier for optimal protein resistance.Citation37 Our work is consistent with this report. Therefore, we focused on FA-PDCT4000-L for a pharmacokinetic and tissue distribution study.
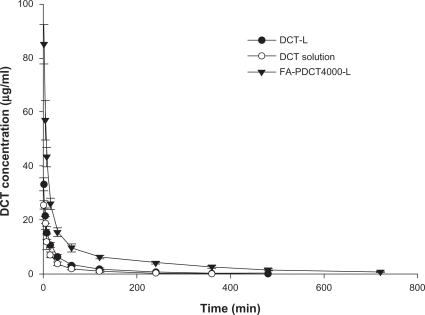
The plasma concentration-time curves for the docetaxel preparations after intravenous administration are shown in , and the pharmacokinetic parameters are listed in . The pharmacokinetic profiles for docetaxel showed significant differences for FA-PDCT4000-L-modified liposomes versus docetaxel-loaded liposomes and docetaxel solution. In the docetaxel solution group, disappearance of docetaxel from the blood circulation was very rapid, with plasma concentrations of only 63 ng/mL at six hours. In contrast, docetaxel in the polymer-modified liposomes was still present in the circulation at 12 hours. Encapsulation of docetaxel into liposomes leads to a significant change in pharmacokinetic parameters. In vitro drug-release studies indicate that docetaxel is released slowly from the liposomes for at least 24 hours, whereas free docetaxel is not readily distributed, which explains the higher initial plasma drug concentrations for docetaxel-loaded liposomes and FA-PDCT4000-L than those for docetaxel solution. As shown in , the AUC for FA-PDCT4000-L increased 6.2-fold and 3.8-fold, while the mean residence time increased 3.3-fold and 2.5-fold compared with the docetaxel solution and docetaxel-loaded liposomes, respectively, with a corresponding decrease in clearance. This effect might be attributed to two factors. First, the negative zeta potential is key to the recognition of nanoparticles by macrophages in the mononuclear phagocyte system. Therefore, a less negative or even neutral zeta potential is expected to be necessary to avoid the macrophage system. As shown in , the reduction of negative zeta potential could be observed in FA-PDCT4000-L in contrast with the docetaxel-loaded liposomes. Second, FALT is an important factor for improving liposome stability and preventing opsonization and macrophage uptake. The FALT of the FA-PDCT4000-L was thicker than that of docetaxel-loaded liposomes.
Table 4 Pharmacokinetic parameters of DCT in three preparations after intravenous administration to rats at a dose of 10 mg/kg
Tissue distribution in sarcoma-180 solid tumor-bearing mice
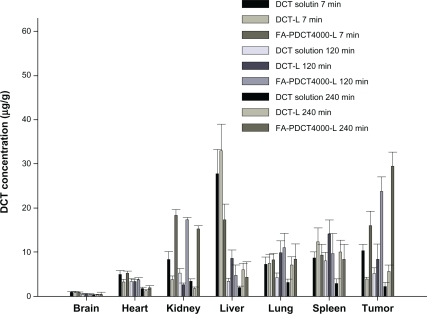
Sarcoma-180 solid tumor-bearing mice were selected as the animal model for our biodistribution study. The distribution profiles for docetaxel solution and liposomes in S180 tumor-bearing mice at seven, 120, and 240 minutes after intravenous administration are shown in . The drug levels in tumor for FA-PDCT4000-L were significantly higher than for the other preparations at seven minutes after administration, and were approximately 0.6-fold and 3.1-fold greater than that for docetaxel solution and docetaxel-loaded liposomes, respectively. At 120 and 240 minutes after dosing, the docetaxel concentration from FA-PDCT4000-L in tumor remained higher in comparison with the other preparations and tissues. In addition, at 120 and 240 minutes after dosing, the tissue distribution of FA-PDCT-L was, in ranked order: tumor > kidney > lung > spleen > liver > heart and brain (P < 0.05). There were no significant differences in docetaxel concentrations for the three formulations in brain and heart tissues at any time point (P > 0.05). The higher concentration of docetaxel found in kidney tumor cells in FA-PDCT4000-L-treated mice could be explained by the folate receptor effect. Folate receptor levels are naturally rich in the kidney and are overexpressed in tumor tissues.Citation38 The clearance behavior and tissue distribution of intravenously injected particulate drug carriers are greatly influenced by their size, surface features, and opsonization. Opsonins are adsorbed on the nanoparticle surface and promote particle recognition by the reticuloendothelial system.Citation39,Citation40 Lipid emulsions are rapidly taken up by the reticuloendothelial system in the liver and spleen after intravenous administration.Citation41 The presence of a long hydrophilic chain in PEG around the shell of FA-PDCT4000-L would lead to a longer retention time in the circulation. As a result, the docetaxel concentration in liver and spleen tissue in the FA-PDCT4000-L-treated group was significantly lower than that for docetaxel-loaded liposomes. Rich blood flow with high capillary permeability could be the reason for the accumulation in lungs. The uptake of modified liposomes by the brain was not improved because of lack of the folate receptor in the inner side walls of brain capillaries.Citation42
Conclusion
A novel polymer, ie, (FA-PEG-PCHL), with a low molecular weight and polydispersity index was successfully synthesized and used to modify docetaxel-loaded liposomes. FA-PDCT-L displayed a sustained release profile, promoted cell cytotoxicity, had a stronger apoptotic effect, prolonged the systemic circulation time, and achieved higher accumulation in tumoral tissue, thus enhancing the therapeutic action of the loading drug. Therefore, the novel polymer FA-PEG-PCHL and its application in liposomal formulations could be a promising solution for delivery of antitumor drugs to cancerous tissue.
Acknowledgements
This research is supported by grants from the Natural Science Fund of Hebei Province and China Shijiazhuang Pharmaceutical Group Co Ltd. (No. C2011319007).
Disclosure
The authors report no conflicts of interest in this work.
References
- FarokhzadOCLangerRImpact of nanotechnology on drug deliveryACS Nano200931162019206243
- ChoKWangXNieSMChenZShinDMTherapeutic nanoparticles for drug delivery in cancerClin Cancer Res20081451310131618316549
- LiuYTLiKPanJLiuBFengSSFolic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of docetaxelBiomaterials201031233033819783040
- ZhangLGuFXChanJMWangAZLangerRSFarokhzadOCNanoparticles in medicine: Therapeutic applications and developmentsClin Pharmacol Ther200883576176917957183
- PulkkinenMPikkarainenJWirthTThree-step tumor targeting of paclitaxel using biotinylated PLA-PEG nanoparticles and avidin-biotin technology: Formulation development and in vitro anticancer activityEur J Pharm Biopharm2008701667418555675
- FarokhzadOCChengJJTeplyBATargeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivoProc Natl Acad Sci U S A2006103166315632016606824
- LiYPPeiYYZhangXYPEGylated PLGA nanoparticles as protein carriers: Synthesis, preparation and biodistribution in ratsJ Control Rel2001712203211
- ZhaoLWeiYMZhongXDPK and tissue distribution of docetaxel in rabbits after i.v. administration of liposomal and injectable formulationsJ Pharm Biomed Anal200949498999619232851
- WangSLZhangJJiangTYProtective effect of coenzyme Q10 against oxidative damage in human lens epithelial cells by novel ocular drug carriersInt J Pharm20114031–221922920971176
- LeamonCPLowPSDelivery of macromolecules into living cells: A method that exploits folate receptor endocytosisProc Natl Acad Sci U S A19918813557255762062838
- LeeRJLowPSFolate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitroBiochim Biophys Acta1995123321341447865538
- LeeEKimHLeeIHJonSIn vivo antitumor effects of chitosan-conjugated docetaxel after oral administrationJ Control Rel200914027985
- LeeRJLowPSDelivery of liposomes into cultured KB cells via folate receptor-mediated endocytosisJ Biol Chem19942695319832048106354
- WongJYKuhlTLIsraelachviliJNMullahNZalipskySDirect measurement of a tethered ligand-receptor interaction potentialScience199727553018208229012346
- ZhaoXBMuthusamyNByrdJCLeeRJCholesterol as a bilayer anchor for PEGylation and targeting ligand in folate-receptor-targeted liposomesJ Pharm Sci20079692424243517588260
- ChernCSChiuHCSynthesis and characterization of amphiphilic graft copolymers with poly(ethylene glycol) and cholesterol side chainsPolym Int2004534420429
- NishikawaTAkiyoshiKSunamotoJSupramolecular assembly between nanoparticles of hydrophobized polysaccharide and soluble protein complexation between the self-aggregate of choles-terolbearing pullulan and a-chymotrypsinMacromolecules1994272676547659
- PeracchiaMTDesmaëleDCouvreurPd’AngeloJSynthesis of a novel poly(MePEG cyanoacrylate-co-alkyl cyanoacrylate) amphiphilic copolymer for nanoparticle technologyMacromolecules1997304846851
- StellaBArpiccoSPeracchiaMTDesign of folic acid-conjugated nanoparticles for drug targetingJ Pharm Sci200089111452146411015690
- YooHSParkTGFolate receptor targeted biodegradable polymeric doxorubicin micellesJ Control Rel2004962273283
- GuoWLeeTSudimackJLeeRJReceptor-specific delivery of liposomes via folate-peg-cholJ Liposome Res2000102–3179195
- ZhangMBLuYZLiXXStudying the cytotoxicity and oxidative stress induced by two kinds of bentonite particles on human B lympho-blast cells in vitroChem Biol Interact2010183339039619948159
- ZhangJWangSLTopical use of coenzyme Q10-loaded liposomes coated with trimethyl chitosan: Tolerance, precorneal retention and anti-cataract effectInt J Pharm20093721–2667519437594
- SadzukaYNakadeAHiramaREffects of mixed polyethyleneglycol modification on fixed aqueous layer thickness and antitumor activity of doxorubicin containing liposomeInt J Pharm20022381–217118011996821
- LiXWangDKZhangJPanWSPreparation and pharmacokinetics of docetaxel based on nanostructured lipid carriersJ Pharm Pharmacol200961111485149219903373
- LiXHeXLZhaoCHWangDKChenYWangYHDetermination of the content and entrapment efficiency of docetaxel in nano-structured lipid carrierChina Pharm2008192217431745
- YooHSParkTGBiodegradable polymeric micelles composed of doxorubicin conjugated PLGA-PEG block copolymerJ Control Rel2001701–26370
- PanJFengSSTargeted delivery of paclitaxel using folate-decorated poly(lactide)-vitamin E TPGS nanoparticlesBiomaterials200829172663267218396333
- MiYLiuYTFengSSFormulation of docetaxel by folic acid-conjugated d-α-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS2k) micelles for targeted and synergistic chemotherapyBiomaterials201132164058406621396707
- LenaertsVCouvreurPChristiaens-LeyhDDegradation of poly (isobutyl cyanoacrylate) nanoparticlesBiomaterials19845265686722249
- VansnickLCouvreurPChristiaens-LeyhDRolandMMolecular weights of free and drug-loaded nanoparticlesPharm Res1985213641
- MukherjeeBPatraBLayekBMukherjeeASustained release of acyclovir from nano-liposomes and nano-niosomes: An in vitro studyInt J Nanomed200722213225
- NeedhamDStoichevaNZhelevDVExchange of monooleoylphos-phatidylcholine as monomer and micelle with membranes containing poly(ethylene glycol)-lipidBiophys J1997735261526299370456
- SulkowskiWWPentakDNowakKSulkowskaAThe influence of temperature, cholesterol content and pH on liposome stabilityJ Mol Struct2005744–747737747
- WangSLowPSFolate-mediated targeting of antineoplastic drugs, imaging agents, and nucleic acids to cancer cellsJ Control Rel1998531–33948
- FabbriFBrigliadoriGCarloniSDocetaxel-ST1481 sequence exerts a potent cytotoxic activity on hormone-resistant prostate cancer cells by reducing drug resistance-related gene expressionProstate201070221922719790230
- GrefRLückMQuellecP“Stealth” corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorptionColloids Surf B Biointerfaces2000183–430131310915952
- WuMExpression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervixCancer Epidemiol Biomarkers Prev19998977578210498396
- MoghimiSMHunterACMurrayJCLong-circulating and target-specific nanoparticles: Theory to practicePharmacol Rev200153228331811356986
- ManjunathKVenkateswarluVPharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administrationJ Control Rel20051072215228
- JiaLJZhangDRLiZYNanostructured lipid carriers for parenteral delivery of silybin: Biodistribution and pharmacokinetic studiesColloids Surf B Biointerfaces201080221321820621458
- KennedyMDJalladKNLuJLowPSBen-AmotzDEvaluation of folate conjugate uptake and transport by the choroid plexus of micePharm Res200320571471912751625